Residual Levels of Mercury, Cadmium, Lead and Arsenic in Some Commercially Key Species from Italian Coasts (Adriatic Sea): Focus on Human Health
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Chemical Analyses and Instrumental Conditions
2.3. Quality Control and Assurance
2.4. Health Risk Assessment
2.4.1. Estimated Intake
2.4.2. Target Hazard Quotient and Hazard Index
2.4.3. Incremental Lifetime Cancer Risk
2.4.4. Estimation of Safe Monthly Consumption Rate
2.5. Statistical Analysis
3. Results and Discussion
3.1. Metal(loid) Levels and Accumulation Versus Fish Length
3.2. Comparison with Literature Data
3.3. Health Risk Assessment
3.3.1. Metal(loid) Concentrations vs. International Dietary Standards
3.3.2. Estimated Intake
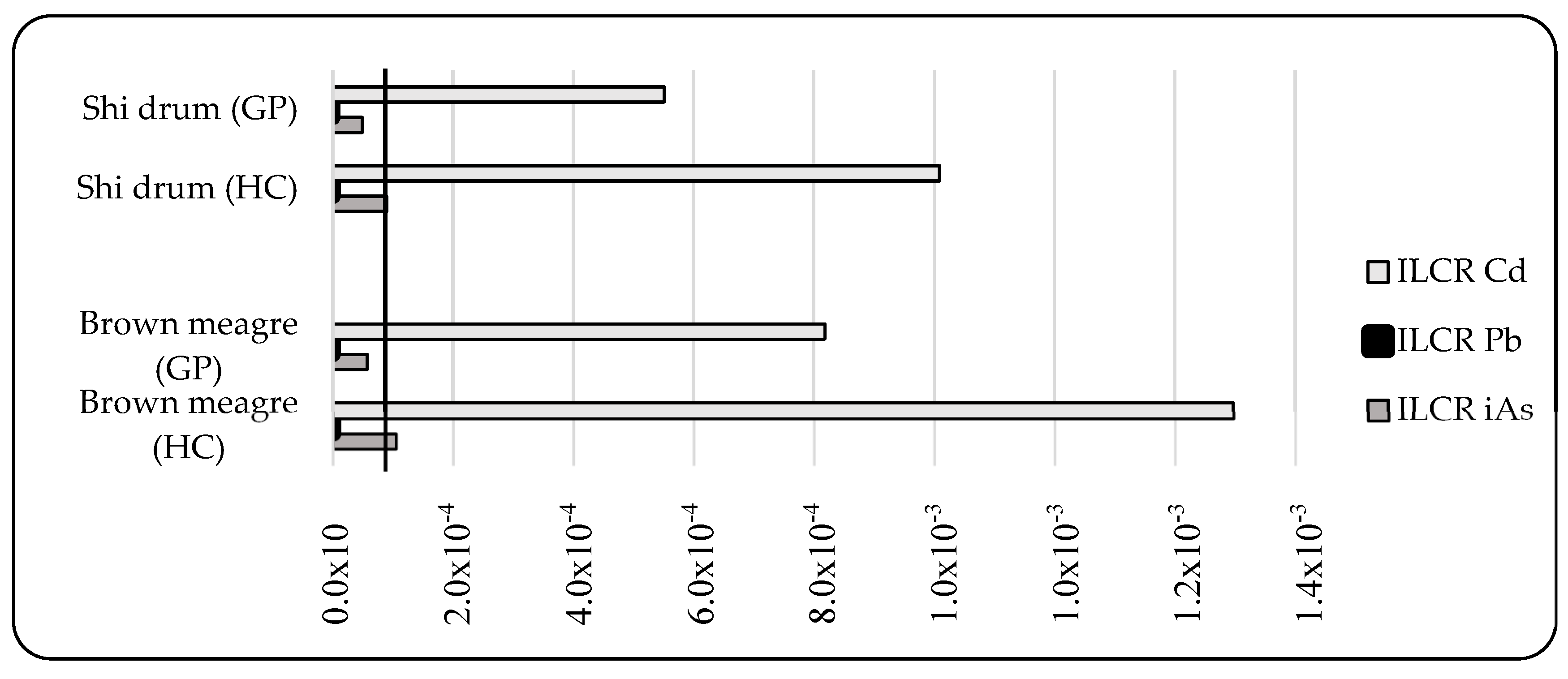
3.3.3. Non-Carcinogenic and Carcinogenic Risk Assessment
3.3.4. Estimation of Safe Monthly Consumption Rate
4. Uncertainties and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Food and Agriculture Organization (FAO). The State of World Fisheries and Aquaculture 2016. Contributing to Food Security and Nutrition for All; FAO: Rome, Italy, 2016; Available online: https://www.fao.org/3/i5555e/i5555e.pdf (accessed on 25 February 2022).
