Basic Exploratory Study of Bisphenol A (BPA) Dietary Administration to Istrian Pramenka Rams and Male Toxicity Investigation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animals and Their Environment
2.3. BPA Exposure Protocol
2.4. Sampling Protocols
2.4.1. Blood Plasma
2.4.2. Gross Morphology and Processing of Organs
2.5. Blood Plasma BPA Determination
2.6. Toxicokinetics
2.7. Histopathology of Testes and Epididymides
Testis Histomorphometry
2.8. Semen Analyses
2.9. Statistical Analyses
3. Results
3.1. Rams’ Exposure to BPA
3.2. Mass Measurements
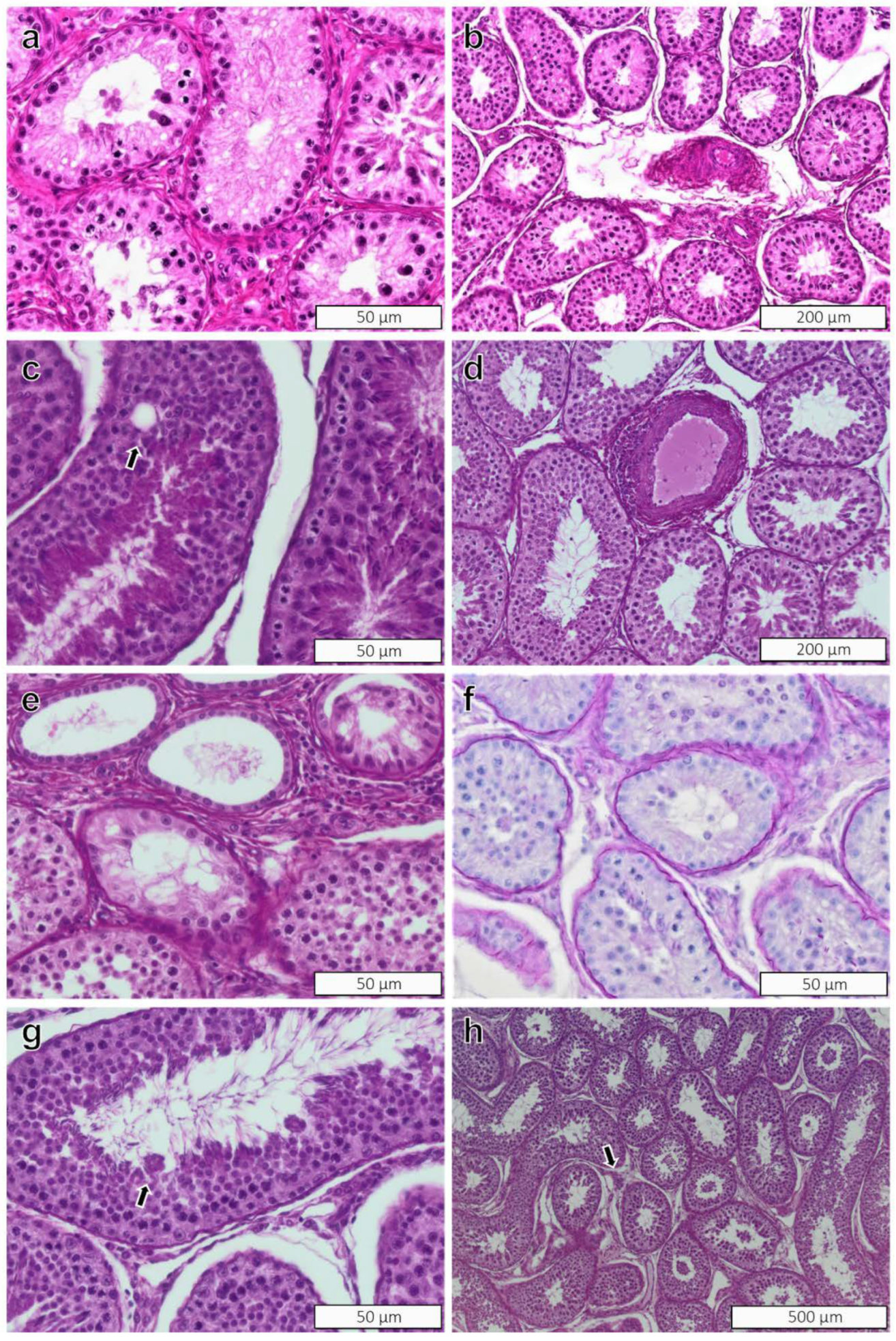
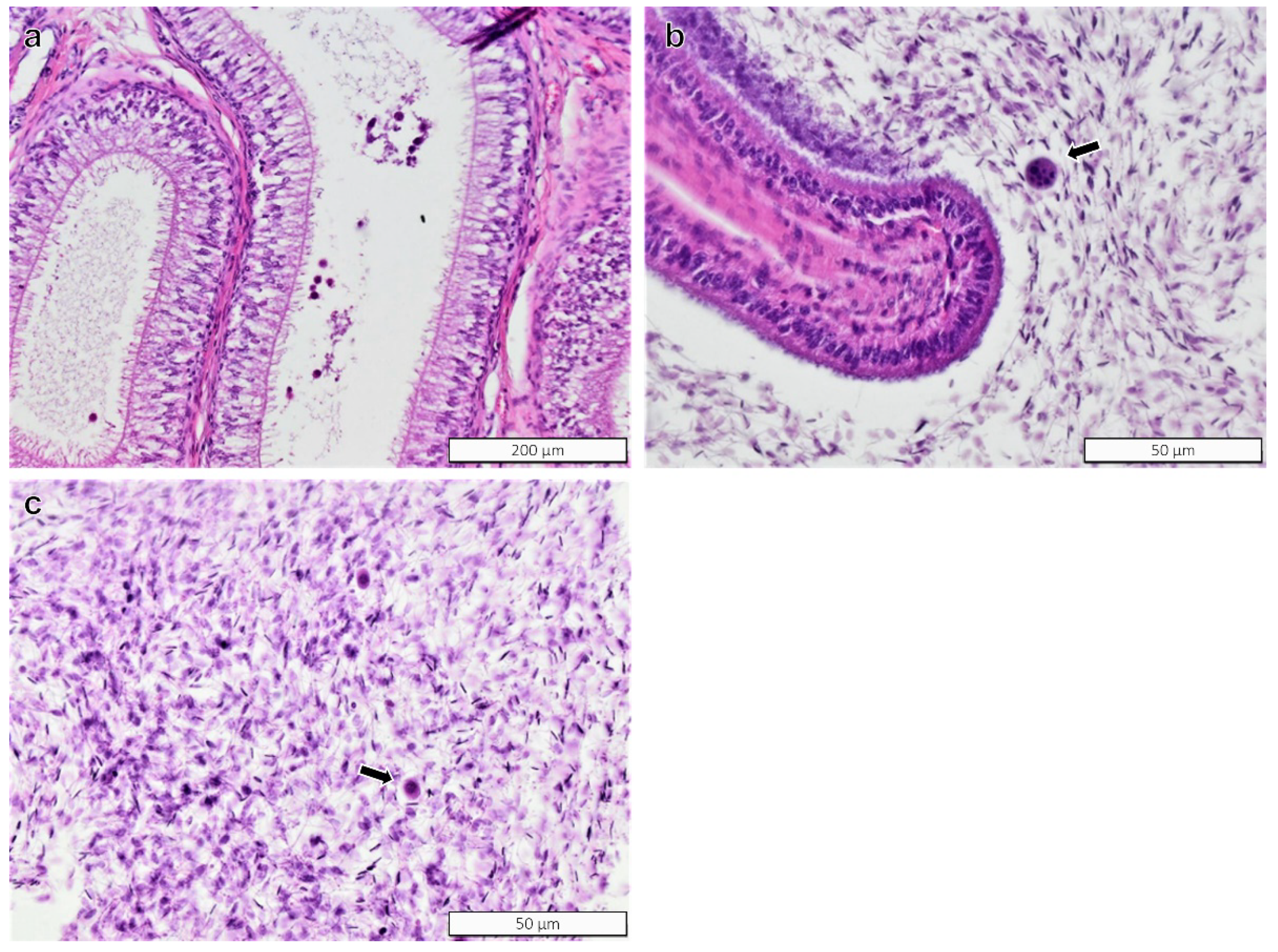
3.3. Histopathology
3.3.1. Testes
3.3.2. Epididymides
3.4. Morphometric Measurements
3.5. Spermatozoa Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sweeney, T.; Fox, J.; Robertson, L.; Kelly, G.; Duffy, P.; Lonergan, P.; O’Doherty, J.; Roche, J.F.; Evans, N.P. Postnatal exposure to octylphenol decreases semen quality in the adult ram. Theriogenology 2007, 67, 1068–1075. [Google Scholar] [CrossRef]
- Staples, C.A.; Dorn, P.B.; Klecka, G.M.; O’Block, S.T.; Harris, L.R. A review of the environmental fate, effects, and exposures of bisphenol A. Chemosphere 1998, 36, 2149–2173. [Google Scholar] [CrossRef]
- Peretz, J.; Vrooman, L.; Ricke, W.A.; Hunt, P.A.; Ehrlich, S.; Hauser, R.; Padmanabhan, V.; Taylor, H.S.; Swan, S.H.; VandeVoort, C.A.; et al. Bisphenol A and Reproductive Health: Update of Experimental and Human Evidence, 2007–2013. Environ. Health Perspect. 2014, 122, 775–786. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF). Scientific Opinion on the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs. EFSA J. 2015, 13, 3978. [Google Scholar] [CrossRef]
- EFSA Bisphenol A: EFSA Draft Opinion Proposes Lowering the Tolerable Daily Intake. Available online: https://www.efsa.europa.eu/en/news/bisphenol-efsa-draft-opinion-proposes-lowering-tolerable-daily-intake (accessed on 27 January 2022).
- Castellini, C.; Totaro, M.; Parisi, A.; D’Andrea, S.; Lucente, L.; Cordeschi, G.; Francavilla, S.; Francavilla, F.; Barbonetti, A. Bisphenol A and Male Fertility: Myths and Realities. Front. Endocrinol. 2020, 11, 353. [Google Scholar] [CrossRef]
