Neuronal and Astrocytic Morphological Alterations Driven by Prolonged Exposure with Δ9-Tetrahydrocannabinol but Not Cannabidiol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Materials
2.3. Preparation of Rat Organotypic Hippocampal Slice Cultures
2.4. Western Blot Analysis
2.5. Fluorescence Immunohistochemistry and Quantitative Analysis
2.6. Statistical Analysis
3. Results
3.1. Acute and Prolonged Administration of Δ9-Tetrahydrocannabinol and Cannabidiol Do Not Produce Death in Mature Organotypic Hippocampal Slices

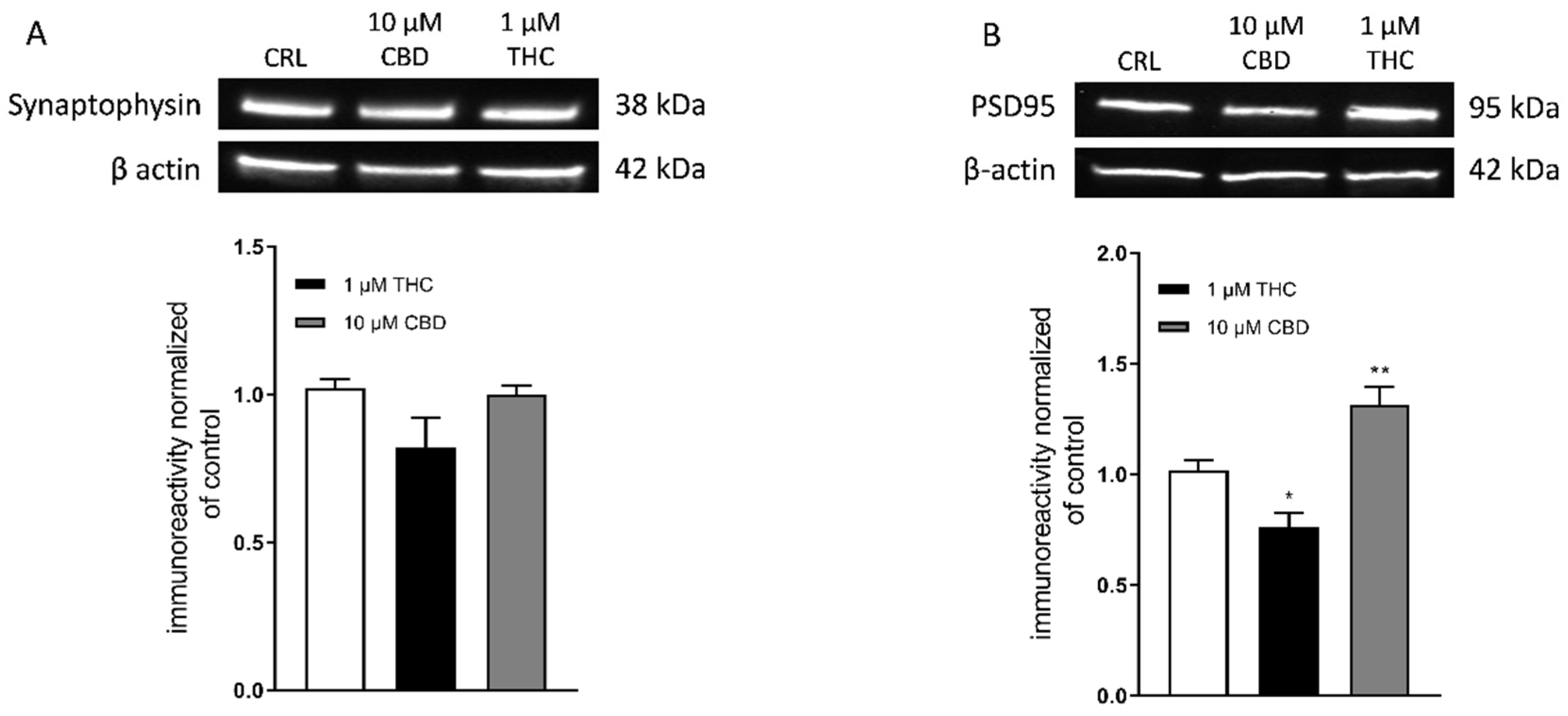
3.2. Effect of THC and CBD on Synaptophysin and PSD95 Levels
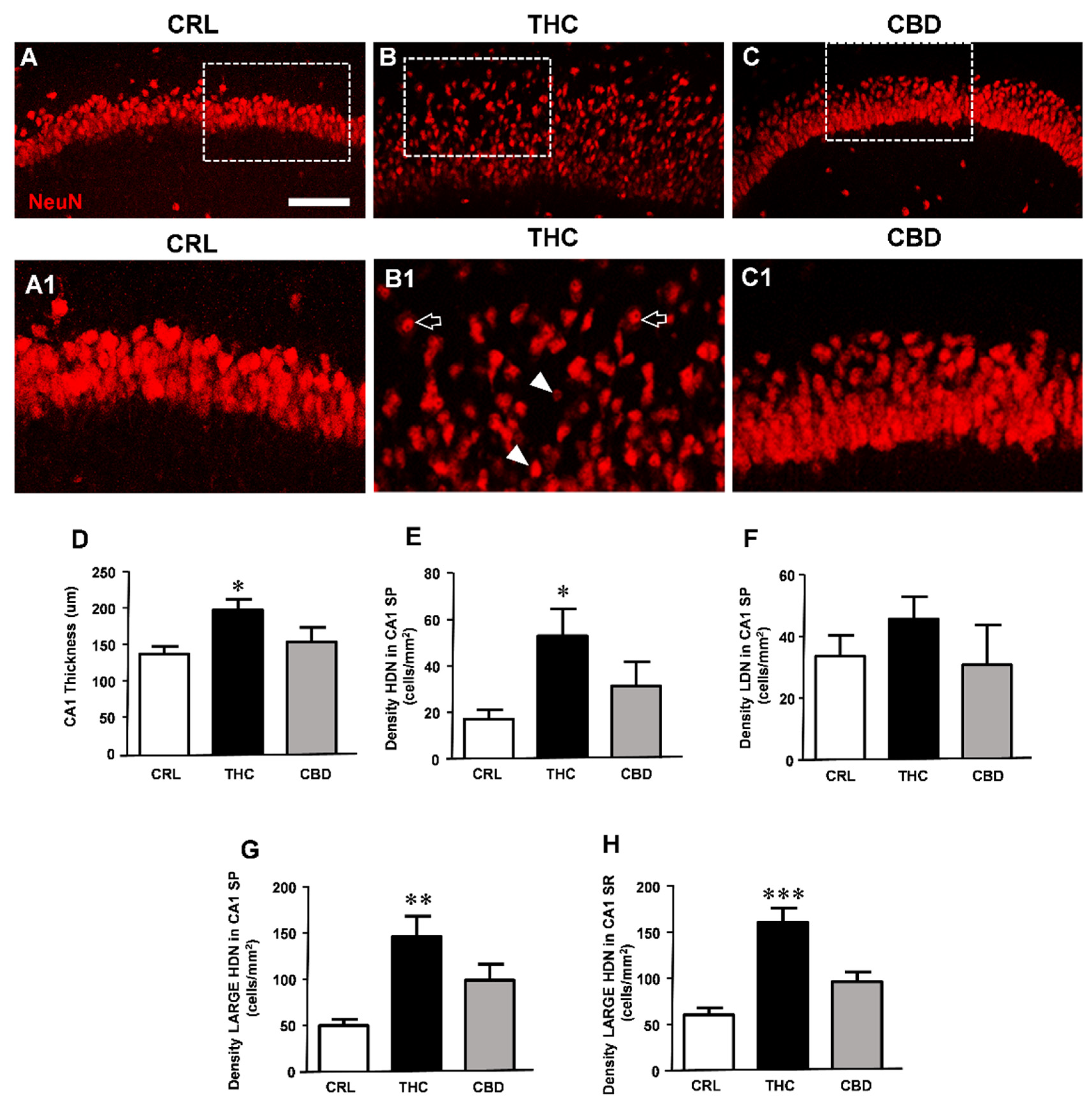
3.3. Prolonged THC or CBD Exposure Effects on Neuronal Viability
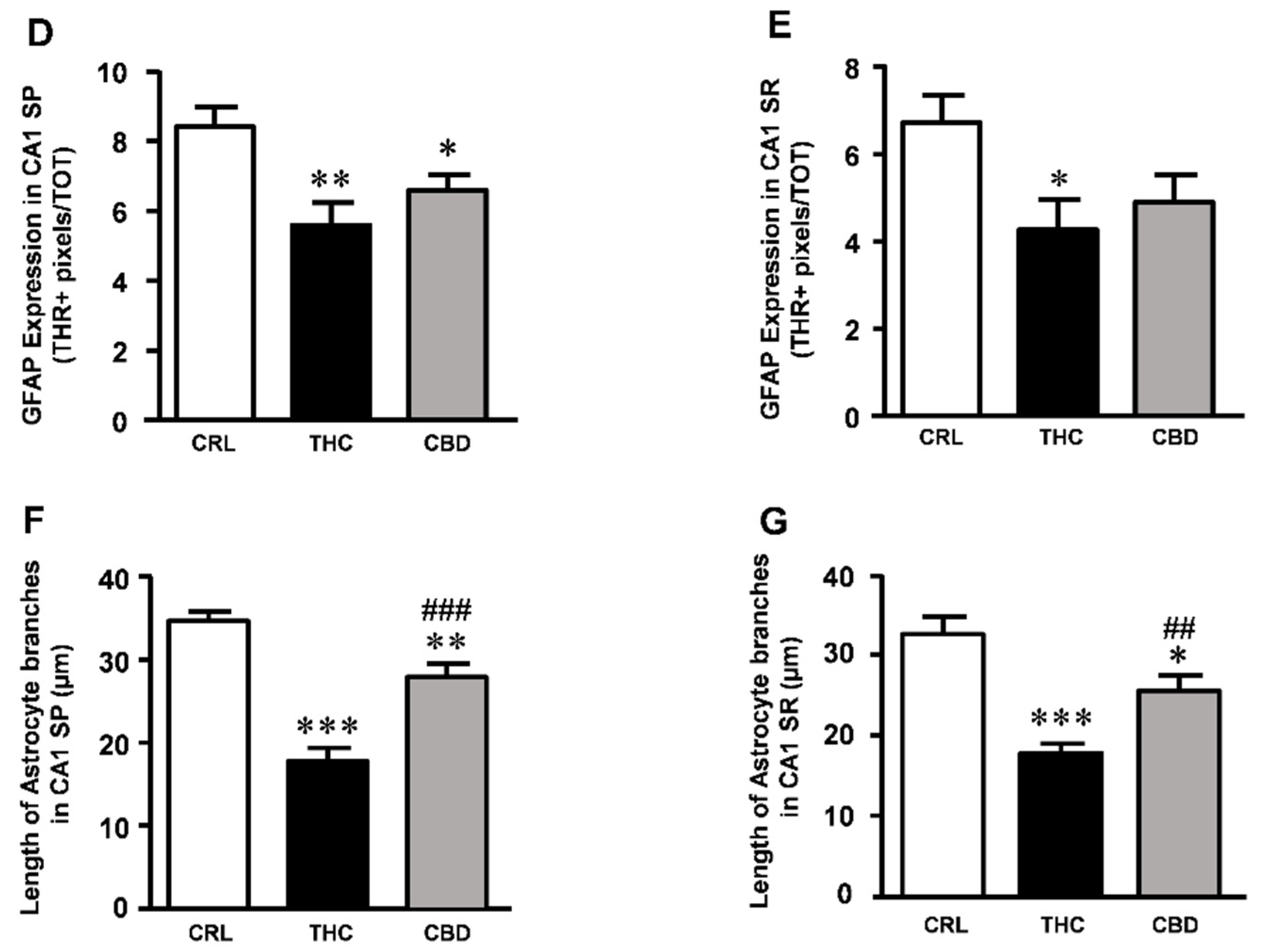
3.4. Effects of THC or CBD Exposure on Astrocytes Viability
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zamberletti, E.; Gabaglio, M.; Grilli, M.; Prini, P.; Catanese, A.; Pittaluga, A.; Marchi, M.; Rubino, T.; Parolaro, D. Long-term hippocampal glutamate synapse and astrocyte dysfunctions underlying the altered phenotype induced by adolescent THC treatment in male rats. Pharmacol. Res. 2016, 111, 459–470. [Google Scholar] [CrossRef]
- Hall, W.; Degenhardt, L. The adverse health effects of chronic cannabis use. Drug Test. Anal. 2014, 6, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Filbey, F.M.; Schacht, J.P.; Myers, U.S.; Chavez, R.S.; Hutchison, K.E. Marijuana craving in the brain. Proc. Natl. Acad. Sci. USA 2009, 106, 13016–13021. [Google Scholar] [CrossRef] [Green Version]
- Weinstein, A.M.; Gorelick, D.A. Pharmacological treatment of Cannabis dependence. Curr. Pharm. Des. 2011, 17, 1351–1358. [Google Scholar] [CrossRef] [PubMed]
- Wölfling, K.; Flor, H.; Grüsser, S.M. Psychophysiological responses to drug-associated stimuli in chronic heavy cannabis use. Eur. J. Neurosci. 2008, 27, 976–983. [Google Scholar] [CrossRef]
- Fratta, W.; Fattore, L. Molecular mechanisms of cannabinoid addiction. Curr. Opin. Neurobiol. 2013, 23, 487–492. [Google Scholar] [CrossRef]
- Murphy, M.; Mills, S.; Winstone, J.; Leishman, E.; Wager-Miller, J.; Bradshaw, H.; Mackie, K. Chronic adolescent Δ 9-Tetrahydrocannabinol treatment of male mice leads to long-term cognitive and behavioral dysfunction, which are prevented by concurrent cannabidiol treatment. Cannabis Cannabinoid Res. 2017, 2, 235–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stark, T.; Di Martino, S.; Drago, F.; Wotjak, C.T.; Micale, V. Phytocannabinoids and schizophrenia: Focus on adolescence as a critical window of enhanced vulnerability and opportunity for treatment. Pharmacol. Res. 2021, 174, 105938. [Google Scholar] [CrossRef]