- Food and Agriculture Organization (FAO). The State of World Fisheries and Aquaculture 2014. Opportunities and Challenges; FAO: Rome, Italy, 2014; Available online: https://www.fao.org/3/i3720e/i3720e.pdf (accessed on 25 February 2022).
- Shibabaw, T. Omega-3 polyunsaturated fatty acids: Anti-inflammatory and anti-hypertriglyceridemia mechanisms in cardiovascular disease. Mol. Cell. Biochem. 2021, 476, 993–1003. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency (US EPA); Food and Drug Administration (FDA). Advice about Eating Fish; US EPA: Washington, DC, USA; FDA: Silver Spring, MD, USA, 2021. Available online: https://www.fda.gov/media/102331/download (accessed on 10 February 2022).
- United Nations Nutrition (UN Nutrition). The Role of Aquatic Foods in Sustainable Healthy Diets. 2021. Available online: https://www.unnutrition.org/wp-content/uploads/FINAL-UN-Nutrition-Aquatic-foods-Paper_EN_pdf (accessed on 12 February 2022).
- World Health Organization (WHO). Preventing Disease through Healthy Environments. In Action Is Needed on Chemicals of Major Public Health Concern. Public Health and Environment; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef] [PubMed]
- González, N.; Marquès, M.; Nadal, M.; Domingo, J.L. Temporal trend of the dietary exposure to metals/metalloids: A case study in Tarragona County, Spain. Food Res. Int. 2021, 147, 110469. [Google Scholar] [CrossRef] [PubMed]
- Serviere-Zaragoza, E.; Lluch-Cota, S.E.; Mazariegos-Villarreal, A.; Balart, E.F.; Valencia-Valdez, H.; Méndez-Rodríguez, L.C. Cadmium, Lead, Copper, Zinc, and Iron Concentration Patterns in Three Marine Fish Species from Two Different Mining Sites inside the Gulf of California, Mexico. Int. J. Environ. Res. Public Health 2021, 18, 844. [Google Scholar] [CrossRef]
- Takarina, N.D.; Purwiyanto, A.I.S.; Suteja, Y. Cadmium (Cd), Copper (Cu), and Zinc (Zn) levels in commercial and non-commercial fishes in the Blanakan River Estuary, Indonesia: A preliminary study. Mar. Pollut. Bull. 2021, 170, 112607. [Google Scholar] [CrossRef] [PubMed]
- Uroko, R.I.; Agbafor, A.; Egba, S.I.; Uchenna, O.N.; Sangodare, R.S.A.; Nwuke, C.P.; Nwanosike, O.K. Heavy metal contents in commercial fishes consumed in Umuahia and their associated human health risks. EQA 2020, 39, 11–19. [Google Scholar]
- Da Silva, C.A.; de Oliveira Santos, S.; Borges Garcia, C.A.; de Pontes, G.C.; Wasserman, J.C. Metals and arsenic in marine fish commercialized in the NE Brazil: Risk to human health. Hum. Ecol. Risk Assess. 2020, 26, 695–712. [Google Scholar] [CrossRef]
- European Market Observatory for Fisheries and Aquaculture Products (EUMOFA). Il Mercato Ittico Dell’ue. 2020. Available online: http://www.eumofa.eu (accessed on 23 February 2022).
