Evaluation of Toxic Effects Induced by Sub-Acute Exposure to Low Doses of α-Cypermethrin in Adult Male Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Compounds
2.2. Animals and Experimental Design
2.3. Collection of Blood and Tissues
2.4. Biochemical Analysis
2.5. DNA Damage Detected by the Alkaline Comet Assay
2.6. Statistical Analysis
3. Results
3.1. Body and Organ Weight
3.2. Biochemical Markers of Toxicity
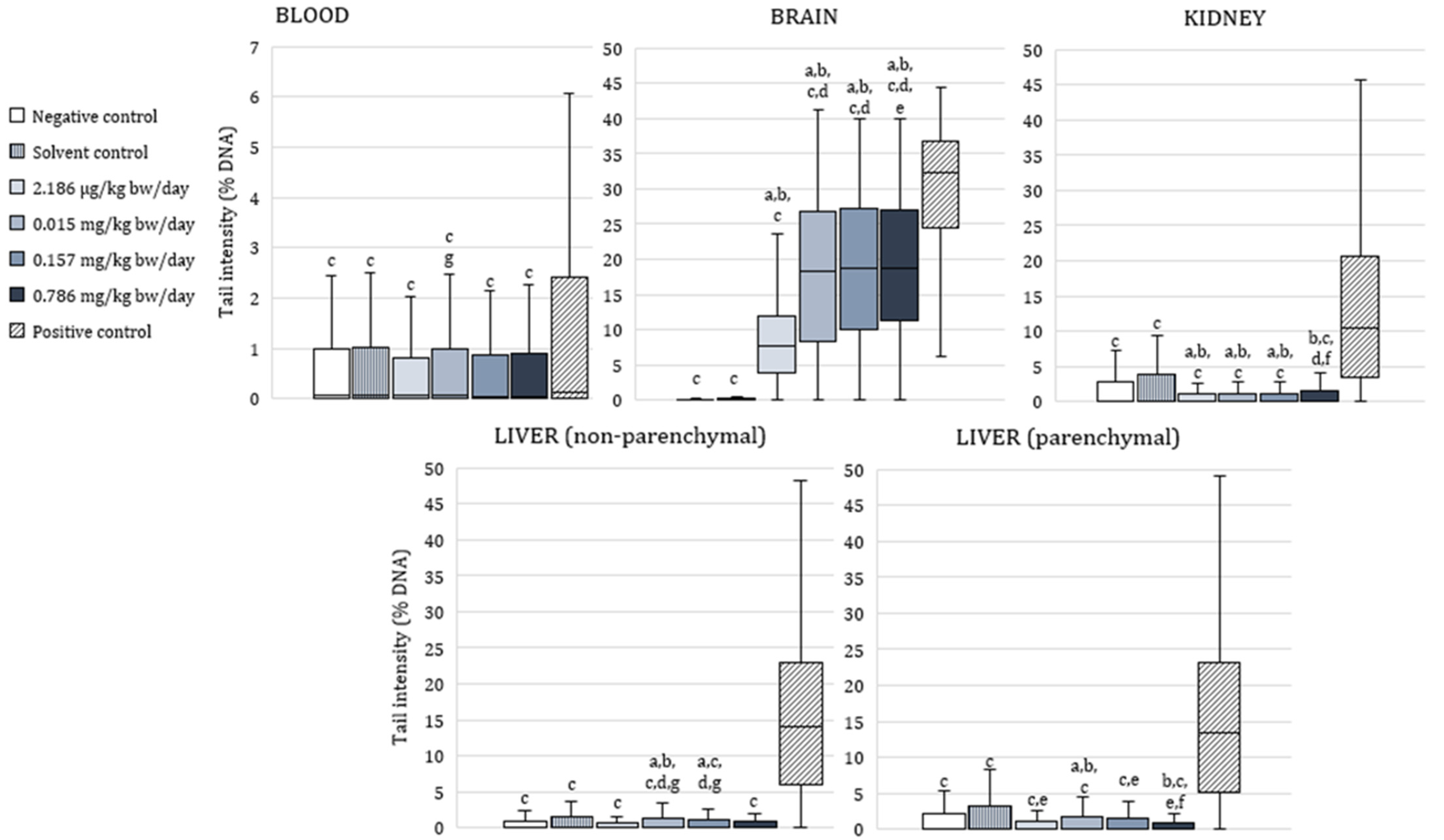
3.3. The Alkaline Comet Assay
4. Discussion
4.1. Effect on Body and Organ Weight
4.2. Cholinesterase Activity
4.3. Oxidative Stress Response
4.4. Genotoxic Effects of Exposure to α-Cypermethrin
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Soderlund, D.M. Toxicology and Mode of Action of Pyrethroid Insecticides. In Hayes’ Handbook of Pesticide Toxicology, 3rd ed.; Krieger, R., Ed.; Academic Press: Cambridge, MA, USA, 2010; pp. 1665–1686. [Google Scholar]
- Rezende-Teixeira, P.; Dusi, R.G.; Jimenez, P.C.; Espindola, L.S.; Costa-Lotufo, L.V. What can we learn from commercial insecticides? Efficacy, toxicity, environmental impacts, and future developments. Environ. Poll. 2022, 300, 118983. [Google Scholar] [CrossRef] [PubMed]
- Sedaghati, E.; Hokmabadi, H.E. Safety of Food and Beverages: Oilseeds and Legumes. In Encyclopedia of Food Safety; Yasmine Motarjemi, Y., Ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 331–339. [Google Scholar]
- Chen, L.; Wang, D.; Zhou, Z.; Diao, J. Comparing alpha-cypermethrin induced dose/gender-dependent responses of lizards in hepatotoxicity and nephrotoxicity in a food chain. Chemosphere 2020, 256, 127069. [Google Scholar] [CrossRef]
- Saillenfait, A.-M.; Ndiaye, D.; Sabaté, J.-P. Pyrethroids: Exposure and health effects—An update. Int. J. Hyg. Environ. Health 2015, 218, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Environmental Protection Agency (EPA). Cypermethrins—Proposed Interim Registration Review Decision. 2020. Docket Number EPA-HQ-OPP-2012-0167. Available online: https://www.regulations.gov/docket/EPA-HQ-OPP-2012-0167/document (accessed on 29 September 2022).
- Clark, J.M.; Brooks, M.W. Role of ion channels and intraterminal calcium homeostasis in the action of deltamethrin at presynaptic nerve terminals. Biochem. Pharmacol. 1989, 38, 2233–2245. [Google Scholar] [CrossRef] [PubMed]
- Vijverberg, H.P.; van den Bercken, J. Neurotoxicological effects and the mode of action of pyrethroid insecticides. Crit. Rev. Toxicol. 1990, 21, 105–126. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, H.; Ghassemi-Barghi, N.; Malakshah, O.; Ashari, S. Pyrethroid exposure and neurotoxicity: A mechanistic approach. Arch. Ind. Hyg. Toxicol. 2019, 70, 74–89. [Google Scholar] [CrossRef] [Green Version]
- Ravula, A.R.; Yenugu, S. Pyrethroid based pesticides—Chemical and biological aspects. Crit. Rev. Toxicol. 2021, 51, 117–140. [Google Scholar] [CrossRef]
- Soderlund, D.M. Molecular mechanisms of pyrethroid insecticide neurotoxicity: Recent advances. Arch. Toxicol. 2012, 86, 165–181. [Google Scholar] [CrossRef] [Green Version]
- Costa, L.G. The neurotoxicity of organochlorine and pyrethroid pesticides. Handb. Clin. Neurol. 2015, 131, 135–148. [Google Scholar]
