Copper Monitoring in Vineyard Soils of Central Italy Subjected to Three Antifungal Treatments, and Effects of Sub-Lethal Copper Doses on the Earthworm Eisenia fetida
Abstract
:1. Introduction
2. Materials and Methods
2.1. Monitoring Campaign
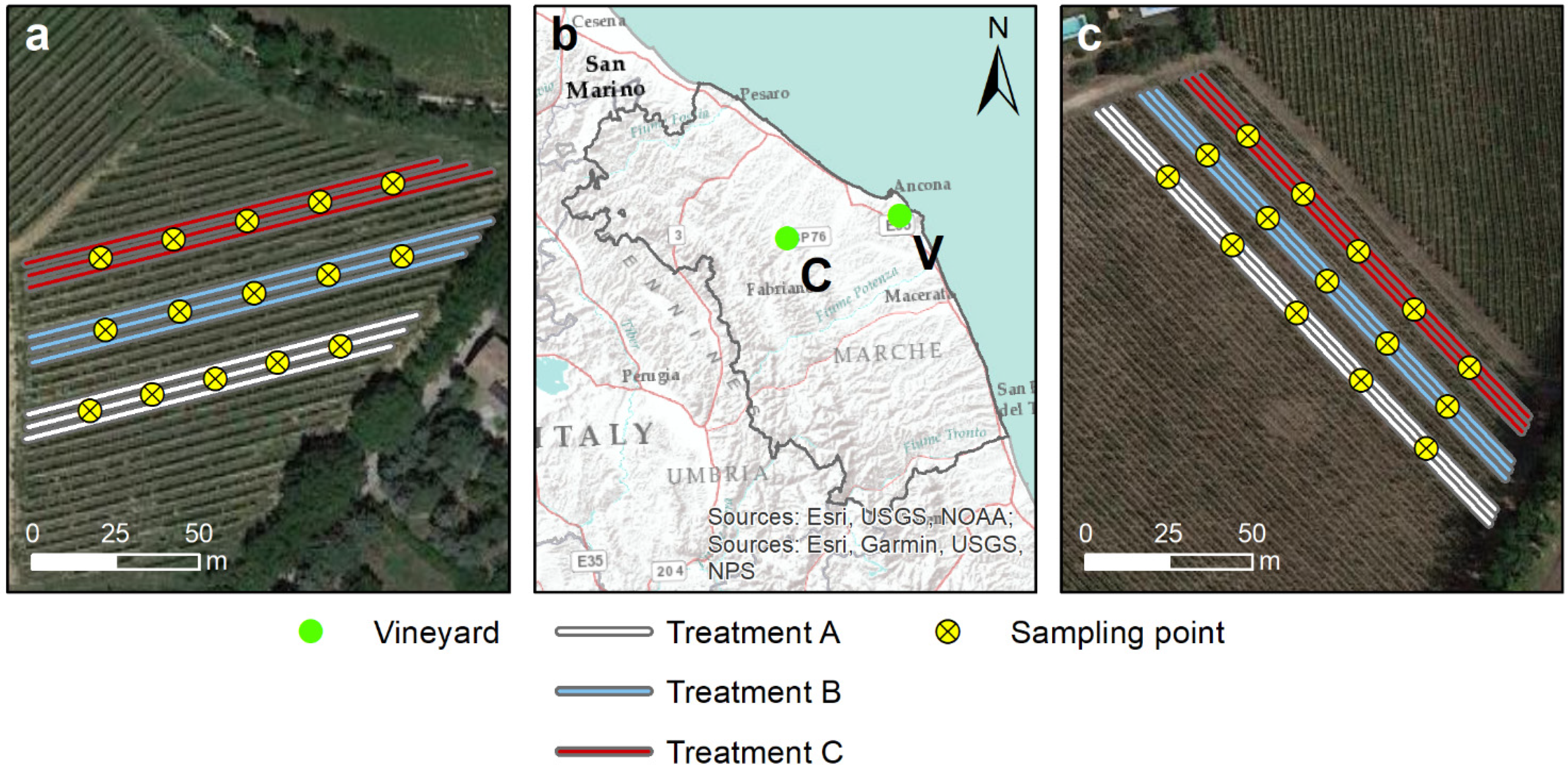
2.1.1. Study Areas
2.1.2. Sampling
- (A)
- Copper treatments (use of copper-based products for the entire growing season);
- (B)
- Alternate treatments (use of copper in the first part of the season and chitosan at 0.5% of p.a. after flowering);
- (C)
- Chitosan treatments (use of 0.5% p.a. chitosan for the entire growing season).