- Renard, J.; Vitalis, T.; Rame, M.; Krebs, M.O.; Lenkei, Z.; Le Pen, G.; Jay, T.M. Chronic cannabinoid exposure during adolescence leads to long-term structural and functional changes in the prefrontal cortex. Eur. Neuropsychopharmacol. 2016, 26, 55–64. [Google Scholar] [CrossRef]
- Gabaglio, M.; Zamberletti, E.; Manenti, C.; Parolaro, D.; Rubino, T. Long-term consequences of adolescent exposure to THC-rich/CBD-poor and CBD-rich/THC-poor combinations: A comparison with pure THC treatment in female rats. Int. J. Mol. Sci. 2021, 22, 8899. [Google Scholar] [CrossRef]
- Schramm-Sapyta, N.L.; Cha, Y.M.; Chaudhry, S.; Wilson, W.A.; Swartzwelder, H.S.; Kuhn, C.M. Differential anxiogenic, aversive, and locomotor effects of THC in adolescent and adult rats. Psychopharmacology 2007, 191, 867–877. [Google Scholar] [CrossRef]
- Dow-Edwards, D.; Izenwasser, S. Pretreatment with Δ9-tetrahydrocannabinol (THC) increases cocaine-stimulated activity in adolescent but not adult male rats. Pharmacol. Biochem. Behav. 2012, 100, 587–591. [Google Scholar] [CrossRef] [Green Version]
- Jacobs, D.S.; Kohut, S.J.; Jiang, S.; Nikas, S.P.; Makriyannis, A.; Bergman, J. Acute and chronic effects of cannabidiol on Δ9-tetrahydrocannabinol (Δ9-THC)-induced disruption in stop signal task performance. Exp. Clin. Psychopharmacol. 2016, 24, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Justinova, Z.; Panlilio, L.V.; Goldberg, S.R. Drug addiction. Curr. Top. Behav. Neurosci. 2009, 1, 309–346. [Google Scholar] [CrossRef]
- Burston, J.J.; Wiley, J.L.; Craig, A.A.; Selley, D.E.; Sim-Selley, L.J. Regional enhancement of cannabinoid CB1 receptor desensitization in female adolescent rats following repeated Delta-tetrahydrocannabinol exposure. Br. J. Pharmacol. 2010, 161, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Fuentes-Verdugo, E.; López-Tolsa, G.E.; Pellón, R.; Miguéns, M. Chronic ∆-9-tetrahydrocannabinol administration delays acquisition of schedule-induced drinking in rats and retains long-lasting effects. Psychopharmacology, 2021; online ahead of print. [Google Scholar] [CrossRef]
