Long-Term Performance of Nitrogen Removal and Microbial Analysis in an Anammox MBBR Reactor with Internal Circulation to Provide Low Concentration DO
Abstract
:1. Introduction
2. Materials and Methods
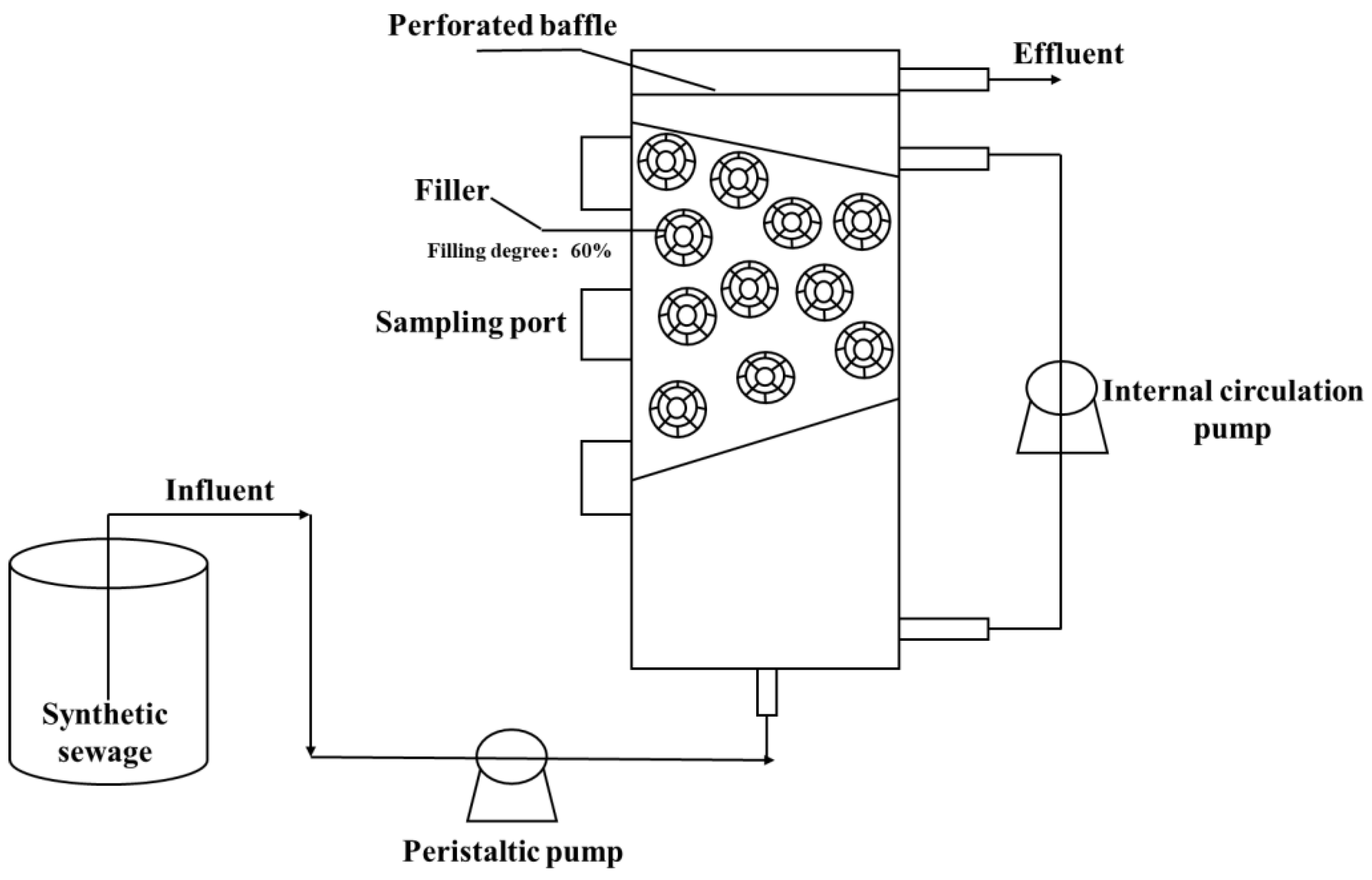
2.1. Reactor Design
2.2. Operation of the Reactor
2.3. Synthetic Sewage, Filler, and Sludge
2.3.1. Contents of Synthetic Sewage
2.3.2. Filler
2.3.3. Sludge
2.4. Chemical Analysis
2.5. Microbial Analysis
2.5.1. DNA Extract
2.5.2. Real-Time Quantification PCR (qPCR)
2.5.3. High-Throughput Sequencing
2.5.4. Statistical Analysis
3. Results
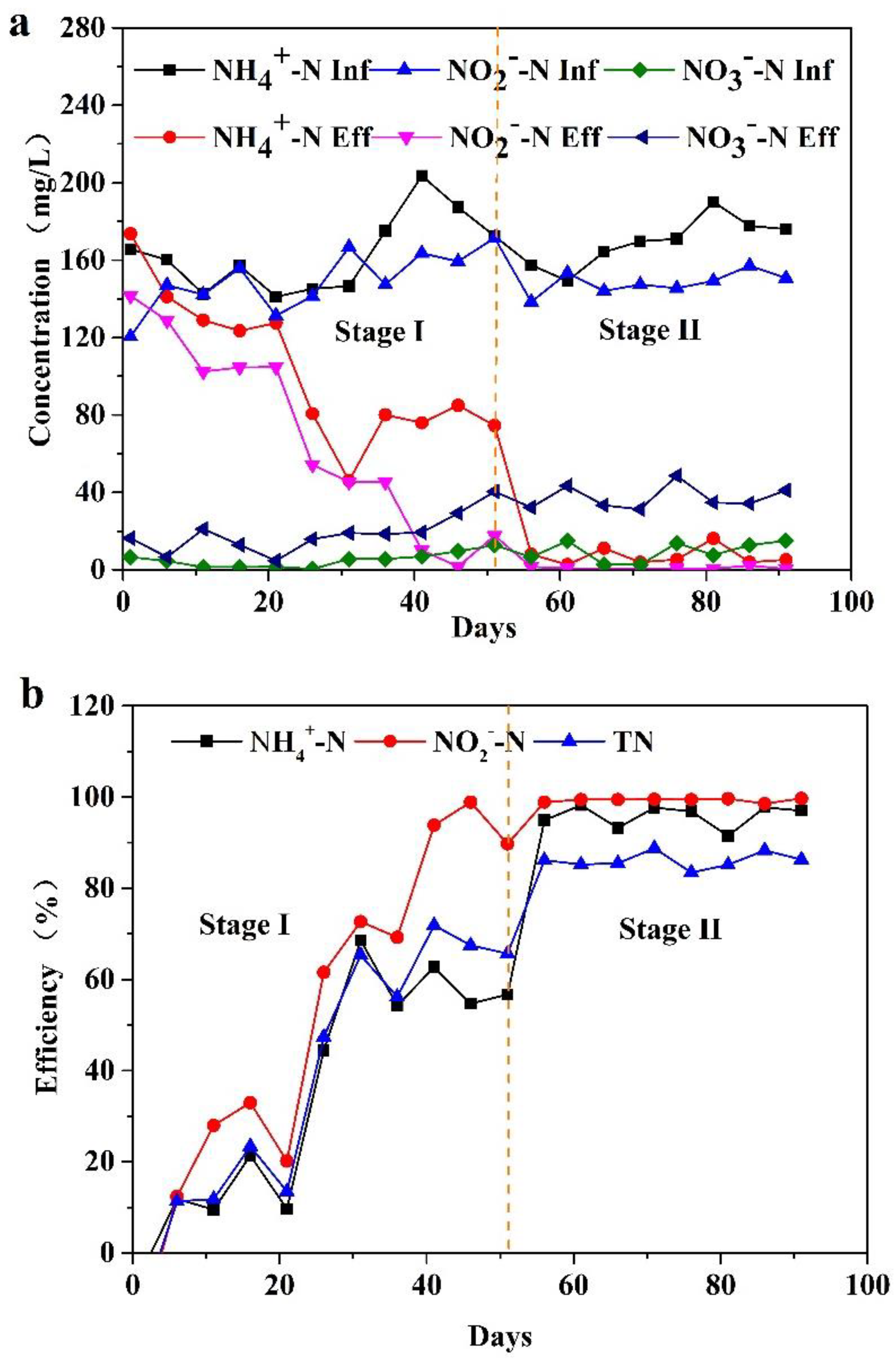
3.1. Nitrogen Removal Performance
3.2. Start up of the PN/A Process
3.3. Abundances of Nitrogen-Metabolism-Related Functional Genes in the MBBR
3.4. Microbial Composition in the Reactor
3.4.1. Phylogenetic Tree
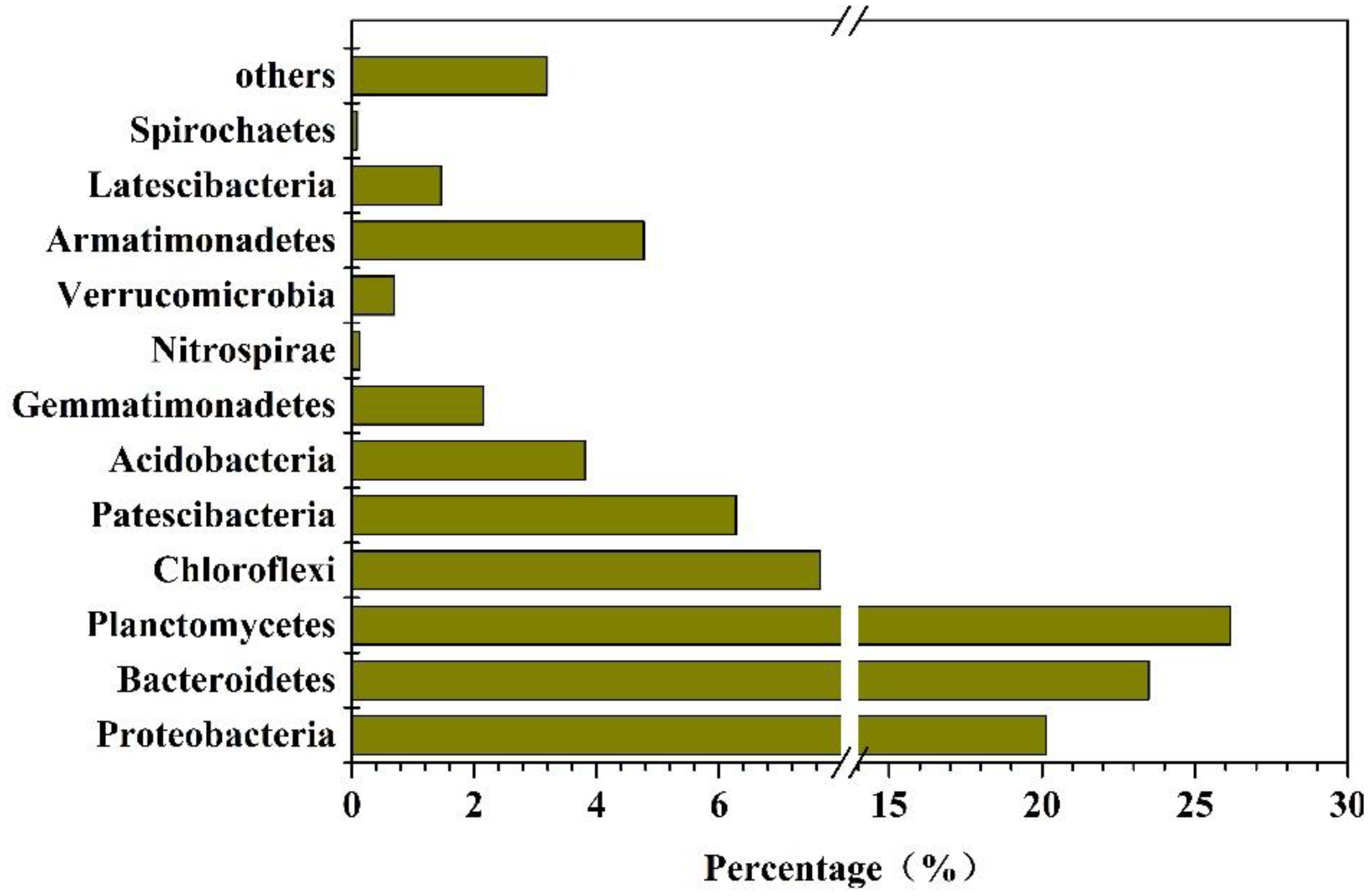
3.4.2. Abundances of Bacteria in the Reactor
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jetten, M.S.; Strous, M.; van de Pas-Schoonen, K.T.; Schalk, J.; van Dongen UG, J.M.; van de Graaf, A.A.; Logemann, S.; Muyzer, G.; van Loosdrecht, M.C.M.; Kuenen, J.G. The anaerobic oxidation of ammonium. FEMS Microbiol. Rev. 1998, 22, 421–437. [Google Scholar] [CrossRef] [PubMed]
- Sri, S.S.; Joseph, K. Nitrogen management in landfill leachate: Application of SHARON, ANAMMOX and combined SHARON-ANAMMOX process. Waste Manag. 2012, 32, 2385–2400. [Google Scholar] [CrossRef]
