Pinus halepensis in Contaminated Mining Sites: Study of the Transfer of Metals in the Plant–Soil System Using the BCR Procedure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling
2.3. Physico-Chemical and Mineralogical Characterizations of the Soil and Root
2.3.1. Physical and Chemical Properties of the Soil
2.3.2. Mineralogical Characterization of the Soil and Root
2.3.3. Total Metal Concentration of the Soil and Root
2.3.4. Bioavailable Concentration of Elements (DTPA)
2.3.5. Sequential Extraction Procedure (BCR)
2.4. Data Analysis
3. Results
3.1. Physical and Chemical Soil Properties
3.2. Mineral Composition in the Soil and Plant Roots
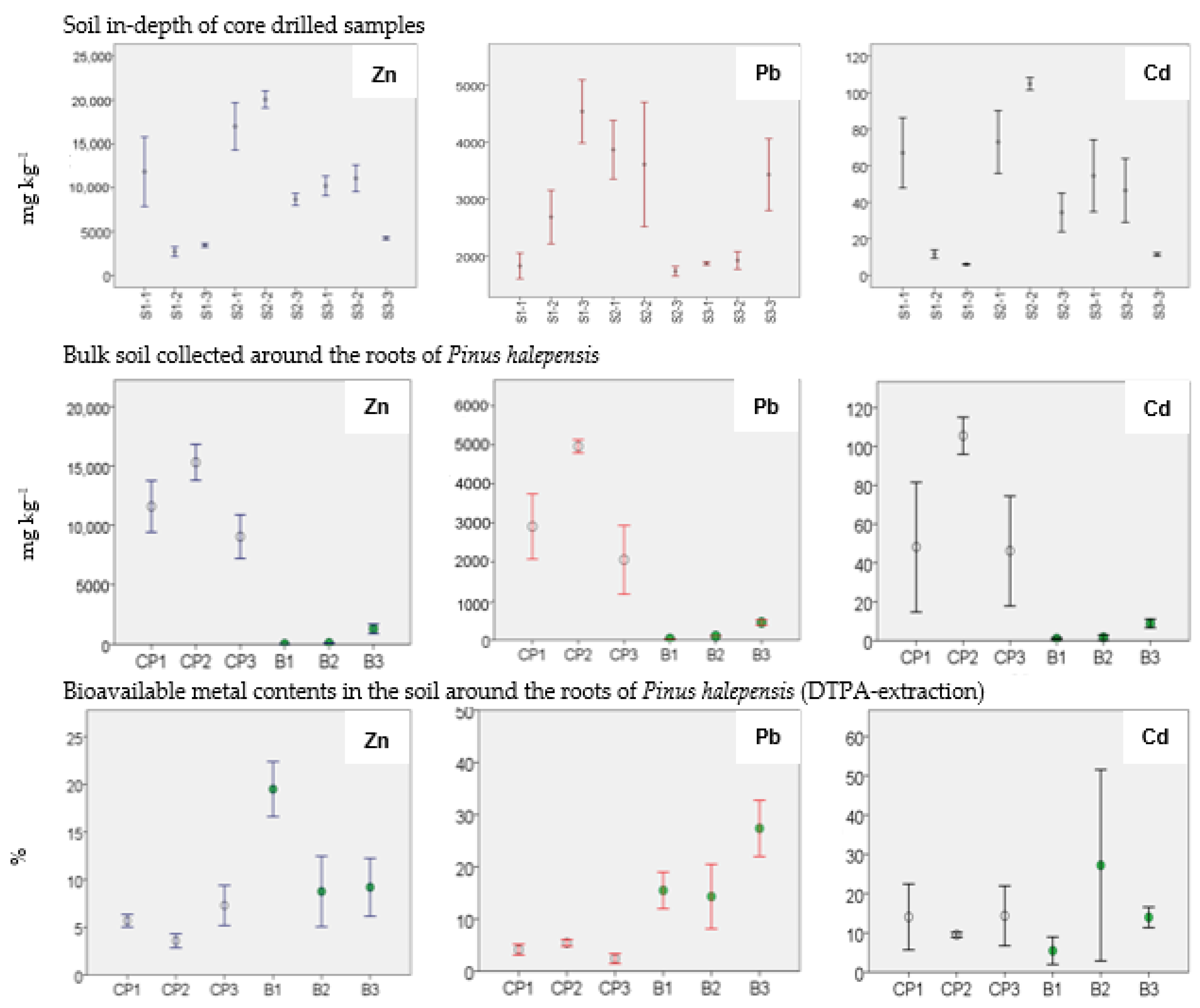
3.3. Total Zn, Pb and Cd Concentrations
3.4. Bioavailable Content of Zn, Pb and Cd (DTPA)
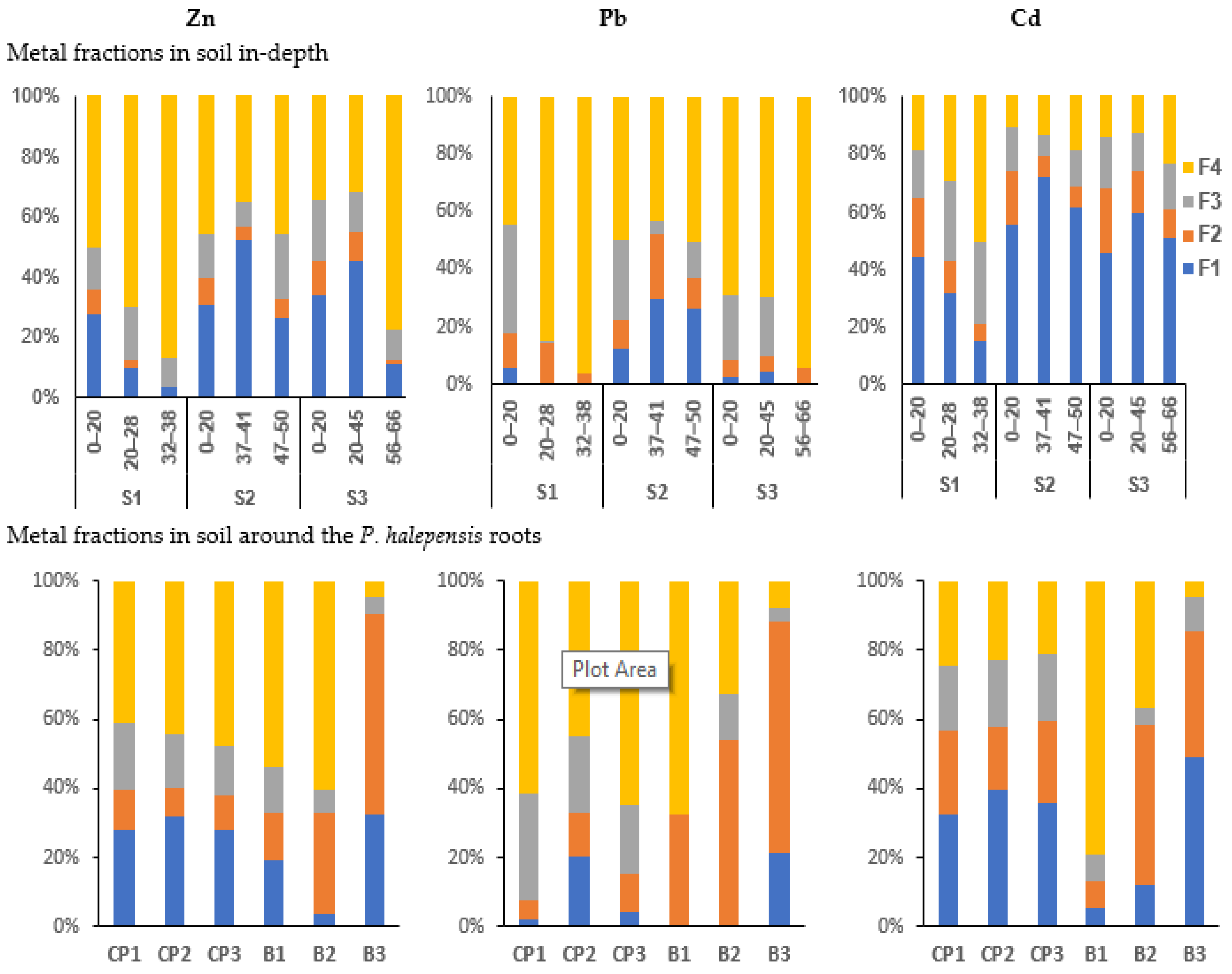
3.5. The BCR Sequential Extraction
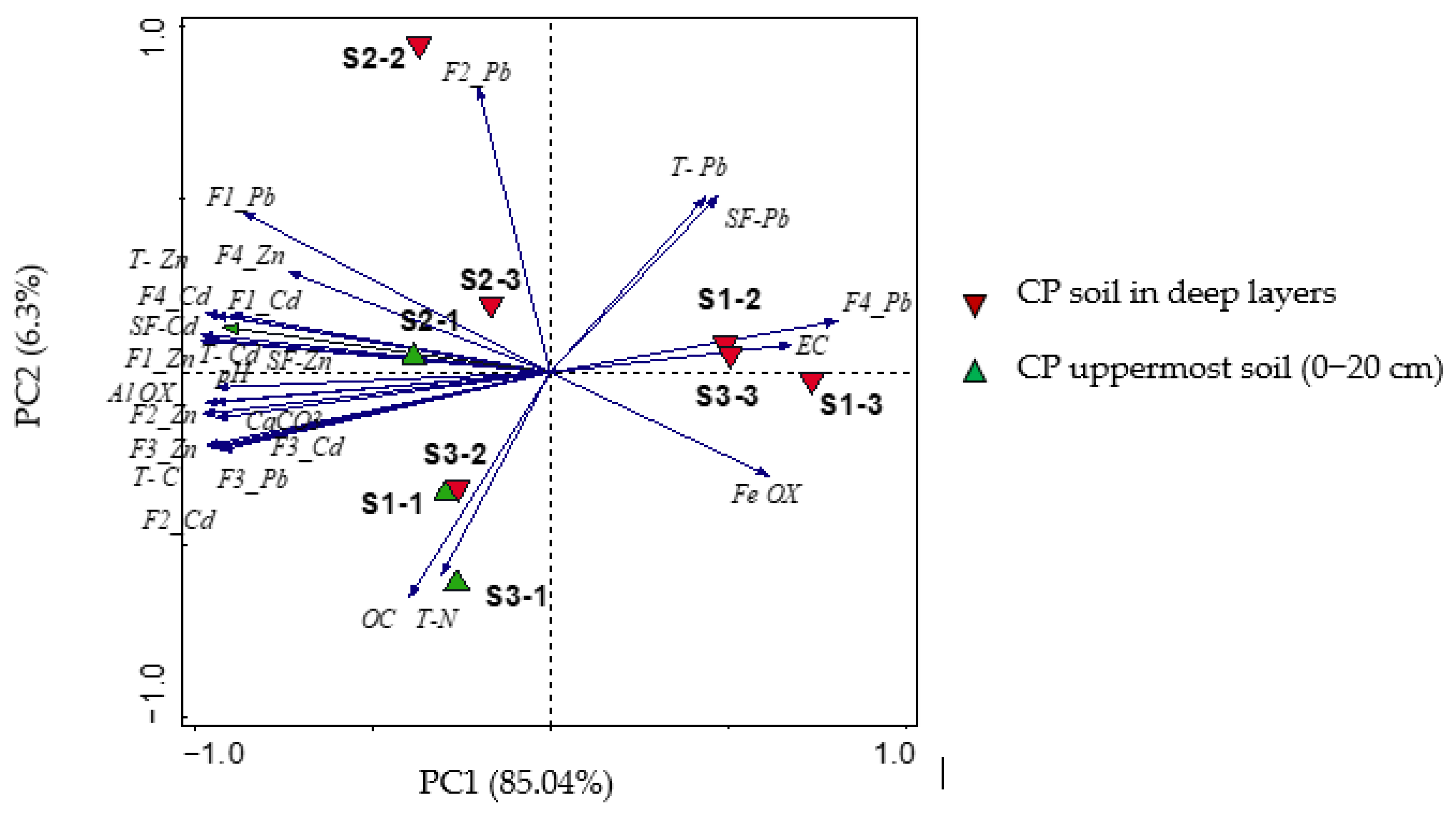
3.6. Correlation between BCR Fractions and Metal Content in the Soil and in the Plant–Root System
4. Discussion
4.1. The Mineralogical Investigation Related to the Soil Properties
4.2. The BCR Fractions Related to the Soil Properties and Mineralogy
4.2.1. Metals in the Exchangeable Fraction (F1)
4.2.2. Metals in Reducible Fraction (F2)
4.2.3. Metals in Oxidizable Fraction (F3)
4.2.4. Metals in Residual Fraction (F4)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mendez, M.O.; Maier, R.M. Phytostabilization of Mine Tailings in Arid and Semiarid Environments—An Emerging Remediation Technology. Environ. Health Perspect. 2008, 116, 278–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Concas, A.; Ardau, C.; Cristini, A.; Zuddas, P.; Cao, G. Mobility of heavy metals from tailings to stream waters in a mining activity contaminated site. Chemosphere 2006, 63, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Barbafieri, M.; Dadea, C.; Tassi, E.; Bretzel, F.; Fanfani, L. Uptake of Heavy Metals by Native Species Growing in a Mining Area in Sardinia, Italy: Discovering Native Flora for Phytoremediation. Int. J. Phytoremediation 2011, 13, 985–997. [Google Scholar] [CrossRef] [PubMed]
- Cao, A.; Cappai, G.; Carucci, A.; Lai, T. Heavy metal bioavailability and chelate mobilization efficiency in an assisted phytoextraction process. Environ. Geochem. Health 2008, 30, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Bacchetta, G.; Cappai, G.; Carucci, A.; Tamburini, E. Use of Native Plants for the Remediation of Abandoned Mine Sites in Mediterranean Semiarid Environments. Bull. Environ. Contam. Toxicol. 2015, 94, 326–333. [Google Scholar] [CrossRef]
