Bone-Marrow-Derived Mesenchymal Stem Cells, Their Conditioned Media, and Olive Leaf Extract Protect against Cisplatin-Induced Toxicity by Alleviating Oxidative Stress, Inflammation, and Apoptosis in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Experimental Animal
2.3. Experimental Design
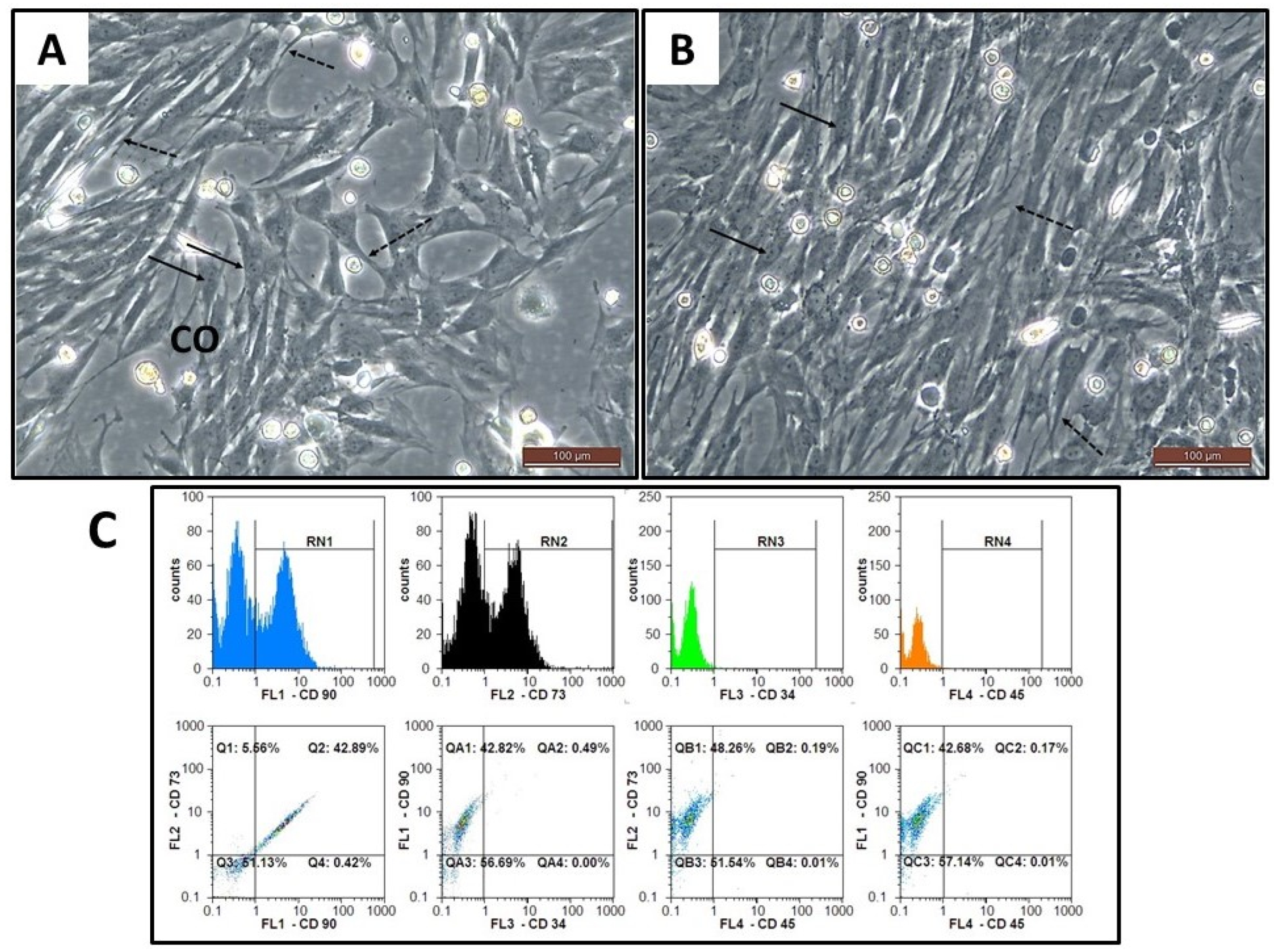
2.4. Stem Cells Isolation, Culture, and Expansion
2.5. Phenotypic Characterization of BM-MSCs
2.6. Assessment of Viability, Cell Count and Transplantation of BM-MSCs
2.7. Collection and Preparation of BM-MSCs Conditioned Media (CM-MSC)
2.8. Tissues Harvesting and Samples Collection
2.9. Assessment of the Body, Liver, and Kidney Weights
2.10. Assessment of Liver Necrosis and Hepatic Functions Markers
2.11. Measurement of Renal Function Markers
2.12. Estimation of the Markers of Oxidative Stress and Antioxidants in Hepatic and Renal Tissues
2.13. Assessment of the Levels of Pro-Inflammatory Markers (IL-1β, IL-6, and TNF-α)
2.14. Quantification of 8-Hydroxy-2-Deoxyguanosine (8-OH-dG) Level in Serum
2.15. Assessment of Lactate Dehydrogenase Enzyme (LDH) Activity
2.16. Assessment of Lysozyme Activity
2.17. Assessment of Nitric Oxide (NO)
2.18. Micronucleus Assay
2.19. Histopathological Investigation
2.20. Immunohistochemical Investigation
2.21. Morphometric Analysis
2.22. Tracking of Donner Male BM-MSCs in Female Tissues
2.23. PCR Analysis for Bcl-2, Bax, and Caspase-3 Genes
2.24. Statistical Analysis
3. Results
3.1. The Effect of Cis, OLE, CM-MSC, and MSC on Body, Liver, and Kidney Weights
3.2. The Effect of Cis, OLE, CM-MSC, and MSC on Liver and Renal Functions Markers
3.3. The Effect of Cis, OLE, CM-MSC, and MSC on Liver and Renal Oxidative/Antioxidants Status
3.4. The Effect of Cis, OLE, CM-MSC, and MSC on Liver and Renal Inflammatory Markers
3.5. The Effect of Cis, OLE, CM-MSC, and MSC on Genotoxicity and Cytotoxicity and Im-Munotoxicity Biomarkers
3.6. Morphological and Phenotypic Characterization of the Isolated BM-MSCs
3.7. Red Blood Cells Micronucleus Test
3.8. Histopathological and Immunohistochemical Assessment of Liver Sections
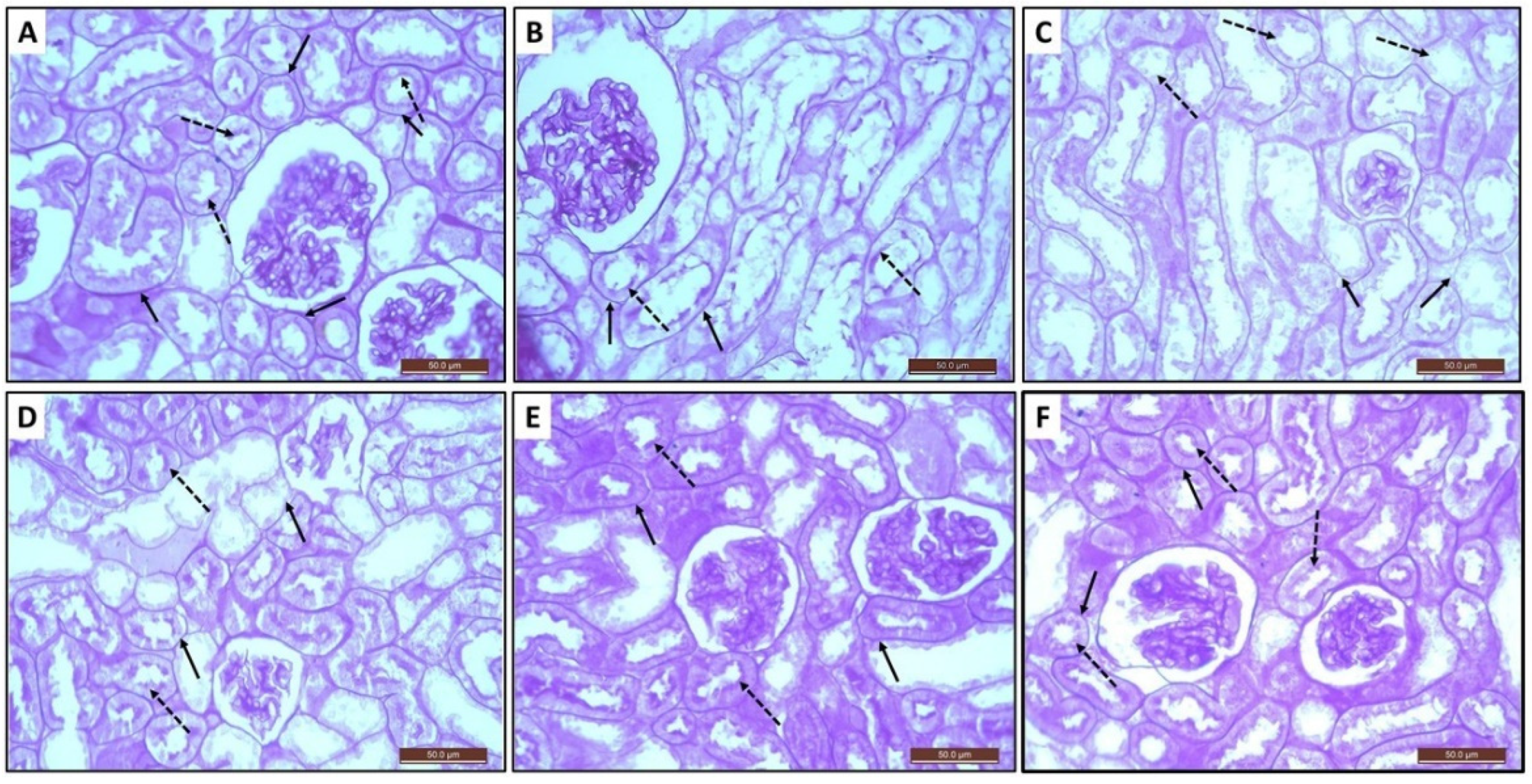
3.9. Histopathological and Immunohistochemical Assessment of Kidney Sections
3.10. Histopathological and Immunohistochemical Assessment of Thymus Sections
3.11. Histopathological and Immunohistochemical Assessment of Spleen Sections
3.12. Quantitative Real-Time Polymerase Chain Reaction (RT-PCR) Analysis of Bcl-2, Bax, and Caspase-3 Genes
3.13. Polymerase Chain Reaction Analysis of Sry Gene
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Said Salem, N.I.; Noshy, M.M.; Said, A.A. Modulatory effect of curcumin against genotoxicity and oxidative stress induced by cisplatin and methotrexate in male mice. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2017, 105, 370–376. [Google Scholar] [CrossRef]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef]
- Roos, W.P.; Kaina, B. DNA damage-induced cell death: From specific DNA lesions to the DNA damage response and apoptosis. Cancer Lett. 2013, 332, 237–248. [Google Scholar] [CrossRef]