2.2. Ecotoxicological Study on Earthworm Eisenia fetida
2.2.1. Experimental Design
2.2.2. Comet Assay
2.3. Copper Analyses
2.4. Statistical Analysis
3. Results
3.1. Monitoring Study
3.2. Ecotoxicological Study on Earthworm Eisenia fetida
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Rai, P.K.; Lee, S.S.; Zhang, M.; Tsang, Y.F.; Kim, K.H. Heavy metals in food crops: Health risks, fate, mechanisms, and management. Environ. Int. 2019, 125, 365–385. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Pandita, S.; Singh Sidhu, G.P.; Sharma, A.; Khanna, K.; Kaur, P.; Bali, A.S.; Setia, R. Copper bioavailability, uptake, toxicity and tolerance in plants: A comprehensive review. Chemosphere 2021, 262, 127810. [Google Scholar] [CrossRef] [PubMed]
- Gaetke, L.M.; Chow-Johnson, H.S.; Chow, C.K. Copper: Toxicological relevance and mechanisms. Arch. Toxicol. 2014, 88, 1929–1938. [Google Scholar] [CrossRef] [PubMed]
- Komárek, M.; Eva, Č.; Chrastný, V.; Bordas, F.; Bollinger, J. Contamination of vineyard soils with fungicides: A review of environmental and toxicological aspects. Environ. Int. 2010, 36, 138–151. [Google Scholar] [CrossRef]
- Merrington, G.; Rogers, S.L.; Van Zwieten, L. The potential impact of long-term copper fungicide usage on soil microbial biomass and microbial activity in an avocado orchard. Soil Res. 2002, 40, 749–759. [Google Scholar] [CrossRef] [Green Version]
- Wightwick, A.M.; Mollah, M.R.; Partington, D.L.; Allinson, G. Copper fungicide residues in Australian vineyard soils. J. Agric. Food Chem. 2008, 56, 2457–2464. [Google Scholar] [CrossRef]
- Azimi, S.; Cambier, P.; Lecuyer, I.; Thevenot, D. Heavy metal determination in atmospheric deposition and other fluxes in northern France agrosystems. Water Air Soil Pollut. 2004, 157, 295–313. [Google Scholar] [CrossRef] [Green Version]
- Ballabio, C.; Panagos, P.; Lugato, E.; Huang, J.H.; Orgiazzi, A.; Jones, A.; Fernández-Ugalde, O.; Borrelli, P.; Montanarella, L. Copper distribution in European topsoils: An assessment based on LUCAS soil survey. Sci. Total Environ. 2018, 636, 282–298. [Google Scholar] [CrossRef]
- Droz, B.; Payraudeau, S.; Rodríguez Martín, J.A.; Tóth, G.; Panagos, P.; Montanarella, L.; Borrelli, P.; Imfeld, G. Copper Content and Export in European Vineyard Soils Influenced by Climate and Soil Properties. Environ. Sci. Technol. 2021, 55, 7327–7334. [Google Scholar] [CrossRef]
