A Tiered Approach for Assessing Individual and Combined Risk of Pyrethroids Using Human Biomonitoring Data
Abstract
1. Introduction
2. Materials and Methods
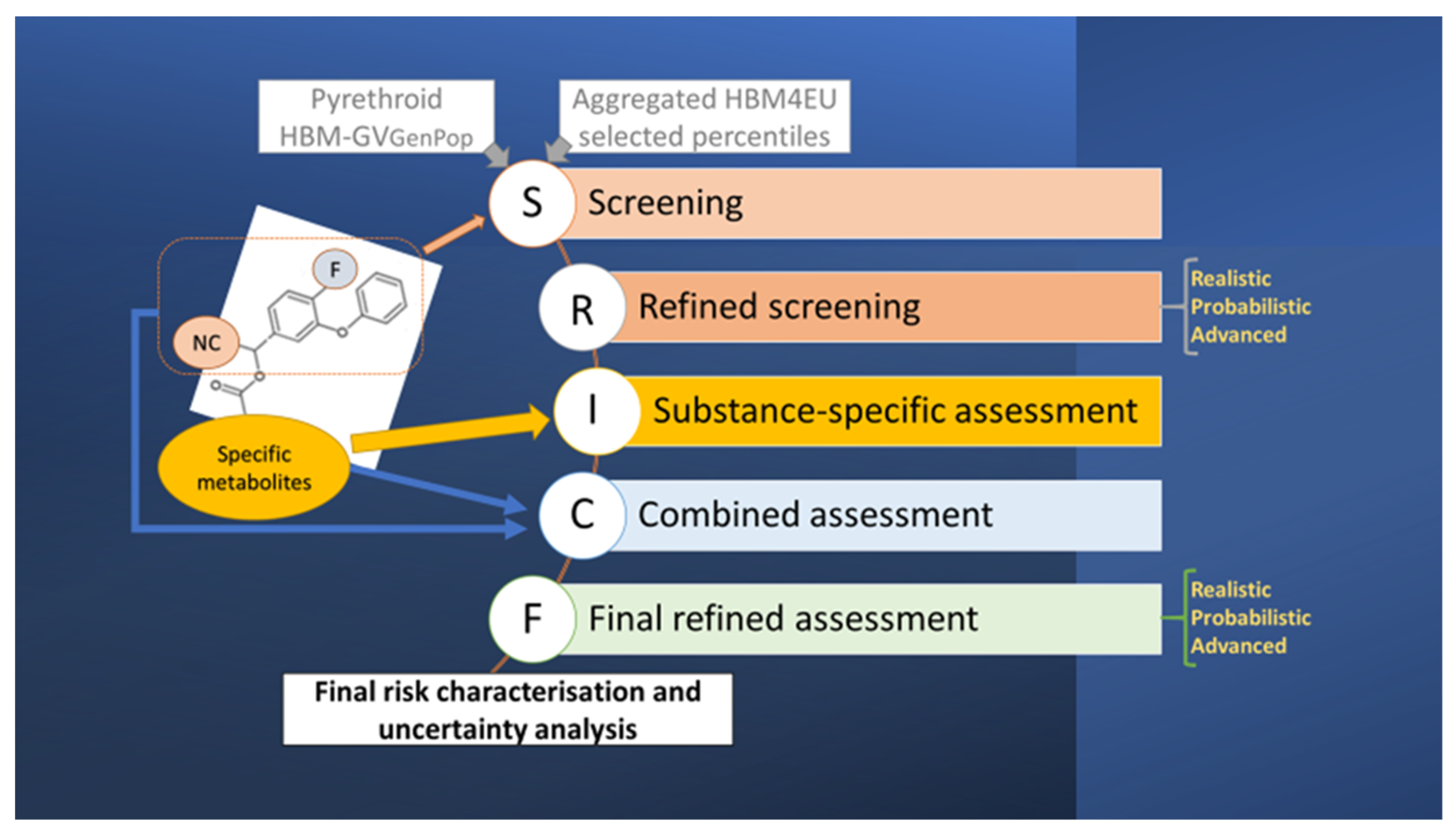
2.1. Conceptual Model and Derivation of Provisional HBM Guidance Values
2.2. Data and Information Sources
3. Results
3.1. Screening and Substance-Specific HBM-GVGenPop
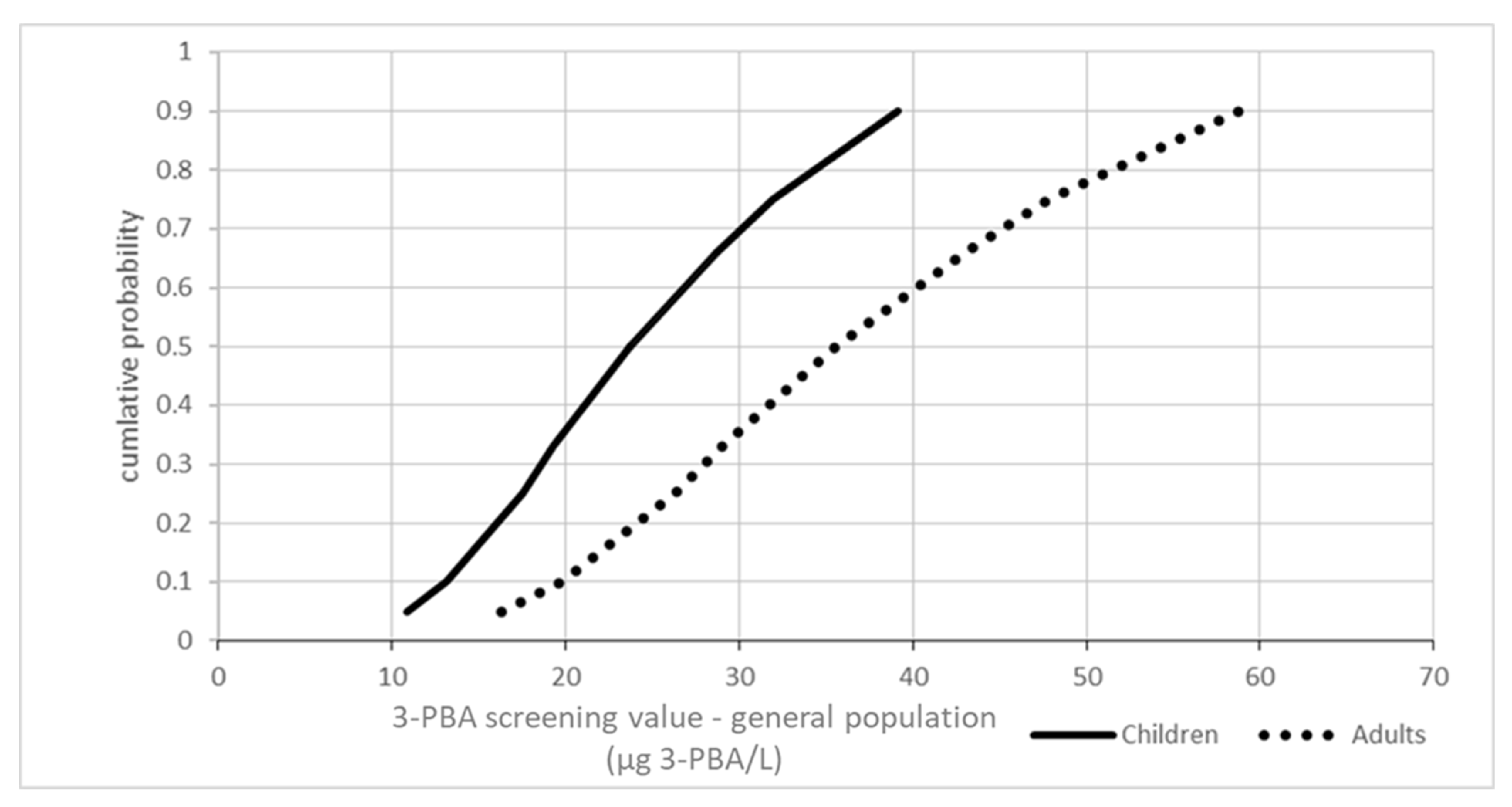
3.1.1. Screening Values for 3-PBA Moiety Metabolites
3.1.2. Deltamethrin
3.1.3. Cyfluthrin
3.1.4. Cypermethrin
3.1.5. Lambda-Cyhalothrin
3.1.6. Permethrin
3.1.7. Bifenthrin
3.1.8. Tau-Fluvalinate
3.1.9. Etofenprox
3.2. Screening and Refined Assessments Based on Common Metabolites
3.3. Substance-Specific Risk Assessments
3.3.1. Deltamethrin
3.3.2. Cyfluthrin
3.3.3. Cypermethrin
3.3.4. Lambda-Cyhalothrin
3.3.5. Permethrin
3.3.6. Bifenthrin
3.3.7. Tau-Fluvalinate
3.4. Combined Risk Assessment
3.5. Overall Discussion, Uncertainty Assessment, and Final Risk Characterisation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jactel, H.; Verheggen, F.; Thiéry, D.; Escobar-Gutiérrez, A.J.; Gachet, E.; Desneux, N. Alternatives to neonicotinoids. Environ. Int. 2019, 129, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Epstein, Y.; Chapron, G.; Verheggen, F. What is an emergency? Neonicotinoids and emergency situations in plant protection in the EU. Ambio 2022, 51, 1764–1771. [Google Scholar] [CrossRef]
- López-García, M.; Romero-González, R.; Frenich, A.G. Monitoring of organophosphate and pyrethroid metabolites in human urine samples by an automated method (TurboFlow™) coupled to ultra-high performance liquid chromatography-Orbitrap mass spectrometry. J. Pharm. Biomed. Anal. 2019, 173, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, X.; McKenzie, J.F.; Mannetje, A.; Cheng, S.; He, C.; Leathem, J.; Pearce, N.; Sunyer, J.; Eskenazi, B.; et al. Pesticide exposure in New Zealand school-aged children: Urinary concentrations of biomarkers and assessment of determinants. Environ. Int. 2022, 163, 107206. [Google Scholar] [CrossRef] [PubMed]
- Pollock, T.; Karthikeyan, S.; Walker, M.; Werry, K.; St-Amand, A. Trends in environmental chemical concentrations in the Canadian population: Biomonitoring data from the Canadian Health Measures Survey 2007–2017. Environ. Int. 2021, 155, 106678. [Google Scholar] [CrossRef]