- Akgul, D.; Aktan, C.K.; Yapsakli, K.; Mertoglu, B. Treatment of landfill leachate using UASB-MBR-SHARON–Anammox configuration. Biodegradation 2013, 24, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zuo, J.; Yang, Y.; Zhu, S.; Kuang, S.; Wang, K. An autotrophic nitrogen removal process: Short-cut nitrification combined with ANAMMOX for treating diluted effluent from an UASB reactor fed by landfill leachate. J Environ. Sci. 2010, 22, 777–783. [Google Scholar] [CrossRef]
- Liang, Y.; Li, D.; Zhang, X.; Zeng, H.; Yang, Z.; Zhang, J. Microbial characteristics and nitrogen removal of simultaneous partial nitrification, anammox and denitrification (SNAD) process treating low C/N ratio sewage. Bioresour. Technol. 2014, 169, 103–109. [Google Scholar] [CrossRef]
- Ibrahim, M.; Yusof, N.; Mohd Yusoff, M.Z.; Hassan, M.A. Enrichment of anaerobic ammonium oxidation (anammox) bacteria for short start-up of the anammox process: A review. Desalination Water Treat. 2016, 57, 13958–13978. [Google Scholar] [CrossRef] [Green Version]
- Isaka, K.; Kimura, Y.; Yamamoto, T.; Osaka, T.; Tsuneda, S. Complete autotrophic denitrification in a single reactor using nitritation and anammox gel carriers. Bioresour. Technol. 2013, 147, 96–101. [Google Scholar] [CrossRef]
- Miao, Y.; Peng, Y.; Liang, Z.; Li, B.; Li, X.; Lei, W.; Wang, S. Partial nitrification-anammox (PNA) treating sewage with intermittent aeration mode: Effect of influent C/N ratios. Chem. Eng. J. 2018, 334, 664–672. [Google Scholar] [CrossRef]
- Ma, B.; Bao, P.; Wei, Y.; Zhu, G.; Yuan, Z.; Peng, Y. Suppressing Nitrite-oxidizing Bacteria Growth to Achieve Nitrogen Removal from Domestic Wastewater via Anammox Using Intermittent Aeration with Low Dissolved Oxygen. Sci. Rep. 2015, 5, 13048. [Google Scholar] [CrossRef] [Green Version]