- Spörndly-Nees, E.; Boberg, J.; Ekstedt, E.; Holm, L.; Fakhrzadeh, A.; Dunder, L.; Kushnir, M.M.; Lejonklou, M.H.; Lind, P.M. Low-dose exposure to Bisphenol A during development has limited effects on male reproduction in midpubertal and aging Fischer 344 rats. Reprod. Toxicol. 2018, 81, 196–206. [Google Scholar] [CrossRef]
- Li, D.K.; Zhou, Z.; Miao, M.; He, Y.; Wang, J.; Ferber, J.; Herrinton, L.J.; Gao, E.; Yuan, W. Urine bisphenol-A (BPA) level in relation to semen quality. Fertil. Steril. 2011, 95, 625–630.e4. [Google Scholar] [CrossRef]
- Chitra, K.C.; Latchoumycandane, C.; Mathur, P.P. Induction of oxidative stress by bisphenol A in the epididymal sperm of rats. Toxicology 2003, 185, 119–127. [Google Scholar] [CrossRef]
- Li, Y.-J.; Song, T.-B.; Cai, Y.-Y.; Zhou, J.-S.; Song, X.; Zhao, X.; Wu, X.-L. Bisphenol A Exposure Induces Apoptosis and Upregulation of Fas/FasL and Caspase-3 Expression in the Testes of Mice. Toxicol. Sci. 2009, 108, 427–436. [Google Scholar] [CrossRef] [Green Version]
- Ullah, A.; Pirzada, M.; Jahan, S.; Ullah, H.; Shaheen, G.; Rehman, H.; Siddiqui, M.F.; Butt, M.A. Bisphenol A and its analogs bisphenol B, bisphenol F, and bisphenol S: Comparative in vitro and in vivo studies on the sperms and testicular tissues of rats. Chemosphere 2018, 209, 508–516. [Google Scholar] [CrossRef]
- Toyama, Y.; Suzuki-Toyota, F.; Maekawa, M.; Ito, C.; Toshimori, K. Adverse effects of bisphenol A to spermiogenesis in mice and rats. Arch. Histol. Cytol. 2004, 67, 373–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Wang, Z.; Liu, F. Chronic exposure of BPA impairs male germ cell proliferation and induces lower sperm quality in male mice. Chemosphere 2021, 262, 127880. [Google Scholar] [CrossRef] [PubMed]
- Wisniewski, P.; Romano, R.M.; Kizys, M.M.; Oliveira, K.C.; Kasamatsu, T.; Giannocco, G.; Chiamolera, M.I.; Dias-da-Silva, M.R.; Romano, M.A. Adult exposure to bisphenol A (BPA) in Wistar rats reduces sperm quality with disruption of the hypothalamic-pituitary-testicular axis. Toxicology 2015, 329, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Kourouma, A.; Duan, P.; Quan, C.; Yamia, M.L.T.; Liu, C.; Wang, C.; Fu, W.; Qi, S.; Yu, T.; Yang, K. Bisphenol A induced reactive oxygen species (ROS) in the liver and affect epididymal semen quality in adults Sprague-Dawley rats. J. Toxicol. Environ. Health Sci. 2014, 6, 103–112. [Google Scholar]