- Ravula, A.R.; Yenugu, S. Pyrethroid based pesticides—Chemical and biological aspects. Crit. Rev. Toxicol. 2021, 51, 117–140. [Google Scholar] [CrossRef]
- Richardson, J.R.; Fitsanakis, V.; Westerink, R.H.S.; Kanthasamy, A.G. Neurotoxicity of pesticides. Acta Neuropathol. 2019, 138, 343–362. [Google Scholar] [CrossRef]
- Abreu-Villaça, Y.; Levin, E.D. Developmental neurotoxicity of succeeding generations of insecticides. Environ. Int. 2017, 99, 55–77. [Google Scholar] [CrossRef]
- Huang, L.; Peng, S.; Li, R.; Huang, D.; Xie, D. Case Report: Fatal Neurotoxicity Following Resmethrin Poisoning in a Child. Front. Pediatr. 2021, 9, 746950. [Google Scholar] [CrossRef]
- Jacob, M.S.; Iyyadurai, R.; Jose, A.; Fleming, J.J.; Rebekah, G.; Zachariah, A.; Hansdak, S.G.; Alex, R.; Chandiraseharan, V.K.; Lenin, A.; et al. Clinical presentation of type 1 and type 2 pyrethroid poisoning in humans. Clin. Toxicol. 2022, 60, 464–471. [Google Scholar] [CrossRef]
- Kim, U.-J.; Hong, M.; Choi, Y.-H. Environmental Pyrethroid Exposure and Cognitive Dysfunction in U.S. Older Adults: The NHANES 2001–2002. Int. J. Environ. Res. Public Health 2021, 18, 12005. [Google Scholar] [CrossRef] [PubMed]
- Burns, C.J.; Pastoor, T.P. Pyrethroid epidemiology: A quality-based review. Crit. Rev. Toxicol. 2018, 48, 297–311. [Google Scholar] [CrossRef] [PubMed]
- Saillenfait, A.M.; Ndiaye, D.; Sabate, J.P. Pyrethroids: Exposure and health effects—An update. Int. J. Hyg. Environ. Health 2015, 218, 281–292. [Google Scholar] [CrossRef]
- Knapke, E.T.; Magalhaes, D.d.P.; Dalvie, M.A.; Mandrioli, D.; Perry, M.J. Environmental and occupational pesticide exposure and human sperm parameters: A Navigation Guide review. Toxicology 2021, 465, 153017. [Google Scholar] [CrossRef] [PubMed]
