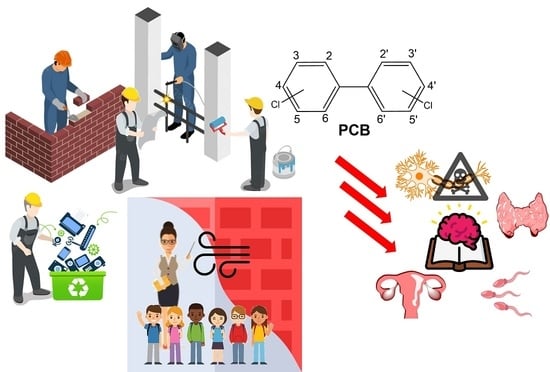
Polychlorinated Biphenyls (PCBs) in the Environment: Occupational and Exposure Events, Effects on Human Health and Fertility
Abstract
1. Introduction
2. Methodology
3. Human Exposure and Bioaccumulation of PCB
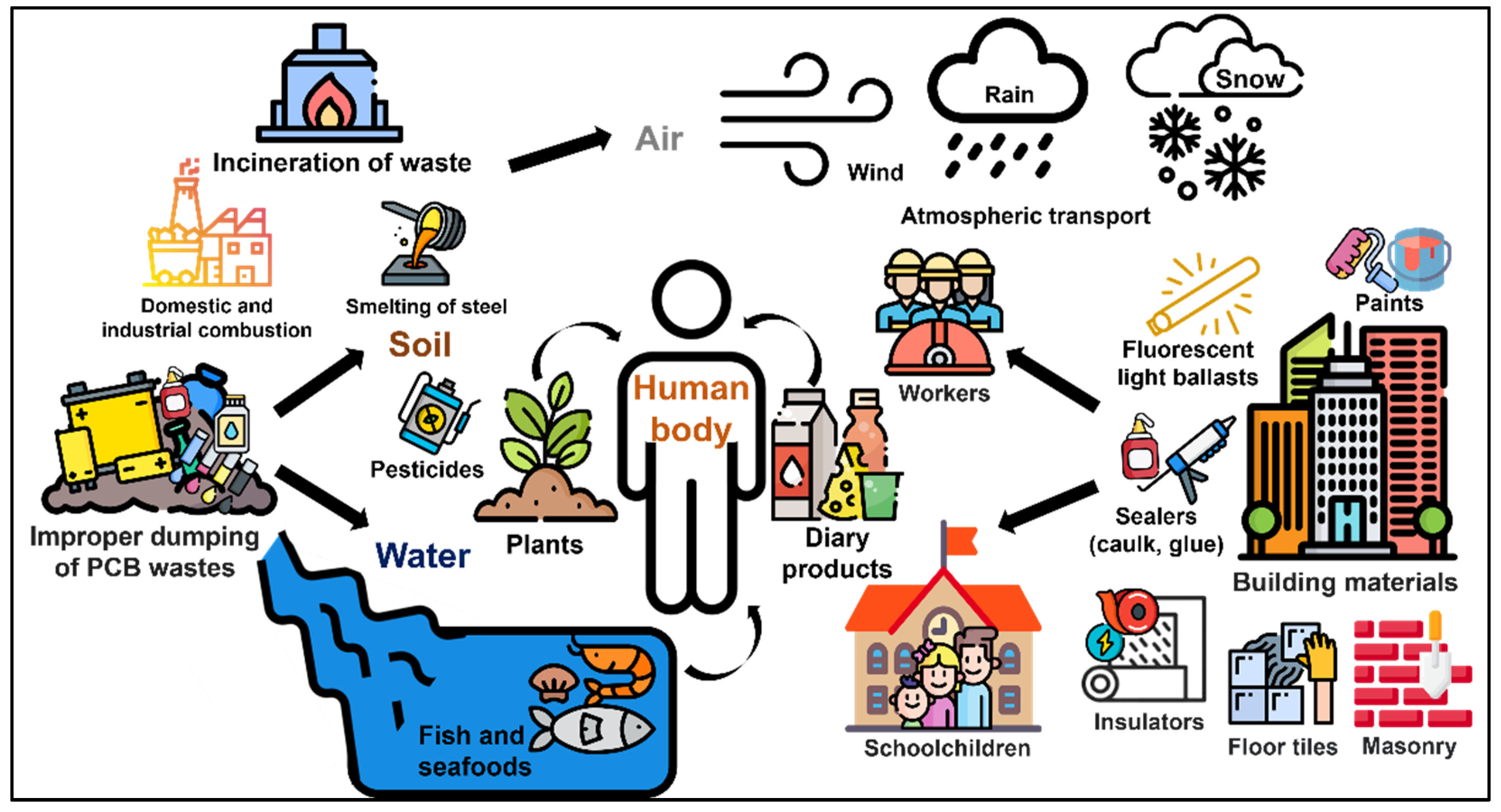
3.1. Routes for Human Exposure
3.2. Occupation and Exposure Events in Workplaces
3.3. Presence of PCB in Human Fluids and Bioaccumulation
4. Effects of PCBs on Human Health
4.1. Nervous System Disorders and Other Dysfunctions
4.2. Endocrine Disrupting Activity and Effects on Reproductive Organs
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jones, K.C.; de Voogt, P. Persistent Organic Pollutants (POPs): State of the Science. Environ. Pollut. 1999, 100, 209–221. [Google Scholar] [CrossRef]
- Alharbi, O.M.L.; Basheer, A.A.; Khattab, R.A.; Ali, I. Health and Environmental Effects of Persistent Organic Pollutants. J. Mol. Liq. 2018, 263, 442–453. [Google Scholar] [CrossRef]
- Pironti, C.; Ricciardi, M.; Proto, A.; Bianco, P.M.; Montano, L.; Motta, O. Endocrine-Disrupting Compounds: An Overview on Their Occurrence in the Aquatic Environment and Human Exposure. Water 2021, 13, 1347. [Google Scholar] [CrossRef]
- Vasseghian, Y.; Hosseinzadeh, S.; Khataee, A.; Dragoi, E.-N. The Concentration of Persistent Organic Pollutants in Water Resources: A Global Systematic Review, Meta-Analysis and Probabilistic Risk Assessment. Sci. Total Environ. 2021, 796, 149000. [Google Scholar] [CrossRef] [PubMed]
- Lallas, P.L. The Stockholm Convention on Persistent Organic Pollutants. Am. J. Int. Law 2001, 95, 692–708. [Google Scholar] [CrossRef]
- Rodrigues, J.P.; Duarte, A.C.; Santos-Echeandía, J.; Rocha-Santos, T. Significance of Interactions between Microplastics and POPs in the Marine Environment: A Critical Overview. TrAC Trends Anal. Chem. 2019, 111, 252–260. [Google Scholar] [CrossRef]
- Ricciardi, M.; Pironti, C.; Motta, O.; Miele, Y.; Proto, A.; Montano, L. Microplastics in the Aquatic Environment: Occurrence, Persistence, Analysis, and Human Exposure. Water 2021, 13, 973. [Google Scholar] [CrossRef]
- Pironti, C.; Ricciardi, M.; Motta, O.; Miele, Y.; Proto, A.; Montano, L. Microplastics in the Environment: Intake through the Food Web, Human Exposure and Toxicological Effects. Toxics 2021, 9, 224. [Google Scholar] [CrossRef]
- James, R.C.; Busch, H.; Tamburro, C.H.; Roberts, S.M.; Schell, J.D.; Harbison, R.D. Polychlorinated Biphenyl Exposure and Human Disease. J. Occup. Environ. Med. 1993, 35, 136–148. [Google Scholar] [CrossRef]
- Salhotra, A.M. Chapter Ten—Human Health Risk Assessment for Contaminated Properties. In Progress in Molecular Biology and Translational Science; Toxicology and Human Environments; Hodgson, E., Ed.; Academic Press: Cambridge, MA, USA, 2012; Volume 112, pp. 285–306. [Google Scholar]
- Vorkamp, K. An Overlooked Environmental Issue? A Review of the Inadvertent Formation of PCB-11 and Other PCB Congeners and Their Occurrence in Consumer Products and in the Environment. Sci. Total Environ. 2016, 541, 1463–1476. [Google Scholar] [CrossRef]
- Mills III, S.A.; Thal, D.I.; Barney, J. A Summary of the 209 PCB Congener Nomenclature. Chemosphere 2007, 68, 1603–1612. [Google Scholar] [CrossRef]
- Grimm, F.A.; Hu, D.; Kania-Korwel, I.; Lehmler, H.-J.; Ludewig, G.; Hornbuckle, K.C.; Duffel, M.W.; Bergman, Å.; Robertson, L.W. Metabolism and Metabolites of Polychlorinated Biphenyls. Crit. Rev. Toxicol. 2015, 45, 245–272. [Google Scholar] [CrossRef] [PubMed]
- Hansen, L.G. Identification of Steady State and Episodic PCB Congeners from Multiple Pathway Exposures. PCBs Recent Adv. Environ. Toxicol. Health Eff. 2001, 245, 47. [Google Scholar]
- Zhao, H.X.; Adamcakova-Dodd, A.; Hu, D.; Hornbuckle, K.C.; Just, C.L.; Robertson, L.W.; Thorne, P.S.; Lehmler, H.-J. Development of a Synthetic PCB Mixture Resembling the Average Polychlorinated Biphenyl Profile in Chicago Air. Environ. Int. 2010, 36, 819–827. [Google Scholar] [CrossRef]
- Robertson, L.W.; Ludewig, G. Polychlorinated Biphenyl (PCB) Carcinogenicity with Special Emphasis on Airborne PCBs. Gefahrst Reinhalt Luft 2011, 71, 25–32. [Google Scholar]
- Audy, O.; Melymuk, L.; Venier, M.; Vojta, S.; Becanova, J.; Romanak, K.; Vykoukalova, M.; Prokes, R.; Kukucka, P.; Diamond, M.L.; et al. PCBs and Organochlorine Pesticides in Indoor Environments—A Comparison of Indoor Contamination in Canada and Czech Republic. Chemosphere 2018, 206, 622–631. [Google Scholar] [CrossRef]
- Venier, M.; Hites, R.A. Time Trend Analysis of Atmospheric POPs Concentrations in the Great Lakes Region Since 1990. Environ. Sci. Technol. 2010, 44, 8050–8055. [Google Scholar] [CrossRef]
- Salamova, A.; Venier, M.; Hites, R.A. Revised Temporal Trends of Persistent Organic Pollutant Concentrations in Air around the Great Lakes. Environ. Sci. Technol. Lett. 2015, 2, 20–25. [Google Scholar] [CrossRef]
- Venier, M.; Salamova, A.; Hites, R.A. How to Distinguish Urban vs. Agricultural Sources of Persistent Organic Pollutants? Curr. Opin. Environ. Sci. Health 2019, 8, 23–28. [Google Scholar] [CrossRef]
- McFarland, V.A.; Clarke, J.U. Environmental Occurrence, Abundance, and Potential Toxicity of Polychlorinated Biphenyl Congeners: Considerations for a Congener-Specific Analysis. Environ. Health Perspect. 1989, 81, 225–239. [Google Scholar] [CrossRef]
- Wethington, D.M.; Hornbuckle, K.C. Milwaukee, WI, as a Source of Atmospheric PCBs to Lake Michigan. Environ. Sci. Technol. 2005, 39, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Lehmler, H.-J.; Martinez, A.; Wang, K.; Hornbuckle, K.C. Atmospheric PCB Congeners across Chicago. Atmos. Environ. 2010, 44, 1550–1557. [Google Scholar] [CrossRef] [PubMed]
- Persoon, C.; Peters, T.M.; Kumar, N.; Hornbuckle, K.C. Spatial Distribution of Airborne Polychlorinated Biphenyls in Cleveland, OH and Chicago, IL. Environ. Sci. Technol. 2010, 44, 2797–2802. [Google Scholar] [CrossRef][Green Version]
- Haarmann-Stemmann, T.; Abel, J. The Arylhydrocarbon Receptor Repressor (AhRR): Structure, Expression, and Function. Biol. Chem. 2006, 387, 1195–1199. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Saktrakulkla, P.; Tuttle, K.; Marek, R.F.; Lehmler, H.-J.; Wang, K.; Hornbuckle, K.C.; Duffel, M.W. Detection and Quantification of Polychlorinated Biphenyl Sulfates in Human Serum. Environ. Sci. Technol. 2021, 55, 2473–2481. [Google Scholar] [CrossRef]
- Klocke, C.; Sethi, S.; Lein, P.J. The Developmental Neurotoxicity of Legacy vs. Contemporary Polychlorinated Biphenyls (PCBs): Similarities and Differences. Environ. Sci. Pollut. Res. 2020, 27, 8885–8896. [Google Scholar] [CrossRef]
- Basu, I.; Arnold, K.A.; Venier, M.; Hites, R.A. Partial Pressures of PCB-11 in Air from Several Great Lakes Sites. Environ. Sci. Technol. 2009, 43, 6488–6492. [Google Scholar] [CrossRef]