- Rodríguez, J.-L.; Ares, I.; Martínez, M.; Martínez-Larrañaga, M.-R.; Anadón, A.; Martínez, M.-A. Bioavailability and nervous tissue distribution of pyrethroid insecticide cyfluthrin in rats. Food Chem. Toxicol. 2018, 118, 220–226. [Google Scholar] [CrossRef]
- Yuan, Y.; Chen, C.; Zheng, C.; Wang, X.; Yang, G.; Wang, Q.; Zhang, Z. Residue of chlorpyrifos and cypermethrin in vegetables and probabilistic exposure assessment for consumers in Zhejiang Province, China. Food Control 2014, 36, 63–68. [Google Scholar] [CrossRef]
- Singh, A.K.; Tiwari, M.N.; Prakash, O.; Pratap Singh, M. A current review of cypermethrin-induced neurotoxicity and nigrostriatal dopaminergic neurodegeneration. Curr. Neuropharmacol. 2012, 10, 64–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sankar, P.; Telang, A.G.; Manimaran, A. Protective effect of curcumin on cypermethrin-induced oxidative stress in Wistar rats. Exp. Toxicol. Pathol. 2012, 64, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Ul Huq, A.; Singh, R. Cypermethrin induced reproductive toxicity in male Wistar rats: Protective role of Tribulus terrestris. J. Environ. Biol. 2013, 34, 857–862. [Google Scholar]
- Zhang, Y.; Kong, C.; Chi, H.; Li, J.; Xing, J.; Wang, F.; Shao, L.; Zhai, Q. Effect of a beta-cypermethrin and emamectin benzoate pesticide mixture on reproductive toxicity in male mice in a greenhouse environment. Toxicol. Mech. Methods 2020, 30, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Abdou, R.H.; Sayed, N. Antioxidant and anti-inflammatory effects of nano-selenium against cypermethrin-induced liver toxicity. CellBio 2019, 8, 53–65. [Google Scholar] [CrossRef] [Green Version]
- Ileriturk, M.; Kandemir, O.; Kandemir, F.M. Evaluation of protective effects of quercetin against cypermethrin-induced lung toxicity in rats via oxidative stress, inflammation, apoptosis, autophagy, and endoplasmic reticulum stress pathway. Environ. Toxicol. 2022, 37, 2639–2650. [Google Scholar] [CrossRef]
- Barón Cuenca, J.; de Oliveira Galvăo, M.F.; Endirlik, B.Ü.; Tirado, N.; Dreij, K. In vitro cytotoxicity and genotoxicity of single and combined pesticides used by Bolivian farmers. Environ. Mol. Mutagen. 2022, 63, 4–17. [Google Scholar] [CrossRef]
- Abdou, H.M.; Hussien, H.M.; Yousef, M.I. Deleterious effects of cypermethrin on rat liver and kidney: Protective role of sesame oil. J. Environ. Sci. Health B 2012, 47, 306–314. [Google Scholar] [CrossRef]
- Kanbur, M.; Siliğ, Y.; Eraslan, G.; Karabacak, M.; Soyer Sarica, Z.; Şahin, S. The toxic effect of cypermethrin, amitraz and combinations of cypermethrin-amitraz in rats. Environ. Sci. Pollut. Res. 2016, 23, 5232–5242. [Google Scholar] [CrossRef]
- Eraslan, G.; Kanbur, M.; Siliğ, Y.; Karabacak, M.; Soyer Sarica, Z.; Şahin, S. The acute and chronic toxic effect of cypermethrin, propetamphos, and their combinations in rats. Environ. Toxicol. 2016, 31, 1415–1429. [Google Scholar] [CrossRef]
- El-Demerdash, F.M. Lipid peroxidation, oxidative stress and acetylcholinesterase in rat brain exposed to organophosphate and pyrethroid insecticides. Food Chem. Toxicol. 2011, 49, 1346–1352. [Google Scholar] [CrossRef]