- Chopin, E.I.B.; Marin, B.; Mkoungafoko, R.; Rigaux, A.; Hopgood, M.J.; Delannoy, E.; Cancès, B.; Laurain, M. Factors affecting distribution and mobility of trace elements (Cu, Pb, Zn) in a perennial grapevine (Vitis vinifera L.) in the Champagne region of France. Environ. Pollut. 2008, 156, 1092–1098. [Google Scholar] [CrossRef]
- Jacobson, A.R.; Dousset, S.; Guichard, N.; Baveye, P.; Andreux, F. Diuron mobility through vineyard soils contaminated with copper. Environ. Pollut. 2005, 138, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Probst, B.; Schüler, C.; Joergensen, R.G. Vineyard soils under organic and conventional management-Microbial biomass and activity indices and their relation to soil chemical properties. Biol. Fertil. Soils 2008, 44, 443–450. [Google Scholar] [CrossRef]
- Pateiro-Moure, M.; Pérez-Novo, C.; Arias-Estévez, M.; López-Periago, E.; Martínez-Carballo, E.; Simal-Gándara, J. Influence of copper on the adsorption and desorption of paraquat, diquat, and difenzoquat in vineyard acid soils. J. Agric. Food Chem. 2007, 55, 6219–6226. [Google Scholar] [CrossRef] [PubMed]
- Nóvoa-Muñoz, J.C.; Queijeiro, J.M.G.; Blanco-Ward, D.; Álvarez-Olleros, C.; Martínez-Cortizas, A.; García-Rodeja, E. Total copper content and its distribution in acid vineyards soils developed from granitic rocks. Sci. Total Environ. 2007, 378, 23–27. [Google Scholar] [CrossRef]
- Romić, M.; Romić, D.; Dolanjski, D.; Stričević, I. Heavy metals accumulation in topsoils from the wine-growing regions. Agric. Conspec. Sci. 2004, 69, 1–10. [Google Scholar]
- Cattani, I.; Fragoulis, G.; Boccelli, R.; Capri, E. Copper bioavailability in the rhizosphere of maize (Zea mays L.) grown in two Italian soils. Chemosphere 2006, 64, 1972–1979. [Google Scholar] [CrossRef]
- Dell’Amico, E.; Mazzocchi, M.; Cavalca, L.; Allievi, L.; Andreoni, V. Assessment of bacterial community structure in a long-term copper-polluted ex-vineyard soil. Microbiol. Res. 2008, 163, 671–683. [Google Scholar] [CrossRef] [Green Version]
- Bretzel, F.; Calderisi, M. Metal contamination in urban soils of coastal Tuscany (Italy). Environ. Monit. Assess. 2006, 118, 319–335. [Google Scholar] [CrossRef]
- ARPAV. Metalli e Metalloidi Nei Suoli del Veneto-Definizione dei Valori di Fondo; Stampa Sud srl: Treviso, Italy, 2019; ISBN 9788875041694. [Google Scholar]
- ARPA Piemonte. Relazione Sullo Stato dell’Ambiente del Piemonte 2014. Available online: http://relazione.ambiente.piemonte.gov.it (accessed on 5 June 2022).