- Bailey, C.H.; Kandel, E.R. Structural changes accompanying memory storage. Annu. Rev. Physiol. 1993, 55, 397–426. [Google Scholar] [CrossRef]
- Moser, M.B. Making more synapses: A way to store information? Cell. Mol. Life Sci. 1999, 55, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Rubino, T.; Realini, N.; Braida, D.; Alberio, T.; Capurro, V.; Viganò, D.; Guidali, C.; Sala, M.; Fasano, M.; Parolaro, D. The depressive phenotype induced in adult female rats by adolescent exposure to THC is associated with cognitive impairment and altered neuroplasticity in the prefrontal cortex. Neurotox. Res. 2009, 15, 291–302. [Google Scholar] [CrossRef]
- Steel, R.W.J.; Miller, J.H.; Sim, D.A.; Day, D.J. Delta-9-tetrahydrocannabinol disrupts hippocampal neuroplasticity and neurogenesis in trained, but not untrained adolescent Sprague-Dawley rats. Brain Res. 2014, 1548, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Suárez, I.; Bodega, G.; Ramos, J.A.; Fernández-Ruiz, J.J.; Fernández, B. Neuronal and astroglial response to pre- and perinatal exposure to delta-9-tetra- hydrocannabinol in the rat substantia nigra. Dev. Neurosci. 2000, 22, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Suárez, I.; Bodega, G.; Fernández-Ruiz, J.J.; Ramos, J.A.; Rubio, M.; Fernández, B. Reduced glial fibrillary acidic protein and glutamine synthetase expression in astrocytes and Bergmann glial cells in the rat cerebellum caused by delta(9)-tetrahydrocannabinol administration during development. Dev. Neurosci. 2002, 24, 300–312. [Google Scholar] [CrossRef] [PubMed]
- Gerace, E.; Landucci, E.; Scartabelli, T.; Moroni, F.; Pellegrini-Giampietro, D.E. Rat hippocampal slice culture models for the evaluation of neuroprotective agents. In Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2012; Volume 846, ISBN 9781617795350. [Google Scholar]
- Landucci, E.; Mazzantini, C.; Buonvicino, D.; Pellegrini-Giampietro, D.E.; Bergonzi, M.C. Neuroprotective effects of Thymoquinone by the modulation of ER stress and apoptotic pathway in in vitro model of excitotoxicity. Molecules 2021, 26, 1592. [Google Scholar] [CrossRef] [PubMed]
- Landucci, E.; Mazzantini, C.; Lana, D.; Davolio, P.L.; Giovannini, M.G.; Pellegrini-Giampietro, D.E. Neuroprotective effects of cannabidiol but not Δ9-Tetrahydrocannabinol in rat hippocampal slices exposed to oxygen-glucose deprivation: Studies with Cannabis extracts and selected cannabinoids. Int. J. Mol. Sci. 2021, 22, 9773. [Google Scholar] [CrossRef]