- Ogo, F.M.; Siervo, G.; Goncalves, G.D.; Cecchini, R.; Guarnier, F.A.; Anselmo-Franci, J.A.; Fernandes, G.S.A. Low doses of bisphenol A can impair postnatal testicular development directly, without affecting hormonal or oxidative stress levels. Reprod. Fertil. Dev. 2017, 29, 2245–2254. [Google Scholar] [CrossRef]
- De Flora, S.; Micale, R.T.; La Maestra, S.; Izzotti, A.; D’Agostini, F.; Camoirano, A.; Davoli, S.A.; Troglio, M.G.; Rizzi, F.; Davalli, P.; et al. Upregulation of Clusterin in Prostate and DNA Damage in Spermatozoa from Bisphenol A-Treated Rats and Formation of DNA Adducts in Cultured Human Prostatic Cells. Toxicol. Sci. 2011, 122, 45–51. [Google Scholar] [CrossRef]
- El-Beshbishy, H.A.; Aly, H.A.; El-Shafey, M. Lipoic acid mitigates bisphenol A-induced testicular mitochondrial toxicity in rats. Toxicol. Ind. Health 2013, 29, 875–887. [Google Scholar] [CrossRef]
- Jiang, X.; Yin, L.; Zhang, N.; Han, F.; Liu, W.-b.; Zhang, X.; Chen, H.-q.; Cao, J.; Liu, J.-y. Bisphenol A induced male germ cell apoptosis via IFNβ-XAF1-XIAP pathway in adult mice. Toxicol. Appl. Pharmacol. 2018, 355, 247–256. [Google Scholar] [CrossRef]
- Dobrzyńska, M.M.; Radzikowska, J. Genotoxicity and reproductive toxicity of bisphenol A and X-ray/bisphenol A combination in male mice. Drug Chem. Toxicol. 2013, 36, 19–26. [Google Scholar] [CrossRef]
- Tian, J.; Ding, Y.; She, R.; Ma, L.; Du, F.; Xia, K.; Chen, L. Histologic study of testis injury after bisphenol A exposure in mice. Toxicol. Ind. Health 2017, 33, 36–45. [Google Scholar] [CrossRef]
- Al-Hiyasat, A.S.; Darmani, H.; Elbetieha, A.M. Effects of bisphenol A on adult male mouse fertility. Eur. J. Oral Sci. 2002, 110, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.F.; Liu, M.; Li, N.; Luo, T.; Zheng, L.P.; Zeng, X.H. Bisphenol A Impairs Mature Sperm Functions by a CatSper-Relevant Mechanism. Toxicol. Sci. 2016, 152, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, D.; Yanagiba, Y.; Duan, Z.; Ito, Y.; Okamura, A.; Asaeda, N.; Tagawa, Y.; Li, C.; Taya, K.; Zhang, S.Y.; et al. Bisphenol A may cause testosterone reduction by adversely affecting both testis and pituitary systems similar to estradiol. Toxicol. Lett. 2010, 194, 16–25. [Google Scholar] [CrossRef]
