Effects of Chronic Inhalation of Electronic Cigarette Vapor Containing Nicotine on Neurobehaviors and Pre/Postsynaptic Neuron Markers
Abstract
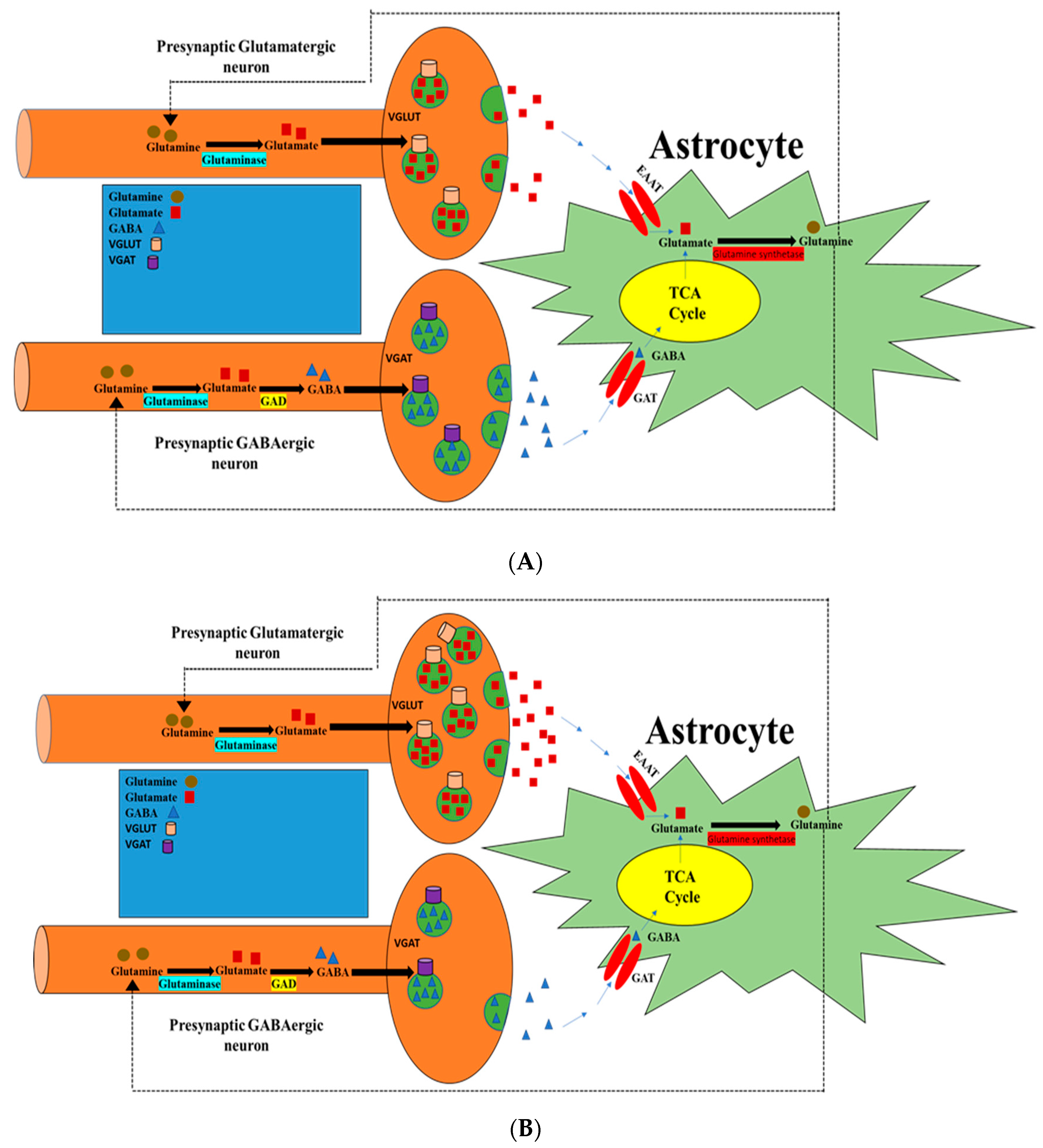
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Experimental Model
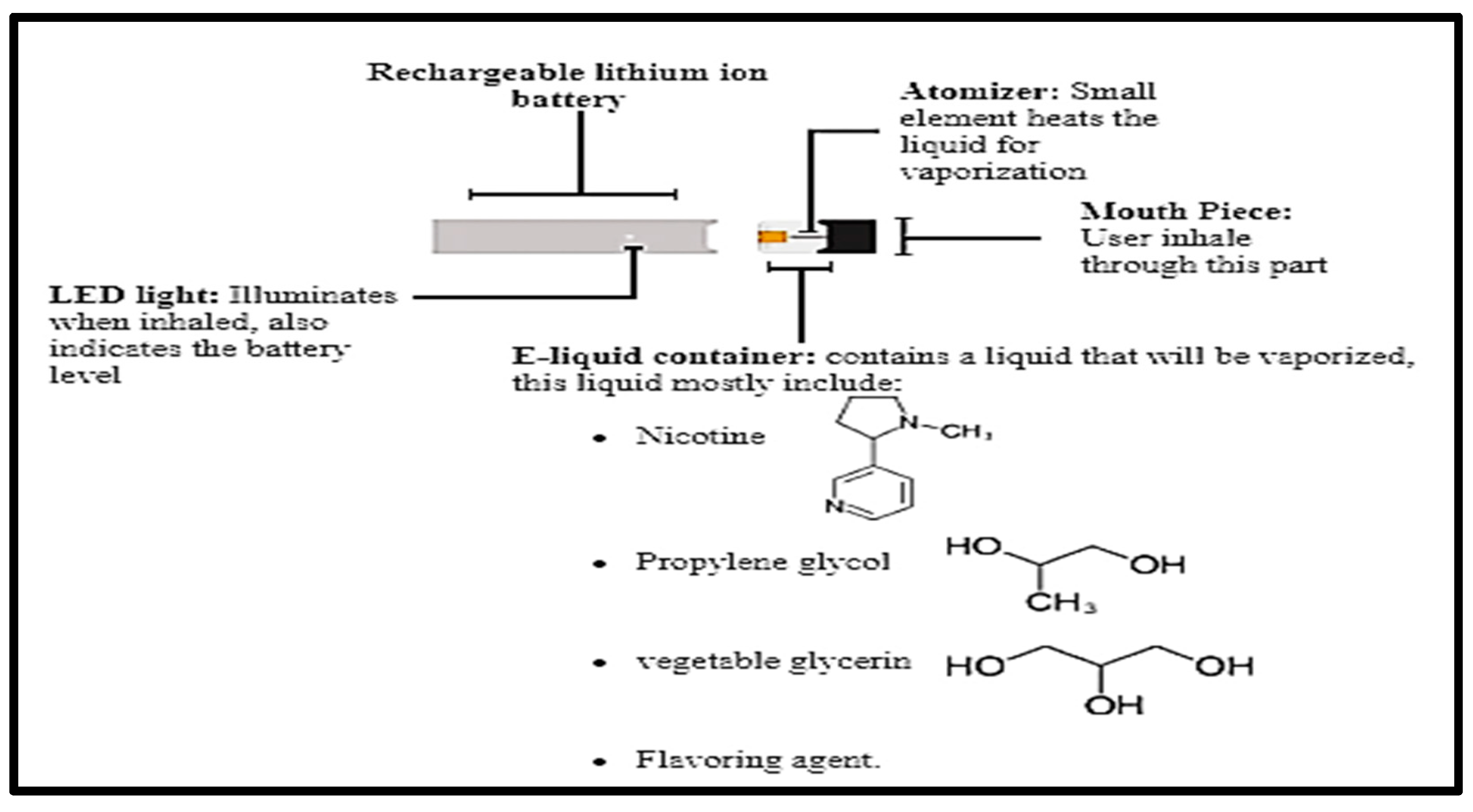
2.3. E-Cigarette and Exposure
2.4. Behavioral Studies
2.5. Brain Harvesting
2.6. Western Blotting
2.7. Multiplex Cytokine Assay
2.8. Statistical Analysis
3. Results
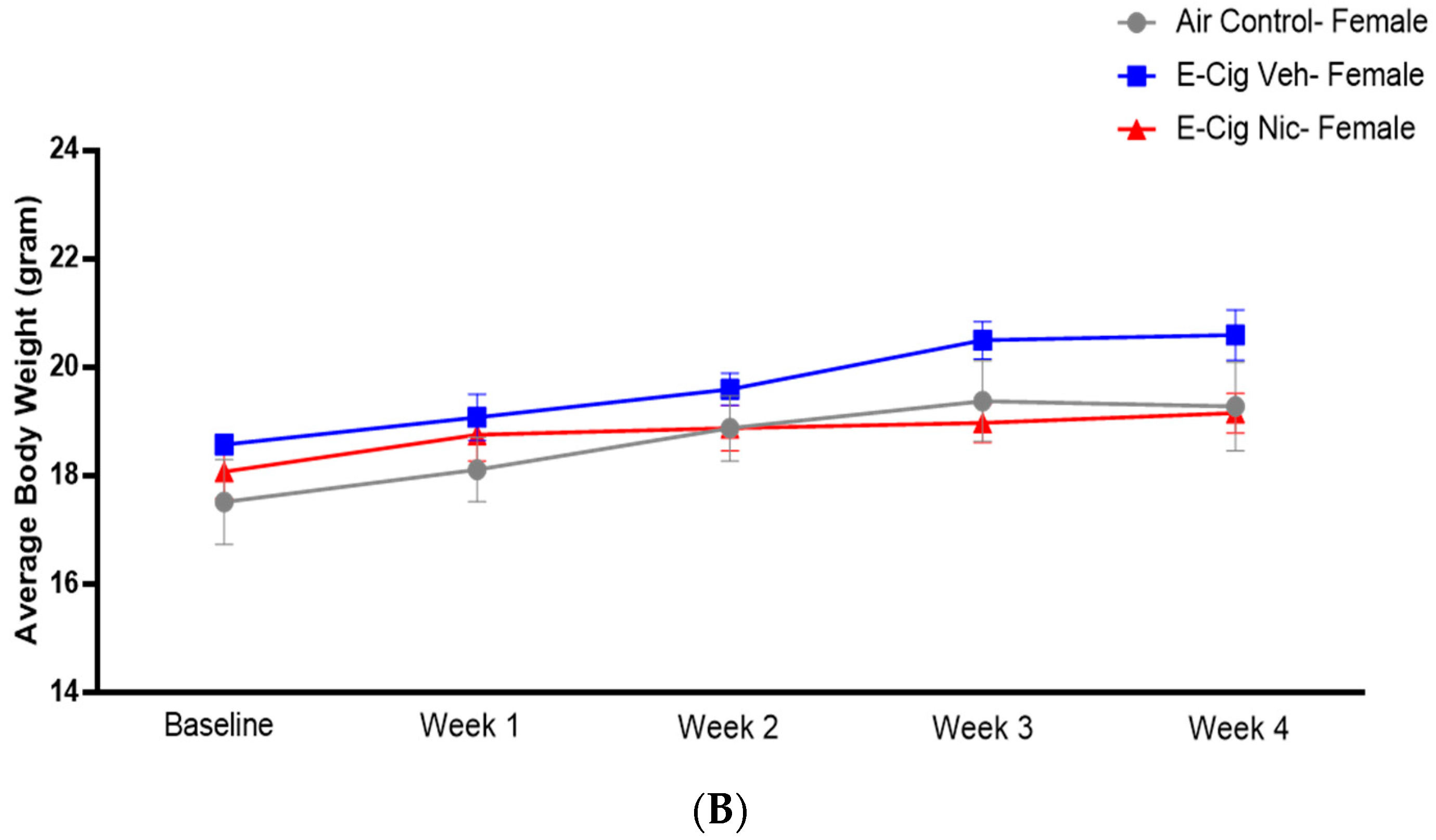
3.1. Effects of E-Cigarette Aerosols Containing Nicotine on Average Body Weight
3.2. Effects of E-Cigarette Aerosols Containing Nicotine on Object Recognition Memory
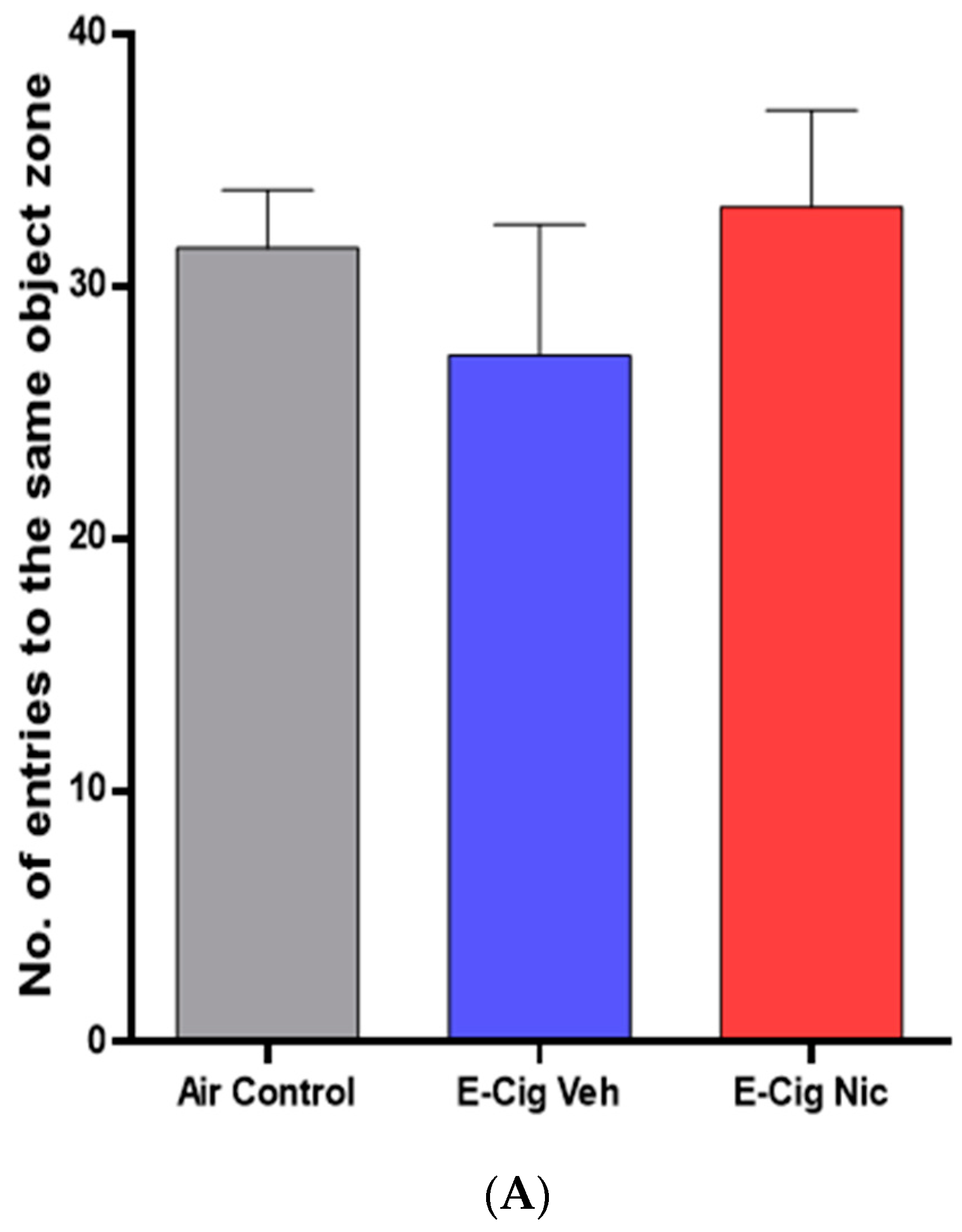
3.2.1. Same Object Recognition Memory
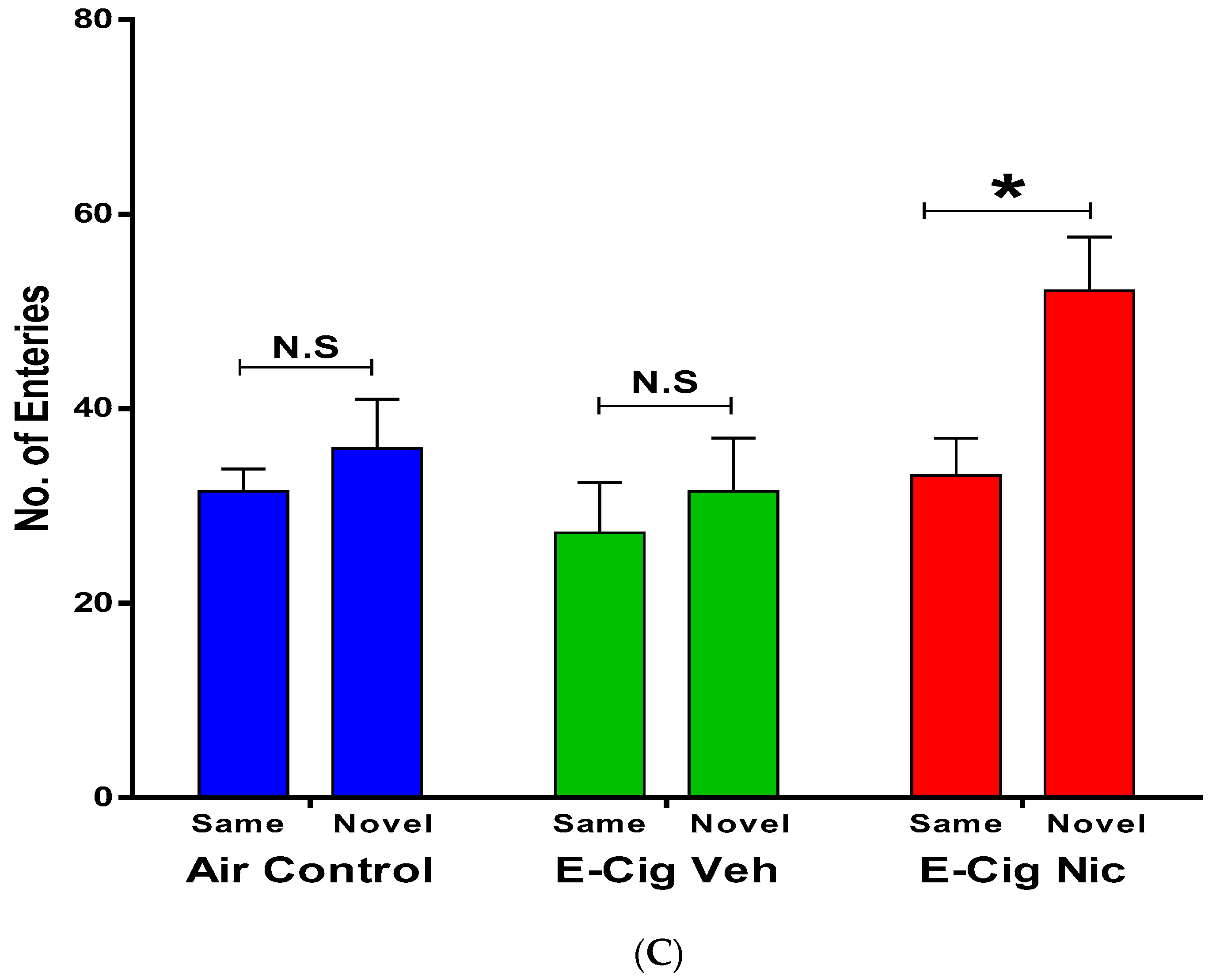
3.2.2. Long-Term Memory Test
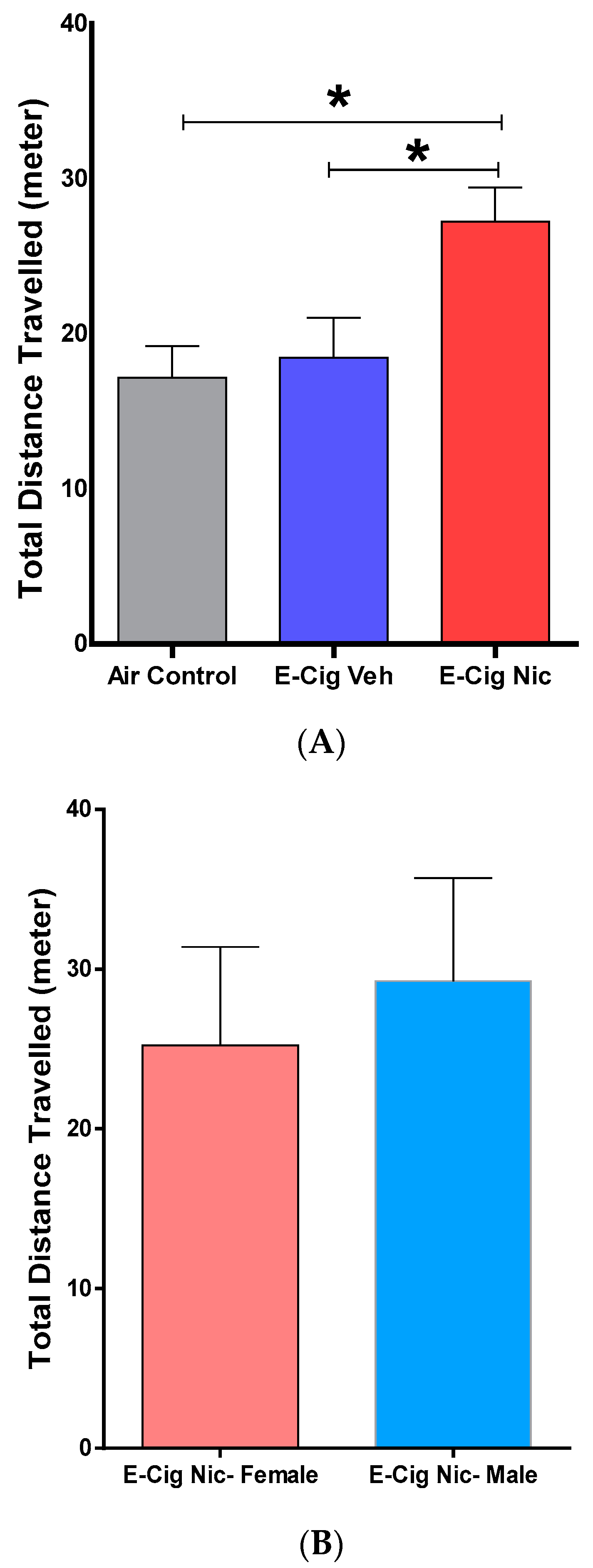
3.3. Effects of E-Cigarette Aerosols Containing Nicotine on Locomotion-Related Behaviors
3.4. Effects of E-Cigarette Aerosols Containing Nicotine on Spatial Memory
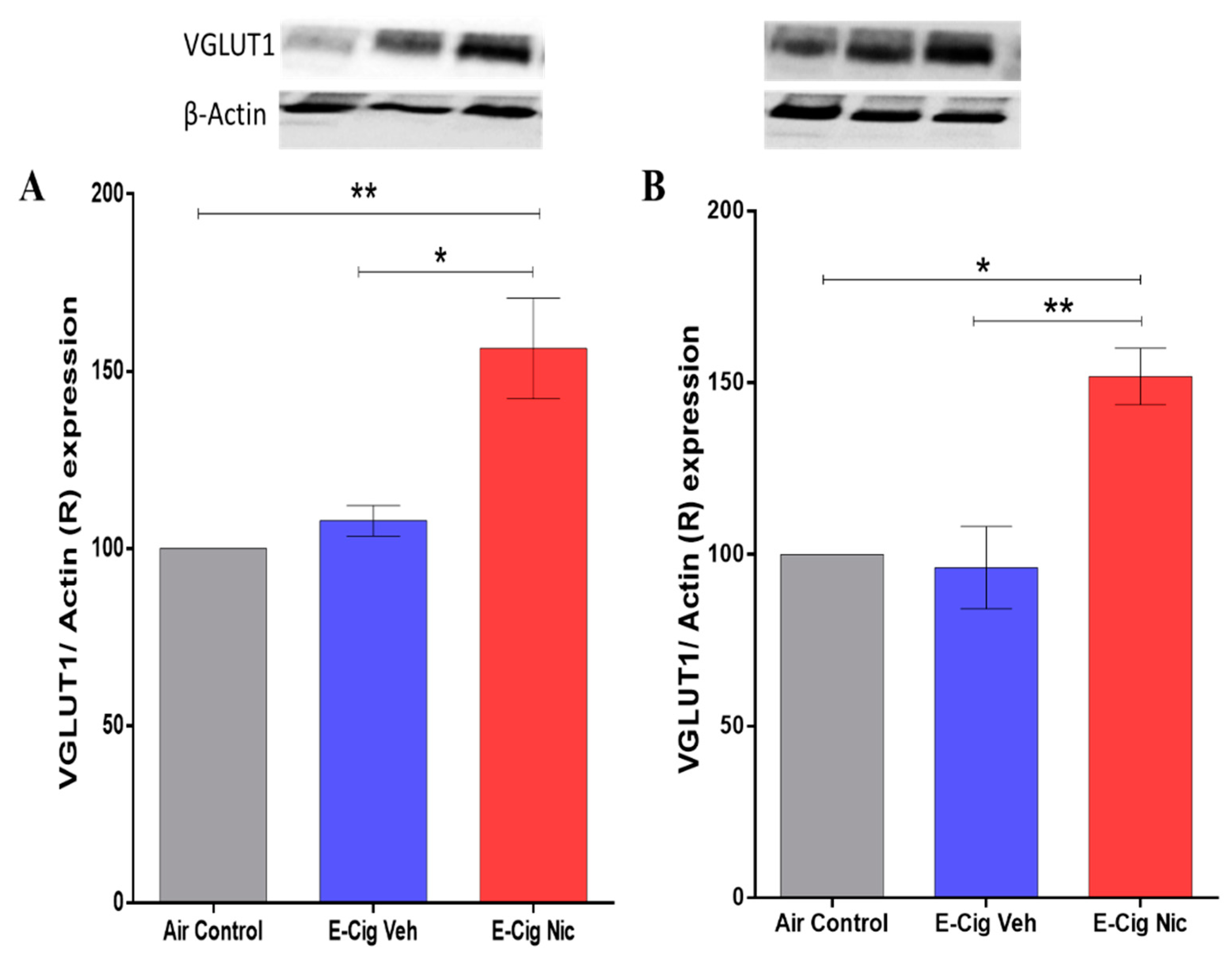
3.5. Effects of E-Cigarette Aerosols Containing Nicotine on VGLUT1 Expression in the Hippocampus
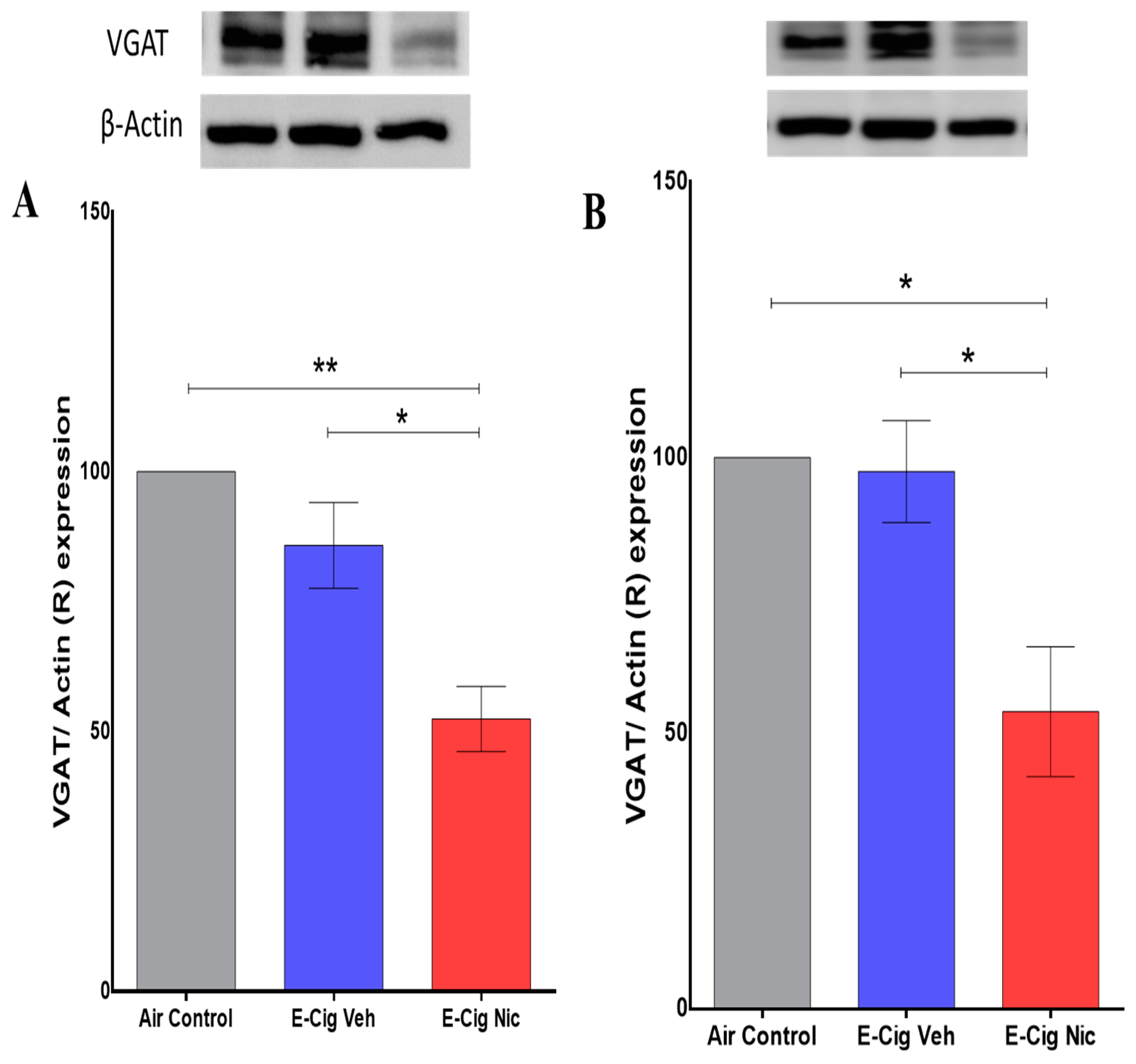
3.6. Effects of E-Cigarette Aerosols Containing Nicotine on VGAT Expression in the Hippocampus
3.7. Effects of E-Cigarette Aerosols Containing Nicotine on PSD-95 Expression in the Hippocampus
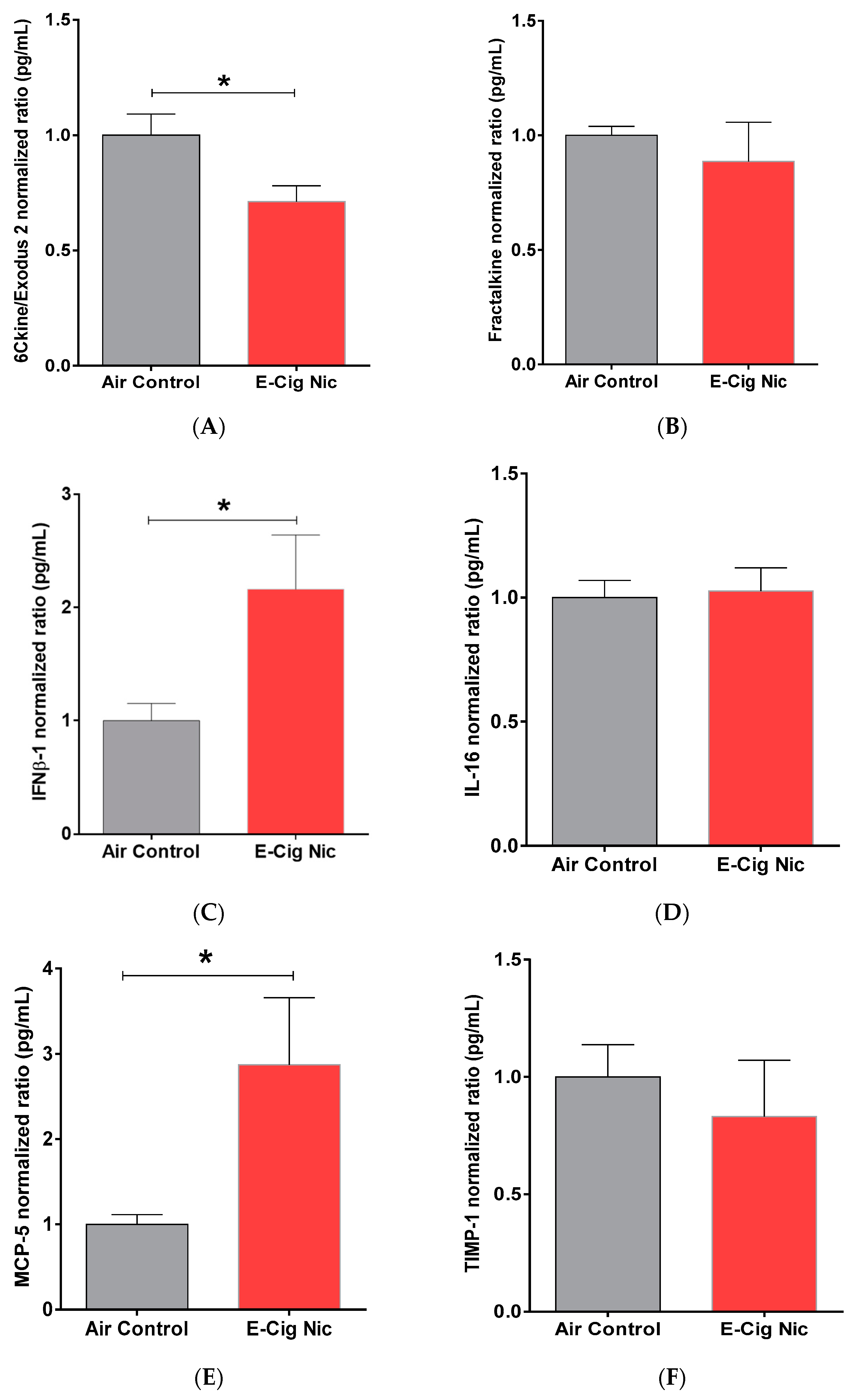
3.8. Effects of E-Cigarette Aerosols Containing Nicotine on Neuro-Inflammatory Cytokines and Chemokines
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Benowitz, N.L.; Burbank, A.D. Cardiovascular toxicity of nicotine: Implications for electronic cigarette use. Trends Cardiovasc. Med. 2016, 26, 515–523. [Google Scholar] [CrossRef] [Green Version]