- Gerace, E.; Cialdai, F.; Sereni, E.; Lana, D.; Nosi, D.; Giovannini, M.G.; Monici, M.; Mannaioni, G. NIR Laser photobiomodulation induces neuroprotection in an in vitro model of cerebral hypoxia/ischemia. Mol. Neurobiol. 2021, 58, 5383–5395. [Google Scholar] [CrossRef] [PubMed]
- Cerbai, F.; Lana, D.; Nosi, D.; Petkova-Kirova, P.; Zecchi, S.; Brothers, H.M.; Wenk, G.L.; Giovannini, M.G. The neuron-astrocyte-microglia triad in normal brain ageing and in a model of neuroinflammation in the rat hippocampus. PLoS ONE 2012, 7, e45250. [Google Scholar] [CrossRef]
- Gerace, E.; Landucci, E.; Totti, A.; Bani, D.; Guasti, D.; Baronti, R.; Moroni, F.; Mannaioni, G.; Pellegrini-Giampietro, D.E. Ethanol toxicity during brain development: Alterations of excitatory synaptic transmission in immature organotypic hippocampal slice cultures. Alcohol. Clin. Exp. Res. 2016, 40, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Landucci, E.; Scartabelli, T.; Gerace, E.; Moroni, F.; Pellegrini-Giampietro, D.E. CB1 receptors and post-ischemic brain damage: Studies on the toxic and neuroprotective effects of cannabinoids in rat organotypic hippocampal slices. Neuropharmacology 2011, 60, 674–682. [Google Scholar] [CrossRef]
- Hulse, R.E.; Winterfield, J.; Kunkler, P.E.; Kraig, R.P. Astrocytic clasmatodendrosis in hippocampal organ culture. Glia 2001, 33, 169–179. [Google Scholar] [CrossRef] [Green Version]
- Kreutz, S.; Koch, M.; Ghadban, C.; Korf, H.W.; Dehghani, F. Cannabinoids and neuronal damage: Differential effects of THC, AEA and 2-AG on activated microglial cells and degenerating neurons in excitotoxically lesioned rat organotypic hippocampal slice cultures. Exp. Neurol. 2007, 203, 246–257. [Google Scholar] [CrossRef]
- Sales, A.J.; Fogaça, M.V.; Sartim, A.G.; Pereira, V.S.; Wegener, G.; Guimarães, F.S.; Joca, S.R.L. Cannabidiol induces rapid and sustained antidepressant-like effects through increased BDNF signaling and synaptogenesis in the prefrontal cortex. Mol. Neurobiol. 2019, 56, 1070–1081. [Google Scholar] [CrossRef]
- Fogaça, M.V.; Campos, A.C.; Coelho, L.D.; Duman, R.S.; Guimarães, F.S. The anxiolytic effects of cannabidiol in chronically stressed mice are mediated by the endocannabinoid system: Role of neurogenesis and dendritic remodeling. Neuropharmacology 2018, 135, 22–33. [Google Scholar] [CrossRef]