- Afolabi, O.K.; Aderibigbe, F.A.; Folarin, D.T.; Arinola, A.; Wusu, A.D. Oxidative stress and inflammation following sub-lethal oral exposure of cypermethrin in rats: Mitigating potential of epicatechin. Heliyon 2019, 5, e02274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saxena, P.N.; Chauhan, L.K.S.; Gupta, S.K. Cytogenetic effects of formulation of cypermethrin in root meristem cells of Allium sativum: Spectroscopic basis of chromosome damage. Toxicology 2005, 216, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Seven, B.; Çavuşoğlu, K.; Yalçin, E.; Acar, A. Investigation of cypermethrin toxicity in Swiss albino mice with physiological, genetic and biochemical approaches. Sci. Rep. 2022, 12, 11439. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, K.; Abdelrahman, S.; Elhakim, H.; Ragab, E. Single or combined exposure to chlorpyrifos and cypermethrin provoke oxidative stress and downregulation in monoamine oxidase and acetylcholinesterase gene expression of the rat’s brain. Environ. Sci. Pollut. Res. 2020, 27, 12692–12703. [Google Scholar] [CrossRef]
- Rajasekaran, G.; Sasipriya, M.; Kumarasamy, P.; Nathiya, N.; Muthukumaravel, K. Effect of cypermethrin on brain acetylcholinesterase activity in the indian major carp labeo rohita. Int. J. Rec. Sci. Res. 2015, 6, 4620–4622. [Google Scholar]
- Sharma, A.; Yadav, B.; Rohatgi, S.; Yadav, B. Cypermethrin toxicity: A review. J. Forensic. Sci. Crim. Investig. 2018, 9, 555767. [Google Scholar]
- Wielgomas, B.; Krechniak, J. Effect of α-cypermethrin and chlorpyrifos in a 28-day study on free radical parameters and cholinesterase activity in Wistar rats. Polish. J. Environ. Stud. 2007, 16, 91–95. [Google Scholar]
- Dave, K.R.; Syal, A.R.; Katyare, S.S. Tissue cholinesterases. A comparative study of their kinetic properties. Z. Naturforsch. C J. Biosci. 2000, 55, 100–108. [Google Scholar] [CrossRef]
- Massoulié, J.; Pezzementi, L.; Bon, S.; Krejci, E.; Vallette, F.-M. Molecular and cellular biology of cholinesterases. Prog. Neurobiol. 1993, 41, 1–91. [Google Scholar]
- Fulton, M.H.; Key, P.B. Acetylcholinesterase inhibition in estuarine fish and invertebrates as an indicator of organophosphorus insecticide exposure and effects. Environ. Toxicol. Chem. 2001, 20, 37–45. [Google Scholar] [CrossRef]
- Masson, P.; Lockridge, O. Butyrylcholinesterase for protection from organophosphorus poisons: Catalytic complexities and hysteretic behaviour. Arch. Biochem. Biophys. 2010, 494, 107–120. [Google Scholar] [CrossRef] [Green Version]
- EFSA (European Food Safety Authority). Conclusion on the peer review of the pesticide risk assessment of the active substance alpha-cypermethrin. EFSA J. 2018, 16, 5403. [Google Scholar]
- EFSA (European Food Safety Authority). Conclusion on the peer review of the pesticide risk assessment of the active substance beta-cypermethrin. EFSA J. 2014, 12, 3717. [Google Scholar]
- Cayir, A.; Coskun, M.; Coskun, M.; Cobanoglu, H. Comet assay for assessment of DNA damage in greenhouse workers exposed to pesticides. Biomarkers 2019, 24, 592–599. [Google Scholar] [CrossRef]
- National Research Council. Guide for the Care and Use of Laboratory Animals, 8th ed.; The National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Pant, K.; Springer, S.; Bruce, S.; Lawlor, T.; Hewitt, N.; Aardema, M. Vehicle and positive control values from the in vivo rodent comet assay and biomonitoring studies using human lymphocytes: Historical database and influence of technical aspects. Environ. Mol. Mutagen. 2014, 55, 633–642. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority; Modification of the existing MRLs for cypermethrin in various crops. EFSA J. 2011, 9, 2280. [CrossRef]