- Aylward, L.L.; Irwin, K.; St-Amand, A.; Nong, A.; Hays, S.M. Screening-level Biomonitoring Equivalents for tiered interpretation of urinary 3-phenoxybenzoic acid (3-PBA) in a risk assessment context. Regul. Toxicol. Pharmacol. 2018, 92, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Heudorf, U.; Butte, W.; Schulz, C.; Angerer, J. Reference values for metabolites of pyrethroid and organophosphorous insec-ticides in urine for human biomonitoring in environmental medicine. Int. J. Hyg. Environ. Health 2006, 209, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Simaremare, S.R.S.; Hung, C.-C.; Hsieh, C.-J.; Yiin, L.-M. Relationship between Organophosphate and Pyrethroid Insecticides in Blood and Their Metabolites in Urine: A Pilot Study. Int. J. Environ. Res. Public Health 2019, 17, 34. [Google Scholar] [CrossRef] [PubMed]
- Côté, J.; Bouchard, M. Dose reconstruction in workers exposed to two major pyrethroid pesticides and determination of biological reference values using a toxicokinetic model. J. Expo. Sci. Environ. Epidemiol. 2018, 28, 599–614. [Google Scholar] [CrossRef]
- Quindroit, P.; Crépet, A.; Brochot, C. Estimating human exposure to pyrethroids’ mixtures from biomonitoring data using physiologically based pharmacokinetic modeling. Environ. Res. 2021, 192, 110281. [Google Scholar] [CrossRef]
- Apel, P.; Rousselle, C.; Lange, R.; Sissoko, F.; Kolossa-Gehring, M.; Ougier, E. Human biomonitoring initiative (HBM4EU)—Strategy to derive human biomonitoring guidance values (HBM-GVs) for health risk assessment. Int. J. Hyg. Environ. Health 2020, 230, 113622. [Google Scholar] [CrossRef]
- Lange, R.; Apel, P.; Rousselle, C.; Charles, S.; Sissoko, F.; Kolossa-Gehring, M.; Ougier, E. The European Human Biomonitoring Initiative (HBM4EU): Human biomonitoring guidance values for selected phthalates and a substitute plasticizer. Int. J. Hyg. Environ. Health 2021, 234, 113722. [Google Scholar] [CrossRef]
- Lamkarkach, F.; Ougier, E.; Garnier, R.; Viau, C.; Kolossa-Gehring, M.; Lange, R.; Apel, P. Human biomonitoring initiative (HBM4EU): Human biomonitoring guidance values (HBM-GVs) derived for cadmium and its compounds. Environ. Int. 2021, 147, 106337. [Google Scholar] [CrossRef] [PubMed]
- Ougier, E.; Zeman, F.; Antignac, J.-P.; Rousselle, C.; Lange, R.; Kolossa-Gehring, M.; Apel, P. Human biomonitoring initiative (HBM4EU): Human biomonitoring guidance values (HBM-GVs) derived for bisphenol A. Environ. Int. 2021, 154, 106563. [Google Scholar] [CrossRef]