- Hu, D.; Hornbuckle, K.C. Inadvertent Polychlorinated Biphenyls in Commercial Paint Pigments. Environ. Sci. Technol. 2010, 44, 2822–2827. [Google Scholar] [CrossRef]
- Rodenburg, L.A.; Du, S.; Fennell, D.E.; Cavallo, G.J. Evidence for Widespread Dechlorination of Polychlorinated Biphenyls in Groundwater, Landfills, and Wastewater Collection Systems. Environ. Sci. Technol. 2010, 44, 7534–7540. [Google Scholar] [CrossRef]
- Grossman, E. Nonlegacy PCBs: Pigment Manufacturing By-Products Get a Second Look. Environ. Health Perspect. 2013, 121, a86–a93. [Google Scholar] [CrossRef]
- Diamond, M.L.; Melymuk, L.; Csiszar, S.A.; Robson, M. Estimation of PCB Stocks, Emissions, and Urban Fate: Will Our Policies Reduce Concentrations and Exposure? Environ. Sci. Technol. 2010, 44, 2777–2783. [Google Scholar] [CrossRef] [PubMed]
- Kaw, H.Y.; Kannan, N. A Review on Polychlorinated Biphenyls (PCBs) and Polybrominated Diphenyl Ethers (PBDEs) in South Asia with a Focus on Malaysia. In Reviews of Environmental Contamination and Toxicology Volume 242; de Voogt, P., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 153–181. ISBN 978-3-319-51243-3. [Google Scholar]
- Meeker, J.D.; Hauser, R. Exposure to Polychlorinated Biphenyls (PCBs) and Male Reproduction. Syst. Biol. Reprod. Med. 2010, 56, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Zani, C.; Magoni, M.; Speziani, F.; Leonardi, L.; Orizio, G.; Scarcella, C.; Gaia, A.; Donato, F. Polychlorinated Biphenyl Serum Levels, Thyroid Hormones and Endocrine and Metabolic Diseases in People Living in a Highly Polluted Area in North Italy: A Population-Based Study. Heliyon 2019, 5, e01870. [Google Scholar] [CrossRef] [PubMed]
- Onozuka, D.; Nakamura, Y.; Tsuji, G.; Furue, M. Mortality in Yusho Patients Exposed to Polychlorinated Biphenyls and Polychlorinated Dibenzofurans: A 50-Year Retrospective Cohort Study. Environ. Health 2020, 19, 119. [Google Scholar] [CrossRef]
- Lee, R.G.M.; Coleman, P.; Jones, J.L.; Jones, K.C.; Lohmann, R. Emission Factors and Importance of PCDD/Fs, PCBs, PCNs, PAHs and PM10 from the Domestic Burning of Coal and Wood in the U.K. Environ. Sci. Technol. 2005, 39, 1436–1447. [Google Scholar] [CrossRef]
- Shen, J.; Yang, L.; Liu, G.; Zhao, X.; Zheng, M. Occurrence, Profiles, and Control of Unintentional POPs in the Steelmaking Industry: A Review. Sci. Total Environ. 2021, 773, 145692. [Google Scholar] [CrossRef] [PubMed]
- Wania, F. Assessing the Potential of Persistent Organic Chemicals for Long-Range Transport and Accumulation in Polar Regions. Environ. Sci. Technol. 2003, 37, 1344–1351. [Google Scholar] [CrossRef]
- Gouin, T.; Mackay, D.; Jones, K.C.; Harner, T.; Meijer, S.N. Evidence for the “Grasshopper” Effect and Fractionation during Long-Range Atmospheric Transport of Organic Contaminants. Environ. Pollut. 2004, 128, 139–148. [Google Scholar] [CrossRef]
- Weitekamp, C.A.; Phillips, L.J.; Carlson, L.M.; DeLuca, N.M.; Cohen Hubal, E.A.; Lehmann, G.M. A State-of-the-Science Review of Polychlorinated Biphenyl Exposures at Background Levels: Relative Contributions of Exposure Routes. Sci. Total Environ. 2021, 776, 145912. [Google Scholar] [CrossRef]
- Schecter, A.; Colacino, J.; Haffner, D.; Patel, K.; Opel, M.; Päpke, O.; Birnbaum, L. Perfluorinated Compounds, Polychlorinated Biphenyls, and Organochlorine Pesticide Contamination in Composite Food Samples from Dallas, Texas, USA. Environ. Health Perspect. 2010, 118, 796–802. [Google Scholar] [CrossRef]
- Feinberg, M.; Soler, L.; Contenot, S.; Verger, P. Assessment of Seasonality in Exposure to Dioxins, Furans and Dioxin-like PCBs by Using Long-Term Food-Consumption Data. Food Addit. Contam. Part A 2011, 28, 502–512. [Google Scholar] [CrossRef]
- Domingo, J.L.; Bocio, A. Levels of PCDD/PCDFs and PCBs in Edible Marine Species and Human Intake: A Literature Review. Environ. Int. 2007, 33, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Weintraub, M.; Birnbaum, L.S. Catfish Consumption as a Contributor to Elevated PCB Levels in a Non-Hispanic Black Subpopulation. Environ. Res. 2008, 107, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Marushka, L.; Hu, X.; Batal, M.; Tikhonov, C.; Sadik, T.; Schwartz, H.; Ing, A.; Fediuk, K.; Chan, H.M. The Relationship between Dietary Exposure to Persistent Organic Pollutants from Fish Consumption and Type 2 Diabetes among First Nations in Canada. Can. J. Public Health 2021, 112, 168–182. [Google Scholar] [CrossRef]
- Lan, T.; Liu, B.; Bao, W.; Thorne, P.S. BMI Modifies the Association between Dietary Intake and Serum Levels of PCBs. Environ. Int. 2021, 156, 106626. [Google Scholar] [CrossRef] [PubMed]
- Saktrakulkla, P.; Lan, T.; Hua, J.; Marek, R.F.; Thorne, P.S.; Hornbuckle, K.C. Polychlorinated Biphenyls in Food. Environ. Sci. Technol. 2020, 54, 11443–11452. [Google Scholar] [CrossRef]
- Undeman, E.; Brown, T.N.; McLachlan, M.S.; Wania, F. Who in the World Is Most Exposed to Polychlorinated Biphenyls? Using Models to Identify Highly Exposed Populations. Environ. Res. Lett. 2018, 13, 064036. [Google Scholar] [CrossRef]
- Ludewig, G.; Lehmann, L.; Esch, H.; Robertson, L.W. Metabolic Activation of PCBs to Carcinogens in vivo—A Review. Environ. Toxicol. Pharmacol. 2008, 25, 241–246. [Google Scholar] [CrossRef]
- Hombrecher, K.; Quass, U.; Leisner, J.; Wichert, M. Significant Release of Unintentionally Produced Non-Aroclor Polychlorinated Biphenyl (PCB) Congeners PCB 47, PCB 51 and PCB 68 from a Silicone Rubber Production Site in North Rhine-Westphalia, Germany. Chemosphere 2021, 285, 131449. [Google Scholar] [CrossRef]
- Ockenden, W.A.; Lohmann, R.; Shears, J.R.; Jones, K.C. The Significance of PCBs in the Atmosphere of the Southern Hemisphere. Env. Sci Pollut Res 2001, 8, 189–194. [Google Scholar] [CrossRef]
- Sun, P.; Basu, I.; Hites, R.A. Temporal Trends of Polychlorinated Biphenyls in Precipitation and Air at Chicago. Environ. Sci. Technol. 2006, 40, 1178–1183. [Google Scholar] [CrossRef] [PubMed]
- Choi, A.L.; Levy, J.I.; Dockery, D.W.; Ryan, L.M.; Tolbert, P.E.; Altshul, L.M.; Korrick, S.A. Does Living Near a Superfund Site Contribute to Higher Polychlorinated Biphenyl (PCB) Exposure? Environ. Health Perspect. 2006, 114, 1092–1098. [Google Scholar] [CrossRef] [PubMed]
- Breivik, K.; Sweetman, A.; Pacyna, J.M.; Jones, K.C. Towards a Global Historical Emission Inventory for Selected PCB Congeners—A Mass Balance Approach: 3. An Update. Sci. Total Environ. 2007, 377, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Melymuk, L.; Robson, M.; Helm, P.A.; Diamond, M.L. PCBs, PBDEs, and PAHs in Toronto Air: Spatial and Seasonal Trends and Implications for Contaminant Transport. Sci. Total Environ. 2012, 429, 272–280. [Google Scholar] [CrossRef] [PubMed]
- US EPA. Great Lakes Integrated Atmospheric Deposition Network. Available online: https://www.epa.gov/great-lakes-monitoring/great-lakes-integrated-atmospheric-deposition-network (accessed on 15 February 2022).