- Kowalski, M.S.; Devlin, T.R.; Oleszkiewicz, J.A. Start-up and long-term performance of anammox moving bed biofilm reactor seeded with granular biomass. Chemosphere 2018, 200, 481–486. [Google Scholar] [CrossRef]
- Van de Graaf, A.A.; Mulder, A.; de Bruijn, P.E.; Jetten, M.S.; Robertson, L.A.; Kuenen, J.G. Anaerobic oxidation of ammonium is a biologically mediated process. Appl. Environ. Microbiol. 1995, 61, 1246–1251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, X.; Zhai, J.; Hu, W.; Li, Y.; Rahaman, M.H.; Mąkinia, J. A fast start-up of the organotrophic anammox process inoculated with constructed wetland sediment. Ecol. Eng. 2019, 138, 454–460. [Google Scholar] [CrossRef]
- Yin, X.; Rahaman, M.H.; Liu, W.; Mąkinia, J.; Zhai, J. Comparison of nitrogen and VFA removal pathways in autotrophic and organotrophic anammox reactors. Environ. Res. 2021, 197, 111065. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Xu, X.; Wei, Y.; Ge, C.; Peng, Y. Recent advances in controlling denitritation for achieving denitratation/anammox in mainstream wastewater treatment plants. Bioresour. Technol. 2020, 299, 122697. [Google Scholar] [CrossRef]
- Li, J.; Zhang, L.; Peng, Y.; Yang, S.; Wang, X.; Li, X.; Zhang, Q. NOB suppression in partial nitritation-anammox (PNA) process by discharging aged flocs: Performance and microbial community dynamics. Chemosphere 2019, 227, 26–33. [Google Scholar] [CrossRef]