- Storelli, A.; Barone, G.; Dambrosio, A.; Garofalo, R.; Busco, A.; Storelli, M.M. Occurrence of trace metals in fish from South Italy: Assessment risk to consumer’s health. J. Food Comp. Anal. 2020, 90, 103487. [Google Scholar] [CrossRef]
- Di Bella, C.; Traina, A.; Giosuè, C.; Carpintieri, D.; Lo Dico, G.M.; Bellante, A.; Del Core, M.; Falco, F.; Gherardi, S.; Uccello, M.M.; et al. Heavy Metals and PAHs in Meat, Milk, and Seafood From Augusta Area (Southern Italy): Contamination Levels, Dietary Intake, and Human Exposure Assessment. Front. Public Health 2020, 8, 273. [Google Scholar] [CrossRef]
- Barone, G.; Storelli, A.; Garofalo, R.; Busco, V.P.; Quaglia, N.C.; Centrone, G.; Storelli, M.M. Assessment of mercury and cadmium via seafood consumption in Italy: Estimated dietary intake (EWI) and target hazard quotient (THQ). Food Add. Contam. Part A 2015, 32, 1277–1286. [Google Scholar] [CrossRef]
- Iamiceli, A.L.; Ubaldi, A.; Lucchetti, D.; Brambilla, G.; Abate, V.; De Felip, E.; De Filippis, S.P.; Dellatte, E.; De Luca, S.; Ferri, F.; et al. Metals in Mediterranean aquatic species. Mar. Pollut. Bull. 2015, 94, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Copat, C.; Arena, G.; Fiore, M.; Ledda, C.; Fallico, R.; Sciacca, S.; Ferrante, M. Heavy metals concentrations in fish and shellfish from eastern Mediterranean Sea: Consumption advisories. Food Chem. Toxicol. 2013, 53, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Picciulin, M.; Bolgan, M.; Corò, A.B.; Calcagno, G.; Malavasi, S. Sound production by the Shi drum Umbrina cirrosa and comparison with the brown meagre Sciaena umbra: A passive acoustic monitoring perspective. J. Fish Biol. 2016, 88, 1655–1660. [Google Scholar] [CrossRef] [PubMed]
- Gazzetta Ufficiale Della Repubblica Italiana (GURI). Metodi di Analisi per la Ricerca di Residui di Metalli Pesanti e Arsenico; GURI: Rome, Italy, 1994. [Google Scholar]
- Leclercq, C.; Arcella, D.; Piccinelli, R.; Sette, S.; Le Donne, C.; Turrini, A. The Italian National Food Consumption Survey INRAN-SCAI 2005-06: Main results in terms of food consumption. Public Health Nutr. 2009, 12, 2504–2532. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency (US EPA). Guidance for Assessing Chemical Contaminant Data for Use in Fish Advisories, Volume 2: Risk Assessment and Fish Consumption Limits, 3rd ed.; United States Environmental Protection Agency (USEPA): Washington, DC, USA, 2000.
- European Food Safety Authority (EFSA). Scientific opinion on the risk for public health related to the presence of mercury and methylmercury in food. EFSA J. 2012, 10, 2985. [Google Scholar]
- Joint FAO/WHO Expert Committee on Food Additives (JECFA). Summary and Conclusions of the Sixty-First Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). 2003. Available online: http://www.who.int/pcs/jecfa/Summary61.pdf (accessed on 20 February 2022).
- Joint FAO/WHO Expert Committee on Food Additives (JECFA). Safety evaluation of certain food additives and contaminants. WHO Food Add. Ser. 2000, 44, 273–312. [Google Scholar]
- Joint FAO/WHO Expert Committee on Food Additives (JECFA). Summary and Conclusions. Issued 24 June 2010. In Proceedings of the Seventy-Third Meeting, Geneva, Switzerland, 8–17 June 2010; Available online: https://www.who.int/foodsafety/publications/chem/summary73.pdf (accessed on 25 February 2022).