- Rudel, R.A.; Perovich, L.J. Endocrine Disrupting Chemicals in Indoor and Outdoor Air. Atmos. Environ. 2009, 43, 170–181. [Google Scholar] [CrossRef]
- Choi, S.-D.; Baek, S.-Y.; Chang, Y.-S.; Wania, F.; Ikonomou, M.G.; Yoon, Y.-J.; Park, B.-K.; Hong, S. Passive Air Sampling of Polychlorinated Biphenyls and Organochlorine Pesticides at the Korean Arctic and Antarctic Research Stations: Implications for Long-Range Transport and Local Pollution. Environ. Sci. Technol. 2008, 42, 7125–7131. [Google Scholar] [CrossRef]
- Anh, H.Q.; Watanabe, I.; Minh, T.B.; Takahashi, S. Unintentionally Produced Polychlorinated Biphenyls in Pigments: An Updated Review on Their Formation, Emission Sources, Contamination Status, and Toxic Effects. Sci. Total Environ. 2021, 755, 142504. [Google Scholar] [CrossRef]
- Gabrio, T.; Piechotowski, I.; Wallenhorst, T.; Klett, M.; Cott, L.; Friebel, P.; Link, B.; Schwenk, M. PCB-Blood Levels in Teachers, Working in PCB-Contaminated Schools. Chemosphere 2000, 40, 1055–1062. [Google Scholar] [CrossRef]
- Schwenk, M.; Gabrio, T.; Päpke, O.; Wallenhorst, T. Human Biomonitoring of Polychlorinated Biphenyls and Polychlorinated Dibenzodioxins and Dibenzofuranes in Teachers Working in a PCB-Contaminated School. Chemosphere 2002, 47, 229–233. [Google Scholar] [CrossRef]
- Liebl, B.; Schettgen, T.; Kerscher, G.; Broding, H.-C.; Otto, A.; Angerer, J.; Drexler, H. Evidence for Increased Internal Exposure to Lower Chlorinated Polychlorinated Biphenyls (PCB) in Pupils Attending a Contaminated School. Int. J. Hyg. Environ. Health 2004, 207, 315–324. [Google Scholar] [CrossRef]
- Fitzgerald, E.F.; Shrestha, S.; Palmer, P.M.; Wilson, L.R.; Belanger, E.E.; Gomez, M.I.; Cayo, M.R.; Hwang, S. Polychlorinated Biphenyls (PCBs) in Indoor Air and in Serum among Older Residents of Upper Hudson River Communities. Chemosphere 2011, 85, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Martinez, A.; Hornbuckle, K.C. Discovery of Non-Aroclor PCB (3,3′-Dichlorobiphenyl) in Chicago Air. Environ. Sci. Technol. 2008, 42, 7873–7877. [Google Scholar] [CrossRef] [PubMed]
- Wolff, M.S. Occupational Exposure to Polychlorinated Biphenyls (PCBs). Environ. Health Perspect. 1985, 60, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Ross, G. The Public Health Implications of Polychlorinated Biphenyls (PCBs) in the Environment. Ecotoxicol. Environ. Saf. 2004, 59, 275–291. [Google Scholar] [CrossRef] [PubMed]
- Pironti, C.; Ricciardi, M.; Proto, A.; Cucciniello, R.; Fiorentino, A.; Fiorillo, R.; Motta, O. New Analytical Approach to Monitoring Air Quality in Historical Monuments through the Isotopic Ratio of CO2. Environ. Sci. Pollut. Res. 2022, 29, 29385–29390. [Google Scholar] [CrossRef]
- Motta, O.; Pironti, C.; Ricciardi, M.; Rostagno, C.; Bolzacchini, E.; Ferrero, L.; Cucciniello, R.; Proto, A. Leonardo Da Vinci’s “Last Supper”: A Case Study to Evaluate the Influence of Visitors on the Museum Preservation Systems. Environ. Sci. Pollut. Res. 2022, 29, 29391–29398. [Google Scholar] [CrossRef]
- Ricciardi, M.; Pironti, C.; Motta, O.; Fiorillo, R.; Camin, F.; Faggiano, A.; Proto, A. Investigations on Historical Monuments’ Deterioration through Chemical and Isotopic Analyses: An Italian Case Study. Envron. Sci. Pollut. Res. 2022, 29, 29409–29418. [Google Scholar] [CrossRef] [PubMed]
- ATSDR. Toxicological Profile for Polychlorinated Biphenyls (Pcbs); U.S. Department of Health and Human Services, Public Health Service Agency for Toxic Substances and Disease Registry, Division of Toxicology: Atlanta, GA, USA, 2000.
- Kohler, M.; Tremp, J.; Zennegg, M.; Seiler, C.; Minder-Kohler, S.; Beck, M.; Lienemann, P.; Wegmann, L.; Schmid, P. Joint Sealants: An Overlooked Diffuse Source of Polychlorinated Biphenyls in Buildings. Environ. Sci. Technol. 2005, 39, 1967–1973. [Google Scholar] [CrossRef] [PubMed]
- Herrick, R.F.; McClean, M.D.; Meeker, J.D.; Baxter, L.K.; Weymouth, G.A. An Unrecognized Source of PCB Contamination in Schools and Other Buildings. Environ. Health Perspect. 2004, 112, 1051–1053. [Google Scholar] [CrossRef]
- Jamshidi, A.; Hunter, S.; Hazrati, S.; Harrad, S. Concentrations and Chiral Signatures of Polychlorinated Biphenyls in Outdoor and Indoor Air and Soil in a Major U.K. Conurbation. Environ. Sci. Technol. 2007, 41, 2153–2158. [Google Scholar] [CrossRef]
- Herrick, R.F. PCBs in School—Persistent Chemicals, Persistent Problems. New Solut. 2010, 20, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Harrad, S.; Goosey, E.; Desborough, J.; Abdallah, M.A.-E.; Roosens, L.; Covaci, A. Dust from U.K. Primary School Classrooms and Daycare Centers: The Significance of Dust As a Pathway of Exposure of Young U.K. Children to Brominated Flame Retardants and Polychlorinated Biphenyls. Environ. Sci. Technol. 2010, 44, 4198–4202. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Diamond, M.L.; Robson, M.; Harrad, S. Sources, Emissions, and Fate of Polybrominated Diphenyl Ethers and Polychlorinated Biphenyls Indoors in Toronto, Canada. Environ. Sci. Technol. 2011, 45, 3268–3274. [Google Scholar] [CrossRef] [PubMed]
- MacIntosh, D.L.; Minegishi, T.; Fragala, M.A.; Allen, J.G.; Coghlan, K.M.; Stewart, J.H.; McCarthy, J.F. Mitigation of Building-Related Polychlorinated Biphenyls in Indoor Air of a School. Environ. Health 2012, 11, 24. [Google Scholar] [CrossRef] [PubMed]
- Marek, R.F.; Thorne, P.S.; Herkert, N.J.; Awad, A.M.; Hornbuckle, K.C. Airborne PCBs and OH-PCBs Inside and Outside Urban and Rural U.S. Schools. Environ. Sci. Technol. 2017, 51, 7853–7860. [Google Scholar] [CrossRef]
- Lee, W.-J.; Su, C.-C.; Sheu, H.-L.; Fan, Y.-C.; Chao, H.-R.; Fang, G.-C. Monitoring and Modeling of PCB Dry Deposition in Urban Area. J. Hazard. Mater. 1996, 49, 57–88. [Google Scholar] [CrossRef]
- Mi, H.-H.; Wu, Z.-S.; Lin, L.-F.; Lai, Y.-C.; Lee, Y.-Y.; Wang, L.-C.; Chang-Chien, G.-P. Atmospheric Dry Deposition of Polychlorinated Dibenzo-p-Dioxins/Dibenzofurans (PCDD/Fs) and Polychlorinated Biphenyls (PCBs) in Southern Taiwan. Aerosol Air Qual. Res. 2012, 12, 1016–1029. [Google Scholar] [CrossRef]
- Fang, M.; Choi, S.-D.; Baek, S.-Y.; Jin, G.; Chang, Y.-S. Deposition of Polychlorinated Biphenyls and Polybrominated Diphenyl Ethers in the Vicinity of a Steel Manufacturing Plant. Atmos. Environ. 2012, 49, 206–211. [Google Scholar] [CrossRef]
- Kang, Y.; Yin, Y.; Man, Y.; Li, L.; Zhang, Q.; Zeng, L.; Luo, J.; Wong, M.H. Bioaccessibility of Polychlorinated Biphenyls in Workplace Dust and Its Implication for Risk Assessment. Chemosphere 2013, 93, 924–930. [Google Scholar] [CrossRef]
- Hu, J.; Zheng, M.; Liu, W.; Li, C.; Nie, Z.; Liu, G.; Xiao, K.; Dong, S. Occupational Exposure to Polychlorinated Dibenzo-p-Dioxins and Dibenzofurans, Dioxin-like Polychlorinated Biphenyls, and Polychlorinated Naphthalenes in Workplaces of Secondary Nonferrous Metallurgical Facilities in China. Environ. Sci. Technol. 2013, 47, 7773–7779. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, J.; Lin, W.; Wang, N.; Li, C.; Luo, P.; Hashmi, M.Z.; Wang, W.; Su, X.; Chen, C.; et al. Health Risk Assessment of Migrant Workers’ Exposure to Polychlorinated Biphenyls in Air and Dust in an e-Waste Recycling Area in China: Indication for a New Wealth Gap in Environmental Rights. Environ. Int. 2016, 87, 33–41. [Google Scholar] [CrossRef] [PubMed]
- He, C.-T.; Zheng, X.-B.; Yan, X.; Zheng, J.; Wang, M.-H.; Tan, X.; Qiao, L.; Chen, S.-J.; Yang, Z.-Y.; Mai, B.-X. Organic Contaminants and Heavy Metals in Indoor Dust from E-Waste Recycling, Rural, and Urban Areas in South China: Spatial Characteristics and Implications for Human Exposure. Ecotoxicol. Environ. Saf. 2017, 140, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Anh, H.Q.; Watanabe, I.; Minh, T.B.; Tue, N.M.; Tuyen, L.H.; Viet, P.H.; Takahashi, S. Polychlorinated Biphenyls in Settled Dusts from an End-of-Life Vehicle Processing Area and Normal House Dusts in Northern Vietnam: Occurrence, Potential Sources, and Risk Assessment. Sci. Total Environ. 2020, 728, 138823. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Cheng, S.M.; Loganath, A.; Chong, Y.S.; Obbard, J.P. Selected Organochlorine Pesticide and Polychlorinated Biphenyl Residues in House Dust in Singapore. Chemosphere 2007, 68, 1675–1682. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, P.; Prithiviraj, B.; Selvaraj, S.; Kumar, B. Polychlorinated Biphenyls in Settled Dust from Informal Electronic Waste Recycling Workshops and Nearby Highways in Urban Centers and Suburban Industrial Roadsides of Chennai City, India: Levels, Congener Profiles and Exposure Assessment. Sci. Total Environ. 2016, 573, 1413–1421. [Google Scholar] [CrossRef] [PubMed]