- Richter, P.; Pechacek, T.; Swahn, M.; Wagman, V. Reducing levels of toxic chemicals in cigarette smoke: A new Healthy People 2010 objective. Public Health Rep. 2008, 123, 30–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tegin, G.; Mekala, H.M.; Sarai, S.K.; Lippmann, S. E-cigarette toxicity. South Med. J. 2018, 111, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Canistro, D.; Vivarelli, F.; Cirillo, S.; Marquillas, C.B.; Buschini, A.; Lazzaretti, M.; Marchi, L.; Cardenia, V.; Rodriguez-Estrada, M.T.; Lodovici, M. E-cigarettes induce toxicological effects that can raise the cancer risk. Sci. Rep. 2017, 7, 2028. [Google Scholar] [CrossRef] [PubMed]
- Rom, O.; Pecorelli, A.; Valacchi, G.; Reznick, A.Z. Are E-cigarettes a safe and good alternative to cigarette smoking? Ann. N. Y. Acad. Sci. 2015, 1340, 65–74. [Google Scholar] [CrossRef]
- Franck, C.; Filion, K.B.; Kimmelman, J.; Grad, R.; Eisenberg, M.J. Ethical considerations of e-cigarette use for tobacco harm reduction. Respir. Res. 2016, 17, 53. [Google Scholar] [CrossRef] [Green Version]
- Behar, R.Z.; Luo, W.; Lin, S.C.; Wang, Y.; Valle, J.; Pankow, J.F.; Talbot, P. Distribution, quantification and toxicity of cinnamaldehyde in electronic cigarette refill fluids and aerosols. Tob. Control 2016, 25, ii94–ii102. [Google Scholar] [CrossRef]
- Hess, C.A.; Olmedo, P.; Navas-Acien, A.; Goessler, W.; Cohen, J.E.; Rule, A.M. E-cigarettes as a source of toxic and potentially carcinogenic metals. Environ. Res. 2017, 152, 221–225. [Google Scholar] [CrossRef] [Green Version]
- Hughes, A.; Hendrickson, R.G. An epidemiologic and clinical description of e-cigarette toxicity. Clin. Toxicol. 2019, 57, 287–293. [Google Scholar] [CrossRef]
- Khlystov, A.; Samburova, V. Flavoring compounds dominate toxic aldehyde production during e-cigarette vaping. Environ. Sci. Technol. 2016, 50, 13080–13085. [Google Scholar] [CrossRef]