- Apel, P.; Lamkarkach, F.; Lange, R.; Sissoko, F.; David, M.; Rousselle, C.; Schoeters, G.; Kolossa-Gehring, M. Human biomonitoring guidance values (HBM-GVs) for priority substances under the HBM4EU Initiative–New values derivation for deltamethrin and cyfluthrin and overall results. Int. J. Hyg. Environ. Health, 2021; submitted. [Google Scholar]
- Tarazona, J.V.; González-Caballero, M.d.C.; Alba-Gonzalez, M.d.; Pedraza-Diaz, S.; Cañas, A.; Dominguez-Morueco, N.; Esteban-López, M.; Cattaneo, I.; Katsonouri, A.; Makris, K.C.; et al. Improving the Risk Assessment of Pesticides through the Integration of Human Biomonitoring and Food Monitoring Data: A Case Study for Chlorpyrifos. Toxics 2022, 10, 313. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Jiménez, S.; Casado, N.; García, M.Á.; Marina, M.L. Enantiomeric analysis of pyrethroids and organophosphorus insecticides. J. Chromatogr. A 2019, 1605, 360345. [Google Scholar] [CrossRef]
- Matsuo, N. Discovery and development of pyrethroid insecticides. Proc. Jpn. Acad. Ser. B 2019, 95, 378–400. [Google Scholar] [CrossRef]
- Kaneko, H.; Miyamoto, J. Chapter 58—Pyrethroid Chemistry and Metabolism. In Handbook of Pesticide Toxicology, 2nd ed.; Krieger, R.I., Krieger, W.C., Eds.; Academic Press: San Diego, CA, USA, 2001; pp. 1263–1288. [Google Scholar]
- Hays, S.M.; Aylward, L.L.; LaKind, J.S.; Bartels, M.J.; Barton, H.A.; Boogaard, P.J.; Brunk, C.; DiZio, S.; Dourson, M.; Goldstein, D.A.; et al. Guidelines for the derivation of Biomonitoring Equivalents: Report from the Biomonitoring Equivalents Expert Workshop. Regul. Toxicol. Pharmacol. 2008, 51, S4–S15. [Google Scholar] [CrossRef]
- Faure, S.; Noisel, N.; Werry, K.; Karthikeyan, S.; Aylward, L.L.; St-Amand, A. Evaluation of human biomonitoring data in a health risk based context: An updated analysis of population level data from the Canadian Health Measures Survey. Int. J. Hyg. Environ. Health 2020, 223, 267–280. [Google Scholar] [CrossRef]
- Govarts, E.; Gilles, L.; Rodriguez-Martin, L.; Santonen, T.; Apel, P.; Alvito, P.; Anastasi, E.; Andersson, A.-M.; Andryskova, L.; Antignac, J.-P.; et al. Human Biomonitoring Data in European children, teenagers and adults: Results from the HBM4EU aligned studies (2014–2021). IJHEH, submitted.