- Harrad, S.; Ibarra, C.; Robson, M.; Melymuk, L.; Zhang, X.; Diamond, M.; Douwes, J. Polychlorinated Biphenyls in Domestic Dust from Canada, New Zealand, United Kingdom and United States: Implications for Human Exposure. Chemosphere 2009, 76, 232–238. [Google Scholar] [CrossRef]
- Holsen, T.M.; Noll, K.E.; Liu, S.P.; Lee, W.J. Dry Deposition of Polychlorinated Biphenyls in Urban Areas. Environ. Sci. Technol. 1991, 25, 1075–1081. [Google Scholar] [CrossRef]
- Tasdemir, Y.; Vardar, N.; Odabasi, M.; Holsen, T.M. Concentrations and Gas/Particle Partitioning of PCBs in Chicago. Environ. Pollut. 2004, 131, 35–44. [Google Scholar] [CrossRef]
- Van Ry, D.A.; Gigliotti, C.L.; Glenn; Nelson, E.D.; Totten, L.A.; Eisenreich, S.J. Wet Deposition of Polychlorinated Biphenyls in Urban and Background Areas of the Mid-Atlantic States. Environ. Sci. Technol. 2002, 36, 3201–3209. [Google Scholar] [CrossRef]
- Gonzalez, J.; Feng, L.; Sutherland, A.; Waller, C.; Sok, H.; Hesse, P.R.; Rosenfeld, P.D.P. PCBs and Dioxins/Furans in Attic Dust Collected near Former PCB Production and Secondary Copper Facilities in Sauget, IL. Procedia Environ. Sci. 2011, 4, 113–125. [Google Scholar] [CrossRef][Green Version]
- Bannavti, M.K.; Jahnke, J.C.; Marek, R.F.; Just, C.L.; Hornbuckle, K.C. Room-to-Room Variability of Airborne Polychlorinated Biphenyls in Schools and the Application of Air Sampling for Targeted Source Evaluation. Environ. Sci. Technol. 2021, 55, 9460–9468. [Google Scholar] [CrossRef]
- Castro-Jiménez, J.; Mariani, G.; Vives, I.; Skejo, H.; Umlauf, G.; Zaldívar, J.M.; Dueri, S.; Messiaen, G.; Laugier, T. Atmospheric Concentrations, Occurrence and Deposition of Persistent Organic Pollutants (POPs) in a Mediterranean Coastal Site (Etang de Thau, France). Environ. Pollut. 2011, 159, 1948–1956. [Google Scholar] [CrossRef] [PubMed]
- Klees, M.; Hombrecher, K.; Gladtke, D. Polychlorinated Biphenyls in the Surrounding of an E-Waste Recycling Facility in North-Rhine Westphalia: Levels in Plants and Dusts, Spatial Distribution, Homologue Pattern and Source Identification Using the Combination of Plants and Wind Direction Data. Sci. Total Environ. 2017, 603–604, 606–615. [Google Scholar] [CrossRef]
- Iwegbue, C.M.A.; Eyengho, S.B.; Egobueze, F.E.; Odali, E.W.; Tesi, G.O.; Nwajei, G.E.; Martincigh, B.S. Polybrominated Diphenyl Ethers and Polychlorinated Biphenyls in Indoor Dust from Electronic Repair Workshops in Southern Nigeria: Implications for Onsite Human Exposure. Sci. Total Environ. 2019, 671, 914–927. [Google Scholar] [CrossRef]
- Abafe, O.A.; Martincigh, B.S. Polybrominated Diphenyl Ethers and Polychlorinated Biphenyls in Indoor Dust in Durban, South Africa. Indoor Air 2015, 25, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Abafe, O.A.; Martincigh, B.S. An Assessment of Polybrominated Diphenyl Ethers and Polychlorinated Biphenyls in the Indoor Dust of E-Waste Recycling Facilities in South Africa: Implications for Occupational Exposure. Environ. Sci. Pollut. Res. 2015, 22, 14078–14086. [Google Scholar] [CrossRef] [PubMed]
- Bozlaker, A.; Odabasi, M.; Muezzinoglu, A. Dry Deposition and Soil–Air Gas Exchange of Polychlorinated Biphenyls (PCBs) in an Industrial Area. Environ. Pollut. 2008, 156, 784–793. [Google Scholar] [CrossRef]
- Korhonen, K.; Liukkonen, T.; Ahrens, W.; Astrakianakis, G.; Boffetta, P.; Burdorf, A.; Heederik, D.; Kauppinen, T.; Kogevinas, M.; Osvoll, P.; et al. Occupational Exposure to Chemical Agents in the Paper Industry. Int. Arch. Occup. Environ. Health 2004, 77, 451–460. [Google Scholar] [CrossRef]
- Barron, M.G.; Yurk, J.J.; Crothers, D.B. Assessment of Potential Cancer Risk from Consumption of PCBs Bioaccumulated in Fish and Shellfish. Environ. Health Perspect. 1994, 102, 562–567. [Google Scholar] [CrossRef]
- Troisi, G.M.; Haraguchi, K.; Kaydoo, D.S.; Nyman, M.; Aguilar, A.; Borrell, A.; Siebert, U.; Mason, C.F. Bioaccumulation of Polychlorinated Biphenyls (PCBs) and Dichlorodiphenylethane (DDE) Methyl Sulfones in Tissues of Seal and Dolphin Morbillivirus Epizootic Victims. J. Toxicol. Environ. Health Part A 2000, 62, 1–8. [Google Scholar] [CrossRef]
- Oregel-Zamudio, E.; Alvarez-Bernal, D.; Franco-Hernandez, M.O.; Buelna-Osben, H.R.; Mora, M. Bioaccumulation of PCBs and PBDEs in Fish from a Tropical Lake Chapala, Mexico. Toxics 2021, 9, 241. [Google Scholar] [CrossRef]
- Link, B.; Gabrio, T.; Zoellner, I.; Piechotowski, I.; Paepke, O.; Herrmann, T.; Felder-Kennel, A.; Maisner, V.; Schick, K.-H.; Schrimpf, M.; et al. Biomonitoring of Persistent Organochlorine Pesticides, PCDD/PCDFs and Dioxin-like PCBs in Blood of Children from South West Germany (Baden-Wuerttemberg) from 1993 to 2003. Chemosphere 2005, 58, 1185–1201. [Google Scholar] [CrossRef] [PubMed]
- Quinete, N.; Esser, A.; Kraus, T.; Schettgen, T. Determination of Hydroxylated Polychlorinated Biphenyls (OH-PCBs) in Human Urine in a Highly Occupationally Exposed German Cohort: New Prospects for Urinary Biomarkers of PCB Exposure. Environ. Int. 2016, 97, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Mari, M.; Schuhmacher, M.; Domingo, J.L. Levels of Metals and Organic Substances in Workers at a Hazardous Waste Incinerator: A Follow-up Study. Int. Arch. Occup. Environ. Health 2009, 82, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Hopf, N.B.; Ruder, A.M.; Succop, P. Background Levels of Polychlorinated Biphenyls in the U.S. Population. Sci. Total Environ. 2009, 407, 6109–6119. [Google Scholar] [CrossRef]
- Consonni, D.; Sindaco, R.; Bertazzi, P.A. Blood Levels of Dioxins, Furans, Dioxin-like PCBs, and TEQs in General Populations: A Review, 1989–2010. Environ. Int. 2012, 44, 151–162. [Google Scholar] [CrossRef]
- Nakamoto, M.; Arisawa, K.; Uemura, H.; Katsuura, S.; Takami, H.; Sawachika, F.; Yamaguchi, M.; Juta, T.; Sakai, T.; Toda, E.; et al. Association between Blood Levels of PCDDs/PCDFs/Dioxin-like PCBs and History of Allergic and Other Diseases in the Japanese Population. Int. Arch. Occup. Environ. Health 2013, 86, 849–859. [Google Scholar] [CrossRef]
- Koh, W.X.; Hornbuckle, K.C.; Thorne, P.S. Human Serum from Urban and Rural Adolescents and Their Mothers Shows Exposure to Polychlorinated Biphenyls Not Found in Commercial Mixtures. Environ. Sci. Technol. 2015, 49, 8105–8112. [Google Scholar] [CrossRef]
- ‘t Mannetje, A.; Eng, A.; Walls, C.; Dryson, E.; McLean, D.; Kogevinas, M.; Fowles, J.; Borman, B.; O’Connor, P.; Cheng, S.; et al. Serum Concentrations of Chlorinated Dibenzo-p-Dioxins, Furans and PCBs, among Former Phenoxy Herbicide Production Workers and Firefighters in New Zealand. Int. Arch. Occup. Environ. Health 2016, 89, 307–318. [Google Scholar] [CrossRef][Green Version]
- Peper, M.; Klett, M.; Morgenstern, R. Neuropsychological Effects of Chronic Low-Dose Exposure to Polychlorinated Biphenyls (PCBs): A Cross-Sectional Study. Environ. Health 2005, 4, 22. [Google Scholar] [CrossRef]
- Pedersen, E.B.; Ebbehøj, N.E.; Göen, T.; Meyer, H.W.; Jacobsen, P. Exposure to 27 Polychlorinated Biphenyls in the Indoor Environment of a Workplace: A Controlled Bio-Monitoring Study. Int. Arch. Occup. Environ. Health 2016, 89, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Pizzini, S.; Sbicego, C.; Corami, F.; Grotti, M.; Magi, E.; Bonato, T.; Cozzi, G.; Barbante, C.; Piazza, R. 3,3′-Dichlorobiphenyl (Non-Aroclor PCB-11) as a Marker of Non-Legacy PCB Contamination in Marine Species: Comparison between Antarctic and Mediterranean Bivalves. Chemosphere 2017, 175, 28–35. [Google Scholar] [CrossRef]
- IARC. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer: Lyon, France, 2012. [Google Scholar]
- Klocke, C.; Lein, P.J. Evidence Implicating Non-Dioxin-Like Congeners as the Key Mediators of Polychlorinated Biphenyl (PCB) Developmental Neurotoxicity. Int. J. Mol. Sci. 2020, 21, 1013. [Google Scholar] [CrossRef] [PubMed]
- Schantz, S.L.; Widholm, J.J.; Rice, D.C. Effects of PCB Exposure on Neuropsychological Function in Children. Environ. Health Perspect. 2003, 111, 357–576. [Google Scholar] [CrossRef]