- Yu, V.; Rahimy, M.; Korrapati, A.; Xuan, Y.; Zou, A.E.; Krishnan, A.R.; Tsui, T.; Aguilera, J.A.; Advani, S.; Alexander, L.E.C. Electronic cigarettes induce DNA strand breaks and cell death independently of nicotine in cell lines. Oral Oncol. 2016, 52, 58–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Etter, J.-F.; Eissenberg, T. Dependence levels in users of electronic cigarettes, nicotine gums and tobacco cigarettes. Drug Alcohol Depend. 2015, 147, 68–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Delaimy, W.K.; Myers, M.G.; Leas, E.C.; Strong, D.R.; Hofstetter, C.R. E-cigarette use in the past and quitting behavior in the future: A population-based study. Am. J. Public Health 2015, 105, 1213–1219. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, M.J.; Heckman, B.W.; Wahlquist, A.E.; Wagener, T.L.; Goniewicz, M.L.; Gray, K.M.; Froeliger, B.; Cummings, K.M. A naturalistic, randomized pilot trial of e-cigarettes: Uptake, exposure, and behavioral effects. Cancer Epidemiol. Prev. Biomark. 2017, 26, 1795–1803. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Mendrek, A.; Cohen, M.S.; Monterosso, J.; Rodriguez, P.; Simon, S.L.; Brody, A.; Jarvik, M.; Domier, C.P.; Olmstead, R. Brain activity in cigarette smokers performing a working memory task: Effect of smoking abstinence. Biol. Psychiatry 2005, 58, 143–150. [Google Scholar] [CrossRef] [Green Version]
- Emre, M.; de Decker, C. Effects of cigarette smoking on motor functions in patients with multiple sclerosis. Arch. Neurol. 1992, 49, 1243–1247. [Google Scholar] [CrossRef]
- Kutlu, M.G.; Gould, T.J. Effects of drugs of abuse on hippocampal plasticity and hippocampus-dependent learning and memory: Contributions to development and maintenance of addiction. Learn. Mem. 2016, 23, 515–533. [Google Scholar] [CrossRef] [Green Version]
- Leal, S.L.; Yassa, M.A. Neurocognitive aging and the hippocampus across species. Trends Neurosci. 2015, 38, 800–812. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Fan, S.J.; Sun, A.B.; Liu, Z.Z.; Liu, L. Prenatal nicotine exposure induces depression-like behavior in adolescent female rats via modulating neurosteroid in the hippocampus. Mol. Med. Rep. 2019, 19, 4185–4194. [Google Scholar] [CrossRef] [Green Version]