- Muggia, F.M.; Bonetti, A.; Hoeschele, J.D.; Rozencweig, M.; Howell, S.B. Platinum Antitumor Complexes: 50 Years Since Barnett Rosenberg’s Discovery. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 4219–4226. [Google Scholar] [CrossRef]
- Aldossary, S.A. Review on pharmacology of cisplatin: Clinical use, toxicity and mechanism of resistance of cisplatin. Biomed. Pharmacol. J. 2019, 12, 7–15. [Google Scholar]
- Ibrahim, M.A.; Bakhaat, G.A.; Tammam, H.G.; Mohamed, R.M.; El-Naggar, S.A. Cardioprotective effect of green tea extract and vitamin E on Cisplatin-induced cardiotoxicity in mice: Toxicological, histological and immunohistochemical studies. Biomed. Pharmacother. 2019, 113, 108731. [Google Scholar]
- Ibrahim, M.A.; Albahlol, I.A.; Wani, F.A.; Abd-Eltawab Tammam, A.; Kelleni, M.T.; Sayeed, M.U.; Abd El-Fadeal, N.M.; Mohamed, A.A. Resveratrol protects against cisplatin-induced ovarian and uterine toxicity in female rats by attenuating oxidative stress, inflammation and apoptosis. Chem. Biol. Interact. 2021, 338, 109402. [Google Scholar] [CrossRef]
- Khalifa, A.M.; Kelleni, M.T.; Ibrahim, M.A.; Farag, M.M.; Bakhaat, G.A. Protective Effects of Blackberry Juice against Cisplatin-Induced Testicular Toxicity in Rats: Up-Regulation of Bcl-2 Proteins and Androgen Receptors. Int. Med. J. 2020, 27, 584–589. [Google Scholar]
- Zhang, K.; Weng, H.; Yang, J.; Wu, C. Protective effect of Liuwei Dihuang Pill on cisplatin-induced reproductive toxicity and genotoxicity in male mice. J. Ethnopharmacol. 2020, 247, 112269. [Google Scholar]
- Al-Eitan, L.N.; Alzoubi, K.H.; Al-Smadi, L.I.; Khabour, O.F. Vitamin E protects against cisplatin-induced genotoxicity in human lymphocytes. Toxicol. Vitr. Int. J. Publ. Assoc. BIBRA 2020, 62, 104672. [Google Scholar]
- Eid, B.G.; El-Shitany, N.A. Captopril downregulates expression of Bax/cytochrome C/caspase-3 apoptotic pathway, reduces inflammation, and oxidative stress in cisplatin-induced acute hepatic injury. Biomed. Pharmacother. Biomed. Pharmacother. 2021, 139, 111670. [Google Scholar] [CrossRef]
- Boroja, T.; Katanić, J.; Rosić, G.; Selaković, D.; Joksimović, J.; Mišić, D.; Stanković, V.; Jovičić, N.; Mihailović, V. Summer savory (Satureja hortensis L.) extract: Phytochemical profile and modulation of cisplatin-induced liver, renal and testicular toxicity. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2018, 118, 252–263. [Google Scholar] [CrossRef]
- Hassan, H.M.; Al-Wahaibi, L.H.; Elmorsy, M.A.; Mahran, Y.F. Suppression of Cisplatin-Induced Hepatic Injury in Rats Through Alarmin High-Mobility Group Box-1 Pathway by Ganoderma lucidum: Theoretical and Experimental Study. Drug Des. Dev. Ther. 2020, 14, 2335–2353. [Google Scholar] [CrossRef]
- Man, Q.; Deng, Y.; Li, P.; Ma, J.; Yang, Z.; Yang, X.; Zhou, Y.; Yan, X. Licorice Ameliorates Cisplatin-Induced Hepatotoxicity Through Antiapoptosis, Antioxidative Stress, Anti-Inflammation, and Acceleration of Metabolism. Front. Pharmacol. 2020, 11, 563750. [Google Scholar] [CrossRef]