- Boucher, O.; Muckle, G.; Bastien, C.H. Prenatal Exposure to Polychlorinated Biphenyls: A Neuropsychologic Analysis. Environ. Health Perspect. 2009, 117, 7–16. [Google Scholar] [CrossRef]
- Berghuis, S.A.; Bos, A.F.; Sauer, P.J.; Roze, E. Developmental Neurotoxicity of Persistent Organic Pollutants: An Update on Childhood Outcome. Arch. Toxicol. 2015, 89, 687–709. [Google Scholar] [CrossRef]
- Pessah, I.N.; Lein, P.J.; Seegal, R.F.; Sagiv, S.K. Neurotoxicity of Polychlorinated Biphenyls and Related Organohalogens. Acta Neuropathol. 2019, 138, 363–387. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.W.; Lonky, E.; Reihman, J.; Pagano, J.; Gump, B.B.; Darvill, T. The Relationship between Prenatal PCB Exposure and Intelligence (IQ) in 9-Year-Old Children. Environ. Health Perspect. 2008, 116, 1416–1422. [Google Scholar] [CrossRef] [PubMed]
- Tatsuta, N.; Nakai, K.; Murata, K.; Suzuki, K.; Iwai-Shimada, M.; Kurokawa, N.; Hosokawa, T.; Satoh, H. Impacts of Prenatal Exposures to Polychlorinated Biphenyls, Methylmercury, and Lead on Intellectual Ability of 42-Month-Old Children in Japan. Environ. Res. 2014, 133, 321–326. [Google Scholar] [CrossRef]
- Kyriklaki, A.; Vafeiadi, M.; Kampouri, M.; Koutra, K.; Roumeliotaki, T.; Chalkiadaki, G.; Anousaki, D.; Rantakokko, P.; Kiviranta, H.; Fthenou, E.; et al. Prenatal Exposure to Persistent Organic Pollutants in Association with Offspring Neuropsychological Development at 4years of Age: The Rhea Mother-Child Cohort, Crete, Greece. Environ. Int. 2016, 97, 204–211. [Google Scholar] [CrossRef]
- Ikeno, T.; Miyashita, C.; Nakajima, S.; Kobayashi, S.; Yamazaki, K.; Saijo, Y.; Kita, T.; Sasaki, S.; Konishi, K.; Kajiwara, J.; et al. Effects of Low-Level Prenatal Exposure to Dioxins on Cognitive Development in Japanese Children at 42months. Sci. Total Environ. 2018, 618, 1423–1430. [Google Scholar] [CrossRef] [PubMed]
- Panesar, H.K.; Kennedy, C.L.; Keil Stietz, K.P.; Lein, P.J. Polychlorinated Biphenyls (PCBs): Risk Factors for Autism Spectrum Disorder? Toxics 2020, 8, 70. [Google Scholar] [CrossRef] [PubMed]
- Sagiv, S.K.; Thurston, S.W.; Bellinger, D.C.; Tolbert, P.E.; Altshul, L.M.; Korrick, S.A. Prenatal Organochlorine Exposure and Behaviors Associated With Attention Deficit Hyperactivity Disorder in School-Aged Children. Am. J. Epidemiol. 2010, 171, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Eubig, P.A.; Aguiar, A.; Schantz, S.L. Lead and PCBs as Risk Factors for Attention Deficit/Hyperactivity Disorder. Environ. Health Perspect. 2010, 118, 1654–1667. [Google Scholar] [CrossRef] [PubMed]
- de Cock, M.; Maas, Y.G.H.; van de Bor, M. Does Perinatal Exposure to Endocrine Disruptors Induce Autism Spectrum and Attention Deficit Hyperactivity Disorders? Review. Acta Paediatr. 2012, 101, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Rosenquist, A.H.; Høyer, B.B.; Julvez, J.; Sunyer, J.; Pedersen, H.S.; Lenters, V.; Jönsson, B.A.G.; Bonde, J.P.; Toft, G. Prenatal and Postnatal PCB-153 and p,p′-DDE Exposures and Behavior Scores at 5–9 Years of Age among Children in Greenland and Ukraine. Environ. Health Perspect. 2017, 125, 107002. [Google Scholar] [CrossRef]
- Taylor, P.R.; Stelma, J.M.; Lawrence, C.J. The Relation of Polychlorinated Biphenyls to Birth Weight and Gestational Age in the Offspring of Occupationally Exposed Mothers. Am. J. Epidemiol. 1989, 129, 395–406. [Google Scholar] [CrossRef]
- Patandin, S.; Koopman-Esseboom, C.; De Ridder, M.A.J.; Weisglas-Kuperus, N.; Sauer, P.J.J. Effects of Environmental Exposure to Polychlorinated Biphenyls and Dioxins on Birth Size and Growth in Dutch Children. Pediatr. Res. 1998, 44, 538–545. [Google Scholar] [CrossRef]
- Baibergenova, A.; Kudyakov, R.; Zdeb, M.; Carpenter, D.O. Low Birth Weight and Residential Proximity to PCB-Contaminated Waste Sites. Environ. Health Perspect. 2003, 111, 1352–1357. [Google Scholar] [CrossRef]
- Hertz-Picciotto, I.; Charles, M.J.; James, R.A.; Keller, J.A.; Willman, E.; Teplin, S. In Utero Polychlorinated Biphenyl Exposures in Relation to Fetal and Early Childhood Growth. Epidemiology 2005, 16, 648–656. [Google Scholar] [CrossRef]
- Govarts, E.; Nieuwenhuijsen, M.; Schoeters, G.; Ballester, F.; Bloemen, K.; de Boer, M.; Chevrier, C.; Eggesbø, M.; Guxens, M.; Krämer, U.; et al. Birth Weight and Prenatal Exposure to Polychlorinated Biphenyls (PCBs) and Dichlorodiphenyldichloroethylene (DDE): A Meta-Analysis within 12 European Birth Cohorts. Environ. Health Perspect. 2012, 120, 162–170. [Google Scholar] [CrossRef]
- Longnecker, M.P.; Klebanoff, M.A.; Brock, J.W.; Guo, X. Maternal Levels of Polychlorinated Biphenyls in Relation to Preterm and Small-for-Gestational-Age Birth. Epidemiology 2005, 16, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Lauritzen, H.B.; Larose, T.L.; Øien, T.; Sandanger, T.M.; Odland, J.Ø.; van de Bor, M.; Jacobsen, G.W. Maternal Serum Levels of Perfluoroalkyl Substances and Organochlorines and Indices of Fetal Growth: A Scandinavian Case—Cohort Study. Pediatr Res 2017, 81, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Govarts, E.; Iszatt, N.; Trnovec, T.; de Cock, M.; Eggesbø, M.; Palkovicova Murinova, L.; van de Bor, M.; Guxens, M.; Chevrier, C.; Koppen, G.; et al. Prenatal Exposure to Endocrine Disrupting Chemicals and Risk of Being Born Small for Gestational Age: Pooled Analysis of Seven European Birth Cohorts. Environ. Int. 2018, 115, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Sable, H.J.K.; Schantz, S.L. Executive Function Following Developmental Exposure to Polychlorinated Biphenyls (PCBs): What Animal Models Have Told Us; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2006. [Google Scholar]
- Yang, D.; Kim, K.H.; Phimister, A.; Bachstetter, A.D.; Ward, T.R.; Stackman, R.W.; Mervis, R.F.; Wisniewski, A.B.; Klein, S.L.; Kodavanti, P.R.S.; et al. Developmental Exposure to Polychlorinated Biphenyls Interferes with Experience-Dependent Dendritic Plasticity and Ryanodine Receptor Expression in Weanling Rats. Environ. Health Perspect. 2009, 117, 426–435. [Google Scholar] [CrossRef]
- Winneke, G. Developmental Aspects of Environmental Neurotoxicology: Lessons from Lead and Polychlorinated Biphenyls. J. Neurol. Sci. 2011, 308, 9–15. [Google Scholar] [CrossRef]
- Gore, A.C.; Krishnan, K.; Reilly, M.P. Endocrine-Disrupting Chemicals: Effects on Neuroendocrine Systems and the Neurobiology of Social Behavior. Horm. Behav. 2019, 111, 7–22. [Google Scholar] [CrossRef]
- Sethi, S.; Keil, K.P.; Chen, H.; Hayakawa, K.; Li, X.; Lin, Y.; Lehmler, H.-J.; Puschner, B.; Lein, P.J. Detection of 3,3′-Dichlorobiphenyl in Human Maternal Plasma and Its Effects on Axonal and Dendritic Growth in Primary Rat Neurons. Toxicol. Sci. 2017, 158, 401–411. [Google Scholar] [CrossRef]
- Mellor, C.L.; Steinmetz, F.P.; Cronin, M.T.D. The Identification of Nuclear Receptors Associated with Hepatic Steatosis to Develop and Extend Adverse Outcome Pathways. Crit. Rev. Toxicol. 2016, 46, 138–152. [Google Scholar] [CrossRef]
- Bock, K.W. Toward Elucidation of Dioxin-Mediated Chloracne and Ah Receptor Functions. Biochem. Pharmacol. 2016, 112, 1–5. [Google Scholar] [CrossRef]
- Wheeler, M.A.; Rothhammer, V.; Quintana, F.J. Control of Immune-Mediated Pathology via the Aryl Hydrocarbon Receptor. J. Biol. Chem. 2017, 292, 12383–12389. [Google Scholar] [CrossRef]
- Silberhorn, E.M.; Glauert, H.P.; Robertson, L.W. Critical Reviews in: Carcinogenicity of Polyhalogenated Biphenyls: PCBs and PBBs. Crit. Rev. Toxicol. 1990, 20, 440–496. [Google Scholar] [CrossRef] [PubMed]
- Loomis, D.; Browning, S.R.; Schenck, A.P.; Gregory, E.; Savitz, D.A. Cancer Mortality among Electric Utility Workers Exposed to Polychlorinated Biphenyls. Occup. Environ. Med. 1997, 54, 720–728. [Google Scholar] [CrossRef] [PubMed]
- National Toxicology Program. NTP Toxicology and Carcinogenesis Studies of 3,3’,4,4’,5-Pentachlorobiphenyl (PCB 126) (CAS No. 57465-28-8) in Female Harlan Sprague-Dawley Rats (Gavage Studies). Natl. Toxicol. Program Tech. Rep. Ser. 2006, 520, 4–246. [Google Scholar]
- National Toxicology Program. Toxicology and Carcinogenesis Studies of 2,3’,4,4’,5-Pentachlorobiphenyl (PCB 118) (CAS No. 31508-00-6) in Female Harlan Sprague-Dawley Rats (Gavage Studies). Natl. Toxicol. Program Tech. Rep. Ser. 2010, 559, 1–174. [Google Scholar]
- Silver, S.R.; Whelan, E.A.; Deddens, J.A.; Steenland, N.K.; Hopf, N.B.; Waters, M.A.; Ruder, A.M.; Prince, M.M.; Yong, L.C.; Hein, M.J.; et al. Occupational Exposure to Polychlorinated Biphenyls and Risk of Breast Cancer. Environ. Health Perspect. 2009, 117, 276–282. [Google Scholar] [CrossRef]