- European Food Safety Authority (EFSA). Scientific opinion on arsenic in food. EFSA J. 2009, 7, 199–1351. [Google Scholar]
- United States Environmental Protection Agency (US EPA). Risk Assessment Guidance for Superfund, Volume I: Human Health Evaluation Manual (Part A), Interim Final; EPA 540/1-89/002; US EPA: Washington, DC, USA, 1989.
- United States Environmental Protection Agency (US EPA). Risk Assessment and Fish Consumption Limits, 3rd ed.; EPA 823-B-00-008. USA, Office of Water; US EPA: Washington, DC, USA, 2000.
- United States Environmental Protection Agency (US EPA). Regional Screening Level (RSL) Summary Table (TR = 1E−06, HQ = 1); United States Environmental Protection Agency: Philadelphia, PA, USA, 2021.
- United States Environmental Protection Agency (US EPA). Risk Assessment Guidance for Superfund, Volume I: Human Health Evaluation Manual (Part E, Supplemental Guidance for Dermal Risk Assessment); EPA/540/R/99/005; USA Office of Emerage and Remedial Response: Washington DC, USA, 2001.
- United States Environmental Protection Agency (US EPA). Supplemental Guidance for Developing Soil Screening Levels for Superfund Sites Office of Solid Waste and Emergency Response; OSWER9355.4-24; US EPA: Washington DC, USA, 2002. Available online: https://rais.ornl.gov/documents/SSG_nonrad_supplemental.pdf (accessed on 18 February 2022).
- Diaz, C.; Galindo, L.; Garcia Montelongo, F. Distribution of metals in some fishes from Santa Cruz de Tenerife, Canary Islands. Bull. Environ. Contam. Toxicol. 1994, 52, 374–381. [Google Scholar] [CrossRef]
- The Commission of the European Communities. Commission Regulation (EU) No. 1881/2006 of 19 December 2006. Setting Maximum Levels for Certain Contaminants in Foodstuffs. Off. J. Eur. Union 2006, 364, 5–24. [Google Scholar]
- The European Commission. Commission Regulation (EU) No. 488/2014 of 12 May 2014 Amending Regulation (EC) No. 1881/2006 as regards maximum levels of cadmium in foodstuffs. Off. J. Eur. Union 2014, 138, 75–79. [Google Scholar]
- The European Commission. Commission Regulation (EU) No. 1005/2015 of 25 June 2015 Amending Regulation (EC) No. 1881/2006 as Regards Maximum Levels of Lead in Certain Foodstuffs. Off. J. Eur. Union 2015, 161, 9–13. [Google Scholar]
- Agência Nacional de Vigilância Sanitária (ANVISA). Limites Máximos de Contaminantes Inorgânicos em Alimentos, No. 168; Diário Oficial da República do Brasil: Brasília, Brazil, 2013. [Google Scholar]
- Food Standards Australia and New Zealand (FSANZ). Australia New Zealand Food Standards Code, Standard 1.4.1, Contaminants and Natural Toxicants. 2013. Available online: http://www.legislation.gov.au/Details/F2013C00140/ (accessed on 17 February 2022).