- Jiménez, M.; Bacchetta, G.; Navarro, F.; Casti, M.; Fernández-Ondoño, E. Native Plant Capacity for Gentle Remediation in Heavily Polluted Mines. Appl. Sci. 2021, 11, 1769. [Google Scholar] [CrossRef]
- Jiménez, M.; Bacchetta, G.; Casti, M.; Navarro, F.B.; Lallena, A.; Fernández-Ondoño, E. Potential use in phytoremediation of three plant species growing on contaminated mine-tailing soils in Sardinia. Ecol. Eng. 2011, 37, 392–398. [Google Scholar] [CrossRef]
- DE Giudici, G.B.; Medas, D.; Meneghini, C.; Casu, M.A.; Gianoncelli, A.; Iadecola, A.; Podda, S.; Lattanzi, P. Microscopic biomineralization processes and Zn bioavailability: A synchrotron-based investigation of Pistacia lentiscus L. roots. Environ. Sci. Pollut. Res. 2015, 22, 19352–19361. [Google Scholar] [CrossRef] [PubMed]
- Conesa, H.M.; Párraga-Aguado, I. Effects of a soil organic amendment on metal allocation of trees for the phytomanagement of mining-impacted soils. Environ. Geochem. Health 2019, 43, 1355–1366. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.; Cappai, G.; Carucci, A.; Bacchetta, C. Phytoremediation of abandoned mining areas using native plant species: A Sardinian case study. Environ. Sci. Technol. 2015, 11, 255–277. [Google Scholar]
- Rodríguez, L.; Ruiz, E.; Alonso-Azcárate, J.; Rincón, J. Heavy metal distribution and chemical speciation in tailings and soils around a Pb–Zn mine in Spain. J. Environ. Manag. 2009, 90, 1106–1116. [Google Scholar] [CrossRef] [PubMed]
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Rauret, G.; López-Sánchez, J.F.; Sahuquillo, A.; Rubio, R.; Davidson, C.; Ure, A.; Quevauviller, P. Improvement of the BCR three step sequential extraction procedure prior to the certification of new sediment and soil reference materials. J. Environ. Monit. 1999, 1, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Gleyzes, C.; Tellier, S.; Astruc, M. Fractionation studies of trace elements in contaminated soils and sediments: A review of sequential extraction procedures. TrAC Trends Anal. Chem. 2002, 21, 451–467. [Google Scholar] [CrossRef]
- Van Der Eijk, W. The activities of the European Community Bureau of Reference—BCR. Anal. Bioanal. Chem. 1979, 297, 10–12. [Google Scholar] [CrossRef]
- Davidson, C.M.; Duncan, A.L.; Littlejohn, D.; Ure, A.M.; Garden, L.M. A critical evaluation of the three-stage BCR sequential extraction procedure to assess the potential mobility and toxicity of heavy metals in industrially-contaminated land. Anal. Chim. Acta 1998, 363, 45–55. [Google Scholar] [CrossRef]