- Flohé, L.; Ötting, F. Superoxide dismutase assays. Methods Enzymol. 1971, 105, 93–104. [Google Scholar]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Belsten, J.L.; Wright, A.J. European Community: FLAIR common assay for whole-blood glutathione peroxidase (GSH-Px); results of an inter-laboratory trial. Eur. J. Clin. Nutr. 1995, 49, 921–927. [Google Scholar] [PubMed]
- Drury, J.A.; Nycyk, J.A.; Cooke, R.W.I. Comparison of urinary and plasma malondialdehyde in preterm infants. Clin. Chim. Acta 1997, 263, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L. A colorimetric method for determining low concentrations of mercaptans. Arch. Biochem. Biophys. 1958, 74, 443–450. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Singh, N.P.; McCoy, M.T.; Tice, R.R.; Schneider, E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 1988, 175, 184–191. [Google Scholar] [CrossRef] [Green Version]
- Recio, L.; Hobbs, C.; Caspary, W.; Witt, K.L. Dose-response assessment of four genotoxic chemicals in a combined mouse and rat micronucleus (MN) and Comet assay protocol. J. Toxicol. Sci. 2010, 35, 149–162. [Google Scholar] [CrossRef] [Green Version]
- Domijan, A.-M.; Želježić, D.; Kopjar, N.; Peraica, M. Standard and Fpg-modified comet assay in kidney cells of ochratoxin A- and fumonisin B1- treated rats. Toxicology 2006, 222, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Trošić, I.; Pavičić, I.; Milković-Kraus, S.; Mladinić, M.; Želježić, D. Effect of electromagnetic radiofrequency radiation on the rats’ brain, liver and kidney cells measured by comet assay. Coll. Antropol. 2011, 35, 1259–1264. [Google Scholar]
- Asahi, J.; Kamo, H.; Baba, R.; Doi, Y.; Yamashita, A.; Murakami, D.; Hanada, A.; Hirano, T. Bisphenol A induces endoplasmic reticulum stress-associated apoptosis in mouse non-parenchymal hepatocytes. Life Sci. 2010, 87, 431–438. [Google Scholar] [CrossRef]
- McNamee, J.P.; Bellier, P.V. Use of a standardized JaCVAM in vivo rat comet assay protocol to assess the genotoxicity of three coded test compounds; ampicillin trihydrate, 1,2-dimethylhydrazine dihydrochloride, and N-nitro-sodimethylamine. Mutat. Res. Toxicol. Environ. Mutagen. 2015, 786, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, Y.; Costa, S.; Collins, A.R.; Azqueta, A. The comet assay, DNA damage, DNA repair and cytotoxicity: Hedgehogs are not always dead. Mutagenesis 2013, 28, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.C.; Adkins, D.J.; Martin, E.A.; O’Donovan, M.R. Recommendations for design of the rat comet assay. Mutagenesis 2008, 23, 233–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michael, B.; Yano, B.; Sellers, R.S.; Perry, R.; Morton, D.; Roome, N.; Johnson, J.K.; Schafer, K. Evaluation of organ weights for rodent and non-rodent toxicity studies: A review of regulatory guidelines and a survey of current practices. Toxicol. Pathol. 2007, 35, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Elbetieha, A.; Da’as, S.I.; Khamas, W.; Darmani, H. Evaluation of the toxic potentials of cypermethrin pesticide on some reproductive and fertility parameters in the male rats. Arch. Environ. Contam. Toxicol. 2001, 41, 522–528. [Google Scholar] [CrossRef]