- Sakaue, M.; Ohsako, S.; Ishimura, R.; Kurosawa, S.; Kurohmaru, M.; Hayashi, Y.; Aoki, Y.; Yonemoto, J.; Tohyama, C. Bisphenol-A Affects Spermatogenesis in the Adult Rat Even at a Low Dose. J. Occup. Health 2001, 43, 185–190. [Google Scholar] [CrossRef] [Green Version]
- Kazemi, S.; Feizi, F.; Aghapour, F.; Joorsaraee, G.A.; Moghadamnia, A.A. Histopathology and Histomorphometric Investigation of Bisphenol A and Nonylphenol on the Male Rat Reproductive System. N. Am. J. Med. Sci. 2016, 8, 215–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srivastava, S.; Gupta, P. Alteration in apoptotic rate of testicular cells and sperms following administration of Bisphenol A (BPA) in Wistar albino rats. Environ. Sci. Pollut. Res. 2018, 25, 21635–21643. [Google Scholar] [CrossRef] [PubMed]
- Gurmeet, K.; Rosnah, I.; Normadiah, M.K.; Das, S.; Mustafa, A.M. Detrimental effects of bisphenol A on development and functions of the male reproductive system in experimental rats. EXCLI J. 2014, 13, 151–160. [Google Scholar]
- Qiu, L.L.; Wang, X.; Zhang, X.H.; Zhang, Z.; Gu, J.; Liu, L.; Wang, Y.; Wang, S.L. Decreased androgen receptor expression may contribute to spermatogenesis failure in rats exposed to low concentration of bisphenol A. Toxicol. Lett. 2013, 219, 116–124. [Google Scholar] [CrossRef]
- Wang, P.; Luo, C.; Li, Q.; Chen, S.; Hu, Y. Mitochondrion-mediated apoptosis is involved in reproductive damage caused by BPA in male rats. Environ. Toxicol. Pharmacol. 2014, 38, 1025–1033. [Google Scholar] [CrossRef]
- Jin, P.; Wang, X.; Chang, F.; Bai, Y.; Li, Y.; Zhou, R.; Chen, L. Low dose bisphenol A impairs spermatogenesis by suppressing reproductive hormone production and promoting germ cell apoptosis in adult rats. J. Biomed. Res. 2013, 27, 135–144. [Google Scholar] [CrossRef]
- Karnam, S.S.; Ghosh, R.C.; Mondal, S.; Mondal, M. Evaluation of subacute bisphenol—A toxicity on male reproductive system. Vet. World 2015, 8, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, D.; Vanage, G. Mutagenic effect of Bisphenol A on adult rat male germ cells and their fertility. Reprod. Toxicol. 