- Wang, X.; Gao, D. In-situ restoration of one-stage partial nitritation-anammox process deteriorated by nitrate build-up via elevated substrate levels. Sci. Rep. 2016, 6, 37500. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; van Loosdrecht MC, M.; Daigger, G.T. Mainstream partial nitritation–anammox in municipal wastewater treatment: Status, bottlenecks, and further studies. Appl. Microbiol. Biotechnol. 2017, 101, 1365–1383. [Google Scholar] [CrossRef]
- Lotti, T.; Kleerebezem, R.; Hu, Z.; Kartal, B.; Jetten, M.; van Loosdrecht, M. Simultaneous partial nitritation and anammox at low temperature with granular sludge. Water Res. 2014, 66, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, E.M.; Agrawal, S.; Karst, S.M.; Horn, H.; Nielsen, P.H.; Lackner, S. Low Temperature Partial Nitritation/Anammox in a Moving Bed Biofilm Reactor Treating Low Strength Wastewater. Environ. Sci. Technol. 2014, 48, 8784–8792. [Google Scholar] [CrossRef]
- Gilbert, E.M.; Agrawal, S.; Brunner, F.; Schwartz, T.; Horn, H.; Lackner, S. Response of Different Nitrospira Species To Anoxic Periods Depends on Operational DO. Environ. Sci. Technol. 2014, 48, 2934–2941. [Google Scholar] [CrossRef]
- Daims, H.; Purkhold, U.; Bjerrum, L.; Arnold, E.; Wilderer, P.A.; Wagner, M. Nitrification in sequencing biofilm batch reactors: Lessons from molecular approaches. Water Sci. Technol. 2001, 43, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Liu, S.; Liu, Q.; Zhang, M.; Liu, Y.; Wen, Y.; Chen, Z.; Zhang, Y.; Yang, Q. Improved performance of simultaneous nitrification and denitrification via nitrite in an oxygen-limited SBR by alternating the DO. Bioresour. Technol. 2019, 275, 153–162. [Google Scholar] [CrossRef] [PubMed]



| Content | Concentration |
|---|---|
| NaHCO3 | 500.0 mg/L |
| KH2PO4 | 27.2 mg/L |