- Miller, M.E.; Hamann, M.; Kroon, F.J. Bioaccumulation and biomagnification of microplastics in marine organisms: A review and meta-analysis of current data. PLoS ONE 2020, 15, e0240792. [Google Scholar] [CrossRef] [PubMed]
- Stergiou, K.I.; Karpouzi, V.S. Feeding habits and trophic levels of Mediterranean fish. Rev. Fish Biol. Fish. 2002, 11, 217–254. [Google Scholar] [CrossRef]
- Lobry, J.; Mourand, L.; Rochard, E.; Elie, P. Structure of the Gironde estuarine fish assemblages: A comparison of European estuaries perspective. Aquat. Living Resour. 2003, 16, 47–58. [Google Scholar] [CrossRef]
- Engin, S.; Seyhan, K. Age, growth, sexual maturity and food composition of Sciaena umbra in the south-eastern Black Sea, Turkey. J. Appl. Ichthyol. 2009, 25, 96–99. [Google Scholar] [CrossRef]
- Adams, D.H.; McMichael, R.H., Jr. Mercury in king mackerel, Scomberomorus cavalla, and Spanish mackerel, S. maculatus, from waters of the south-eastern USA: Regional and historical trends. Mar. Freshw. Res. 2007, 58, 187–193. [Google Scholar] [CrossRef]
- Dali Youcef, N.; Benguedda, W.; Youbi, F. Levels of heavy metals (Zn, Pb, Cu, Cd, Ni) in tissues of Sarpa salpa from Honaine bay in the Western part of the Algerian Coast. Desalin. Water Treat. 2014, 52, 2850–2855. [Google Scholar] [CrossRef]
- Noël, L.; Chekri, R.; Millour, S.; Merlo, M.; Leblanc, J.-C.; Guérin, T. Distribution and relationships of As, Cd, Pb and Hg in freshwater fish from five French fishing areas. Chemosphere 2013, 90, 1900–1910. [Google Scholar] [CrossRef]
- Rainbow, P.S.; White, S.L. Comparative strategies of heavy metal accumulation by crustaceans: Zinc, copper and cadmium in a decapod, an amphipod and a barnacle. Hydrobiologia 1989, 174, 245–262. [Google Scholar] [CrossRef]
- Annibaldi, A.; Truzzi, C.; Illuminati, S.; Scarponi, G. Recent sudden decrease of lead in Adriatic coastal seawater during the years 2000–2004 in parallel with the phasing out of leaded gasoline in Italy. Mar. Chem. 2009, 113, 238–249. [Google Scholar] [CrossRef]
- Vieira, C.; Morais, S.; Ramos, S.; Delerue-Matos, C.; Oliveira, M.B.P.P. Mercury, cadmium, lead and arsenic levels in three pelagic fish species from the Atlantic Ocean: Intra- and inter-specific variability and human health risks for consumption. Food Chem. Toxicol. 2011, 49, 923–932. [Google Scholar] [CrossRef] [PubMed]
- Ourgaud, M.; Ruitton, S.; Bourgogne, H.; Bustamante, P.; Churlaud, C.; Guillou, G.; Lebreton, B.; Harmelin-Vivien, M.L. Trace elements in a Mediterranean scorpaenid fish: Bioaccumulation processes and spatial variations. Prog. Oceanogr. 2018, 163, 184–195. [Google Scholar] [CrossRef]
- Ramos-Miras, J.J.; Sanchez-Muros, M.J.; Morote, E.; Torrijos, M.; Gil, C.; Zamani-Ahmadm, R. Potentially toxic elements in commonly consumed fish species from the western Mediterranean Sea (Almería Bay): Bioaccumulation in liver and muscle tissues in relation to biometric parameters. Sci. Tot. Environ. 2019, 671, 280–287. [Google Scholar] [CrossRef]