2013, 40, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Duan, W.; Li, R.; Xu, S.; Zhang, L.; Chen, C.; He, M.; Lu, Y.; Wu, H.; Pi, H.; et al. Exposure to bisphenol A disrupts meiotic progression during spermatogenesis in adult rats through estrogen-like activity. Cell Death Dis. 2013, 4, e676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Cruz, S.C.; Jubendradass, R.; Mathur, P.P. Bisphenol A induces oxidative stress and decreases levels of insulin receptor substrate 2 and glucose transporter 8 in rat testis. Reprod. Sci. 2012, 19, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Vijaykumar, T.; Singh, D.; Vanage, G.R.; Dhumal, R.V.; Dighe, V.D. Bisphenol A-induced ultrastructural changes in the testes of common marmoset. Indian J. Med. Res. 2017, 146, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Gotardo, A.T.; Pavanelli, E.L.; Carvalho, H.F.; Lemes, K.M.; Arruda, R.P.; Kempinas, W.G.; Górniak, S.L. Endocrine disrupter action in ruminants: A study of the effects of Ipomoea carnea in adult male goats. Small Rumin. Res. 2014, 119, 81–87. [Google Scholar] [CrossRef]
- Kacew, S.; Ruben, Z.; McConnell, R.F. Strain as a determinant factor in the differential responsiveness of rats to chemicals. Toxicol. Pathol. 1995, 23, 701–714. [Google Scholar] [CrossRef] [Green Version]
- Vandenberg, L.N.; Hauser, R.; Marcus, M.; Olea, N.; Welshons, W.V. Human exposure to bisphenol A (BPA). Reprod. Toxicol. 2007, 24, 139–177. [Google Scholar] [CrossRef]
- Guignard, D.; Gauderat, G.; Gayrard, V.; Lacroix, M.Z.; Picard-Hagen, N.; Puel, S.; Toutain, P.L.; Viguie, C. Characterization of the contribution of buccal absorption to internal exposure to bisphenol A through the diet. Food Chem. Toxicol. 2016, 93, 82–88. [Google Scholar] [CrossRef]
- Guignard, D.; Gayrard, V.; Lacroix, M.Z.; Puel, S.; Picard-Hagen, N.; Viguie, C. Evidence for bisphenol A-induced disruption of maternal thyroid homeostasis in the pregnant ewe at low level representative of human exposure. Chemosphere 2017, 182, 458–467. [Google Scholar] [CrossRef]
- Collet, S.H.; Picard-Hagen, N.; Lacroix, M.Z.; Puel, S.; Viguie, C.; Bousquet-Melou, A.; Toutain, P.L.; Gayrard, V. Allometric scaling for predicting human clearance of bisphenol A. Toxicol. Appl. Pharmacol. 2015, 284, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Vandenberg, L.N.; Chahoud, I.; Heindel, J.J.; Padmanabhan, V.; Paumgartten, F.J.; Schoenfelder, G. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environ. Health Perspect. 2010, 118, 1055–1070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Šturm, S.; Grabnar, I.; Škibin, A.; Pogačnik, M.; Cerkvenik-Flajs, V. Preliminary toxicokinetic study of BPA in lactating dairy sheep after repeated dietary and subcutaneous administration. Sci. Rep. 2020, 10, 6498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Republic of Slovenia. Slovenian Animal Protection Act (Zakon o zaščiti živali). Uradni list Republike Slovenije. Off. Gaz. Rep. Slov. 2013, 13, 4455–4466. [Google Scholar]
- European Union. Directive 2010/63/EU on the protection of animals used for scientific purposes. Off. J. Eur. Union 2010, L276, 33–79. [Google Scholar]
- Berndston, W.E.; Desjardins, C. The cycle of the seminiferous epithelium and spermatogenesis in the bovine testis. Am. J. Anat. 1974, 140, 167–179. [Google Scholar] [CrossRef]
- Clermont, Y.; Leblond, C.P. Spermiogenesis of man, monkey, ram and other mammals as shown by the periodic acid-Schiff technique. Am. J. Anat. 1955, 96, 229–253. [Google Scholar] [CrossRef]
- Šturm, S.; Švara, T.; Spörndly-Nees, E.; Cerkvenik-Flajs, V.; Gombač, M.; Weber, A.L.; Weber, K. Seminiferous epithelium cycle staging based on the development of the acrosome in ram testis. J. Toxicol. Pathol. 2021, 34, 331–338. [Google Scholar] [CrossRef]
- Lanning, L.L.; Creasy, D.M.; Chapin, R.E.; Mann, P.C.; Barlow, N.J.; Regan, K.S.; Goodman, D.G. Recommended approaches for the evaluation of testicular and epididymal toxicity. Toxicol. Pathol. 2002, 30, 507–520. [Google Scholar] [CrossRef] [Green Version]
- Creasy, D.; Bube, A.; de Rijk, E.; Kandori, H.; Kuwahara, M.; Masson, R.; Nolte, T.; Reams, R.; Regan, K.; Rehm, S.; et al. Proliferative and nonproliferative lesions of the rat and mouse male reproductive system. Toxicol. Pathol. 2012, 40, 40s–121s. [Google Scholar] [CrossRef] [Green Version]
- Premrov Bajuk, B.; Pihlar, T.; Pogačnik, N.; Klinc, P. Dialysis of the goat semen and its effect on the quality of frozen/thawed spermatozoa processed in the presence of egg yolk. Anim. Reprod. Sci. 2018, 198, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Team, R.C. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 8 February 2022).