- Luecke, S.; Backlund, M.; Jux, B.; Esser, C.; Krutmann, J.; Rannug, A. The Aryl Hydrocarbon Receptor (AHR), a Novel Regulator of Human Melanogenesis. Pigment. Cell Melanoma Res. 2010, 23, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, R.P.; MacArthur, A.C.; Lee, T.K.; Weber, J.-P.; Leblanc, A.; Mark Elwood, J.; Borugian, M.; Abanto, Z.; Spinelli, J.J. Plasma Levels of Polychlorinated Biphenyls and Risk of Cutaneous Malignant Melanoma: A Preliminary Study. Int. J. Cancer 2011, 128, 1872–1880. [Google Scholar] [CrossRef] [PubMed]
- Lauby-Secretan, B.; Loomis, D.; Grosse, Y.; El Ghissassi, F.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Baan, R.; Mattock, H.; Straif, K. Carcinogenicity of Polychlorinated Biphenyls and Polybrominated Biphenyls. Lancet Oncol. 2013, 14, 287–288. [Google Scholar] [CrossRef]
- Ludewig, G.; Robertson, L.W. Polychlorinated Biphenyls (PCBs) as Initiating Agents in Hepatocellular Carcinoma. Cancer Lett. 2013, 334, 46–55. [Google Scholar] [CrossRef]
- Raffetti, E.; Donato, F.; De Palma, G.; Leonardi, L.; Sileo, C.; Magoni, M. Polychlorinated Biphenyls (PCBs) and Risk of Dementia and Parkinson Disease: A Population-Based Cohort Study in a North Italian Highly Polluted Area. Chemosphere 2020, 261, 127522. [Google Scholar] [CrossRef] [PubMed]
- Schantz, S.L.; Gasior, D.M.; Polverejan, E.; McCaffrey, R.J.; Sweeney, A.M.; Humphrey, H.E.; Gardiner, J.C. Impairments of Memory and Learning in Older Adults Exposed to Polychlorinated Biphenyls via Consumption of Great Lakes Fish. Environ. Health Perspect. 2001, 109, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, E.F.; Belanger, E.E.; Gomez, M.I.; Hwang, S.; Jansing, R.L.; Hicks, H.E. Environmental Exposures to Polychlorinated Biphenyls (PCBs) among Older Residents of Upper Hudson River Communities. Environ. Res. 2007, 104, 352–360. [Google Scholar] [CrossRef]
- Haase, R.F.; McCaffrey, R.J.; Santiago-Rivera, A.L.; Morse, G.S.; Tarbell, A. Evidence of an Age-Related Threshold Effect of Polychlorinated Biphenyls (PCBs) on Neuropsychological Functioning in a Native American Population. Environ. Res. 2009, 109, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Heilmann, C.; Grandjean, P.; Weihe, P.; Nielsen, F.; Budtz-Jørgensen, E. Reduced Antibody Responses to Vaccinations in Children Exposed to Polychlorinated Biphenyls. PLoS Med. 2006, 3, e311. [Google Scholar] [CrossRef]
- Selgrade, M.K. Immunotoxicity—The Risk Is Real. Toxicol. Sci. 2007, 100, 328–332. [Google Scholar] [CrossRef]
- Park, H.-Y.; Hertz-Picciotto, I.; Petrik, J.; Palkovicova, L.; Kocan, A.; Trnovec, T. Prenatal PCB Exposure and Thymus Size at Birth in Neonates in Eastern Slovakia. Environ. Health Perspect. 2008, 116, 104–109. [Google Scholar] [CrossRef]
- Hennig, B.; Reiterer, G.; Majkova, Z.; Oesterling, E.; Meerarani, P.; Toborek, M. Modification of Environmental Toxicity by Nutrients. Cardiovasc. Toxicol. 2005, 5, 153–160. [Google Scholar] [CrossRef]
- Dziennis, S.; Yang, D.; Cheng, J.; Anderson, K.A.; Alkayed, N.J.; Hurn, P.D.; Lein, P.J. Developmental Exposure to Polychlorinated Biphenyls Influences Stroke Outcome in Adult Rats. Environ. Health Perspect. 2008, 116, 474–480. [Google Scholar] [CrossRef]
- Everett, C.J.; Mainous, A.G.; Frithsen, I.L.; Player, M.S.; Matheson, E.M. Association of Polychlorinated Biphenyls with Hypertension in the 1999–2002 National Health and Nutrition Examination Survey. Environ. Res. 2008, 108, 94–97. [Google Scholar] [CrossRef]
- Humblet, O.; Birnbaum, L.; Rimm, E.; Mittleman, M.A.; Hauser, R. Dioxins and Cardiovascular Disease Mortality. Environ. Health Perspect. 2008, 116, 1443–1448. [Google Scholar] [CrossRef] [PubMed]
- Helyar, S.G.; Patel, B.; Headington, K.; El Assal, M.; Chatterjee, P.K.; Pacher, P.; Mabley, J.G. PCB-Induced Endothelial Cell Dysfunction: Role of Poly(ADP-Ribose) Polymerase. Biochem. Pharmacol. 2009, 78, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Raffetti, E.; Donato, F.; De Palma, G.; Leonardi, L.; Sileo, C.; Magoni, M. Polychlorinated Biphenyls (PCBs) and Risk of Hypertension: A Population-Based Cohort Study in a North Italian Highly Polluted Area. Sci. Total Environ. 2020, 714, 136660. [Google Scholar] [CrossRef] [PubMed]
- Samsó, M.; Feng, W.; Pessah, I.N.; Allen, P.D. Coordinated Movement of Cytoplasmic and Transmembrane Domains of RyR1 upon Gating. PLoS Biol. 2009, 7, e1000085. [Google Scholar] [CrossRef]
- Pessah, I.N.; Cherednichenko, G.; Lein, P.J. Minding the Calcium Store: Ryanodine Receptor Activation as a Convergent Mechanism of PCB Toxicity. Pharmacol. Ther. 2010, 125, 260–285. [Google Scholar] [CrossRef]
- Howard, A.S.; Fitzpatrick, R.; Pessah, I.; Kostyniak, P.; Lein, P.J. Polychlorinated Biphenyls Induce Caspase-Dependent Cell Death in Cultured Embryonic Rat Hippocampal but Not Cortical Neurons via Activation of the Ryanodine Receptor. Toxicol. Appl. Pharmacol. 2003, 190, 72–86. [Google Scholar] [CrossRef]
- Murugesan, P.; Kanagaraj, P.; Yuvaraj, S.; Balasubramanian, K.; Aruldhas, M.M.; Arunakaran, J. The Inhibitory Effects of Polychlorinated Biphenyl Aroclor 1254 on Leydig Cell LH Receptors, Steroidogenic Enzymes and Antioxidant Enzymes in Adult Rats. Reprod. Toxicol. 2005, 20, 117–126. [Google Scholar] [CrossRef]
- Glauert, H.P.; Tharappel, J.C.; Lu, Z.; Stemm, D.; Banerjee, S.; Chan, L.S.; Lee, E.Y.; Lehmler, H.-J.; Robertson, L.W.; Spear, B.T. Role of Oxidative Stress in the Promoting Activities of PCBs. Environ. Toxicol. Pharmacol. 2008, 25, 247–250. [Google Scholar] [CrossRef]
- Lyng, G.D.; Seegal, R.F. Polychlorinated Biphenyl-Induced Oxidative Stress in Organotypic Co-Cultures: Experimental Dopamine Depletion Prevents Reductions in GABA. NeuroToxicology 2008, 29, 301–308. [Google Scholar] [CrossRef][Green Version]
- Duntas, L.H. Environmental Factors and Autoimmune Thyroiditis. Nat. Rev. Endocrinol. 2008, 4, 454–460. [Google Scholar] [CrossRef]
- Liu, N.; Rizzi, N.; Boveri, L.; Priori, S.G. Ryanodine Receptor and Calsequestrin in Arrhythmogenesis: What We Have Learnt from Genetic Diseases and Transgenic Mice. J. Mol. Cell. Cardiol. 2009, 46, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-M.; Jacobs, D.R., Jr.; Lee, D.-H. Persistent Organic Pollutants and Type 2 Diabetes: A Critical Review of Review Articles. Front. Endocrinol. 2018, 9, 712. [Google Scholar] [CrossRef] [PubMed]
- Buha Djordjevic, A.; Antonijevic, E.; Curcic, M.; Milovanovic, V.; Antonijevic, B. Endocrine-Disrupting Mechanisms of Polychlorinated Biphenyls. Curr. Opin. Toxicol. 2020, 19, 42–49. [Google Scholar] [CrossRef]
- Curtis, S.W.; Terrell, M.L.; Jacobson, M.H.; Cobb, D.O.; Jiang, V.S.; Neblett, M.F.; Gerkowicz, S.A.; Spencer, J.B.; Marder, M.E.; Barr, D.B.; et al. Thyroid Hormone Levels Associate with Exposure to Polychlorinated Biphenyls and Polybrominated Biphenyls in Adults Exposed as Children. Environ. Health 2019, 18, 75. [Google Scholar] [CrossRef] [PubMed]
- Buck Louis, G.M.; Sundaram, R.; Schisterman, E.F.; Sweeney, A.M.; Lynch, C.D.; Gore-Langton, R.E.; Maisog, J.; Kim, S.; Chen, Z.; Barr, D.B. Persistent Environmental Pollutants and Couple Fecundity: The LIFE Study. Environ. Health Perspect. 2013, 121, 231–236. [Google Scholar] [CrossRef] [PubMed]
- He, Q.-L.; Zhang, L.; Liu, S.-Z. Effects of Polychlorinated Biphenyls on Animal Reproductive Systems and Epigenetic Modifications. Bull. Environ. Contam. Toxicol. 2021, 107, 398–405. [Google Scholar] [CrossRef]
- Klenov, V.; Flor, S.; Ganesan, S.; Adur, M.; Eti, N.; Iqbal, K.; Soares, M.J.; Ludewig, G.; Ross, J.W.; Robertson, L.W.; et al. The Aryl Hydrocarbon Receptor Mediates Reproductive Toxicity of Polychlorinated Biphenyl Congener 126 in Rats. Toxicol. Appl. Pharmacol. 2021, 426, 115639. [Google Scholar] [CrossRef]
- Axelrad, D.A.; Goodman, S.; Woodruff, T.J. PCB Body Burdens in US Women of Childbearing Age 2001–2002: An Evaluation of Alternate Summary Metrics of NHANES Data. Environ. Res. 2009, 109, 368–378. [Google Scholar] [CrossRef]
- Meeker, J.D.; Maity, A.; Missmer, S.A.; Williams, P.L.; Mahalingaiah, S.; Ehrlich, S.; Berry, K.F.; Altshul, L.; Perry, M.J.; Cramer, D.W.; et al. Serum Concentrations of Polychlorinated Biphenyls in Relation to in Vitro Fertilization Outcomes. Environ. Health Perspect. 2011, 119, 1010–1016. [Google Scholar] [CrossRef]