- Huang, Y.C.; Tsai, M.S.; Hsieh, P.C.; Shih, J.H.; Wang, T.S.; Wang, Y.C.; Lin, T.H.; Wang, S.H. Galangin ameliorates cisplatin-induced nephrotoxicity by attenuating oxidative stress, inflammation and cell death in mice through inhibition of ERK and NF-kappaB signaling. Toxicol. Appl. Pharm. 2017, 329, 128–139. [Google Scholar] [CrossRef]
- Un, H.; Ugan, R.A.; Gurbuz, M.A.; Bayir, Y.; Kahramanlar, A.; Kaya, G.; Cadirci, E.; Halici, Z. Phloretin and phloridzin guard against cisplatin-induced nephrotoxicity in mice through inhibiting oxidative stress and inflammation. Life Sci. 2021, 266, 118869. [Google Scholar] [CrossRef]
- Yousef, M.I.; Hussien, H.M. Cisplatin-induced renal toxicity via tumor necrosis factor-α, interleukin 6, tumor suppressor P53, DNA damage, xanthine oxidase, histological changes, oxidative stress and nitric oxide in rats: Protective effect of ginseng. Food Chem. Toxicol. 2015, 78, 17–25. [Google Scholar]
- El-Shitany, N.A.; Eid, B. Proanthocyanidin protects against cisplatin-induced oxidative liver damage through inhibition of inflammation and NF-κβ/TLR-4 pathway. Environ. Toxicol. 2017, 32, 1952–1963. [Google Scholar]
- Santos, N.A.; Catão, C.S.; Martins, N.M.; Curti, C.; Bianchi, M.L.; Santos, A.C. Cisplatin-induced nephrotoxicity is associated with oxidative stress, redox state unbalance, impairment of energetic metabolism and apoptosis in rat kidney mitochondria. Arch. Toxicol. 2007, 81, 495–504. [Google Scholar] [CrossRef]
- Yao, X.; Panichpisal, K.; Kurtzman, N.; Nugent, K. Cisplatin nephrotoxicity: A review. Am. J. Med. Sci. 2007, 334, 115–124. [Google Scholar] [CrossRef]
- Chu, Y.H.; Sibrian-Vazquez, M.; Escobedo, J.O.; Phillips, A.R.; Dickey, D.T.; Wang, Q.; Ralle, M.; Steyger, P.S.; Strongin, R.M. Systemic Delivery and Biodistribution of Cisplatin in Vivo. Mol. Pharm. 2016, 13, 2677–2682. [Google Scholar] [CrossRef]
- Gómez-Sierra, T.; Eugenio-Pérez, D.; Sánchez-Chinchillas, A.; Pedraza-Chaverri, J. Role of food-derived antioxidants against cisplatin induced-nephrotoxicity. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2018, 120, 230–242. [Google Scholar] [CrossRef]
- Kim, H.J.; Park, D.J.; Kim, J.H.; Jeong, E.Y.; Jung, M.H.; Kim, T.H.; Yang, J.I.; Lee, G.W.; Chung, H.J.; Chang, S.H. Glutamine protects against cisplatin-induced nephrotoxicity by decreasing cisplatin accumulation. J. Pharmacol. Sci. 2015, 127, 117–126. [Google Scholar] [CrossRef]
- Saleh, S.; El-Demerdash, E. Protective effects of L-arginine against cisplatin-induced renal oxidative stress and toxicity: Role of nitric oxide. Basic Clin. Pharm. Toxicol. 2005, 97, 91–97. [Google Scholar] [CrossRef]
- Nisar, S.; Feinfeld, D.A. N-acetylcysteine as salvage therapy in cisplatin nephrotoxicity. Ren. Fail. 2002, 24, 529–533. [Google Scholar] [CrossRef]
- Squillaro, T.; Peluso, G.; Galderisi, U. Clinical Trials With Mesenchymal Stem Cells: An Update. Cell Transplant. 2016, 25, 829–848. [Google Scholar] [CrossRef]