- Prins, G.S.; Patisaul, H.B.; Belcher, S.M.; Vandenberg, L.N. CLARITY-BPA academic laboratory studies identify consistent low-dose Bisphenol A effects on multiple organ systems. Basic Clin. Pharmacol. Toxicol. 2019, 125 (Suppl. S3), 14–31. [Google Scholar] [CrossRef] [PubMed]
- Volkel, W. Why did researchers not use realistic doses in animal studies of bisphenol A? Arch. Toxicol. 2017, 91, 1519–1522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, T.; Cao, Y.; Wang, H.; Wang, P.; Wang, X.; Niu, H.; Shao, C. The Effect of Exposure to Bisphenol A on Spermatozoon and the Expression of Tight Junction Protein Occludin in Male Mice. Dose-Response A Publ. Int. Hormesis Soc. 2020, 18, 1559325820926745. [Google Scholar] [CrossRef]
- Chitra, K.C.; Rao, K.R.; Mathur, P.P. Effect of bisphenol A and co-administration of bisphenol A and vitamin C on epididymis of adult rats: A histological and biochemical study. Asian J. Androl. 2003, 5, 203–208. [Google Scholar]
- Camacho, L.; Lewis, S.M.; Vanlandingham, M.M.; Olson, G.R.; Davis, K.J.; Patton, R.E.; Twaddle, N.C.; Doerge, D.R.; Churchwell, M.I.; Bryant, M.S.; et al. A two-year toxicology study of bisphenol A (BPA) in Sprague-Dawley rats: CLARITY-BPA core study results. Food Chem. Toxicol. 2019, 132, 110728. [Google Scholar] [CrossRef]
- Dere, E.; Anderson, L.M.; Huse, S.M.; Spade, D.J.; McDonnell-Clark, E.; Madnick, S.J.; Hall, S.J.; Camacho, L.; Lewis, S.M.; Vanlandingham, M.M.; et al. Effects of continuous bisphenol A exposure from early gestation on 90 day old rat testes function and sperm molecular profiles: A CLARITY-BPA consortium study. Toxicol. Appl. Pharmacol. 2018, 347, 1–9. [Google Scholar] [CrossRef]
- Brankovič, J.; Jakob, L.; Šturm, S.; Cerkvenik Flajs, V.; Osredkar, J.; Šterpin, S.; Pogorevc, E.; Antolinc, D.; Vrecl, M. Experimental exposure to bisphenol A at low doses has minimal effects on bone tissue in growing rams. Correspondence University of Ljubljana, Veterinary Faculty, Ljubljana, Slovenia. 2022; to be submitted. [Google Scholar]
- Affinisep, 53. 2015. Available online: www.affinisep.com (accessed on 23 March 2022).
- European Union. Commission Decision 2002/657/EC of 12 August 2002 Implementing Council Directive 96/23/EC concerning the Performance of Analytical Methods and the Interpretation of Results. Off. J. Eur. Commun. 2002, L221, 8–36. [Google Scholar]


| Control (n = 7) Mean ± SD | Treated (n = 7) Mean ± SD | |
|---|---|---|
| Body weight (kg) | 54.2 ± 7.4 | 52.6 ± 4.1 |
| Testis, left (g) | 150.4 ± 74.1 | 120.8 ± 27.2 |
| Testis, right (g) | 150.8 ± 69.3 | 118.4 ± 27.2 |
| Right Testis | Left Testis | Rete Testis | |||||
|---|---|---|---|---|---|---|---|
| Endpoint Examined | Control Group | Treated Group | Control Group | Treated Group | Endpoint Examined | Control Group | Treated Group |
| Perivasculitis | 0/6 | 0/6 | 0/6 | 1/6 | Mineralization | 1/6 | 0/6 |
| Sertoli-only tubules | 0/6 | 0/6 | 0/6 | 0/6 | Multinucleated cells | 1/6 | 0/6 |
| Segmental hypoplasia | 1/6 | 1/6 | 1/6 | 0/6 | Mononuclear infiltrates | 0/6 | 0/6 |
| Vacuolation of Sertoli cells | 0/6 | 0/6 | 1/6 | 0/6 | Fibrosis | 0/6 | 0/6 |
| Multinucleated cells | 2/6 | 1/6 | 4/6 | 1/6 | |||
| Mononuclear infiltrates | 5/6 | 5/6 | 4/6 | 6/6 | |||