- Lanno, R.; Wells, J.; Conder, J.; Bradham, K.; Basta, N. The bioavailability of chemicals in soil for earthworms. Ecotoxicol. Environ. Saf. 2004, 57, 39–47. [Google Scholar] [CrossRef]
- Wang, Q.Y.; Sun, J.Y.; Xu, X.J.; Yu, H.W. Integration of chemical and toxicological tools to assess the bioavailability of copper derived from different copper-based fungicides in soil. Ecotoxicol. Environ. Saf. 2018, 161, 662–668. [Google Scholar] [CrossRef]
- Puglisi, E.; Vernile, P.; Bari, G.; Spagnuolo, M.; Trevisan, M.; de Lillo, E.; Ruggiero, P. Bioaccessibility, bioavailability and ecotoxicity of pentachlorophenol in compost amended soils. Chemosphere 2009, 77, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Bari, G.; Vernile, P.; Spagnuolo, M.; de Lillo, E.; Pizzigallo, M.D.R.; Ruggiero, P. The influence of aging and compost amendment on bioaccessibility, bioavailability and toxicity of phenanthrene in contaminated soils. Fresenius Environ. Bull. 2010, 19, 1727–1734. [Google Scholar]
- Spagnuolo, M.; Puglisi, E.; Vernile, P.; Bari, G.; de Lillo, E.; Trevisan, M.; Ruggiero, P. Soil monitoring of pentachlorophenol by bioavailability and ecotoxicity measurements. J. Environ. Monit. 2010, 12, 1575–1581. [Google Scholar] [CrossRef]
- Vischetti, C.; Casucci, C.; De Bernardi, A.; Monaci, E.; Tiano, L.; Marcheggiani, F.; Ciani, M.; Comitini, F.; Marini, E.; Taskin, E.; et al. Sub-Lethal Effects of Pesticides on the DNA of Soil Organisms as Early Ecotoxicological Biomarkers. Front. Microbiol. 2020, 11, 1892. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.Y.; Zhou, D.M.; Cang, L. Bioavailability of Soil Copper from Different Sources: Integrating Chemical Approaches with Biological Indicators. Pedosphere 2014, 24, 145–152. [Google Scholar] [CrossRef]
- European Commission. Commission Implementing Regulation (EU) 2015/408 of 11 March 2015 on implementing Article 80(7) of Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market and establishin. Off. J. Eur. Union 2015, 67, 18–22. [Google Scholar]
- European Commission. Commission Implementing Regulation (EU) 2018/1981 of 13 December 2018 renewing the approval of the active substances copper compounds, as candidates for substitution, in accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council. Off. J. Eur. Union 2018, 317, 16–20. [Google Scholar]
- Karimi, B.; Cahurel, J.Y.; Gontier, L.; Charlier, L.; Chovelon, M.; Mahé, H.; Ranjard, L. A meta-analysis of the ecotoxicological impact of viticultural practices on soil biodiversity. Environ. Chem. Lett. 2020, 18, 1947–1966. [Google Scholar] [CrossRef]
- Strumpf, T.; Strassemeyer, J.; Krück, S.; Horney, P.; Hommel, B.; Felgentreu, D.; Herwig, N. Methodological aspects in the collection of earthworm communities in quality viticulture. J. Für Kult. 2015, 67, 5–21. [Google Scholar]
- Karimi, B.; Masson, V.; Guilland, C.; Leroy, E.; Pellegrinelli, S.; Giboulot, E.; Maron, P.A.; Ranjard, L. Ecotoxicity of copper input and accumulation for soil biodiversity in vineyards. Environ. Chem. Lett. 2021, 19, 2013–2030. [Google Scholar] [CrossRef]
- Ramadass, K.; Palanisami, T.; Smith, E.; Mayilswami, S.; Megharaj, M.; Naidu, R. Earthworm Comet Assay for Assessing the Risk of Weathered Petroleum Hydrocarbon Contaminated Soils: Need to Look Further than Target Contaminants. Arch. Environ. Contam. Toxicol. 2016, 71, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Smit, M.G.D.; Bechmann, R.K.; Hendriks, A.J.; Skadsheim, A.; Larsen, B.K.; Baussant, T.; Bamber, S.; Sanni, S. Relating biomarkers to whole-organism effects using species sensitivity distributions: A pilot study for marine species exposed to oil. Environ. Toxicol. Chem. An. Int. J. 2009, 28, 1104–1109. [Google Scholar] [CrossRef] [PubMed]
- Mincarelli, L.; Tiano, L.; Craft, J.; Marcheggiani, F.; Vischetti, C. Evaluation of gene expression of different molecular biomarkers of stress response as an effect of copper exposure on the earthworm EIsenia Andrei. Ecotoxicology 2019, 28, 938–948. [Google Scholar] [CrossRef] [PubMed]