- Fernández, E.; Jiménez, R.; Lallena, A.; Aguilar, J. Evaluation of the BCR sequential extraction procedure applied for two unpolluted Spanish soils. Environ. Pollut. 2004, 131, 355–364. [Google Scholar] [CrossRef]
- Bacon, J.R.; Davidson, C.M. Is there a future for sequential chemical extraction? Analyst 2007, 133, 25–46. [Google Scholar] [CrossRef]
- Cabała, J.; Teper, L. Metalliferous constituents of rhizosphere soils contaminated by Zn-Pb mining in Southern Poland. Water Air Soil Pollut. 2007, 178, 351–362. [Google Scholar] [CrossRef]
- Nowak-Winiarska, K.; Wrobel, S.; Sienkiewicz-Cholewa, U. Application of sequential analysis with the BCR method in the estimation of effects of chemical remediation of soil polluted with copper. Chem. Speciat. Bioavailab. 2012, 24, 53–59. [Google Scholar] [CrossRef]
- Fernández-Ondoño, E.; Bacchetta, G.; Lallena, A.M.; Navarro, F.B.; Ortiz, I.; Jiménez, M.N. Use of BCR sequential extraction procedures for soils and plant metal transfer predictions in contaminated mine tailings in Sardinia. J. Geochem. Explor. 2017, 172, 133–141. [Google Scholar] [CrossRef]
- Ure, A.M.; Quevauviller, P.; Muntau, H.; Griepink, B. Speciation of Heavy Metals in Soils and Sediments. An Account of the Improvement and Harmonization of Extraction Techniques Undertaken Under the Auspices of the BCR of the Commission of the European Communities. Int. J. Environ. Anal. Chem. 1993, 51, 135–151. [Google Scholar] [CrossRef]
- Gabarrón, M.; Zornoza, R.; Martínez-Martínez, S.; Muñoz, V.A.; Faz, Á.; Acosta, J.A. Effect of land use and soil properties in the feasibility of two sequential extraction procedures for metals fractionation. Chemosphere 2018, 218, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Bacchetta, G.; Cao, A.; Cappai, G.; Carucci, A.; Casti, M.; Fercia, M.L.; Lonis, R.; Mola, F. A field experiment on the use of Pistacia lentiscus L. and Scrophularia canina L. subsp. bicolor(Sibth. et Sm.) Greuter for the phytoremediation of abandoned mining areas. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2012, 146, 1054–1063. [Google Scholar] [CrossRef]
- Bacchetta, G.; Boi, M.E.; Cappai, G.; De Giudici, G.; Piredda, M.; Porceddu, M. Metal Tolerance Capability of Helichrysum microphyllum Cambess. subsp. tyrrhenicum Bacch., Brullo & Giusso: A Candidate for Phytostabilization in Abandoned Mine Sites. Bull. Environ. Contam. Toxicol. 2018, 101, 758–765. [Google Scholar] [CrossRef]