| Sperm retention | 0/6 | 0/6 | 0/6 | 0/6 | |||
| Sperm head phagocytosis in the basal Sertoli cell cytoplasm | 0/6 | 0/6 | 0/6 | 0/6 | |||
| Leydig cells atrophy | 0/6 | 0/6 | 0/6 | 0/6 | |||
| Leydig cells hypertrophy/hyperplasia | 0/6 | 0/6 | 0/6 | 0/6 | |||
| Leydig cell vacuolation | 0/6 | 0/6 | 0/6 | 0/6 | |||
| Endpoint Examined | Incidence of Findings in Control Rams | Incidence of Findings in Treated Rams | |
|---|---|---|---|
| Head of epididymis | Mononuclear infiltrates | 3/6 | 4/6 |
| Pyknotic sperm | 0/6 | 0/6 | |
| Sloughed epithelial cells | 0/6 | 0/6 | |
| Intraepithelial fusion cysts | 0/6 | 1/6 | |
| Sperm granuloma | 0/6 | 0/6 | |
| Body of epididymis | Mononuclear infiltrates | 4/6 | 5/6 |
| Pyknotic sperm | 0/6 | 0/6 | |
| Sloughed epithelial cells | 0/6 | 0/6 | |
| Intraepithelial fusion cysts | 0/6 | 0/6 | |
| Sperm granuloma | 0/6 | 0/6 | |
| Tail of epididymis | Mononuclear infiltrates | 2/6 | 3/6 |
| Pyknotic sperm | 0/6 | 2/6 | |
| Sloughed epithelial cells | 0/6 | 1/6 | |
| Intraepithelial fusion cysts | 0/6 | 0/6 | |
| Sperm granuloma | 0/6 | 0/6 |
| Control (n = 6) Mean ± SD | Treated (n = 6) Mean ± SD | |
|---|---|---|
| Seminiferous epithelial height (μm) | 58.44 ± 6.97 | 48.71 ± 2.34 |
| Seminiferous tubule diameter (μm) | 92.28 ± 9.04 | 89.48 ± 6.35 |
| Seminiferous tubular area (mm2) | 26,988 ± 5301 | 25,255 ± 3453 |
| Head of Epididymis | Body of Epididymis | Tail of Epididymis | Ductus deferens | |||||
|---|---|---|---|---|---|---|---|---|
| Parameter | Control (n = 6) | Treated (n = 6) | Control (n = 6) | Treated (n = 6) | Control (n = 6) | Treated (n = 6) | Control (n = 6) | Treated (n = 6) |
| Concentration (×108) | 1.2 ± 0.9 | 0.9 ± 0.3 | 1.4 ± 1.6 | 1.1 ± 0.7 | 6.4 ± 1.6 | 4.4 ± 0.4 | 0.4 ± 0.4 | 0.3 ± 0.2 |
| HOST (% of live sperm) | 54 ± 6 | 56 ± 11 | 64 ± 9 | 64 ± 11 | 71 ± 8 | 67 ± 8 | 61 ± 7 | 56 ± 17 |
| Motility (%) | 4.6 ± 5.2 | 1.1 ± 0.6 | 25.7 ± 15.1 | 24.1 ± 22.1 | 96.1 ± 1.1 | 84.7 ± 16.4 | 78.7 ± 27.5 | 55.3 ± 28.6 |
| Progressive motility (%) | 0.2 ± 0.4 | 0 ± 0 | 2.9 ± 3.5 | 3.8 ± 3.7 | 35.1 ± 5.1 | 30.4 ± 14.8 | 29.1 ± 11.3 | 22 ± 16.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šturm, S.; Weber, K.; Klinc, P.; Spörndly-Nees, E.; Fakhrzadeh, A.; Knific, T.; Škibin, A.; Fialová, V.; Okazaki, Y.; Razinger, T.; et al. Basic Exploratory Study of Bisphenol A (BPA) Dietary Administration to Istrian Pramenka Rams and Male Toxicity Investigation. Toxics 2022, 10, 224. https://doi.org/10.3390/toxics10050224
Šturm S, Weber K, Klinc P, Spörndly-Nees E, Fakhrzadeh A, Knific T, Škibin A, Fialová V, Okazaki Y, Razinger T, et al. Basic Exploratory Study of Bisphenol A (BPA) Dietary Administration to Istrian Pramenka Rams and Male Toxicity Investigation. Toxics. 2022; 10(5):224. https://doi.org/10.3390/toxics10050224
Chicago/Turabian StyleŠturm, Sabina, Klaus Weber, Primož Klinc, Ellinor Spörndly-Nees, Azadeh Fakhrzadeh, Tanja Knific, Andrej Škibin, Věra Fialová, Yoshimasa Okazaki, Tanja Razinger, and et al. 2022. "Basic Exploratory Study of Bisphenol A (BPA) Dietary Administration to Istrian Pramenka Rams and Male Toxicity Investigation" Toxics 10, no. 5: 224. https://doi.org/10.3390/toxics10050224