- Jezierska, B.; Witeska, M. The metal uptake and accumulation in fish living in polluted waters. In Soil and Water Pollution Monitoring, Protection and Remediation; Twardowska, I., Allen, H.E., Häggblom, M.M., Stefaniak, S., Eds.; NATO Science Series, 69; Springer: Dordrecht, The Netherlands, 2006. [Google Scholar]
- Luoma, S.N.; Rainbow, P.S. Why is metal bioaccumulation so variable? Biodynamics as a unifying concept. Environ. Sci. Technol. 2005, 39, 1921–1931. [Google Scholar] [CrossRef] [PubMed]
- Al-Asadi, M.K.K. Evaluation of marine pollution by mercury from petrochemical hot spot, west of Libya. Mesopot. J. Mar. Sci. 2018, 33, 49–56. [Google Scholar]
- Banana, A.A.S.; Radin Mohamed, R.M.S.; Al-Gheethi, A.A.S. Mercury pollution for marine environment at Farwa Island, Libya. J. Environ. Health Sci. Eng. 2016, 14, 5. [Google Scholar] [CrossRef]
- Bat, L.; Sezgin, M.; Üstün, F.; Şahin, F. Heavy Metal Concentrations in Ten Species of Fishes Caught in Sinop Coastal Waters of the Black Sea, Turkey. Turk. J. Fish. Aquat. Sci. 2012, 12, 371–376. [Google Scholar] [CrossRef]
- Türkmen, M.; Türkmen, A.; Tepe, Y.; Ateş, A.; Gökkuş, K. Determination of metal contaminations in sea foods from Marmara, Aegean and Mediterranean seas: Twelve fish species. Food Chem. 2008, 108, 794–800. [Google Scholar] [CrossRef]
- Celik, U.; Cakli, S.; Oehlenschlager, J. Determination of the lead and cadmium burden in some northeastern Atlantic and Mediterranean fish species by DPSAV. Eur. Food Res. Technol. 2004, 218, 298–305. [Google Scholar] [CrossRef]
- Kütter, V.T.; Moreira, V.A.; Kütter, M.T.; Silva-Filho, E.V.; Marques, E.D.; Garnier, J.; Bidone, E.D. Metal bioavailability and distribution in the fish community in a tropical estuary, Sepetiba Bay, Rio de Janeiro, Brazil. Geochim. Bras. 2021, 35, 17–38. [Google Scholar] [CrossRef]
- Moreau, M.F.; Surico-Bennett, J.; Vicario-Fisher, M.; Gerads, R.; Gersberg, R.M.; Hurlbert, S.H. Selenium, arsenic, DDT and other contaminants in four fish species in the Salton Sea, California, their temporal trends, and their potential impact on human consumers and wildlife. Lake Reserv. Manag. 2007, 23, 536–569. [Google Scholar] [CrossRef][Green Version]
- Greene, R.; Crecelius, E. Total and Inorganic Arsenic in Mid-Atlantic Marine Fish and Shellfish and Implications for Fish Advisories. Integr. Environ. Assess. Manag. 2006, 2, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Agency for Toxic Substances & Disease Registry (ATSDR). Public Health Assessment Guidance Manual. Appendix G: Calculating Exposure Doses. 2005. Available online: http://www.atsdr.cdc.gov/hac/phamanual/appg.html (accessed on 9 March 2022).
- Joint FAO/WHO Expert Committee on Food Additives (JECFA). In Proceedings of the FAO/WHO Expert Committee on Food Additives. Summary and Conclusions, Seventy-second Meeting, Rome, Italy, 16–25 February 2010; Available online: http://www.fao.org/3/a-at868e.pdf (accessed on 20 February 2022).
- International Agency for Research on Cancer (IARC). Agents Classified by the IARC Monographs; IARC: Lyon, France, 2016; pp. 1–116. Available online: http://monographs.iarc.fr/ENG/Classification/latest_classif.php (accessed on 23 February 2022).

| Length Range (cm) | N° Specimens | Hg | Cd | Pb | As | ||