- Medas, D.; De Giudici, G.; Casu, M.A.; Musu, E.; Gianoncelli, A.; Iadecola, A.; Meneghini, C.; Tamburini, E.; Sprocati, A.R.; Turnau, K.; et al. Microscopic Processes Ruling the Bioavailability of Zn to Roots of Euphorbia pithyusa L. Pioneer Plant. Environ. Sci. Technol. 2015, 49, 1400–1408. [Google Scholar] [CrossRef] [PubMed]
- Medas, D.; De Giudici, G.; Pusceddu, C.; Casu, M.A.; Birarda, G.; Vaccari, L.; Gianoncelli, A.; Meneghini, C. Impact of Zn excess on biomineralization processes in Juncus acutus grown in mine polluted sites. J. Hazard. Mater. 2019, 370, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Pulford, I.D.; Watson, C. Phytoremediation of heavy metal-contaminated land by trees-A review. Environ. Int. 2003, 29, 529–540. [Google Scholar] [CrossRef]
- Querejeta, J.I.; Barberá, G.G.; Granados, A.; Castillo, V.M. Afforestation method affects the isotopic composition of planted Pinus halepensis in a semiarid region of Spain. For. Ecol. Manag. 2008, 254, 56–64. [Google Scholar] [CrossRef]
- Párraga-Aguado, I.; Álvarez-Rogel, J.; González-Alcaraz, M.N.; Jiménez-Cárceles, F.J.; Conesa, H.M. Assessment of metal(loid)s availability and their uptake by Pinus halepensis in a Mediterranean forest impacted by abandoned tailings. Ecol. Eng. 2013, 58, 84–90. [Google Scholar] [CrossRef]
- Sardans, J.; Peñuelas, J.; Rodà, F. Changes in nutrient use efficiency, status and retranslocation in young post-fire regeneration Pinus halepensis in response to sudden N and P input, irrigation and removal of competing vegetation. Trees 2005, 19, 233–250. [Google Scholar] [CrossRef]
- Kharazian, P.; Bacchetta, G.; Cappai, G.; Piredda, M.; De Giudici, G. An integrated geochemical and mineralogical investigation on soil-plant system of Pinus halepensis pioneer tree growing on heavy metal polluted mine tailing. Plant Biosyst. Int. J. Deal. all Asp. Plant Biol. 2022, 1–14. [Google Scholar] [CrossRef]
- Bacchetta, G.; Bagella, S.; Biondi, E.; Farris, E.; Filigheddu, R.; Mossa, L. Vegetazione forestale e serie divegetazione della Sardegna (con rappresentazione cartografica alla scala 1:350.000). Fitosociologia 2009, 46, 3–82. [Google Scholar]
- Bechstädt, T.; Boni, M. Sedimentological, Stratigraphical and Ore Deposits Field Guide of the Autochthonous CambroOrdovician of Southwestern Sardinia; Servizio Geologico d’Italia Memorie Descritive Carta Geologica d’Italia, Istituto Superiore per la Protezione e la Ricerca Ambientale: Roma, Italy, 1994; Volume 48, p. 434.