- Amiri, F.; Molaei, S.; Bahadori, M.; Nasiri, F.; Deyhim, M.R.; Jalili, M.A.; Nourani, M.R.; Habibi Roudkenar, M. Autophagy-Modulated Human Bone Marrow-Derived Mesenchymal Stem Cells Accelerate Liver Restoration in Mouse Models of Acute Liver Failure. Iran. Biomed. J. 2016, 20, 135–144. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, X.; He, J.; Zhao, W. Influence of erythropoietin on microvesicles derived from mesenchymal stem cells protecting renal function of chronic kidney disease. Stem Cell Res. Ther. 2015, 6, 100. [Google Scholar] [CrossRef]
- Li, X.-W.; Feng, L.-X.; Zhu, X.-J.; Liu, Q.; Wang, H.-S.; Wu, X.; Yan, P.; Duan, X.-J.; Xiao, Y.-Q.; Cheng, W. Human umbilical cord blood mononuclear cells protect against renal tubulointerstitial fibrosis in cisplatin-treated rats. Biomed. Pharm. 2020, 121, 109310. [Google Scholar]
- Lotfinegad, P.; Shamsasenjan, K.; Movassaghpour, A.; Majidi, J.; Baradaran, B. Immunomodulatory nature and site specific affinity of mesenchymal stem cells: A hope in cell therapy. Adv. Pharm. Bull. 2014, 4, 5–13. [Google Scholar] [CrossRef]
- Bahsoun, S.; Coopman, K.; Akam, E.C. The impact of cryopreservation on bone marrow-derived mesenchymal stem cells: A systematic review. J. Transl. Med. 2019, 17, 397. [Google Scholar] [CrossRef]
- Sherif, I.O.; Sabry, D.; Abdel-Aziz, A.; Sarhan, O.M. The role of mesenchymal stem cells in chemotherapy-induced gonadotoxicity. Stem Cell Res. Ther. 2018, 9, 196. [Google Scholar]
- Luo, J.; Zhao, X.; Tan, Z.; Su, Z.; Meng, F.; Zhang, M. Mesenchymal-like progenitors derived from human embryonic stem cells promote recovery from acute kidney injury via paracrine actions. Cytotherapy 2013, 15, 649–662. [Google Scholar] [CrossRef]
- Wang, Y.H.; Wu, D.B.; Chen, B.; Chen, E.Q.; Tang, H. Progress in mesenchymal stem cell-based therapy for acute liver failure. Stem Cell Res. Ther. 2018, 9, 227. [Google Scholar] [CrossRef]
- Gunawardena, T.N.A.; Rahman, M.T.; Abdullah, B.J.J.; Abu Kasim, N.H. Conditioned media derived from mesenchymal stem cell cultures: The next generation for regenerative medicine. J. Tissue Eng. Regen. Med. 2019, 13, 569–586. [Google Scholar] [CrossRef]
- Semedo, P.; Palasio, C.G.; Oliveira, C.D.; Feitoza, C.Q.; Gonçalves, G.M.; Cenedeze, M.A.; Wang, P.M.; Teixeira, V.P.; Reis, M.A.; Pacheco-Silva, A.; et al. Early modulation of inflammation by mesenchymal stem cell after acute kidney injury. Int. Immunopharmacol. 2009, 9, 677–682. [Google Scholar] [CrossRef]
- Zhang, Y.; Ye, C.; Wang, G.; Gao, Y.; Tan, K.; Zhuo, Z.; Liu, Z.; Xia, H.; Yang, D.; Li, P. Kidney-targeted transplantation of mesenchymal stem cells by ultrasound-targeted microbubble destruction promotes kidney repair in diabetic nephropathy rats. Biomed Res. Int. 2013, 2013, 526367. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, H.; Xu, W.; Wang, B.; Wu, H.; Tao, Y.; Zhang, B.; Wang, M.; Mao, F.; Yan, Y. Exosomes released by human umbilical cord mesenchymal stem cells protect against cisplatin-induced renal oxidative stress and apoptosis in vivo and in vitro. Stem Cell Res. Ther. 2013, 4, 34. [Google Scholar]
- Şahin, S.; Bilgin, M. Olive tree (Olea europaea L.) leaf as a waste by-product of table olive and olive oil industry: A review. J. Sci. Food Agric. 2018, 98, 1271–1279. [Google Scholar] [CrossRef]