- Hu, J.; Li, Y.; Li, J.; Pan, C.; He, Z.; Dong, H.; Xu, L. Toxic effects of cypermethrin on the male reproductive system: With emphasis on the androgen receptor. J. Appl. Toxicol. 2013, 33, 576–585. [Google Scholar] [CrossRef]
- Mansour, S.A.; Mohamed, R.I.; Ali, A.R.; Farrag, A.H. The protective effect of Moringa tea against cypermethrin-induced hepatorenal dysfunction, oxidative stress, and histopathological alterations in female rats. Asian J. Pharm. Clin. Res. 2018, 11, 111–117. [Google Scholar] [CrossRef]
- Kalra, S.; Sangha, G.K. Free radical mediated neurotoxicity induced by cypermethrin in rats. J. Entomol. Zool. Stud. 2018, 6, 1087–1092. [Google Scholar]
- Sharma, P.; Firdous, S.; Singh, R. Neurotoxic effect of cypermethrin and protective role of resveratrol in Wistar rats. Int. J. Nutr. Pharmacol. Neurol. Dis. 2014, 4, 104–111. [Google Scholar]
- García-Ayllón, S.; Silveyra, X.; Candela, A.; Compañ, A.; Clària, J.; Jover, R.; Pérez-Mateo, M.; Felipo, V.; Martínez, S.; Galcerán, J.; et al. Changes in liver and plasma acetylcholinesterase in rats with cirrhosis induced by bile duct ligation. Hepatology 2006, 43, 444–453. [Google Scholar] [CrossRef]
- Soreq, H.; Seidman, S. Acetylcholinesterase-new roles for an old actor. Nat. Rev. Neurosci. 2001, 2, 294–302. [Google Scholar] [CrossRef]
- Ahmed, O.; Mastan, S.A.; Rabia Banu, S.; Indira, P. Sub-lethal effect of cypermethrin on acetylcholinesterase (AChE) activity and acetylcholine (Ach) content in selected tissues of Channa striatus (Bloch.). J. Toxicol. Environ. Health Sci. 2015, 7, 31–37. [Google Scholar]
- Zaheer, K.; Ghazala, Y. Effect of Sandaphos and β-cypermethrin exposure on cholinesterase and alkaline phosphatase activity in liver, kidney and brain of Euphlyctis cyanophlyctis. Can. J. Pure Appl. Sci. 2008, 2, 511–519. [Google Scholar]
- Rao, G.V.; Jagannatha Rao, K.S. Modulation in acetylcholinesterase of rat brain by pyrethroids in vivo and an in vitro kinetic study. J. Neurochem. 1995, 65, 2259–2266. [Google Scholar] [CrossRef]
- Pezzementi, L.; Chatonnet, A. Evolution of cholinesterases in the animal kingdom. Chem. Biol. Interact 2010, 187, 27–33. [Google Scholar] [CrossRef]
- Agarwal, A.; Sharma, B. Pesticides induced oxidative stress in mammalian systems: A review. Int. J. Biol. Med. Res. 2010, 1, 90–104. [Google Scholar]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell B 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Ghani, M.A.; Barril, C.; Bedgood, D.R.; Prenzler, P.D. Measurement of antioxidant activity with the thiobarbituric acid reactive substances assay. Food Chem. 2017, 230, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Sochor, J.; Ruttkay-Nedecky, B.; Babula, P.; Vojtech, A.; Hubalek, J.; Kizek, R. Automation of methods for determination of lipid peroxidation. In Lipid peroxidation; Catala, A., Ed.; InTech: London, UK, 2012; pp. 131–154. [Google Scholar]
- Yang, H.Y.; Lee, T.H. Antioxidant enzymes as redox-based biomarkers: A brief review. BMB Rep. 2015, 48, 200–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soliman, M.; Attia, H.F.; El-Ella, G.A. Genetic and histopathological alterations induced by cypermethrin in rat kidney and liver: Protection by sesame oil. Int. J. Immunopathol. Pharmacol. 2015, 28, 508–520. [Google Scholar] [CrossRef] [PubMed]