- Aversa, G.; Balassone, G.; Boni, M.; Amalfitano, C. The mineralogy of the «calamine» Ores in SW Sardinia (Italy): Preliminary results. Period. Mineral. 2002, 71, 201–218. [Google Scholar]
- Boni, M.; Costabile, S.; De Vivo, B.; Gasparrini, M. Potential environmental hazard in the mining district of southern Iglesiente (SW Sardinia, Italy). J. Geochem. Explor. 1999, 67, 417–430. [Google Scholar] [CrossRef]
- Boi, M.E.; Porceddu, M.; Cappai, G.; De Giudici, G.; Bacchetta, G. Effects of zinc and lead on seed germination of Helichrysum microphyllum subsp. tyrrhenicum, a metal-tolerant plant. Int. J. Environ. Sci. Technol. 2019, 17, 1917–1928. [Google Scholar] [CrossRef]
- Soil Conservation Service. Soil Conservation Service. Soil survey laboratory methods and procedures for collecting soils samples. In Soil Survey Report 1; U.S.D.A.: Washington, DC, USA, 1972. [Google Scholar]
- Ussiri, D.A.N.; Lal, R. Method for Determining Coal Carbon in the Reclaimed Minesoils Contaminated with Coal. Soil Sci. Soc. Am. J. 2008, 72, 231–237. [Google Scholar] [CrossRef]
- Schwertmann, U.; Taylor, R.M. Iron oxides. In Minerals in Soil Environments; Dixon, J.B., Webb, S.B., Eds.; Soil Science Society of America: Madison, WI, USA, 1977; pp. 148–180. [Google Scholar]
- Barbafieri, M.; Lubrano, L.; Petruzzelli, G. Characterization of pollution in sites contaminated by heavy metals: A proposal. J. Ann. Di Chim. 1996, 86, 585–594. [Google Scholar]
- Lindsay, W.L.; Norvell, W.A. Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Sci. Soc. Am. J. 1978, 42, 421–428. [Google Scholar] [CrossRef]
- Guan, T.; He, H.; Zhang, X.; Bai, Z. Cu fractions, mobility and bioavailability in soil-wheat system after Cu-enriched livestock manure applications. Chemosphere 2010, 82, 215–222. [Google Scholar] [CrossRef]
- Feng, M.-H.; Shan, X.-Q.; Zhang, S.-Z.; Wen, B. Comparison of a rhizosphere-based method with other one-step extraction methods for assessing the bioavailability of soil metals to wheat. Chemosphere 2005, 59, 939–949. [Google Scholar] [CrossRef] [PubMed]
- Mossop, K.F.; Davidson, C.M. Comparison of original and modified BCR sequential extraction procedures for the fractionation of copper, iron, lead, manganese and zinc in soils and sediments. Anal. Chim. Acta 2003, 478, 111–118. [Google Scholar] [CrossRef] [Green Version]
- Van Her-reweghe, S.; Swennen, R.; Vandecasteele, C.; Cappuyns, V. Solid phase speciation of arsenic by sequential extraction in standard reference materials and industrially contaminated soil samples. Environ. Pollut. 2003, 122, 323–342. [Google Scholar] [CrossRef]
- Kabala, C.; Karczewska, A.; Szopka, K.; Wilk, J. Copper, Zinc, and Lead Fractions in Soils Long-Term Irrigated with Municipal Wastewater. Commun. Soil Sci. Plant Anal. 2011, 42, 905–919. [Google Scholar] [CrossRef]
- Cappuyns, V.; Swennen, R.; Niclaes, M. Application of the BCR sequential extraction scheme to dredged pond sediments contaminated by Pb–Zn mining: A combined geochemical and mineralogical approach. J. Geochem. Explor. 2007, 93, 78–90. [Google Scholar] [CrossRef]
- Ter Braak, C.J.F.; Smilauer, P. CANOCO Reference Manual and CanoDraw for Windows User’s Guide: Software for Canonical Community Ordination, version 4.5; Biometris; Wageningen University and Research: Wageningen, The Netherlands, 2002. [Google Scholar]
- Lepš, J.; Šmilauer, P. Multivariate Analysis of Ecological Data Using CANOCO; Cambridge University Press: Cambridge, UK, 2003; 268p. [Google Scholar] [CrossRef]
- Cidu, R.; Fanfani, L. Overview of the environmental geochemistry of mining districts in southwestern Sardinia, Italy. Geochem. Explor. Environ. Anal. 2002, 2, 243–251. [Google Scholar] [CrossRef]
- Jambor, J.L.; Nordstrom, D.K.; Alpers, C.N. Metal-sulfate salts from sulfide mineral oxidation. Rev. Mineral. Geochem. 2000, 40, 303–350. [Google Scholar] [CrossRef]
- Jerz, J.K.; Rimstidt, J.D. Efflorescent iron sulfate minerals: Paragenesis, relative stability, and environmental impact. Am. Miner. 2003, 88, 1919–1932. [Google Scholar] [CrossRef]
- Martín, F.; García, I.; Díez, M.; Sierra, M.; Simon, M.; Dorronsoro, C. Soil alteration by continued oxidation of pyrite tailings. Appl. Geochem. 2008, 23, 1152–1165. [Google Scholar] [CrossRef]