- Serreli, G.; Deiana, M. Biological Relevance of Extra Virgin Olive Oil Polyphenols Metabolites. Antioxidants 2018, 7, 170. [Google Scholar] [CrossRef] [Green Version]
- Xie, P.-J.; Huang, L.-X.; Zhang, C.-H.; Zhang, Y.-L. Phenolic compositions, and antioxidant performance of olive leaf and fruit (Olea europaea L.) extracts and their structure–activity relationships. J. Funct. Foods 2015, 16, 460–471. [Google Scholar]
- Castro, A.J.; Lima-Cabello, E.; Alché, J.D. Identification of seed storage proteins as the major constituents of the extra virgin olive oil proteome. Food Chem. X 2020, 7, 100099. [Google Scholar] [CrossRef]
- Al-Quraishy, S.; Othman, M.S.; Dkhil, M.A.; Abdel Moneim, A.E. Olive (Olea europaea) leaf methanolic extract prevents HCl/ethanol-induced gastritis in rats by attenuating inflammation and augmenting antioxidant enzyme activities. Biomed. Pharmacother. Biomed. Pharmacother. 2017, 91, 338–349. [Google Scholar] [CrossRef]
- Geyikoglu, F.; Emir, M.; Colak, S.; Koc, K.; Turkez, H.; Bakir, M.; Hosseinigouzdagani, M.; Cerig, S.; Keles, O.N.; Ozek, N.S. Effect of oleuropein against chemotherapy drug-induced histological changes, oxidative stress, and DNA damages in rat kidney injury. J. Food Drug Anal. 2017, 25, 447–459. [Google Scholar]
- Maalej, A.; Mahmoudi, A.; Bouallagui, Z.; Fki, I.; Marrekchi, R.; Sayadi, S. Olive phenolic compounds attenuate deltamethrin-induced liver and kidney toxicity through regulating oxidative stress, inflammation and apoptosis. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2017, 106, 455–465. [Google Scholar] [CrossRef]
- Sarriá, B.; Mateos, R.; Gallardo, E.; Ramos, S.; Martín, M.; Bravo, L.; Goya, L. Nitroderivatives of olive oil phenols protect HepG2 cells against oxidative stress. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2012, 50, 3752–3758. [Google Scholar] [CrossRef]
- Benavente-Garcıa, O.; Castillo, J.; Lorente, J.; Ortuño, A.; Del Rio, J. Antioxidant activity of phenolics extracted from Olea europaea L. leaves. Food Chem. 2000, 68, 457–462. [Google Scholar]
- Elgebaly, H.A.; Mosa, N.M.; Allach, M.; El-Massry, K.F.; El-Ghorab, A.H.; Al Hroob, A.M.; Mahmoud, A.M. Olive oil and leaf extract prevent fluoxetine-induced hepatotoxicity by attenuating oxidative stress, inflammation and apoptosis. Biomed. Pharmacother. Biomed. Pharmacother. 2018, 98, 446–453. [Google Scholar] [CrossRef]
- Al-Attar, A.M.; Alrobai, A.A.; Almalki, D.A. Protective effect of olive and juniper leaves extracts on nephrotoxicity induced by thioacetamide in male mice. Saudi J. Biol. Sci. 2017, 24, 15–22. [Google Scholar] [CrossRef]
- Chen, Y.-X.; Zeng, Z.-C.; Sun, J.; Zeng, H.-Y.; Huang, Y.; Zhang, Z.-Y. Mesenchymal stem cell–conditioned medium prevents radiation-induced liver injury by inhibiting inflammation and protecting sinusoidal endothelial cells. J. Radiat. Res. 2015, 56, 700–708. [Google Scholar]
- Abd El Zaher, F.; El Shawarby, A.; Hammouda, G.; Bahaa, N. Role of mesenchymal stem cells versus their conditioned medium on cisplatin-induced acute kidney injury in albino rat. A histological and immunohistochemical study. Egypt. J. Histol. 2017, 40, 37–51. [Google Scholar]