- Bruel-Jungerman, E.; Rampon, C.; Laroche, S. Adult hippocampal neurogenesis, synaptic plasticity and memory: Facts and hypotheses. Rev. Neurosci. 2007, 18, 93–114. [Google Scholar] [CrossRef]
- Zeid, D.; Kutlu, M.G.; Gould, T.J. Differential effects of nicotine exposure on the hippocampus across lifespan. Curr. Neuropharmacol. 2018, 16, 388–402. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Ding, N.; Guo, M.; Wang, S.; Wang, Z.; Liu, H.; Yang, J.; Li, Y.; Ren, J.; Jiang, J. Analysis of learning and memory ability in an Alzheimer’s disease mouse model using the Morris water maze. JoVE 2019, e60055. [Google Scholar] [CrossRef] [PubMed]
- Alasmari, F.; Alexander, L.E.C.; Nelson, J.A.; Schiefer, I.T.; Breen, E.; Drummond, C.A.; Sari, Y. Effects of chronic inhalation of electronic cigarettes containing nicotine on glial glutamate transporters and α-7 nicotinic acetylcholine receptor in female CD-1 mice. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2017, 77, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Stagg, C.; Bestmann, S.; Constantinescu, A.; Moreno Moreno, L.; Allman, C.; Mekle, R.; Woolrich, M.; Near, J.; Johansen-Berg, H.; Rothwell, J. Relationship between physiological measures of excitability and levels of glutamate and GABA in the human motor cortex. J. Physiol. 2011, 589, 5845–5855. [Google Scholar] [CrossRef]
- Bojesen, K.B.; Broberg, B.V.; Fagerlund, B.; Jessen, K.; Thomas, M.B.; Sigvard, A.; Tangmose, K.; Nielsen, M.Ø.; Andersen, G.S.; Larsson, H.B.W. Associations between cognitive function and levels of glutamatergic metabolites and gamma-aminobutyric acid in antipsychotic-naïve patients with schizophrenia or psychosis. Biol. Psychiatry 2021, 89, 278–287. [Google Scholar] [CrossRef]