- Glei, M.; Schneider, T.; Schlörmann, W. Comet assay: An essential tool in toxicological research. Arch. Toxicol. 2016, 90, 2315–2336. [Google Scholar] [CrossRef]
- de Lapuente, J.; Lourenço, J.; Mendo, S.A.; Borràs, M.; Martins, M.G.; Costa, P.M.; Pacheco, M. The Comet Assay and its applications in the field of ecotoxicology: A mature tool that continues to expand its perspectives. Front. Genet. 2015, 6, 180. [Google Scholar] [CrossRef]
- Romanazzi, G.; Mancini, V.; Foglia, R.; Marcolini, D.; Kavari, M.; Piancatelli, S. Use of Chitosan and Other Natural Compounds Alone or in Different Strategies with Copper Hydroxide for Control of Grapevine Downy Mildew. Plant Dis. 2021, 105, 3261–3268. [Google Scholar] [CrossRef]
- Romanazzi, G.; Feliziani, E.; Sivakumar, D. Chitosan, a biopolymer with triple action on postharvest decay of fruit and vegetables: Eliciting, antimicrobial and film-forming properties. Front. Microbiol. 2018, 9, 2745. [Google Scholar] [CrossRef]
- Rivas-Martìnez, S. Bases para una nueva clasificación bioclimática de la tierra. Universidad Complutense de Madrid. Folia Bot. Matritensis 1993, 10, 23. [Google Scholar]
- Pesaresi, S.; Biondi, E.; Casavecchia, S. Bioclimates of italy. J. Maps 2017, 13, 955–960. [Google Scholar] [CrossRef]
- ASSAM. Suoli e Paesaggi Delle Marche: Programma Interregionale Agricoltura e Qualità, Misura 5, Carta dei Suoli, Scala 1:250.000 (dD.G.R. n. 2805 del 18/12/2000); ASSAM: Ancona, Italy, 2006. [Google Scholar]
- Italian Official Gazette Ministry. Italian Official Gazette Ministerial Decree: Approval of the Official Methods of Soil Chemical Analysis; Italian Official Gazette Ministry: Rome, Italy, 1999; Volume 248. [Google Scholar]
- SIRMIP ON-LINE Regione Marche-Servizio Protezione Civile. Available online: http://app.protezionecivile.marche.it/sol/indexjs.sol?lang=it (accessed on 18 March 2022).
- OECD. OECD Guideline for Testing of Chemicals “Earthworm Reproduction Test (Eisenia Fetida/Eisenia Andrei)” 222; OECD: Paris, France, 2016. [Google Scholar]
- OECD. OECD Guideline for Testing of Chemicals “Earthworm, Acute Toxicity Tests” 207; OECD: Paris, France, 1984. [Google Scholar] [CrossRef]
- Eyambe, G.S.; Goven, A.J.; Fitzpatrick, L.C.; Venables, B.J.; Cooper, E.L. A non-invasive technique for sequential collection of earthworm (Lumbricus terrestris) leukocytes during subchronic immunotoxicity studies. Lab. Anim. 1991, 25, 61–67. [Google Scholar] [CrossRef] [Green Version]
- Mincarelli, L.; Vischetti, C.; Craft, J.; Tiano, L. DNA damage in different Eisenia andrei coelomocytes sub-populations after in vitro exposure to hydrogen peroxide. Springerplus 2016, 5, 302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valverde, M.; Ostrosky-wegman, P.; Rojas, E.; Fortoul, T.N.M.; Meneses, F.M.C.; Ramírez, M.M.C.; Díaz-Barriga, F.; Cebrian, M. The application of single cell gel electrophoresis or comet assay to human monitoring studies. SciELO Public Health. 1999, 41, 109–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orlando, P.; Silvestri, S.; Galeazzi, R.; Antonicelli, R.; Marcheggiani, F.; Cirilli, I.; Bacchetti, T.; Tiano, L. Effect of ubiquinol supplementation on biochemical and oxidative stress indexes after intense exercise in young athletes. Redox Rep. 2018, 23, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Tiano, L.; Littarru, G.P.; Principi, F.; Orlandi, M.; Santoro, L.; Carnevali, P.; Gabrielli, O. Assessment of DNA damage in Down Syndrome patients by means of a new, optimised single cell gel electrophoresis technique. BioFactors 2005, 25, 187–195. [Google Scholar] [CrossRef]
- UNI EN 12457-2; Characterisation of Waste Leaching-Compliance Test for Leaching of Granular Waste Materials and Sludges Part 2; iTeh, Inc.: Newark, DE, USA, 2004.