- López, M.E.; Göen, T.; Mol, H.; Nübler, S.; Haji-Abbas-Zarrabi, K.; Koch, H.M.; Kasper-Sonnenberg, M.; Dvorakova, D.; Hajslova, J.; Antignac, J.-P.; et al. The European human biomonitoring platform—Design and implementation of a laboratory quality assurance/quality control (QA/QC) programme for selected priority chemicals. Int. J. Hyg. Environ. Health 2021, 234, 113740. [Google Scholar] [CrossRef] [PubMed]
- Gilles, L.; Govarts, E.; Martin, L.R.; Andersson, A.-M.; Appenzeller, B.M.R.; Barbone, F.; Castaño, A.; Coertjens, D.; Hond, E.D.; Dzhedzheia, V.; et al. Harmonization of Human Biomonitoring Studies in Europe: Characteristics of the HBM4EU-Aligned Studies Participants. Int. J. Environ. Res. Public Health 2022, 19, 6787. [Google Scholar] [CrossRef] [PubMed]
- Greene, T.; Salley, J.; Polcher, A.; Gentry, R.; Rücker, T. Literature review on pyrethroid common metabolites. EFSA Support. Publ. 2021, 18, EN-7064. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority). Conclusion on the peer review of the pesticide risk assessment of the active substance lambda-cyhalothrin. EFSA J. 2014, 12, 3677. [Google Scholar] [CrossRef]
- JMPR (Joint Meeting on Pesticide Residues). Pesticide Residues in Food: Permethrin. Available online: https://www.fao.org/fileadmin/templates/agphome/documents/Pests_Pesticides/JMPR/Reports_1991-2006/REPORT1999.pdf (accessed on 25 May 2022).
- Sams, C.; Jones, K. Biological monitoring for exposure to deltamethrin: A human oral dosing study and background levels in the UK general population. Toxicol. Lett. 2012, 213, 35–38. [Google Scholar] [CrossRef]
- Côté, J.; Bonvalot, Y.; Carrier, G.; Lapointe, C.; Fuhr, U.; Tomalik-Scharte, D.; Wachall, B.; Bouchard, M. A Novel Toxicokinetic Modeling of Cypermethrin and Permethrin and Their Metabolites in Humans for Dose Reconstruction from Biomarker Data. PLoS ONE 2014, 9, e88517. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Woollen, B.H.; Marsh, J.R.; Laird, W.J.D.; Lesser, J.E. The metabolism of cypermethrin in man: Differences in uri-nary metabolite profiles following oral and dermal administration. Xenobiotica 1992, 22, 983–991. [Google Scholar] [CrossRef]
- Ratelle, M.; Côté, J.; Bouchard, M. Toxicokinetics of permethrin biomarkers of exposure in orally exposed volunteers. Toxicol. Lett. 2015, 232, 369–375. [Google Scholar] [CrossRef]
- Ratelle, M.; Coté, J.; Bouchard, M. Time profiles and toxicokinetic parameters of key biomarkers of exposure to cypermethrin in orally exposed volunteers compared with previously available kinetic data following permethrin exposure. J. Appl. Toxicol. 2015, 35, 1586–1593. [Google Scholar] [CrossRef]
- JMPR Pesticide Residues in Food 2000: Deltamethrin. Evaluation of the Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food (JMPR) and the Environment and the WHO Core Assessment Group. First Draft Prepared by D.B. McGregor, International Agency for Research on Cancer, Lyon, France. Available online: http://www.inchem.org/documents/jmpr/jmpmono/v00pr04.htm (accessed on 25 July 2022).
- Leng, G. Pyrethrum and pyrethroids (e.g., allethrin, cyfluthrin, cypermethrin, deltamethrin, permethrin, resmethrin, phe-nothrin, tetramethrin)—Evaluation of study results in biological material. Assessment Values in Biological Material—Translation of the German version from 2008. MAK Collect Occup. Health Saf. 2021, 930, 15. [Google Scholar] [CrossRef]
- Leng, G.; Kühn, K.-H.; Idel, H. Biological monitoring of pyrethroids in blood and pyrethroid metabolites in urine: Applications and limitations. Sci. Total Environ. 1997, 199, 173–181. [Google Scholar] [CrossRef]
- Hays, S.M.; Aylward, L.L.; Gagné, M.; Krishnan, K. Derivation of Biomonitoring Equivalents for cyfluthrin. Regul. Toxicol. Pharmacol. 2009, 55, 268–275. [Google Scholar] [CrossRef] [PubMed]
- EC. Final Renewal Report for the Active Substance Beta-Cyfluthrin. Standing Committee on Plants, Animals, Food and Feed. SANTE/12798/2019 Rev 1. European Commission Food and Feed Safety, Innovation. Pesticides and Biocides. 2020. Available online: https://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/active-substances/?event=as.details&as_id=458 (accessed on 25 May 2022).
- EC. Evaluation of Active Substances: Assessment Report Cyfluthrin, Product-Type 18. Overall Summary and Conclusion. 2018. Available online: https://echa.europa.eu/documents/10162/965f85c8-07b0-dad7-83dc-ce32039307db (accessed on 25 May 2022).