- Ahmed, K.T.; Amin, M.R.; Shah, P.; Ali, D.W. Motor neuron development in zebrafish is altered by brief (5-hr) exposures to THC (∆ 9-tetrahydrocannabinol) or CBD (cannabidiol) during gastrulation. Sci. Rep. 2018, 8, 10518. [Google Scholar] [CrossRef] [Green Version]
- Volterra, A.; Meldolesi, J. Astrocytes, from brain glue to communication elements: The revolution continues. Nat. Rev. Neurosci. 2005, 6, 626–640. [Google Scholar] [CrossRef] [PubMed]
- Maragakis, N.J.; Rothstein, J.D. Mechanisms of Disease: Astrocytes in neurodegenerative disease. Nat. Clin. Pract. Neurol. 2006, 2, 679–689. [Google Scholar] [CrossRef]
- Takuma, K.; Baba, A.; Matsuda, T. Astrocyte apoptosis: Implications for neuroprotection. Prog. Neurobiol. 2004, 72, 111–127. [Google Scholar] [CrossRef]
- Pekny, M.; Wilhelmsson, U.; Pekna, M. The dual role of astrocyte activation and reactive gliosis. Neurosci. Lett. 2014, 565, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Tilleux, S.; Hermans, E. Neuroinflammation and regulation of glial glutamate uptake in neurological disorders. J. Neurosci. Res. 2007, 85, 2059–2070. [Google Scholar] [CrossRef] [PubMed]
- Navarrete, M.; Araque, A. Endocannabinoids potentiate synaptic transmission through stimulation of astrocytes. Neuron 2010, 68, 113–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salio, C.; Doly, S.; Fischer, J.; Franzoni, M.F.; Conrath, M. Neuronal and astrocytic localization of the cannabinoid receptor-1 in the dorsal horn of the rat spinal cord. Neurosci. Lett. 2002, 329, 13–16. [Google Scholar] [CrossRef]
- Parpura, V.; Basarsky, T.A.; Liu, F.; Jeftinija, K.; Jeftinija, S.; Haydon, P.G. Glutamate-mediated astrocyte-neuron signalling. Nature 1994, 369, 744–747. [Google Scholar] [CrossRef]
- Perea, G.; Araque, A. Astrocytes potentiate transmitter release at single hippocampal synapses. Science 2007, 317, 1083–1086. [Google Scholar] [CrossRef]
- Araque, A.; Carmignoto, G.; Haydon, P.G.; Oliet, S.H.R.; Robitaille, R.; Volterra, A. Gliotransmitters travel in time and space. Neuron 2014, 81, 728–739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hegyi, Z.; Oláh, T.; Koszeghy, Á.; Pisticelli, F.; Holló, K.; Pál, B.; Csernoch, L.; Di Marzo, V.; Antal, M. CB 1 receptor activation induces intracellular Ca 2+ mobilization and 2-arachidonoylglycerol release in rodent spinal cord astrocytes. Sci. Rep. 2018, 8, 10562. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.J.; Kim, J.E.; Yeo, S.I.; Kang, T.C. p65/RelA-Ser529 NF-κB subunit phosphorylation induces autophagic astroglial death (Clasmatodendrosis) following status epilepticus. Cell. Mol. Neurobiol. 