- Roila, F.; Molassiotis, A.; Herrstedt, J.; Aapro, M.; Gralla, R.; Bruera, E.; Clark-Snow, R.; Dupuis, L.; Einhorn, L.; Feyer, P. 2016 MASCC and ESMO guideline update for the prevention of chemotherapy-and radiotherapy-induced nausea and vomiting and of nausea and vomiting in advanced cancer patients. Ann. Oncol. 2016, 27, v119–v133. [Google Scholar]
- Yi, L.; Ju, Y.; He, Y.; Yin, X.; Xu, Y.; Weng, T. Intraperitoneal injection of Desferal® alleviated the age-related bone loss and senescence of bone marrow stromal cells in rats. Stem Cell Res. Ther. 2021, 12, 45. [Google Scholar] [CrossRef]
- Angoulvant, D.; Ivanes, F.; Ferrera, R.; Matthews, P.G.; Nataf, S.; Ovize, M. Mesenchymal stem cell conditioned media attenuates in vitro and ex vivo myocardial reperfusion injury. J. Heart Lung Transplant. Off. Publ. Int. Soc. Heart Transplant. 2011, 30, 95–102. [Google Scholar] [CrossRef]
- Reitman, S.; Frankel, S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol. 1957, 28, 56–63. [Google Scholar]
- Otto, A.; Oliver, H.; Jane, M. A method for the rapid determination of alkaline phosphatase with five cubic millimeters of serum. J. Biol. Chem. 1946, 164, 321–329. [Google Scholar]
- Kingsley, G.R. The direct biuret method for the determination of serum proteins as applied to photoelectric and visual colorimetry. J. Lab. Clin. Med. 1942, 27, 840–845. [Google Scholar]
- Pinnell, A.E.; Northam, B.E. New automated dye-binding method for serum albumin determination with bromcresol purple. Clin. Chem. 1978, 24, 80–86. [Google Scholar]
- Perry, B.; Doumas, B.; Buffone, G.; Glick, M.; Ou, C.; Ryder, K. Measurement of total bilirubin by use of bilirubin oxidase. Clin. Chem. 1986, 32, 329–332. [Google Scholar]
- Banday, A.A.; Farooq, N.; Priyamvada, S.; Yusufi, A.N.; Khan, F. Time dependent effects of gentamicin on the enzymes of carbohydrate metabolism, brush border membrane and oxidative stress in rat kidney tissues. Life Sci. 2008, 82, 450–459. [Google Scholar] [CrossRef]
- Buchanan, M.; Isdale, I.; Rose, B. Serum uric acid estimation: Chemical and enzymatic methods compared. Ann. Rheum. Dis. 1965, 24, 285. [Google Scholar]
- Beutler, E. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 1963, 61, 882–888. [Google Scholar]
- Masayasu, M.; Hiroshi, Y. A simplified assay method of superoxide dismutase activity for clinical use. Clin. Chim. Acta 1979, 92, 337–342. [Google Scholar]
- Aebi, H. [13] Catalase in vitro. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1984; Volume 105, pp. 121–126. [Google Scholar]
- Kei, S. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin. Chim. Acta 1978, 90, 37–43. [Google Scholar]
- Breton, J.; Sichel, F.; Bianchini, F.; Prevost, V. Measurement of 8-hydroxy-2′-deoxyguanosine by a commercially available ELISA test: Comparison with HPLC/electrochemical detection in calf thymus DNA and determination in human serum. Anal. Lett. 2003, 36, 123–134. [Google Scholar]