- Hertz, L.; Chen, Y. Integration between glycolysis and glutamate-glutamine cycle flux may explain preferential glycolytic increase during brain activation, requiring glutamate. Front. Integr. Neurosci. 2017, 11, 18. [Google Scholar] [CrossRef] [Green Version]
- Alasmari, F.; Crotty Alexander, L.; Hammad, A.; Bojanowski, C.M.; Moshensky, A.; Sari, Y. Effects of chronic inhalation of electronic cigarette vapor containing nicotine on neurotransmitters in the frontal cortex and striatum of C57BL/6 mice. Front. Pharmacol. 2019, 10, 885. [Google Scholar] [CrossRef] [Green Version]
- Alhaddad, H.; Wong, W.; Sari, A.T.; Crotty Alexander, L.E.; Sari, Y. Effects of 3-month exposure to e-cigarette aerosols on glutamatergic receptors and transporters in mesolimbic brain regions of female C57BL/6 mice. Toxics 2020, 8, 95. [Google Scholar] [CrossRef]
- Konradsson-Geuken, Å.; Gash, C.R.; Alexander, K.; Pomerleau, F.; Huettl, P.; Gerhardt, G.A.; Bruno, J.P. Second-by-second analysis of alpha 7 nicotine receptor regulation of glutamate release in the prefrontal cortex of awake rats. Synapse 2009, 63, 1069–1082. [Google Scholar] [CrossRef] [Green Version]
- Bortz, D.; Mikkelsen, J.; Bruno, J. Localized infusions of the partial alpha 7 nicotinic receptor agonist SSR180711 evoke rapid and transient increases in prefrontal glutamate release. Neuroscience 2013, 255, 55–67. [Google Scholar] [CrossRef]
- Mansvelder, H.D.; Keath, J.R.; McGehee, D.S. Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron 2002, 33, 905–919. [Google Scholar] [CrossRef] [Green Version]
- Pidoplichko, V.I.; Noguchi, J.; Areola, O.O.; Liang, Y.; Peterson, J.; Zhang, T.; Dani, J.A. Nicotinic cholinergic synaptic mechanisms in the ventral tegmental area contribute to nicotine addiction. Learn. Mem. 2004, 11, 60–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alrouji, M.; Manouchehrinia, A.; Gran, B.; Constantinescu, C.S. Effects of cigarette smoke on immunity, neuroinflammation and multiple sclerosis. J. Neuroimmunol. 2019, 329, 24–34. [Google Scholar] [CrossRef]