2011, 31, 1071. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.S.B.; Bayir, H.; Chu, C.T.; Alber, S.M.; Kochanek, P.M.; Watkins, S.C. Autophagy is increased in mice after traumatic brain injury and is detectable in human brain after trauma and critical illness. Autophagy 2008, 4, 88–90. [Google Scholar] [CrossRef] [Green Version]
- Navarrete, M.; Perea, G.; de Sevilla, D.F.; Gómez-Gonzalo, M.; Núñez, A.; Martín, E.D.; Araque, A. Astrocytes mediate in vivo cholinergic-induced synaptic plasticity. PLoS Biol. 2012, 10, e1001259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verkhratsky, A.; Parpura, V.; Rodríguez, J.J. Where the thoughts dwell: The physiology of neuronal-glial “diffuse neural net”. Brain Res. Rev. 2011, 66, 133–151. [Google Scholar] [CrossRef]
- Kucukdereli, H.; Allen, N.J.; Lee, A.T.; Feng, A.; Ozlu, M.I.; Conatser, L.M.; Chakraborty, C.; Workman, G.; Weaver, M.; Sage, E.H.; et al. Control of excitatory CNS synaptogenesis by astrocyte-secreted proteins hevin and SPARC. Proc. Natl. Acad. Sci. USA 2011, 108, E440–E449. [Google Scholar] [CrossRef] [Green Version]
- Christopherson, K.S.; Ullian, E.M.; Stokes, C.C.A.; Mullowney, C.E.; Hell, J.W.; Agah, A.; Lawler, J.; Mosher, D.F.; Bornstein, P.; Barres, B.A. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell 2005, 120, 421–433. [Google Scholar] [CrossRef] [Green Version]
- Diniz, L.P.; Almeida, J.C.; Tortelli, V.; Lopes, C.V.; Setti-Perdigão, P.; Stipursky, J.; Kahn, S.A.; Romão, L.F.; De Miranda, J.; Alves-Leon, S.V.; et al. Astrocyte-induced synaptogenesis is mediated by transforming growth factor β signaling through modulation of D-serine levels in cerebral cortex neurons. J. Biol. Chem. 2012, 287, 41432–41445. [Google Scholar] [CrossRef]
- Diniz, L.P.; Matias, I.C.P.; Garcia, M.N.; Gomes, F.C.A. Astrocytic control of neural circuit formation: Highlights on TGF-beta signaling. Neurochem. Int. 2014, 78, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Diniz, L.P.; Tortelli, V.; Matias, I.; Morgado, J.; Araujo, A.P.B.; Melo, H.M.; Seixas da Silva, G.S.; Alves-Leon, S.V.; de Souza, J.M.; Ferreira, S.T.; et al. Astrocyte transforming growth factor beta 1 protects synapses against Aβ oligomers in Alzheimer’s disease Model. J. Neurosci. 2017, 37, 6797–6809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sofroniew, M.V.; Vinters, H.V. Astrocytes: Biology and pathology. Acta Neuropathol. 2010, 119, 7–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.J.; Mannaioni, G.; Yuan, H.; Woo, D.H.; Gingrich, M.B.; Traynelis, S.F. Astrocytic control of synaptic NMDA receptors. J. Physiol. 2007, 581, 1057–1081. [Google Scholar] [CrossRef] [PubMed]