- Kachmar, J.F.; Moss, D.W. Enzymes in Fundamentals of Clinical Chemistry; Tiez, N.W., Ed.; Saunders: Philladelphia, PA, USA, 1976; pp. 652–660. [Google Scholar]
- Ellis, A.E. Lysozyme assays. Tech. Fish Immunol. 1990, 1, 101–103. [Google Scholar]
- Rajaraman, V.; Nonnecke, B.; Franklin, S.; Hammell, D.; Horst, R. Effect of vitamins A and E on nitric oxide production by blood mononuclear leukocytes from neonatal calves fed milk replacer. J. Dairy Sci. 1998, 81, 3278–3285. [Google Scholar]
- Holden, H.E.; Majeska, J.B.; Studwell, D. A direct comparison of mouse and rat bone marrow and blood as target tissues in the micronucleus assay. Mutat. Res. 1997, 391, 87–89. [Google Scholar] [CrossRef]
- Bancroft, J.; Stevens, A.; Turner, D. Theory and Practice of Histological Techniques, 4th ed.; Churchill Living Stone: New York, NY, USA; Edinburgh, UK, 1996. [Google Scholar]
- Ramos-Vara, J.A.; Kiupel, M.; Baszler, T.; Bliven, L.; Brodersen, B.; Chelack, B.; Czub, S.; Del Piero, F.; Dial, S.; Ehrhart, E.J.; et al. Suggested guidelines for immunohistochemical techniques in veterinary diagnostic laboratories. J. Vet. Diagn. Investig. 2008, 20, 393–413. [Google Scholar] [CrossRef]
- Mangi, A.A.; Noiseux, N.; Kong, D.; He, H.; Rezvani, M.; Ingwall, J.S.; Dzau, V.J. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat. Med. 2003, 9, 1195–1201. [Google Scholar]
- Toma, C.; Pittenger, M.F.; Cahill, K.S.; Byrne, B.J.; Kessler, P.D. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation 2002, 105, 93–98. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Longchar, A.; Prasad, S.B. Biochemical changes associated with ascorbic acid-cisplatin combination therapeutic efficacy and protective effect on cisplatin-induced toxicity in tumor-bearing mice. Toxicol. Rep. 2015, 2, 489–503. [Google Scholar] [CrossRef]
- Vasaikar, N.; Mahajan, U.; Patil, K.R.; Suchal, K.; Patil, C.R.; Ojha, S.; Goyal, S.N. D-pinitol attenuates cisplatin-induced nephrotoxicity in rats: Impact on pro-inflammatory cytokines. Chem. Biol. Interact. 2018, 290, 6–11. [Google Scholar] [CrossRef]
- Ishikawa, T.; Ali-Osman, F. Glutathione-associated cis-diamminedichloroplatinum(II) metabolism and ATP-dependent efflux from leukemia cells. Molecular characterization of glutathione-platinum complex and its biological significance. J. Biol. Chem. 1993, 268, 20116–20125. [Google Scholar]
- Zsengellér, Z.K.; Ellezian, L.; Brown, D.; Horváth, B.; Mukhopadhyay, P.; Kalyanaraman, B.; Parikh, S.M.; Karumanchi, S.A.; Stillman, I.E.; Pacher, P. Cisplatin nephrotoxicity involves mitochondrial injury with impaired tubular mitochondrial enzyme activity. J. Histochem. Cytochem. Off. J. Histochem. Soc. 2012, 60, 521–529. [Google Scholar] [CrossRef]
- Jiang, M.; Wang, C.Y.; Huang, S.; Yang, T.; Dong, Z. Cisplatin-induced apoptosis in p53-deficient renal cells via the intrinsic mitochondrial pathway. Am. J. Physiol. Ren. Physiol. 2009, 296, F983–F993. [Google Scholar] [CrossRef]