- Gonçalves, R.; Coletta, R.; Silvério, K.; Benevides, L.; Casati, M.; Da Silva, J.; Nociti, F. Impact of smoking on inflammation: Overview of molecular mechanisms. Inflamm. Res. 2011, 60, 409–424. [Google Scholar] [CrossRef]
- Crotty Alexander, L.E.; Drummond, C.A.; Hepokoski, M.; Mathew, D.; Moshensky, A.; Willeford, A.; Das, S.; Singh, P.; Yong, Z.; Lee, J.H.; et al. Chronic inhalation of e-cigarette vapor containing nicotine disrupts airway barrier function and induces systemic inflammation and multiorgan fibrosis in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 314, R834–R847. [Google Scholar] [CrossRef] [Green Version]
- Church, J.S.; Chace-Donahue, F.; Blum, J.L.; Ratner, J.R.; Zelikoff, J.T.; Schwartzer, J.J. Neuroinflammatory and Behavioral Outcomes Measured in Adult Offspring of Mice Exposed Prenatally to E-Cigarette Aerosols. Environ. Health Perspect. 2020, 128, 47006. [Google Scholar] [CrossRef]
- Alasmari, F.; Alexander, L.E.C.; Hammad, A.M.; Horton, A.; Alhaddad, H.; Schiefer, I.T.; Shin, J.; Moshensky, A.; Sari, Y. E-cigarette aerosols containing nicotine modulate nicotinic acetylcholine receptors and astroglial glutamate transporters in mesocorticolimbic brain regions of chronically exposed mice. Chem.-Biol. Interact. 2021, 333, 109308. [Google Scholar] [CrossRef]
- Moshensky, A.; Brand, C.S.; Alhaddad, H.; Shin, J.; Masso-Silva, J.A.; Advani, I.; Gunge, D.; Sharma, A.; Mehta, S.; Jahan, A. Effects of mango and mint pod-based e-cigarette aerosol inhalation on inflammatory states of the brain, lung, heart, and colon in mice. eLife 2022, 11, e67621. [Google Scholar] [CrossRef]
- Bai, J.; Trinh, T.L.; Chuang, K.H.; Qiu, A. Atlas-based automatic mouse brain image segmentation revisited: Model complexity vs. image registration. Magn. Reson. Imaging 2012, 30, 789–798. [Google Scholar] [CrossRef]