- Kasassi, A.; Rakimbei, P.; Karagiannidis, A.; Zabaniotou, A.; Tsiouvaras, K.; Nastis, A.; Tzafeiropoulou, K. Soil contamination by heavy metals: Measurements from a closed unlined landfill. Bioresour. Technol. 2008, 99, 8578–8584. [Google Scholar] [CrossRef]
- U.S. EPA. Method 6010D (SW-846): Inductively coupled plasma—optical emission spectrometry. In Computers and Industrial Engineering; Elsevier: Washington, DC, 2014; Volume 2, p. 6. ISBN 9781538643426. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; The R Foundation for Statistical Computing: Indianapolis, IN, USA, 2022. [Google Scholar]
- Dunn, O.J. Multiple comparisons using rank sums. Technometrics 1964, 6, 241–252. [Google Scholar] [CrossRef]
- Mangiafico, S.; Mangiafico, M.S. Package ‘rcompanion’. Cran. Repos. 2017, 20, 1–71. [Google Scholar]
- Lamichhane, J.R.; Osdaghi, E.; Behlau, F.; Köhl, J.; Jones, J.B.; Aubertot, J.N. Thirteen decades of antimicrobial copper compounds applied in agriculture. A review. Agron. Sustain. Dev. 2018, 38, 28. [Google Scholar] [CrossRef] [Green Version]
- Romić, M.; Matijevi, L.; Baki, H.; Romi, D. Copper Accumulation in Vineyard Soils: Distribution, Fractionation and Bioavailability Assessment; In Environmental Risk Assessment of Soil Contamination; IntechOpen: London, UK, 2014. [Google Scholar] [CrossRef] [Green Version]
- U.E Commissione. REGOLAMENTO DI ESECUZIONE (UE) 2018/1981 DELLA COMMISSIONE-del 13 dicembre 2018-che rinnova l’approvazione delle sostanze attive composti di rame, come sostanze candidate alla sostituzione, in conformità al regolamento (CE) n. 1107/ 2009 del Parlamen. Eur. Off. Gazzette 2018, 2018, 1–5. [Google Scholar]
- Italian Official Gazette. Italian Official Gazette Decree 1 March 2019 N 46. Ital. Off. Gaz. 2019. [Google Scholar]
- Italian Official Gazette. Italian Official Gazette Legislative Decree 27 January N 99. Ital. Off. Gaz. 1992. [Google Scholar]
- Reimann, C.; Birke, M.; Demetriades, A.; Filzmoser, P.; O’Connor, P. Chemistry of Europe’s agricultural soils, part A. In Methodology and Interpretation of the JEMAS Data Set. Geol. Jahrb. (Reihe B 102); Schweizerbarth: Hannover, Germany, 2014; p. 528. [Google Scholar]
- Polish Ministry. Polish Ministry of the Environment Decree 9th September. Permissible limit values in agricultural and grazing land soil. J. Laws No. 165 2002. [Google Scholar]
- Danish Environmental Protection Agency. Danish Environmental Protection Agency List of Quality Criteria in Relation to Polluted Soil and Quality Criteria for Drinking Water; Danish Environmental Protection Agency: København, Denmark, 2015. [Google Scholar]
- Ministério Do Ambiente, Do Ordenamento Do Território E Do Desenvolvimento. Regional Decreto-Lei N.276/2009 Regime jurídico de utilização agrícola das lamas de depuração em solos agrícolas. In Diário da República no 192, Série I 2 Outubro 2009; pp. 7154–7165.