| MgSO4·7H2O | 300.0 mg/L |
| CaC12·2H2O | 180.0 mg/L |
| Trace element I | 1 mL/L |
| Trace element II | 1 mL/L |
| Genera | 90 d (%) | Genera | 90 d (%) |
|---|---|---|---|
| DNB | DNB | ||
| Rhodanobacteraceae | 0.77 | Devosia | 0.01 |
| Thiobacillus | 0.63 | Denitratisoma | 3.76 |
| Thermomonas | 0.98 | Defluviimonas | 0.03 |
| Thauera | 0.03 | Dechloromonas | 0.03 |
| Terrimonas | 1.97 | Arenimonas | 0.35 |
| Roseomonas | 0.009 | Bdellovibrio | 0.38 |
| Pseudomonas | 0.009 | Total DNB | 9.60 |
| Pedobacter | 0.38 | NOB | |
| Opitutus | 0.03 | Nitrospira | 0.11 |
| Nakamurella | 0.01 | AOB | |
| Mesorhizobium | 0.005 | Nitrosomonas | 0.63 |
| Haliangium | 0.17 | AnAOB | |
| Dokdonella | 0.05 | Candidatus Brocadia | 23.23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, X.; Wen, J.; Zhang, Y.; Zhang, X.; Zhao, J. Long-Term Performance of Nitrogen Removal and Microbial Analysis in an Anammox MBBR Reactor with Internal Circulation to Provide Low Concentration DO. Toxics 2022, 10, 640. https://doi.org/10.3390/toxics10110640
Yin X, Wen J, Zhang Y, Zhang X, Zhao J. Long-Term Performance of Nitrogen Removal and Microbial Analysis in an Anammox MBBR Reactor with Internal Circulation to Provide Low Concentration DO. Toxics. 2022; 10(11):640. https://doi.org/10.3390/toxics10110640
Chicago/Turabian StyleYin, Xuejiao, Jiaxin Wen, Yihang Zhang, Xin Zhang, and Jujiao Zhao. 2022. "Long-Term Performance of Nitrogen Removal and Microbial Analysis in an Anammox MBBR Reactor with Internal Circulation to Provide Low Concentration DO" Toxics 10, no. 11: 640. https://doi.org/10.3390/toxics10110640