|---|---|---|---|---|---|---|---|
| Umbrina cirrosa (Shi drum) | 28.5–30.4 | 40 | 0.11 ± 0.03 | 0.05 ± 0.02 | 0.12 ± 0.03 | 0.58 ± 0.08 | |
| 30.5–32.4 | 36 | 0.18 ± 0.05 | 0.06 ± 0.02 | 0.09 ± 0.02 | 0.61 ± 0.09 | ||
| 32.5–34.4 | 38 | 0.22 ± 0.04 | 0.09 ± 0.02 | 0.12 ± 0.03 | 0.49 ± 0.07 | ||
| 34.5–36.4 | 23 | 0.18 ± 0.05 | 0.06 ± 0.02 | 0.08 ± 0.02 | 0.66 ± 0.10 | ||
| 36.5–38.4 | 10 | 0.20 ± 0.05 | 0.07 ± 0.03 | 0.10 ± 0.03 | 0.60 ± 0.10 | ||
| Total 147 | Min–Max Average ± St. Dev. | 0.11–0.22 0.18 ± 0.04 | 0.05–0.09 0.07 ± 0.02 | 0.08–0.12 0.10 ± 0.02 | 0.49–0.66 0.59 ± 0.06 | ||
| Sciaena umbra (Brown meagre) | 20.5–22.4 | 32 | 0.14 ± 0.03 | 0.12 ± 0.03 | 0.12 ± 0.02 | 0.66 ± 0.09 | |
| 22.5–23.4 | 34 | 0.09 ± 0.02 | 0.13 ± 0.03 | 0.12 ± 0.03 | 0.58 ± 0.07 | ||
| 23.5–24.4 | 23 | 0.18 ± 0.03 | 0.10 ± 0.03 | 0.16 ± 0.03 | 0.78 ± 0.11 | ||
| 24.5–25.4 | 25 | 0.21 ± 0.04 | 0.07 ± 0.02 | 0.10 ± 0.03 | 0.61 ± 0.10 | ||
| 25.5–26.4 | 12 | 0.33 ± 0.04 | 0.07 ± 0.02 | 0.09 ± 0.02 | 0.80 ± 0.12 | ||
| Total 126 | Min-Max Average ± St. Dev. | 0.09–0.33 0.19 ± 0.09 | 0.07–0.13 0.10 ± 0.03 | 0.09–0.16 0.12 ± 0.03 | 0.58–0.80 0.69 ± 0.10 | ||
| MPLs | 0.50 1 | 0.05 1 | 0.30 1 | 1.0 2, 2.0 3 |
| EWIHg | PTMICd | EWIPb | EDIiAs | |||||
|---|---|---|---|---|---|---|---|---|
| GP | HC | GP | HC | GP | HC | GP | HC | |
| Umbrina cirrosa (Shi drum) | 0.43–0.86 | 0.78–1.57 | 0.84–1.50 | 1.53–2.75 | 0.31–0.47 | 0.57–0.86 | 0.03–0.04 | 0.05–0.07 |
| 0.69 ± 0.16 | 1.27 ± 0.30 | 1.10 ± 0.25 | 2.02 ± 0.46 | 0.40 ± 0.07 | 0.73 ± 0.13 | 0.03 ± 0.003 | 0.06 ± 0.01 | |
| Sciaena umbra (Brown meagre) | 0.35–1.29 | 0.64–2.35 | 1.17–2.17 | 2.14–3.97 | 0.35–0.62 | 0.64–1.14 | 0.03–0.04 | 0.06–0.08 |
| 0.74 ± 0.35 | 1.35 ± 0.64 | 1.64 ± 0.46 | 2.99 ± 0.85 | 0.46 ± 0.10 | 0.84 ± 0.19 | 0.04 ± 0.01 | 0.07 ± 0.01 | |
| PTWI/PTMI | Hg 1:4; MeHg 2:1.6 | 25 | 25 | - | ||||
| BMDL01 | - | - | - | 0.3–8 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barone, G.; Storelli, A.; Garofalo, R.; Mallamaci, R.; Storelli, M.M. Residual Levels of Mercury, Cadmium, Lead and Arsenic in Some Commercially Key Species from Italian Coasts (Adriatic Sea): Focus on Human Health. Toxics 2022, 10, 223. https://doi.org/10.3390/toxics10050223
Barone G, Storelli A, Garofalo R, Mallamaci R, Storelli MM. Residual Levels of Mercury, Cadmium, Lead and Arsenic in Some Commercially Key Species from Italian Coasts (Adriatic Sea): Focus on Human Health. Toxics. 2022; 10(5):223. https://doi.org/10.3390/toxics10050223
Chicago/Turabian StyleBarone, Grazia, Arianna Storelli, Rita Garofalo, Rosanna Mallamaci, and Maria Maddalena Storelli. 2022. "Residual Levels of Mercury, Cadmium, Lead and Arsenic in Some Commercially Key Species from Italian Coasts (Adriatic Sea): Focus on Human Health" Toxics 10, no. 5: 223. https://doi.org/10.3390/toxics10050223
APA StyleBarone, G., Storelli, A., Garofalo, R., Mallamaci, R., & Storelli, M. M. (2022). Residual Levels of Mercury, Cadmium, Lead and Arsenic in Some Commercially Key Species from Italian Coasts (Adriatic Sea): Focus on Human Health. Toxics, 10(5), 223. https://doi.org/10.3390/toxics10050223