- Albanese, S.; Sadeghi, M.; Lima, A.; Cicchella, D.; Dinelli, E.; Valera, P.; Falconi, M.; Demetriades, A.; De Vivo, B. The GEMASProject Team GEMAS: Cobalt, Cr, Cu and Ni distribution in agricultural and grazing land soil of Europe. J. Geochem. Explor. 2015, 154, 81–93. [Google Scholar] [CrossRef]
- European Commission. European Commission Protection of the Environment, and in particular of the soil, when sewage sludge is used in agriculture. Off. J. Eur. Communities 1986, 4, 6–12. [Google Scholar]
- Garlato, A. I casi di studio e le problematiche aperte: Ulteriori approfondimenti in corso e prospettive future. 2019. Available online: arpa.veneto.it (accessed on 5 June 2022).
- Kabata-Pendias, A.; Pendias, H. Biogeochemistry of Trace Elements; Nova Science Publishers: New York, NY, USA, 2001; Volume 2, ISBN 0849315751. [Google Scholar]
- Mackie, K.A.; Müller, T.; Zikeli, S.; Kandeler, E. Long-term copper application in an organic vineyard modifies spatial distribution of soil micro-organisms. Soil Biol. Biochem. 2013, 65, 245–253. [Google Scholar] [CrossRef]
- Duan, X.; Xu, M.; Zhou, Y.; Yan, Z.; Du, Y.; Zhang, L.; Zhang, C.; Bai, L.; Nie, J.; Chen, G.; et al. Effects of soil properties on copper toxicity to earthworm Eisenia fetida in 15 Chinese soils. Chemosphere 2016, 145, 185–192. [Google Scholar] [CrossRef]
- Singh, B.K.; Quince, C.; Macdonald, C.A.; Khachane, A.; Thomas, N.; Al-Soud, W.A.; Sørensen, S.J.; He, Z.; White, D.; Sinclair, A.; et al. Loss of microbial diversity in soils is coincident with reductions in some specialized functions. Environ. Microbiol. 2014, 16, 2408–2420. [Google Scholar] [CrossRef]
- Keiblinger, K.M.; Schneider, M.; Gorfer, M.; Paumann, M.; Deltedesco, E.; Berger, H.; Jöchlinger, L.; Mentler, A.; Zechmeister-Boltenstern, S.; Soja, G.; et al. Assessment of Cu applications in two contrasting soils—effects on soil microbial activity and the fungal community structure. Ecotoxicology 2018, 27, 217–233. [Google Scholar] [CrossRef] [Green Version]
- La Torre, A.; Iovino, V.; Caradonia, F. Field suppression of Fusarium wilt disease in banana by the combined application of native endophytic and rhizospheric bacterial isolates possessing multiple functions. Phytopathol. Mediterr. 2018, 54, 241–252. [Google Scholar] [CrossRef]









| Vineyards | Thesis | N° of Cu Treatments/Year | Year |
|---|---|---|---|
| Varano (V) | A | 9 | 2020 |
| 7 | 2021 | ||
| B | 4 | 2020 | |
| 5 | 2021 | ||
| Castelplanio (C) | A | 9 | 2020 |
| 5 | 2021 | ||
| B | 4 | 2020 | |
| 4 | 2021 |
| Sample Code 1 | pH (H2O) | Texture | Sand | Silt | Clay | Conductivity | OM | CEC |
|---|---|---|---|---|---|---|---|---|