- EFSA (European Food Safety Authority). Peer review of the pesticide risk assessment of the active substance cypermethrin. EFSA J. 2018, 16, 5402. [Google Scholar] [CrossRef]
- Khemiri, R.; Côté, J.; Fetoui, H.; Bouchard, M. Documenting the kinetic time course of lambda-cyhalothrin metabolites in orally exposed volunteers for the interpretation of biomonitoring data. Toxicol. Lett. 2017, 276, 115–121. [Google Scholar] [CrossRef] [PubMed]
- EFSA (European Food Safety Authority). Conclusion on the peer review of the pesticide risk assessment of the active substance bifenthrin. EFSA J. 2011, 9, 2159. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority). Conclusion on the peer review of the pesticide risk assessment of the active substance tau-fluvalinate. EFSA J. 2010, 8, 1645. [Google Scholar] [CrossRef]
- Remer, T.; Neubert, A.; Maser-Gluth, C. Anthropometry-based reference values for 24-h urinary creatinine excretion during growth and their use in endocrine and nutritional research. Am. J. Clin. Nutr. 2002, 75, 561–569. [Google Scholar] [CrossRef]
- Van Haarst, E.P.; Heldeweg, E.A.; Newling, D.W.; Schlatmann, T.J. The 24-h frequency-volume chart in adults reporting no voiding complaints: Defining reference values and analysing variables. Br. J. Urol. 2004, 93, 1257–1261. [Google Scholar] [CrossRef]
- Yusà, V.; Fernández, S.F.; Dualde, P.; López, A.; Lacomba, I.; Coscollà, C. Exposure to non-persistent pesticides in the Spanish population using biomonitoring: A review. Environ. Res. 2022, 205, 112437. [Google Scholar] [CrossRef]
- Morgan, M.K. Children’s Exposures to Pyrethroid Insecticides at Home: A Review of Data Collected in Published Exposure Measurement Studies Conducted in the United States. Int. J. Environ. Res. Public Health 2012, 9, 2964–2985. [Google Scholar] [CrossRef]
- Lehmler, H.-J.; Simonsen, D.; Liu, B.; Bao, W. Environmental exposure to pyrethroid pesticides in a nationally representative sample of U.S. adults and children: The National Health and Nutrition Examination Survey 2007–2012. Environ. Pollut. 2020, 267, 115489. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.-H.; Lee, J.-Y.; Huh, D.-A.; Moon, K.W. Urinary 3-phenoxybenzoic acid (3-PBA) levels and changes in hematological parameters in Korean adult population: A Korean National Environmental Health Survey (KoNEHS) 2012–2014 analysis. Int. J. Hyg. Environ. Health 2022, 243, 113988. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Pan, W.; Liu, J. Variability of urinary pyrethroid biomarkers in Chinese young-aged men and women over one year. Environ. Pollut. 2021, 269, 116155. [Google Scholar] [CrossRef]
- Klimowska, A.; Amenda, K.; Rodzaj, W.; Wileńska, M.; Jurewicz, J.; Wielgomas, B. Evaluation of 1-year urinary excretion of eight metabolites of synthetic pyrethroids, chlorpyrifos, and neonicotinoids. Environ. Int. 2020, 145, 106119. [Google Scholar] [CrossRef]
- Couture, C.; Fortin, M.-C.; Carrier, G.; Dumas, P.; Tremblay, C.; Bouchard, M. Assessment of Exposure to Pyrethroids and Pyrethrins in a Rural Population of the Montérégie Area, Quebec, Canada. J. Occup. Environ. Hyg. 2009, 6, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Glorennec, P.; Serrano, T.; Fravallo, M.; Warembourg, C.; Monfort, C.; Cordier, S.; Viel, J.-F.; Le Gléau, F.; Le Bot, B.; Chevrier, C. Determinants of children’s exposure to pyrethroid insecticides in western France. Environ. Int. 2017, 104, 76–82. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA); Carrasco Cabrera, C.L.; Pastor, P.M. The 2019 European Union report on pesticide residues in food. EFSA J. 2021, 19, e06491. [Google Scholar] [CrossRef]