- Pastor-Jáuregui, R.; Paniagua-López, M.; Martínez-Garzón, J.; Martín-Peinado, F.; Sierra-Aragón, M. Evolution of the Residual Pollution in Soils after Bioremediation Treatments. Appl. Sci. 2020, 10, 1006. [Google Scholar] [CrossRef] [Green Version]
- Simón, M.; Martín, F.; Ortiz, I.; García, I.; Fernández, E.; Dorronsoro, C.; Aguilar, J. Soil pollution by oxidation of tailings from toxic spill of a pyrite mine. Sci. Total Environ. 2001, 279, 63–74. [Google Scholar] [CrossRef]
- Wong, M.H. Ecological restoration of mine degraded soils, with emphasis on metal contaminated soils. Chemosphere 2003, 50, 775–780. [Google Scholar] [CrossRef]
- Young, I.; Renault, S.; Markham, J. Low levels organic amendments improve fertility and plant cover on non-acid generating gold mine tailings. Ecol. Eng. 2015, 74, 250–257. [Google Scholar] [CrossRef]
- Luo, Y.; Wu, Y.; Qiu, J.; Wang, H.; Yang, L. Suitability of four woody plant species for the phytostabilization of a zinc smelting slag site after 5 years of assisted revegetation. J. Soils Sediments 2018, 19, 702–715. [Google Scholar] [CrossRef]
- Clemente, R.; Bernal, M.P. Fractionation of heavy metals and distribution of organic carbon in two contaminated soils amended with humic acids. Chemosphere 2006, 64, 1264–1273. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Vila, A.; Asensio, V.; Forján, R.; Covelo, E.F. Assessing the influence of technosol and biochar amendments combined with Brassica juncea L. on the fractionation of Cu, Ni, Pb and Zn in a polluted mine soil. J. Soils Sediments 2015, 16, 339–348. [Google Scholar] [CrossRef]
- García-Carmona, M.; Romero-Freire, A.; Aragón, M.S.; Peinado, F.M. Effectiveness of ecotoxicological tests in relation to physicochemical properties of Zn and Cu polluted Mediterranean soils. Geoderma 2019, 338, 259–268. [Google Scholar] [CrossRef]
- Garcíia, I.; Diez, M.; Martíin, F.; Simóon, M.; Dorronsoro, C. Mobility of Arsenic and Heavy Metals in a Sandy-Loam Textured and Carbonated Soil. Pedosphere 2009, 19, 166–175. [Google Scholar] [CrossRef]
- Jacquat, O.; Voegelin, A.; Villard, A.; Marcus, M.A.; Kretzschmar, R. Formation of Zn-rich phyllosilicate, Zn-layered double hydroxide and hydrozincite in contaminated calcareous soils. Geochim. et Cosmochim. Acta 2008, 72, 5037–5054. [Google Scholar] [CrossRef] [Green Version]
- Aragón, M.S.; Nakamaru, Y.M.; García-Carmona, M.; Garzón, F.J.M.; Peinado, F.J.M. The role of organic amendment in soils affected by residual pollution of potentially harmful elements. Chemosphere 2019, 237, 124549. [Google Scholar] [CrossRef]
- Mohamed, I.; Ahamadou, B.; Li, M.; Gong, C.; Cai, P.; Liang, W.; Huang, Q. Fractionation of copper and cadmium and their binding with soil organic matter in a contaminated soil amended with organic materials. J. Soils Sediments 2010, 10, 973–982. [Google Scholar] [CrossRef]
- Cambier, P.; Michaud, A.; Paradelo, R.; Germain, M.; Mercier, V.; Guérin-Lebourg, A.; Revallier, A.; Houot, S. Trace metal availability in soil horizons amended with various urban waste composts during 17 years—Monitoring and modelling. Sci. Total Environ. 2019, 651, 2961–2974. [Google Scholar] [CrossRef]
- Swęd, M.; Uzarowicz, Ł.; Duczmal-Czernikiewicz, A.; Kwasowski, W.; Pędziwiatr, A.; Siepak, M.; Niedzielski, P. Forms of metal(loid)s in soils derived from historical calamine mining waste and tailings of the Olkusz Zn–Pb ore district, southern Poland: A combined pedological, geochemical and mineralogical approach. Appl. Geochem. 2022, 139, 105218. [Google Scholar] [CrossRef]
- Favas, P.J.C.; Pratas, J.; Gomes, M.E.P.; Cala, V. Selective chemical extraction of heavy metals in tailings and soils contaminated by mining activity: Environmental implications. J. Geochem. Explor. 2011, 111, 160–171. [Google Scholar] [CrossRef]
- Jerzykowska, I.; Majzlan, J.; Michalik, M.; Göttlicher, J.; Steininger, R.; Błachowski, A.; Ruebenbauer, K. Mineralogy and speciation of Zn and As in Fe-oxide-clay aggregates in the mining waste at the MVT Zn–Pb deposits near Olkusz, Poland. Geochemistry 2014, 74, 393–406. [Google Scholar] [CrossRef]