| % | dS/m | % | Meq/100 g | |||||
| VAZ | 8.1 | Silty Clay Loam | 19.5 | 51.0 | 29.5 | 0.644 | 2.53 | 24.5 |
| VAQ | 8.2 | Clay Loam | 20.8 | 49.0 | 30.2 | 0.587 | 1.26 | 25.6 |
| VBZ | 8.2 | 21.5 | 45.6 | 32.9 | 0.61 | 2.05 | 30.9 | |
| VBQ | 8.2 | 20.4 | 47.3 | 32.3 | 0.536 | 1.50 | 30.6 | |
| VCZ | 8.2 | Silty Clay Loam | 19.9 | 48.5 | 31.6 | 0.627 | 2.02 | 29.1 |
| VCQ | 8.2 | 15.4 | 50.6 | 34.0 | 0.516 | 1.69 | 29.6 | |
| CAZ | 8.1 | Loam | 44.3 | 29.8 | 25.9 | 0.638 | 2.22 | 25.4 |
| CAQ | 8.2 | Sandy Clay Loam | 47.6 | 27.1 | 25.3 | 0.597 | 1.18 | 24.5 |
| CBZ | 8.1 | Loam | 41.0 | 33.6 | 25.4 | 0.869 | 2.28 | 22.4 |
| CBQ | 8.2 | 42.4 | 31.5 | 26.1 | 0.731 | 1.33 | 22.0 | |
| CCZ | 8.1 | 43.3 | 31.5 | 25.2 | 0.614 | 2.02 | 24.1 | |
| CCQ | 8.1 | 41.3 | 33.4 | 25.3 | 0.717 | 1.38 | 21.9 | |
| Sample Codes 1 | Cu Percentage Variation between Two Years (%) | ||
|---|---|---|---|
| Tot | Bio | Sol | |
| VAZ | −8.64 | −8.61 | +53.27 ** |
| VBZ | +5.68 | +32.15 * | +59.12 ** |
| VCZ | −3.07 | +26.71 | +48.24 ** |
| VAQ | +4.54 | +15.59 | +76.05 ** |
| VBQ | −0.14 | +4.87 | +59.12 ** |
| VCQ | −8.59 | +1.19 | +52.66 ** |
| CAZ | −10.40 | −33.75 | +21.31 ** |
| CBZ | −0.66 | +10.40 | +50.97 ** |
| CCZ | −0.26 | +3.22 | +44.13 ** |
| CAQ | −24.44 * | −51.51 * | +13.80 |
| CBQ | −7.11 | −25.03 | +43.73 * |
| CCQ | −5.47 | −15.04 | +34.56 * |
| Treatments | Time (Days) | ||||
|---|---|---|---|---|---|
| 2 | 7 | 14 | 21 | 28 | |
| VAZ | 83% | 78% | 80% | 72% | 78% |
| VAZ90 | 78% * | 72% * | 77% | 76% | 80% |
| VAZ150 | 83% | 42% **** | 74% *** | 75% | 35% **** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Bernardi, A.; Marini, E.; Casucci, C.; Tiano, L.; Marcheggiani, F.; Vischetti, C. Copper Monitoring in Vineyard Soils of Central Italy Subjected to Three Antifungal Treatments, and Effects of Sub-Lethal Copper Doses on the Earthworm Eisenia fetida. Toxics 2022, 10, 310. https://doi.org/10.3390/toxics10060310
De Bernardi A, Marini E, Casucci C, Tiano L, Marcheggiani F, Vischetti C. Copper Monitoring in Vineyard Soils of Central Italy Subjected to Three Antifungal Treatments, and Effects of Sub-Lethal Copper Doses on the Earthworm Eisenia fetida. Toxics. 2022; 10(6):310. https://doi.org/10.3390/toxics10060310
Chicago/Turabian StyleDe Bernardi, Arianna, Enrica Marini, Cristiano Casucci, Luca Tiano, Fabio Marcheggiani, and Costantino Vischetti. 2022. "Copper Monitoring in Vineyard Soils of Central Italy Subjected to Three Antifungal Treatments, and Effects of Sub-Lethal Copper Doses on the Earthworm Eisenia fetida" Toxics 10, no. 6: 310. https://doi.org/10.3390/toxics10060310