- Calatayud-Vernich, P.; Calatayud, F.; Simó, E.; Picó, Y. Pesticide residues in honey bees, pollen and beeswax: Assessing beehive exposure. Environ. Pollut. 2018, 241, 106–114. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority); Carrasco Cabrera, L.; Medina Pastor, P. The 2020 European Union report on pesticide residues in food. EFSA J. 2022, 20, 7215. [Google Scholar] [CrossRef]
- Maule, A.L.; Scarpaci, M.M.; Proctor, S.P. Urinary concentrations of permethrin metabolites in US Army personnel in comparison with the US adult population, occupationally exposed cohorts, and other general populations. Int. J. Hyg. Environ. Health 2019, 222, 355–363. [Google Scholar] [CrossRef]
- Khambay, B.P.S.; Jewess, P.J. 6.1—Pyrethroids. In Comprehensive Molecular Insect Science; Gilbert, L.I., Ed.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 1–29. [Google Scholar]
- Watkins, D.J.; Fortenberry, G.Z.; Sánchez, B.N.; Barr, D.B.; Panuwet, P.; Schnaas, L.; Osorio-Valencia, E.; Solano-González, M.; Ettinger, A.S.; Hernández-Ávila, M.; et al. Urinary 3-phenoxybenzoic acid (3-PBA) levels among pregnant women in Mexico City: Distribution and relationships with child neurodevelopment. Environ. Res. 2016, 147, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Norén, E.; Lindh, C.; Rylander, L.; Glynn, A.; Axelsson, J.; Littorin, M.; Faniband, M.; Larsson, E.; Nielsen, C. Concentrations and temporal trends in pesticide biomarkers in urine of Swedish adolescents, 2000–2017. J. Expo. Sci. Environ. Epidemiol. 2020, 30, 756–767. [Google Scholar] [CrossRef] [PubMed]
- Andersen, H.R.; David, A.; Freire, C.; Fernández, M.F.; D’Cruz, S.C.; Reina-Pérez, I.; Fini, J.-B.; Blaha, L. Pyrethroids and developmental neurotoxicity—A critical review of epidemiological studies and supporting mechanistic evidence. Environ. Res. 2022, 214, 113935. [Google Scholar] [CrossRef] [PubMed]

| Study Acronym | Location | Geographical Coverage | Region | Study Design | Sampling Period | Age Range |
|---|---|---|---|---|---|---|
| Children | ||||||
| 3xG | Belgium | Regional | Dessel, Mol, Retie | Longitudinal | 01/2019–06/2021 | 6–8 |
| ESTEBAN | France | National | Mainland | Cross-sectional | 04/2014–03/2016 | 6–11 |
| ORGANIKO | Cyprus | Regional | Limassol | Cross-over | 01/2017–04/2017 | 10–11 |
| RAV MABAT | Israel | National | - | Cross-sectional | 2015–2016 | 4–11 |
| SLO CRP | Slovenia | Regional | Mura region | Cross-sectional | 01/2018–06/2018 | 7–10 |
| SPECIMEn-NL | The Netherlands | Regional | Central-East | Cross-sectional | 01/2020–03/2020 | 6–11 |
| Adults | ||||||
| ESB | Germany | Regional | Münster | Cross-sectional | Earliest samples from 1981, ongoing study | 20–29 |
| ESTEBAN | France | National | Mainland France | Cross-sectional | 04/2014–03/2016 | 18–74 |
| HBM4EU-study for Switzerland | Switzerland | Regional | Basel | Cross-sectional | 01/2020–10/2020 | 20–39 |
| RAV MABAT | Israel | National | - | Cross-sectional | 2015–2016 | 20–39 |
| Biomarker | Name | Parent Pyrethroids |
|---|---|---|
| 3-PBA | 3-phenoxybenzoic acid | Many, e.g., cypermethrin, deltamethrin, etofenprox, fenpropathrin, fenvalerate, esfenvalerate, lambda-cyhalothrin, permethrin, tau-fluvalinate |
| 4-FPBA | 4-fluoro-3-phenoxybenzoic acid | cyfluthrin |
| CIF3CA * | cis-3-[2-chloro-3,3,3-trifluoroprop-1-enyl]-2,2-dimethylcyclopropanecarboxylic acid | bifenthrin, lambda-cyhalothrin and tefluthrin |
| DBCA (cis isomer) | cis-3-(2,2-dibromovinyl)-2,2-dimethylcyclopropane-1-carboxylic acid | deltamethrin |
| DCCA (sum of cis and trans) | 3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane-1-carboxylic acid | cyfluthrin, cypermethrin, and permethrin |
| Active Substance | ADI mg/kg bw Source * | Biomarker | Fue * % | Proposed HBM-GVGenPop µg Metabolite/L Urine | Comments |
|---|---|---|---|---|---|
| Deltamethrin | 0.01 HBM4EU | DBCA | 45 | 90 (children) † 130 (adults) † | Specific metabolite # |