- Sutley, S.; Gustkiewicz, M.-S.; Mayer, W.; Leach, D.L. Mineralogy and Chemistry of Oxidized Ores from the Upper Silesia Mississippi Valley-Type Zinc-Lead Deposits, Poland. In USGS Science for a Changing World, Open File Report; US Dept. of the Interior, US Geological Survey: Washington, DC, USA, 1999. [Google Scholar] [CrossRef]
- Monterroso, C.; Rodríguez, F.; Chaves, R.; Diez, J.; Becerra-Castro, C.; Kidd, P.; Macías, F. Heavy metal distribution in mine-soils and plants growing in a Pb/Zn-mining area in NW Spain. Appl. Geochem. 2014, 44, 3–11. [Google Scholar] [CrossRef]
- Simón, M.; Diez, M.; Gonzalez, V.; García, I.; Martín, F.; De Haro, S. Use of liming in the remediation of soils polluted by sulphide oxidation: A leaching-column study. J. Hazard. Mater. 2010, 180, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Rozek, D.; Nadłonek, W.; Cabała, J. Forms of heavy metals (Zn, Pb, Cd) occurring in rhizosphere from the areas of former and contemporary Zn-Pb mining. Mineral. Sci. 2015, 22, 125–138. [Google Scholar]
- Swęd, M.; Potysz, A.; Duczmal-Czernikiewicz, A.; Siepak, M.; Bartz, W. Bioweathering of Zn–Pb-bearing rocks: Experimental exposure to water, microorganisms, and root exudates. Appl. Geochem. 2021, 130, 104966. [Google Scholar] [CrossRef]
- Kumpiene, J.; Lagerkvist, A.; Maurice, C. Stabilization of As, Cr, Cu, Pb and Zn in soil using amendments—A review. Waste Manag. 2008, 28, 215–225. [Google Scholar] [CrossRef]
- García-Carmona, M.; García-Robles, H.; Torrano, C.T.; Ondoño, E.F.; Moreno, J.L.; Aragón, M.S.; Peinado, F.M. Residual pollution and vegetation distribution in amended soils 20 years after a pyrite mine tailings spill (Aznalcóllar, Spain). Sci. Total Environ. 2018, 650, 933–940. [Google Scholar] [CrossRef]
- Romero-Freire, A.; Peinado, F.M.; van Gestel, C. Effect of soil properties on the toxicity of Pb: Assessment of the appropriateness of guideline values. J. Hazard. Mater. 2015, 289, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Coppola, E.; Capra, G.; Odierna, P.; Vacca, S.; Buondonno, A. Lead distribution as related to pedological features of soils in the Volturno River low Basin (Campania, Italy). Geoderma 2010, 159, 342–349. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4th edition, CRC Press, Taylor and Francis Group, 6000 Broken Sound Parkway NW, Suite 300, Boca Raton, FL 33487-2742, United States of America, Florida. S. Afr. J. Bot. 2011, 80, 118. [Google Scholar] [CrossRef]


| Soil | Soil Depth (cm) | Soil Properties | Mean Value (mg kg−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| pH | EC (dS m−1) | CaCO3 (%) | Total Carbon (%) | Total Nitrogen (%) | Organic Carbon (%) | Fe-Oxide | Al-Oxide | ||
| Bulk soil in-depth | |||||||||
| S1-1 | 0–20 | 6.8 | 12.05 | 52.07 ± 0.7 | 8.9 ± 0.1 | 0.17 ± 0.01 | 1.9 ± 0.1 | 10,565.3 ± 301.3 | 160.4 ± 6.2 |
| S1-2 | 20–28 | 4.5 | 15.3 | 4.05 ± 0.1 | 0.71 ± 0.04 | 0.010 ± 0.001 | 0.22 ± 0.01 | 29,658.3 ± 3026.05 | 17.2 ± 2.9 |
| S1-3 | 32–38 | 2.3 | 29.3 | 0.1 ± 0.08 | 0.36 ± 0.001 | 0.03 ± 0.01 | 0.020 ± 0.001 | 14,920.9 ± 751.9 | 3.4 ± 1.7 |
| S2-1 | 0–20 | 7.3 | 1.9 | 59.4 ± 0.4 | 8.3 ± 0.1 | 0.04 ± 0.02 | 0.35 ± 0.02 | 19,160.8 ± 537.9 | 189.5 ± 10.3 |
| S2-2 | 37–41 | 6.7 | 14.2 | 37.5 ± 0.5 | 5.00 ± 0.05 | 0.020 ± 0.0003 | 0.020 ± 0.0004 | 5909.6 ± 128.6 | 101.5 ± 18.3 |
| S2-3 | 47–50 | 7.6 | 11.3 | 47.6 ± 0.7 | 6.59 ± 0.02 | 0.002 ± 0.001 | 0.180 ± 0.003 | 11,498.5 ± 326.8 | 37.2 ± 4.9 |
| S3-1 | 0–20 | 6.9 | 10.7 | 53.6 ± 1.5 | 8.6 ± 0.2 | 0.15 ± 0.02 | 1.5 ± 0.2 | 12,334.9 ± 99.6 | 112.9 ± 6.7 |
| S3-2 | 20–45 | 7.1 | 11.2 | 55.5 ± 0.2 | 7.62 ± 0.08 | 0.002 ± 0.001 | 0.140 ± 0.005 | 17,411.7 ± 2030.7 | 61.2 ± 6.3 |
| S3-3 | 56–66 | 2.5 | 19.1 | 1.40 ± 0.02 | 0.38 ± 0.005 | 0.02 ± 0.01 | 0.190 ± 0.002 | 42,948.9 ± 5084.9 | 14.3 ± 3.9 |
| Bulk soil around the roots of P. halepensis | |||||||||
| CP1 | 7.6 | 8.3 | 49.7 ± 1.7 | 9.97 ± 0.05 | 0.70 ± 0.08 | 0.35 ± 0.03 | 9194.4 ± 129.9 | 211.95 ± 7.08 | |
| CP2 | 7.7 | 2.2 | 45.4 ± 1.2 | 5.7 ± 0.1 | 0.27 ± 0.02 | 0.060 ± 0.002 | 18,265.6 ± 159.4 | 112.8 ± 9.8 | |
| CP3 | 7.4 | 8.8 | 41.03 ± 1.03 | 8.9 ± 0.2 | 0.51 ± 0.03 | 0.33 ± 0.04 | 10,936.2 ± 269.4 | 245.6 ± 2.8 | |