- Pfrieger, F.W. Roles of glial cells in synapse development. Cell. Mol. Life Sci. 2009, 66, 2037–2047. [Google Scholar] [CrossRef] [Green Version]
- Heneka, M.T.; Rodríguez, J.J.; Verkhratsky, A. Neuroglia in neurodegeneration. Brain Res. Rev. 2010, 63, 189–211. [Google Scholar] [CrossRef]
- Rodríguez, J.J.; Olabarria, M.; Chvatal, A.; Verkhratsky, A. Astroglia in dementia and Alzheimer’s disease. Cell Death Differ. 2009, 16, 378–385. [Google Scholar] [CrossRef] [Green Version]
- Jouroukhin, Y.; Zhu, X.; Shevelkin, A.V.; Hasegawa, Y.; Abazyan, B.; Saito, A.; Pevsner, J.; Kamiya, A.; Pletnikov, M.V. Adolescent Δ 9-Tetrahydrocannabinol exposure and astrocyte-specific genetic vulnerability converge on nuclear factor-κB-cyclooxygenase-2 signaling to impair memory in adulthood. Biol. Psychiatry 2019, 85, 891–903. [Google Scholar] [CrossRef]
- de Assis Lima, I.V.; Bellozi, P.M.Q.; Batista, E.M.; Vilela, L.R.; Brandão, I.L.; Ribeiro, F.M.; Moraes, M.F.D.; Moreira, F.A.; de Oliveira, A.C.P. Cannabidiol anticonvulsant effect is mediated by the PI3Kγ pathway. Neuropharmacology 2020, 176, 108156. [Google Scholar] [CrossRef]
- Esposito, G.; Scuderi, C.; Valenza, M.; Togna, G.I.; Latina, V.; de Filippis, D.; Cipriano, M.; Carratù, M.R.; Iuvone, T.; Steardo, L. Cannabidiol reduces Aβ-induced neuroinflammation and promotes hippocampal neurogenesis through PPARγ involvement. PLoS ONE 2011, 6, e28668. [Google Scholar] [CrossRef]
- Zhang, Y.; Barres, B.A. Astrocyte heterogeneity: An underappreciated topic in neurobiology. Curr. Opin. Neurobiol. 2010, 20, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Khakh, B.S.; Sofroniew, M.V. Diversity of astrocyte functions and phenotypes in neural circuits. Nat. Neurosci. 2015, 18, 942–952. [Google Scholar] [CrossRef]
- Ben Haim, L.; Rowitch, D.H. Functional diversity of astrocytes in neural circuit regulation. Nat. Rev. Neurosci. 2017, 18, 31–41. [Google Scholar] [CrossRef]
- Khakh, B.S.; Deneen, B. The emerging nature of astrocyte diversity. Annu. Rev. Neurosci. 2019, 42, 187–207. [Google Scholar] [CrossRef]