- Hammad, M.A.; Ziada, R.M. Toxicological effects and histopathological alterations of Diazinon and alpha Cypermethrin on male albino rats. Asian J. Res. Med. Pharm. Sci. 2019, 6, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Taha, M.A.I.; Badawy, M.E.I.; Abdel-Razik, R.K.; Younis, H.M.; Abo-El-Saad, M.M. Mitochondrial dysfunction and oxidative stress in liver of male albino rats after exposing to sub-chronic intoxication of chlorpyrifos, cypermethrin, and imidacloprid. Pestic. Biochem. Phys. 2021, 178, 104938. [Google Scholar] [CrossRef]
- Davico, C.E.; Loteste, A.; Parma, M.J.; Poletta, G.; Simoniello, M.F. Stress oxidative and genotoxicity in Prochilodus lineatus (Valenciennes, 1836) exposed to commercial formulation of insecticide cypermethrin. Drug Chem. Toxicol. 2018, 43, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Piancini, L.D.S.; Guiloski, I.C.; de Assis, H.C.S.; Cestari, M.M. Mesotrione herbicide promotes biochemical changes and DNA damage in two fish species. Toxicol. Rep. 2015, 2, 1157–1163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tice, R.R.; Agurell, E.; Anderson, D.; Burlinson, B.; Hartmann, A.; Kobayashi, H.; Miyamae, Y.; Rojas, E.; Ryu, J.; Sasaki, Y.F. Single cell gel/comet assay: Guidelines for in vitro and in vivo genetic toxicology testing. Environ. Mol. Mutagen. 2000, 35, 206–221. [Google Scholar] [CrossRef]
- Hartmann, A.; Speit, G. Genotoxic effects of chemicals in the single cell gel (SCG) test with human blood cells in relation to the induction of sister-chromatid exchanges (SCE). Mutat. Res. Lett. 1995, 346, 49–56. [Google Scholar] [CrossRef]
- Kopjar, N.; Žunec, S.; Mendaš, G.; Micek, V.; Kašuba, V.; Mikolić, A.; Tariba Lovaković, B.; Pavičić, I.; Marjanović Čermak, A.M.; Pizent, A.; et al. Evaluation of chlorpyrifos toxicity through a 28-day study: Cholinesterase activity, oxidative stress responses, parent compound/metabolite levels, and primary DNA damage in blood and brain tissue of adult male Wistar rats. Chem. Biol. Interact. 2018, 279, 51–63. [Google Scholar] [CrossRef]
- Tariba Lovaković, B.; Kašuba, V.; Katić, A.; Kopjar, N.; Marjanović Čermak, A.M.; Micek, V.; Milić, M.; Pavičić, I.; Pizent, A.; Žunec, S.; et al. Evaluation of oxidative stress responses and primary DNA damage in blood and brain of rats exposed to low levels of tembotrione. Chemosphere 2020, 253, 126643. [Google Scholar] [CrossRef] [PubMed]
- Žunec, S.; Kašuba, V.; Pavičić, I.; Marjanović, A.M.; Tariba, B.; Milić, M.; Kopjar, N.; Pizent, A.; Lucić Vrdoljak, A.; Rozgaj, R.; et al. Assessment of oxidative stress responses and the cytotoxic and genotoxic potential of the herbicide tembotrione in HepG2 cells. Food Chem. Toxicol. 2016, 94, 64–74. [Google Scholar] [CrossRef]
- Taha, M.A.I.; Badawy, M.E.I.; Abdel-Razik, R.K.; Younis, H.M.; Abo-El-Saad, M.M. Effects of sub-chronic exposure of male albino rats to chlorpyrifos, cypermethrin, and imidacloprid on mitochondrial dysfunction and oxidative stress in the kidney with molecular docking. J. Cell. Neurosci. Oxid Stress 2021, 13, 1014–1030. [Google Scholar]