- Wolter, M.; Huff, E.; Speigel, T.; Winters, B.D.; Leri, F. Cocaine, nicotine, and their conditioned contexts enhance consolidation of object memory in rats. Learn. Mem. 2019, 26, 46–55. [Google Scholar] [CrossRef] [Green Version]
- Vieira-Brock, P.L.; McFadden, L.M.; Nielsen, S.M.; Smith, M.D.; Hanson, G.R.; Fleckenstein, A.E. Nicotine administration attenuates methamphetamine-induced novel object recognition deficits. Int. J. Neuropsychopharmacol. 2015, 18, pyv073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, S.; Pan, S.; You, Y. Nicotine enhances the reconsolidation of novel object recognition memory in rats. Pharmacol. Biochem. Behav. 2015, 129, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Esaki, H.; Izumi, S.; Fukao, A.; Ito, S.; Nishitani, N.; Deyama, S.; Kaneda, K. Nicotine Enhances Object Recognition Memory via Stimulating α4β2 and α7 Nicotinic Acetylcholine Receptors in the Medial Prefrontal Cortex of Mice. Biol. Pharm. Bull. 2021, 44, 1007–1013. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.M.; Lopez, B.; Nathan, D.; Wilson, J.; Bankole, E.; Tumoyan, H.; Munoz, A.; Espinoza-Derout, J.; Hasan, K.M.; Chang, S. A mouse model for chronic intermittent electronic cigarette exposure exhibits nicotine pharmacokinetics resembling human vapers. J. Neurosci. Methods 2019, 326, 108376. [Google Scholar] [CrossRef] [PubMed]
- Floresco, S.B.; Todd, C.L.; Grace, A.A. Glutamatergic afferents from the hippocampus to the nucleus accumbens regulate activity of ventral tegmental area dopamine neurons. J. Neurosci. 2001, 21, 4915–4922. [Google Scholar] [CrossRef] [Green Version]
- Sarawagi, A.; Soni, N.D.; Patel, A.B. Glutamate and GABA homeostasis and neurometabolism in major depressive disorder. Front. Psychiatry 2021, 12, 419. [Google Scholar] [CrossRef]
- Fenster, C.P.; Hicks, J.H.; Beckman, M.L.; Covernton, P.J.; Quick, M.W.; Lester, R.A. Desensitization of nicotinic receptors in the central nervous system. Ann. N. Y. Acad. Sci. 1999, 868, 620–623. [Google Scholar] [CrossRef]