- Pestana, F.; Edwards-Faret, G.; Belgard, T.G.; Martirosyan, A.; Holt, M.G. No longer underappreciated: The emerging concept of astrocyte heterogeneity in neuroscience. Brain Sci. 2020, 10, 168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, A.P.; Shah, A.S.; Aperi, B.V.; Kurpad, S.N.; Stemper, B.D.; Glavaski-Joksimovic, A. Acute death of astrocytes in blast-exposed rat organotypic hippocampal slice cultures. PLoS ONE 2017, 12, e0173167. [Google Scholar] [CrossRef] [Green Version]
- Burda, J.E.; Sofroniew, M.V. Reactive gliosis and the multicellular response to CNS damage and disease. Neuron 2014, 81, 229–248. [Google Scholar] [CrossRef] [Green Version]
- Kesner, A.J.; Lovinger, D.M. Cannabis use, abuse, and withdrawal: Cannabinergic mechanisms, clinical, and preclinical findings. J. Neurochem. 2021, 157, 1674–1696. [Google Scholar] [CrossRef]
- Morales, P.; Jagerovic, N. Synthetic and natural derivatives of cannabidiol. Adv. Exp. Med. Biol. 2021, 1297, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Vitale, R.M.; Iannotti, F.A.; Amodeo, P. The (poly)pharmacology of cannabidiol in neurological and neuropsychiatric disorders: Molecular mechanisms and targets. Int. J. Mol. Sci. 2021, 22, 4876. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Landucci, E.; Mazzantini, C.; Lana, D.; Giovannini, M.G.; Pellegrini-Giampietro, D.E. Neuronal and Astrocytic Morphological Alterations Driven by Prolonged Exposure with Δ9-Tetrahydrocannabinol but Not Cannabidiol. Toxics 2022, 10, 48. https://doi.org/10.3390/toxics10020048
Landucci E, Mazzantini C, Lana D, Giovannini MG, Pellegrini-Giampietro DE. Neuronal and Astrocytic Morphological Alterations Driven by Prolonged Exposure with Δ9-Tetrahydrocannabinol but Not Cannabidiol. Toxics. 2022; 10(2):48. https://doi.org/10.3390/toxics10020048
Chicago/Turabian StyleLanducci, Elisa, Costanza Mazzantini, Daniele Lana, Maria Grazia Giovannini, and Domenico E. Pellegrini-Giampietro. 2022. "Neuronal and Astrocytic Morphological Alterations Driven by Prolonged Exposure with Δ9-Tetrahydrocannabinol but Not Cannabidiol" Toxics 10, no. 2: 48. https://doi.org/10.3390/toxics10020048
APA StyleLanducci, E., Mazzantini, C., Lana, D., Giovannini, M. G., & Pellegrini-Giampietro, D. E. (2022). Neuronal and Astrocytic Morphological Alterations Driven by Prolonged Exposure with Δ9-Tetrahydrocannabinol but Not Cannabidiol. Toxics, 10(2), 48. https://doi.org/10.3390/toxics10020048