- Muhammed, R.E.; El-Desouky, M.A.; Abo-Seda, S.B.; Nahas, A.A.; Elhakim, H.K.A.; Alkhalaf, M.I. The protecting role of Moringa oleifera in cypermethrin-induced mitochondrial dysfunction and apoptotic events in rats’ brain. J. King Saud. Univ. Sci. 2020, 32, 2717–2722. [Google Scholar] [CrossRef]
- Đikić, D.; Mojsović-Ćuić, A.; Čupor, I.; Benković, V.; Horvat-Knežević, A.; Lisičić, D.; Oršolić, N. Carbendazim combined with imazalil or cypermethrin potentiate DNA damage in hepatocytes of mice. Hum. Exp. Toxicol. 2012, 31, 492–505. [Google Scholar] [CrossRef]
- Patel, S.; Pandey, A.K.; Bajpayee, M.; Parmar, D.; Dhawan, A. Cypermethrin-induced DNA damage in organs and tissues of the mouse: Evidence from the comet assay. Mutat. Res. 2006, 607, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Guo, J.; Xu, B.; Chen, Z. Potential of chlorpyrifos and cypermethrin forming DNA adducts. Mutat. Res. 2006, 604, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Hussien, H.M.; Abdou, H.M.; Yousef, M.I. Cypermethrin induced damage in genomic DNA and histopathological changes in brain and haematotoxicity in rats: The protective effect of sesame oil. Brain Res. Bull. 2013, 92, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Do Nascimento Marinho, K.S.; Cavalcanti Lapa Neto, C.J.; Duarte de Sousa Coelho, I.D.; da Silva, M.A.; Gomes Melo, M.E.; Pereira dos Santos, K.R.; Chagas, K.A.; Coelho Teixeira, A.A.; Wanderley Teixeira, V. Evaluation of the protective effect on exogenous melatonin in adult rats and their offspring exposed to insecticides methomyl and cypermethrin during pregnancy. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2019, 848, 503107. [Google Scholar] [CrossRef]
- Nair, S.; Engelbrecht, M.; Miles, X.; Ndimba, R.; Fisher, R.; du Plessis, P.; Bolcaen, J.; Niento-Camero, J.; de Kock, E.; Vandervoorde, C. The impact of dose rate on DNA double-strand break formation and repair in human lymphocytes exposed to fast neutron irradiation. Int. J. Mol. Sci. 2019, 20, 5350. [Google Scholar] [CrossRef] [Green Version]
- Badding, M.A.; Barraj, L.; Williams, A.L.; Scrafford, C.; Reiss, R. CLARITY-BPA Core Study: Analysis for non-monotonic dose-responses and biological relevance. Food Chem. Toxicol. 2019, 131, 110554. [Google Scholar] [CrossRef]
- Liu, C.; Wu, M.; Qu, J.; Huang, X.; Zeng, Q.; Ha, M. JNK and Jag1/Notch2 co-regulate CXCL16 to facilitate cypermethrin-induced kidney damage. Ecotox Environ. Safe 2022, 238, 113582. [Google Scholar] [CrossRef]

| Parameters | Negative Control | Positive Control | Solvent Control | 2.186 µg/kg bw/Day | 0.015 mg/kg bw/Day | 0.157 mg/kg bw/Day | 0.786 mg/kg bw/Day |
|---|---|---|---|---|---|---|---|
| Initial weight/g | 333 ± 2 | 312 ± 3 | 307 ± 3 | 357 ± 8 | 337 ± 3 | 360 ± 6 | 342 ± 7 |
| Final weight/g | 409 ± 8 c | 341 ± 6 a,b,d | 388 ± 6 c,e | 392 ± 8 c,e | 346 ± 7 a,b,d | 369 ± 9 a | 366 ± 9 a |
| Weight change/g | 76.6 ± 9.5 c | 29.0 ± 5.8 | 80.4 ± 7.00 c,d,e,f,g | 35.6 ± 2.6 a,b,e,f | 9.40 ± 4.58 a,b,d | 8.80 ± 4.99 a,b,d | 24.0 ± 2.81 a,b |
| Liver weight/g | 12.4 ± 0.5 c,e,f | 9.24 ± 0.18 a,b,d | 11.41 ± 0.34 c,e,f | 11.70 ± 0.47 c,e,f | 8.39 ± 0.34 a,b,d,g | 8.68 ± 0.45 a,b,d,g | 10.96 ± 0.41 e,f |
| Relative liver weight | 3.03 ± 0.09 e,f | 2.71 ± 0.04 f | 2.94 ± 0.09 e,f | 2.98 ± 0.07 e,f | 2.42 ± 0.06 a,b,d,g | 2.35 ± 0.07 a,b,c,d,g | 2.99 ± 0.08 e,f |