| Cyfluthrin | 0.01 HBM4EU | 4-FPBA | 47 | 80 (children) † 130 (adults) † | Screening and specific metabolite # |
| Cypermethrin | 0.005 EFSA | DCCA | 36 | 30 (children) 45 (adults) | Sum of cis/trans-DCCA |
| Lambda-cyhalothrin | 0.0025 EFSA | CIF3CA | 21 | 9 (children) 14 (adults) | |
| Permethrin | 0.05 ECHA | DCCA | 36 | 320 (children) 480 (adults) | Sum of cis/trans-DCCA |
| Bifenthrin | 0.015 EFSA | CIF3CA | 21 | 60 (children) 90 (adults) | Fue inferred from lambda-cyhalothrin |
| Tau-fluvalinate | 0.005 EFSA | 3-PBA | 9–31 | 6.4–22 (children) 9.6–33 (adults) | Screening and specific metabolite # |
| Etofenprox | 0.03 EFSA | 3-PBA | 1 | low reliability | Excluded from this risk assessment |
| Aylward et al. [15] | Côté et al. [38] | Quindroit et al. [19] | Côté and Bouchard [18] | |||||
|---|---|---|---|---|---|---|---|---|
| Reported Value | Original Reference | Reported Value | Original Reference | Reported Value | Original Reference | Reported Value | Original Reference | |
| Cypermethrin | 0.13 0.27 | [39,40] | 0.129 | [39] | trans 0.39 cis 0.16 | [39,40] | 0.05–0.55 | [40] |
| Deltamethrin | 0.09 | [37] | 0.15 | [37] | ||||
| Lambda-cyhalothrin | 0.251 | Marsh et al. 1994 # | ||||||
| Permethrin | 0.457 | [41] | 0.129 * | [39] | trans 0.85 cis 0.37 | [37] | 0.32–0.78 | [41] |
| Biomarker | ClF3CA | DBCA | DCCA | 4-FPBA | ||
|---|---|---|---|---|---|---|
| Active Substance | Lambda-Cyhalothrin | Bifenthrin | Deltamethrin | Cypermethrin | Permethrin | Cyfluthrin |
| Children (age 6 to 11 years) | ||||||
| Israel | 0.085 | 0.013 | 0.011 | 0.17 | 0.016 | 0.013 |
| Netherlands | 0.142 | 0.021 | 0.042 | 0.15 | 0.014 | NR |
| Belgium | 0.086 | 0.013 | 0.034 | 0.25 | 0.02 | NR |
| Cyprus | 0.029 | 0.004 | 0.044 | 0.20 | 0.02 | NR |
| France | - | - | 0.059 | 0.108 | 0.01 | 0.004 |
| Slovenia | NR | NR | NR | NR | NR | 0.018 |
| Adults | ||||||
| Switzerland | 0.031 | 0.005 | 0.0068 | 0.036 | 0.003 | NR |
| Germany | 0.019 | 0.003 | 0.0041 | 0.019 | 0.003 | NR |
| Israel | 0.075 | 0.012 | 0.0032 | 0.068 | 0.006 | NR |
| France | - | - | 0.041 | 0.053 | 0.005 | 0.003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarazona, J.V.; Cattaneo, I.; Niemann, L.; Pedraza-Diaz, S.; González-Caballero, M.C.; de Alba-Gonzalez, M.; Cañas, A.; Dominguez-Morueco, N.; Esteban-López, M.; Castaño, A.; et al. A Tiered Approach for Assessing Individual and Combined Risk of Pyrethroids Using Human Biomonitoring Data. Toxics 2022, 10, 451. https://doi.org/10.3390/toxics10080451
Tarazona JV, Cattaneo I, Niemann L, Pedraza-Diaz S, González-Caballero MC, de Alba-Gonzalez M, Cañas A, Dominguez-Morueco N, Esteban-López M, Castaño A, et al. A Tiered Approach for Assessing Individual and Combined Risk of Pyrethroids Using Human Biomonitoring Data. Toxics. 2022; 10(8):451. https://doi.org/10.3390/toxics10080451
Chicago/Turabian StyleTarazona, Jose V., Irene Cattaneo, Lars Niemann, Susana Pedraza-Diaz, Maria Carmen González-Caballero, Mercedes de Alba-Gonzalez, Ana Cañas, Noelia Dominguez-Morueco, Marta Esteban-López, Argelia Castaño, and et al. 2022. "A Tiered Approach for Assessing Individual and Combined Risk of Pyrethroids Using Human Biomonitoring Data" Toxics 10, no. 8: 451. https://doi.org/10.3390/toxics10080451
APA StyleTarazona, J. V., Cattaneo, I., Niemann, L., Pedraza-Diaz, S., González-Caballero, M. C., de Alba-Gonzalez, M., Cañas, A., Dominguez-Morueco, N., Esteban-López, M., Castaño, A., Borges, T., Katsonouri, A., Makris, K. C., Ottenbros, I., Mol, H., De Decker, A., Morrens, B., Berman, T., Barnett-Itzhaki, Z., ... Santonen, T. (2022). A Tiered Approach for Assessing Individual and Combined Risk of Pyrethroids Using Human Biomonitoring Data. Toxics, 10(8), 451. https://doi.org/10.3390/toxics10080451