- Huang, Y.; Yan, M.; Nie, H.; Wang, W.; Wang, J. Persistent Halogenated Organic Pollutants in Follicular Fluid of Women Undergoing in vitro Fertilization from China: Occurrence, Congener Profiles, and Possible Sources. Environ. Pollut. 2019, 244, 1–8. [Google Scholar] [CrossRef]
- Bloom, M.S.; Fujimoto, V.Y.; Storm, R.; Zhang, L.; Butts, C.D.; Sollohub, D.; Jansing, R.L. Persistent Organic Pollutants (POPs) in Human Follicular Fluid and in vitro Fertilization Outcomes, a Pilot Study. Reprod. Toxicol. 2017, 67, 165–173. [Google Scholar] [CrossRef]
- Patel, S.; Zhou, C.; Rattan, S.; Flaws, J.A. Effects of Endocrine-Disrupting Chemicals on the Ovary. Biol. Reprod. 2015, 93, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Axmon, A.; Rylander, L.; Strömberg, U.; Hagmar, L. Altered Menstrual Cycles in Women with a High Dietary Intake of Persistent Organochlorine Compounds. Chemosphere 2004, 56, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Buck Louis, G.M.; Dmochowski, J.; Lynch, C.; Kostyniak, P.; McGuinness, B.M.; Vena, J.E. Polychlorinated Biphenyl Serum Concentrations, Lifestyle and Time-to-Pregnancy. Hum. Reprod. 2009, 24, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Toft, G.; Thulstrup, A.M.; Jönsson, B.A.; Pedersen, H.S.; Ludwicki, J.K.; Zvezday, V.; Bonde, J.P. Fetal Loss and Maternal Serum Levels of 2,2′,4,4′,5,5′-Hexachlorbiphenyl (CB-153) and 1,1-Dichloro-2,2-Bis(p-Chlorophenyl)Ethylene (p,p′-DDE) Exposure: A Cohort Study in Greenland and Two European Populations. Environ. Health 2010, 9, 22. [Google Scholar] [CrossRef]
- Grindler, N.M.; Allsworth, J.E.; Macones, G.A.; Kannan, K.; Roehl, K.A.; Cooper, A.R. Persistent Organic Pollutants and Early Menopause in U.S. Women. PLoS ONE 2015, 10, e0116057. [Google Scholar] [CrossRef]
- Neblett, M.F.; Curtis, S.W.; Gerkowicz, S.A.; Spencer, J.B.; Terrell, M.L.; Jiang, V.S.; Marder, M.E.; Barr, D.B.; Marcus, M.; Smith, A.K. Examining Reproductive Health Outcomes in Females Exposed to Polychlorinated Biphenyl and Polybrominated Biphenyl. Sci. Rep. 2020, 10, 3314. [Google Scholar] [CrossRef]
- Hauser, R.; Altshul, L.; Chen, Z.; Ryan, L.; Overstreet, J.; Schiff, I.; Christiani, D.C. Environmental Organochlorines and Semen Quality: Results of a Pilot Study. Environ. Health Perspect. 2002, 110, 229–233. [Google Scholar] [CrossRef]
- Dallinga, J.W.; Moonen, E.J.C.; Dumoulin, J.C.M.; Evers, J.L.H.; Geraedts, J.P.M.; Kleinjans, J.C.S. Decreased Human Semen Quality and Organochlorine Compounds in Blood. Hum. Reprod. 2002, 17, 1973–1979. [Google Scholar] [CrossRef]
- Mumford, S.L.; Kim, S.; Chen, Z.; Gore-Langton, R.E.; Boyd Barr, D.; Buck Louis, G.M. Persistent Organic Pollutants and Semen Quality: The LIFE Study. Chemosphere 2015, 135, 427–435. [Google Scholar] [CrossRef]
- Sumner, R.N.; Tomlinson, M.; Craigon, J.; England, G.C.W.; Lea, R.G. Independent and Combined Effects of Diethylhexyl Phthalate and Polychlorinated Biphenyl 153 on Sperm Quality in the Human and Dog. Sci. Rep. 2019, 9, 3409. [Google Scholar] [CrossRef]
- Jensen, T.K. Endocrine Disrupters, Semen Quality and Anogenital Distance. Curr. Opin. Endocr. Metab. Res. 2019, 7, 34–42. [Google Scholar] [CrossRef]
- Stukenborg, J.-B.; Mitchell, R.T.; Söder, O. Endocrine Disruptors and the Male Reproductive System. Best Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101567. [Google Scholar] [CrossRef]
- Paoli, D.; Giannandrea, F.; Gallo, M.; Turci, R.; Cattaruzza, M.S.; Lombardo, F.; Lenzi, A.; Gandini, L. Exposure to Polychlorinated Biphenyls and Hexachlorobenzene, Semen Quality and Testicular Cancer Risk. J. Endocrinol. Investig. 2015, 38, 745–752. [Google Scholar] [CrossRef]
- Paul, R.; Moltó, J.; Ortuño, N.; Romero, A.; Bezos, C.; Aizpurua, J.; Gómez-Torres, M.J. Relationship between Serum Dioxin-like Polychlorinated Biphenyls and Post-Testicular Maturation in Human Sperm. Reprod. Toxicol. 2017, 73, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Goncharov, A.; Rej, R.; Negoita, S.; Schymura, M.; Santiago-Rivera, A.; Morse, G.; Akwesasne Task Force on the Environment; Carpenter, D.O. Lower Serum Testosterone Associated with Elevated Polychlorinated Biphenyl Concentrations in Native American Men. Environ. Health Perspect. 2009, 117, 1454–1460. [Google Scholar] [CrossRef]
- Paul, R.; Romero, A.; Moltó, J.; Ortuño, N.; Aizpurua, J.; Gómez-Torres, M.J. Associations of Paternal Serum Dioxin-like Polychlorinated Biphenyl Concentrations with IVF Success: A Pilot Study. Environ. Res. 2021, 206, 112248. [Google Scholar] [CrossRef] [PubMed]
- Bush, B.; Bennett, A.H.; Snow, J.T. Polychlorobiphenyl Congeners, p,p′-DDE, and Sperm Function in Humans. Arch. Environ. Contam. Toxicol. 1986, 15, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Bräuner, E.V.; Lim, Y.-H.; Koch, T.; Uldbjerg, C.S.; Gregersen, L.S.; Pedersen, M.K.; Frederiksen, H.; Petersen, J.H.; Coull, B.A.; Andersson, A.-M.; et al. Endocrine Disrupting Chemicals and Risk of Testicular Cancer: A Systematic Review and Meta-Analysis. J. Clin. Endocrinol. Metab. 2021, 106, e4834–e4860. [Google Scholar] [CrossRef]
- Swan, S.H.; Colino, S.A. Count Down—How Our Modern World Is Threatening Sperm Counts, Altering Male and Female Reproductive Development, and Imperiling the Future of the Human Race; Scribner: New York, NY, USA, 2021; ISBN 978-1-982113-67-4. [Google Scholar]
- Crinnion, W.J. Toxic Effects of the Easily Avoidable Phthalates and Parabens. Altern. Med. Rev. 2010, 15, 190–196. [Google Scholar]
- Meeker, J.D.; Ehrlich, S.; Toth, T.L.; Wright, D.L.; Calafat, A.M.; Trisini, A.T.; Ye, X.; Hauser, R. Semen Quality and Sperm DNA Damage in Relation to Urinary Bisphenol A among Men from an Infertility Clinic. Reprod. Toxicol. 2010, 30, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Meeker, J.D.; Yang, T.; Ye, X.; Calafat, A.M.; Hauser, R. Urinary Concentrations of Parabens and Serum Hormone Levels, Semen Quality Parameters, and Sperm DNA Damage. Environ. Health Perspect. 2011, 119, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Li, D.-K.; Zhou, Z.; Miao, M.; He, Y.; Wang, J.; Ferber, J.; Herrinton, L.J.; Gao, E.; Yuan, W. Urine Bisphenol-A (BPA) Level in Relation to Semen Quality. Fertil. Steril. 2011, 95, 625–630.e4. [Google Scholar] [CrossRef] [PubMed]
- Mori, C.; Nakamura, N.; Todaka, E.; Fujisaki, T.; Matsuno, Y.; Nakaoka, H.; Hanazato, M. Correlation between Human Maternal–Fetal Placental Transfer and Molecular Weight of PCB and Dioxin Congeners/Isomers. Chemosphere 2014, 114, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Mennigen, J.A.; Thompson, L.M.; Bell, M.; Tellez Santos, M.; Gore, A.C. Transgenerational Effects of Polychlorinated Biphenyls: 1. Development and Physiology across 3 Generations of Rats. Environ. Health 2018, 17, 18. [Google Scholar] [CrossRef] [PubMed]
- Gore, A.C.; Thompson, L.M.; Bell, M.; Mennigen, J.A. Transgenerational Effects of Polychlorinated Biphenyls: 2. Hypothalamic Gene Expression in Rats. Biol. Reprod. 2021, 105, 690–704. [Google Scholar] [CrossRef] [PubMed]
- Gillette, R.; Son, M.J.; Ton, L.; Gore, A.C.; Crews, D. Passing Experiences on to Future Generations: Endocrine Disruptors and Transgenerational Inheritance of Epimutations in Brain and Sperm. Epigenetics 2018, 13, 1106–1126. [Google Scholar] [CrossRef]
- Kezios, K.L.; Liu, X.; Cirillio, P.M.; Kalantzi, O.I.; Wang, Y.; Petreas, M.X.; Park, J.-S.; Bradwin, G.; Cohn, B.A.; Factor-Litvak, P. Prenatal Polychlorinated Biphenyl Exposure Is Associated with Decreased Gestational Length but Not Birth Weight: Archived Samples from the Child Health and Development Studies Pregnancy Cohort. Environ. Health 2012, 11, 49. [Google Scholar] [CrossRef]
- Lignell, S.; Aune, M.; Darnerud, P.O.; Hanberg, A.; Larsson, S.C.; Glynn, A. Prenatal Exposure to Polychlorinated Biphenyls (PCBs) and Polybrominated Diphenyl Ethers (PBDEs) May Influence Birth Weight among Infants in a Swedish Cohort with Background Exposure: A Cross-Sectional Study. Environ. Health 2013, 12, 44. [Google Scholar] [CrossRef]
- Papadopoulou, E.; Caspersen, I.H.; Kvalem, H.E.; Knutsen, H.K.; Duarte-Salles, T.; Alexander, J.; Meltzer, H.M.; Kogevinas, M.; Brantsæter, A.L.; Haugen, M. Maternal Dietary Intake of Dioxins and Polychlorinated Biphenyls and Birth Size in the Norwegian Mother and Child Cohort Study (MoBa). Environ. Int. 2013, 60, 209–216. [Google Scholar] [CrossRef]