| Kidney weight/g | 1.29 ± 0.05 a,e,f | 1.10 ± 0.03 a | 1.15 ± 0.03 | 1.22 ± 0.03 e | 1.01 ± 0.02 a,d | 1.06 ± 0.04 a | 1.16 ± 0.05 |
| Relative kidney weight | 0.31 ± 0.01 | 0.32 ± 0.01 | 0.30 ± 0.01 | 0.31 ± 0.01 | 0.29 ± 0.01 | 0.29 ± 0.01 | 0.31 ± 0.01 |
| Brain weight/g | 1.38 ± 0.05 | 1.33 ± 0.04 | 1.44 ± 0.04 | 1.52 ± 0.03 | 1.41 ± 0.04 | 1.44 ± 0.05 | 1.47 ± 0.06 |
| Relative brain weight | 0.34 ± 0.02 e,g | 0.39 ± 0.01 | 0.37 ± 0.01 | 0.39 ± 0.002 | 0.41 ± 0.01 a | 0.39 ± 0.01 a | 0.40 ± 0.01 |
| Liver | Kidney | Brain | Plasma | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ChE | AChE | BChE | ChE | AChE | BChE | AChE | ChE | AChE | BChE | |
| Negative control | 0.467 0.412–0.549 | 0.215 0.170–0.304 | 0.241 0.227–0.274 | 0.204 0.163–0.227 | 0.124 0.095–0.148 | 0.084 0.039–0.112 | 23.9 20.0–35.9 | 0.079 0.077–0.108 | 0.063 0.056–0.084 | 0.023 0.014–0.030 |
| Solvent control | 0.244 a 0.212–0.274 | 0.169 0.138–0.188 | 0.074 a 0.056–0.125 | 0.262 0.166–0.282 | 0.180 0.122–0.219 | 0.062 0.039–0.090 | 22.5 13.6–31.5 | 0.046 c 0.042–0.089 | 0.030 a 0.024–0.054 | 0.019 0.010–0.035 |
| 2.186 µg/kg bw/day | 0.245 a 0.207–0.276 | 0.101 a 0.073–0.153 | 0.134 0.092–0.167 | 0.096 a,b 0.084–0.111 | 0.079 0.058–0.106 | 0.019 0.0–0.049 | 25.6 22.3–34.7 | 0.068 0.044–0.089 | 0.047 0.032–0.060 | 0.016 0.012–0.032 |
| 0.015 mg/kg bw/day | 0.284 0.212–0.413 | 0.155 0.139–0.264 | 0.129 a 0.073–0.149 | 0.136 0.102–0.188 | 0.066 b 0.048–0.083 | 0.088 0.031–0.105 | 25.4 20.0–30.9 | 0.067 0.043–0.081 | 0.043 0.031–0.072 | 0.012 c 0.007–0.029 |
| 0.157 mg/kg bw/day | 0.259 0.235–0.333 | 0.125 0.098–0.182 | 0.137 0.131–0.159 | 0.128 0.101–0.142 | 0.064 b 0.052–0.074 | 0.069 0.037–0.087 | 27.1 25.6–33.9 | 0.053 0.051–0.081 | 0.048 0.042–0.056 | 0.008 c 0.0–0.027 |
| 0.786 mg/kg bw/day | 0.247 a 0.216–0.264 | 0.094 a 0.072–0.122 | 0.151 0.132–0.158 | 0.098 a,b 0.090–0.101 | 0.052 a,b 0.047–0.067 | 0.038 0.032–0.053 | 29.5 26.0–32.2 | 0.117 0.087–0.142 | 0.059 0.047–0.073 | 0.059 0.034–0.069 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kašuba, V.; Tariba Lovaković, B.; Lucić Vrdoljak, A.; Katić, A.; Kopjar, N.; Micek, V.; Milić, M.; Pizent, A.; Želježić, D.; Žunec, S. Evaluation of Toxic Effects Induced by Sub-Acute Exposure to Low Doses of α-Cypermethrin in Adult Male Rats. Toxics 2022, 10, 717. https://doi.org/10.3390/toxics10120717
Kašuba V, Tariba Lovaković B, Lucić Vrdoljak A, Katić A, Kopjar N, Micek V, Milić M, Pizent A, Želježić D, Žunec S. Evaluation of Toxic Effects Induced by Sub-Acute Exposure to Low Doses of α-Cypermethrin in Adult Male Rats. Toxics. 2022; 10(12):717. https://doi.org/10.3390/toxics10120717
Chicago/Turabian StyleKašuba, Vilena, Blanka Tariba Lovaković, Ana Lucić Vrdoljak, Anja Katić, Nevenka Kopjar, Vedran Micek, Mirta Milić, Alica Pizent, Davor Želježić, and Suzana Žunec. 2022. "Evaluation of Toxic Effects Induced by Sub-Acute Exposure to Low Doses of α-Cypermethrin in Adult Male Rats" Toxics 10, no. 12: 717. https://doi.org/10.3390/toxics10120717
APA StyleKašuba, V., Tariba Lovaković, B., Lucić Vrdoljak, A., Katić, A., Kopjar, N., Micek, V., Milić, M., Pizent, A., Želježić, D., & Žunec, S. (2022). Evaluation of Toxic Effects Induced by Sub-Acute Exposure to Low Doses of α-Cypermethrin in Adult Male Rats. Toxics, 10(12), 717. https://doi.org/10.3390/toxics10120717