| B1 | 6.7 | 0.3 | 0.32 ± 0.02 | 1.2 ± 0.2 | 0.06 ± 0.01 | 0.77 ± 0.07 | 726.8 ± 116.9 | 215.2 ± 14.3 | |
| B2 | 7.9 | 3.9 | 4.07 ± 0.30 | 10.07 ± 0.04 | 0.66 ± 0.01 | 7.61 ± 0.04 | 992.2 ± 26.8 | 2323.9 ± 37.9 | |
| B3 | 8.8 | 1.1 | 19.03 ± 0.50 | 3.83 ± 0.08 | 0.080 ± 0.005 | 0.78 ± 0.06 | 467.6 ± 62.5 | 195.2 ± 8.4 | |
| Samples | F1 | F2 | F3 | F4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zn | Pb | Cd | Zn | Pb | Cd | Zn | Pb | Cd | Zn | Pb | Cd | |
| Bulk soil around the roots of P. halepensis | ||||||||||||
| T-Zn | 0.96 | 0.86 | 0.90 | 0.81 | - | - | 0.89 | 0.92 | 0.93 | 0.88 | - | 0.80 |
| T-Pb | 0.98 | 0.92 | 0.94 | 0.76 | 0.85 | - | 0.86 | 0.95 | 0.97 | 0.89 | 0.83 | - |
| T-Cd | 0.92 | 0.95 | 0.96 | - | 0.92 | - | - | 0.96 | 0.94 | 0.87 | 0.87 | - |
| Zn-R | 0.96 | 0.98 | .99 | - | 0.95 | - | - | 0.98 | 0.99 | 0.87 | 0.90 | - |
| Pb-R | 0.94 | 0.99 | 0.99 | - | 0.97 | - | - | 0.97 | 0.97 | 0.86 | 0.93 | - |
| Cd-R | 0.93 | - | 0.82 | 0.9 | - | - | 0.98 | 0.84 | 0.88 | 0.80 | - | - |
| DTPA-Zn | 0.83 | - | 0.88 | 0.80 | - | 0.97 | 0.88 | - | 0.92 | 0.85 | 0.82 | 0.89 |
| DTPA-Pb | 0.82 | 0.83 | - | 0.85 | 0.87 | - | - | 0.81 | - | - | - | - |
| DTPA-Cd | 0.85 | - | 0.89 | 0.80 | - | 0.98 | 0.89 | - | 0.93 | 0.87 | 0.84 | 0.90 |
| SF-Zn | 0.99 | - | 0.98 | 0.86 | - | 0.95 | 0.98 | 0.97 | 0.95 | 0.96 | 0.96 | 0.98 |
| SF-Pb | 0.96 | 0.91 | 0.95 | 0.73 | 0.92 | 0.82 | 0.87 | 0.99 | 0.92 | 0.94 | 0.98 | 0.89 |
| SF-Cd | 0.98 | 0.92 | 0.99 | 0.85 | - | 0.98 | 0.97 | 0.94 | 0.99 | 0.98 | 0.96 | 0.98 |
| pH | 0.86 | - | - | - | - | - | - | 0.84 | 0.82 | 0.89 | - | 0.94 |
| EC | - | - | - | - | - | - | - | - | - | - | - | - |
| CaCO3 | 0.88 | - | 0.80 | 0.92 | - | 0.98 | 0.92 | 0.80 | 0.94 | 0.88 | 0.83 | 0.94 |
| Total C | - | - | 0.92 | - | - | - | - | - | - | - | - | - |
| Total N | - | - | - | - | - | - | - | - | - | - | - | - |
| OC | - | - | - | - | - | - | - | - | - | - | - | - |
| Fe-oxide | 0.97 | 0.80 | 0.98 | 0.74 | 0.82 | 0.90 | 0.93 | 0.98 | 0.98 | 0.99 | 0.94 | - |
| Al-oxide | - | - | - | - | - | - | - | - | - | - | - | - |
| Bulk soil in-depth | ||||||||||||
| T-Zn | 0.85 | - | 0.96 | - | - | - | - | - | - | 0.89 | - | 0.90 |
| T-Pb | - | - | - | - | - | - | - | - | - | - | - | - |
| T-Cd | 0.86 | - | 0.93 | - | - | - | - | - | - | 0.89 | - | 0.94 |
| SF-Zn | 0.97 | 0.97 | .95 | 0.81 | - | - | - | - | - | 0.89 | - | 0.91 |
| SF-Pb | - | - | - | - | - | - | - | - | - | - | 0.80 | - |
| SF-Cd | 0.90 | - | 0.97 | 0.80 | - | - | - | - | - | 0.88 | - | 0.93 |
| pH | - | - | - | 0.85 | - | - | 0.89 | - | - | - | - | - |
| EC | - | - | - | - | - | - | - | - | - | - | - | - |
| CaCO3 | - | - | - | 0.92 | - | 0.85 | 0.89 | 0.83 | 0.89 | - | - | - |
| Total C | - | - | - | 0.91 | - | 0.88 | 0.87 | 0.85 | 0.90 | - | - | - |
| Total N | - | - | - | - | - | - | - | - | - | - | - | - |
| OC | - | - | - | - | - | - | - | - | - | - | - | - |
| Fe-oxide | - | - | - | - | - | - | - | - | - | - | - | - |
| Al-oxide | - | - | - | - | - | 0.94 | - | 0.92 | 0.92 | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kharazian, P.; Fernández-Ondoño, E.; Jiménez, M.N.; Sierra Aragón, M.; Aguirre-Arcos, A.; Bacchetta, G.; Cappai, G.; De Giudici, G. Pinus halepensis in Contaminated Mining Sites: Study of the Transfer of Metals in the Plant–Soil System Using the BCR Procedure. Toxics 2022, 10, 728. https://doi.org/10.3390/toxics10120728
Kharazian P, Fernández-Ondoño E, Jiménez MN, Sierra Aragón M, Aguirre-Arcos A, Bacchetta G, Cappai G, De Giudici G. Pinus halepensis in Contaminated Mining Sites: Study of the Transfer of Metals in the Plant–Soil System Using the BCR Procedure. Toxics. 2022; 10(12):728. https://doi.org/10.3390/toxics10120728
Chicago/Turabian StyleKharazian, Pegah, Emilia Fernández-Ondoño, María Noelia Jiménez, Manuel Sierra Aragón, Antonio Aguirre-Arcos, Gianluigi Bacchetta, Giovanna Cappai, and Giovanni De Giudici. 2022. "Pinus halepensis in Contaminated Mining Sites: Study of the Transfer of Metals in the Plant–Soil System Using the BCR Procedure" Toxics 10, no. 12: 728. https://doi.org/10.3390/toxics10120728