- Caspersen, I.H.; Haugen, M.; Schjølberg, S.; Vejrup, K.; Knutsen, H.K.; Brantsæter, A.L.; Meltzer, H.M.; Alexander, J.; Magnus, P.; Kvalem, H.E. Maternal Dietary Exposure to Dioxins and Polychlorinated Biphenyls (PCBs) Is Associated with Language Delay in 3year Old Norwegian Children. Environ. Int. 2016, 91, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, S.L.; Ramlau-Hansen, C.H.; Ernst, E.; Olsen, S.F.; Bonde, J.P.; Vested, A.; Halldorsson, T.I.; Rantakokko, P.; Kiviranta, H.; Toft, G. Prenatal Exposure to Persistent Organochlorine Pollutants and Female Reproductive Function in Young Adulthood. Environ. Int. 2016, 92–93, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.L.; Hsu, P.-C.; Hsu, C.-C.; Lambert, G.H. Semen Quality after Prenatal Exposure to Polychlorinated Biphenyls and Dibenzofurans. Lancet 2000, 356, 1240–1241. [Google Scholar] [CrossRef]
- Lessard, M.; Herst, P.M.; Charest, P.L.; Navarro, P.; Joly-Beauparlant, C.; Droit, A.; Kimmins, S.; Trasler, J.; Benoit-Biancamano, M.-O.; MacFarlane, A.J.; et al. Prenatal Exposure to Environmentally-Relevant Contaminants Perturbs Male Reproductive Parameters Across Multiple Generations That Are Partially Protected by Folic Acid Supplementation. Sci. Rep. 2019, 9, 13829. [Google Scholar] [CrossRef]
- Sheinberg, R.; Siegel, E.L.; Keidar, R.; Mandel, D.; Lubetzky, R.; Kohn, E.; Livneh, A.; Tovbin, J.; Betser, M.; Moskovich, M.; et al. Associations between Intrauterine Exposure to Polychlorinated Biphenyls on Neonatal Ano-Genital Distance. Reprod. Toxicol. 2020, 96, 67–75. [Google Scholar] [CrossRef]
- Raffetti, E.; Speziani, F.; Donato, F.; Leonardi, L.; Orizio, G.; Scarcella, C.; Apostoli, P.; Magoni, M. Temporal Trends of Polychlorinated Biphenyls Serum Levels in Subjects Living in a Highly Polluted Area from 2003 to 2015: A Follow-up Study. Int. J. Hydrog. Energy Health 2017, 220, 461–467. [Google Scholar] [CrossRef]
- Spanò, M.; Toft, G.; Hagmar, L.; Eleuteri, P.; Rescia, M.; Rignell-Hydbom, A.; Tyrkiel, E.; Zvyezday, V.; Bonde, J.P.; INUENDO. Exposure to PCB and p, P′-DDE in European and Inuit Populations: Impact on Human Sperm Chromatin Integrity. Hum. Reprod. 2005, 20, 3488–3499. [Google Scholar] [CrossRef]
- Lettieri, G.; D’Agostino, G.; Mele, E.; Cardito, C.; Esposito, R.; Cimmino, A.; Giarra, A.; Trifuoggi, M.; Raimondo, S.; Notari, T.; et al. Discovery of the Involvement in DNA Oxidative Damage of Human Sperm Nuclear Basic Proteins of Healthy Young Men Living in Polluted Areas. Int. J. Mol. Sci. 2020, 21, 4198. [Google Scholar] [CrossRef]
- Lettieri, G.; Marra, F.; Moriello, C.; Prisco, M.; Notari, T.; Trifuoggi, M.; Giarra, A.; Bosco, L.; Montano, L.; Piscopo, M. Molecular Alterations in Spermatozoa of a Family Case Living in the Land of Fires—A First Look at Possible Transgenerational Effects of Pollutants. Int. J. Mol. Sci. 2020, 21, 6710. [Google Scholar] [CrossRef]
- Montano, L.; Donato, F.; Bianco, P.M.; Lettieri, G.; Guglielmino, A.; Motta, O.; Bonapace, I.M.; Piscopo, M. Semen Quality as a Potential Susceptibility Indicator to SARS-CoV-2 Insults in Polluted Areas. Environ. Sci. Pollut. Res. 2021, 28, 37031–37040. [Google Scholar] [CrossRef]
- Montano, L.; Donato, F.; Bianco, P.M.; Lettieri, G.; Guglielmino, A.; Motta, O.; Bonapace, I.M.; Piscopo, M. Air Pollution and COVID-19: A Possible Dangerous Synergy for Male Fertility. Int. J. Environ. Res. Public Health 2021, 18, 6846. [Google Scholar] [CrossRef] [PubMed]
- Raimondo, S.; Gentile, M.; Esposito, G.; Gentile, T.; Ferrara, I.; Crescenzo, C.; Palmieri, M.; Cuomo, F.; De Filippo, S.; Lettieri, G.; et al. Could Kallikrein-Related Serine Peptidase 3 Be an Early Biomarker of Environmental Exposure in Young Women? Int. J. Environ. Res. Public Health 2021, 18, 8833. [Google Scholar] [CrossRef] [PubMed]
- Longo, V.; Forleo, A.; Radogna, A.V.; Siciliano, P.; Notari, T.; Pappalardo, S.; Piscopo, M.; Montano, L.; Capone, S. A Novel Human Biomonitoring Study by Semiconductor Gas Sensors in Exposomics: Investigation of Health Risk in Contaminated Sites. Environ. Pollut. 2022, 304, 119119. [Google Scholar] [CrossRef] [PubMed]
- Montano, L.; Maugeri, A.; Volpe, M.G.; Micali, S.; Mirone, V.; Mantovani, A.; Navarra, M.; Piscopo, M. Mediterranean Diet as a Shield against Male Infertility and Cancer Risk Induced by Environmental Pollutants: A Focus on Flavonoids. Int. J. Mol. Sci. 2022, 23, 1568. [Google Scholar] [CrossRef]


| Continent | Country | Location | Type of Site | Concentration | Reference |
|---|---|---|---|---|---|
| Asia | Taiwan | Tainan | Urban | 4.730 ng m−2 day−1 | [80] |
| Urban/industrial/rural | 0.57–0.65 ng m−2 day−1 | [81] | |||
| South Korea | Pohang | Industrial | 2.1 ng m−2 day−1 | [82] | |
| Japan | Hong Kong | Office | 52.5–589 ng g−1 | [83] | |
| Electronic factory | 47–249 ng g−1 | ||||
| Manufacturing plan | 709 ng g−1 | ||||
| Electronic factory, commercial office, hospital, school and shopping store | 107–233 ng g−1 | ||||
| China | - | Nonferrous Metallurgical Facilities | 0.0155–0.770 ng m−3 | [84] | |
| Taizhou | E-waste recycling site | 37.75–65.83 ng m−3 | [85] | ||
| Urban | 5.28–21.48 ng m−3 | ||||
| Quingyuan and Guangzhou | E-waste recycling site | 568–11,500 ng g−1 | [86] | ||
| Rural | 55.3–658 ng g−1 | ||||
| Urban | 38.6–226 ng g−1 | ||||
| Industrial | 0.94–1665 ng g−1 | ||||
| Vietnam | - | Home | 11–1900 ng g−1 | [87] | |
| Singapore | Singapore | Home | 5.6 ng g−1 | [88] | |
| India | Chennai | E-waste recycling site | 3.6–53 ng g−1 | [89] | |
| suburban industrial roadsides | 1.6 ng g−1 | ||||
| America | Canada | Toronto | Home | 56–820 ng g−1 | [90] |
| Home air | 0.11–5.11 ng m−3 | [17] | |||
| Home dust | <LOD-521 ng g−1 | ||||
| United States | Chicago | Urban | 4500 ng m−2 day−1 | [91] | |
| Resident | 190 ng m−2 day−1 | [92] | |||
| Urban-industrial | 0.075–5.5 ng m−3 | [23] | |||
| New Jersey | Urban | 10–40 ng m−2 day−1 | [93] | ||
| Suburban | 0.9–3 ng m−2 day−1 | ||||
| Background | 0.8–2 ng m−2 day−1 | ||||
| Texas | Home | 47–620 ng g−1 | [90] | ||
| Illinois | Dwelling and church | 199–43,540 ng g−1 | [94] | ||
| Iowa | School | 39.2–1.24 ng m−3 | [95] | ||
| Indiana and Iowa | School | 0.5–194 ng m−3 | [79] | ||
| Europe | United Kingdom | Birmingham | Home | 57–860 ng g−1 | [90] |
| France | Thau lagoon | Rural | 0.715 ng m−2 day−1 | [96] | |
| Germany | Stuttgart | School | 3643–13,561 ng m−3 | [61] | |
| North-Rhine Westphalia | E-waste recycling site | 8000–330,000 ng g−1 | [97] | ||
| Czech Republic | Brno | Home air | 0.14–4.23 ng m−3 | [17] | |
| Home dust | 11.4–358 ng g−1 | ||||
| Africa | Nigeria | Abraka and Warri | Office | 96.6–3949 ng g−1 | [98] |
| Lagos | Power Station office | 0.02–2.20 ng m−2 day−1 | [99] | ||
| South Africa | Durban | E-waste recycling site | 50–490 ng g−1 | [100] | |
| Office | 923–1040 ng g−1 | ||||
| Computer laboratory | 360–1880 ng g−1 | ||||
| Oceania | New Zealand | Wellington | Home | 46 ng g−1 | [90] |
| - | Turkey | Izmir | Industrial | 409 ng m−2 day−1 | [101] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montano, L.; Pironti, C.; Pinto, G.; Ricciardi, M.; Buono, A.; Brogna, C.; Venier, M.; Piscopo, M.; Amoresano, A.; Motta, O. Polychlorinated Biphenyls (PCBs) in the Environment: Occupational and Exposure Events, Effects on Human Health and Fertility. Toxics 2022, 10, 365. https://doi.org/10.3390/toxics10070365
Montano L, Pironti C, Pinto G, Ricciardi M, Buono A, Brogna C, Venier M, Piscopo M, Amoresano A, Motta O. Polychlorinated Biphenyls (PCBs) in the Environment: Occupational and Exposure Events, Effects on Human Health and Fertility. Toxics. 2022; 10(7):365. https://doi.org/10.3390/toxics10070365
Chicago/Turabian StyleMontano, Luigi, Concetta Pironti, Gabriella Pinto, Maria Ricciardi, Amalia Buono, Carlo Brogna, Marta Venier, Marina Piscopo, Angela Amoresano, and Oriana Motta. 2022. "Polychlorinated Biphenyls (PCBs) in the Environment: Occupational and Exposure Events, Effects on Human Health and Fertility" Toxics 10, no. 7: 365. https://doi.org/10.3390/toxics10070365
APA StyleMontano, L., Pironti, C., Pinto, G., Ricciardi, M., Buono, A., Brogna, C., Venier, M., Piscopo, M., Amoresano, A., & Motta, O. (2022). Polychlorinated Biphenyls (PCBs) in the Environment: Occupational and Exposure Events, Effects on Human Health and Fertility. Toxics, 10(7), 365. https://doi.org/10.3390/toxics10070365