- Grieder, T.E.; Herman, M.A.; Contet, C.; Tan, L.A.; Vargas-Perez, H.; Cohen, A.; Chwalek, M.; Maal-Bared, G.; Freiling, J.; Schlosburg, J.E.; et al. VTA CRF neurons mediate the aversive effects of nicotine withdrawal and promote intake escalation. Nat. Neurosci. 2014, 17, 1751–1758. [Google Scholar] [CrossRef] [Green Version]
- Pfeffer, L.M. The role of nuclear factor κB in the interferon response. J. Interferon Cytokine Res. 2011, 31, 553–559. [Google Scholar] [CrossRef] [Green Version]
- Komiyama, M.; Takanabe, R.; Ono, K.; Shimada, S.; Wada, H.; Yamakage, H.; Satoh-Asahara, N.; Morimoto, T.; Shimatsu, A.; Takahashi, Y. Association between monocyte chemoattractant protein-1 and blood pressure in smokers. J. Int. Med. Res. 2018, 46, 965–974. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alasmari, F.; Alotibi, F.M.; Alqahtani, F.; Alshammari, T.K.; Kadi, A.A.; Alghamdi, A.M.; Allahem, B.S.; Alasmari, A.F.; Alsharari, S.D.; Al-Rejaie, S.S.; et al. Effects of Chronic Inhalation of Electronic Cigarette Vapor Containing Nicotine on Neurobehaviors and Pre/Postsynaptic Neuron Markers. Toxics 2022, 10, 338. https://doi.org/10.3390/toxics10060338
Alasmari F, Alotibi FM, Alqahtani F, Alshammari TK, Kadi AA, Alghamdi AM, Allahem BS, Alasmari AF, Alsharari SD, Al-Rejaie SS, et al. Effects of Chronic Inhalation of Electronic Cigarette Vapor Containing Nicotine on Neurobehaviors and Pre/Postsynaptic Neuron Markers. Toxics. 2022; 10(6):338. https://doi.org/10.3390/toxics10060338
Chicago/Turabian StyleAlasmari, Fawaz, Farraj M. Alotibi, Faleh Alqahtani, Tahani K. Alshammari, Aban A. Kadi, Abdullah M. Alghamdi, Bassil S. Allahem, Abdullah F. Alasmari, Shakir D. Alsharari, Salim S. Al-Rejaie, and et al. 2022. "Effects of Chronic Inhalation of Electronic Cigarette Vapor Containing Nicotine on Neurobehaviors and Pre/Postsynaptic Neuron Markers" Toxics 10, no. 6: 338. https://doi.org/10.3390/toxics10060338