- Neamatallah, T.; El-Shitany, N.A.; Abbas, A.T.; Ali, S.S.; Eid, B.G. Honey protects against cisplatin-induced hepatic and renal toxicity through inhibition of NF-κB-mediated COX-2 expression and the oxidative stress dependent BAX/Bcl-2/caspase-3 apoptotic pathway. Food Funct. 2018, 9, 3743–3754. [Google Scholar] [CrossRef]
- Almeer, R.S.; Abdel Moneim, A.E. Evaluation of the Protective Effect of Olive Leaf Extract on Cisplatin-Induced Testicular Damage in Rats. Oxidative Med. Cell. Longev. 2018, 2018, 8487248. [Google Scholar] [CrossRef]
- Kim, J. Poly(ADP-ribose) polymerase activation induces high mobility group box 1 release from proximal tubular cells during cisplatin nephrotoxicity. Physiol. Res. 2016, 65, 333–340. [Google Scholar] [CrossRef]
- Badr, A.; Fouad, D. Anti-apoptotic and anti-inflammatory effects of olive leaf extract against cisplatin-induced nephrotoxicity in male rats. Int. J. Pharmacol. 2016, 12, 675–688. [Google Scholar]
- Abdel-Gayoum, A.A.; Al-Hassan, A.A.; Ginawi, I.A.; Alshankyty, I.M. The ameliorative effects of virgin olive oil and olive leaf extract on amikacin-induced nephrotoxicity in the rat. Toxicol. Rep. 2015, 2, 1327–1333. [Google Scholar] [CrossRef] [Green Version]
- Kumral, A.; Giris, M.; Soluk-Tekkesin, M.; Olgac, V.; Dogru-Abbasoglu, S.; Turkoglu, U.; Uysal, M. Effect of olive leaf extract treatment on doxorubicin-induced cardiac, hepatic and renal toxicity in rats. Pathophysiology 2015, 22, 117–123. [Google Scholar] [CrossRef]
- Vezza, T.; Rodríguez-Nogales, A.; Algieri, F.; Garrido-Mesa, J.; Romero, M.; Sánchez, M.; Toral, M.; Martín-García, B.; Gómez-Caravaca, A.M.; Arráez-Román, D.; et al. The metabolic and vascular protective effects of olive (Olea europaea L.) leaf extract in diet-induced obesity in mice are related to the amelioration of gut microbiota dysbiosis and to its immunomodulatory properties. Pharmacol. Res. 2019, 150, 104487. [Google Scholar]
- Rostamzadeh, A.; Amini-Khoei, H.; Mardani Korani, M.J.; Rahimi-Madiseh, M. Comparison effects of olive leaf extract and oleuropein compounds on male reproductive function in cyclophosphamide exposed mice. Heliyon 2020, 6, e03785. [Google Scholar] [CrossRef]
- Rosenberg, M.E. Cell-based therapies in kidney disease. Kidney Int. Suppl. 2013, 3, 364–367. [Google Scholar]
- Biancone, L.; Bruno, S.; Deregibus, M.C.; Tetta, C.; Camussi, G. Therapeutic potential of mesenchymal stem cell-derived microvesicles. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2012, 27, 3037–3042. [Google Scholar] [CrossRef]
- Anglani, F.; Forino, M.; Prete, D.D.; Tosetto, E.; Torregrossa, R.; D’Angelo, A. In search of adult renal stem cells. J. Cell. Mol. Med. 2004, 8, 474–487. [Google Scholar]
- Zaahkouk, S.; Bakry, S.; Mansour, A.; Ibrahim, R. Therapeutic role of mesenchymal stem cells in cisplatin induced renal failure in adult male rats. Adv. Biol. Res. 2015, 9, 201–209. [Google Scholar]
- Li, L.; Truong, P.; Igarashi, P.; Lin, F. Renal and bone marrow cells fuse after renal ischemic injury. J. Am. Soc. Nephrol. 2007, 18, 3067–3077. [Google Scholar]
- Stoorvogel, W.; Kleijmeer, M.J.; Geuze, H.J.; Raposo, G. The biogenesis and functions of exosomes. Traffic 2002, 3, 321–330. [Google Scholar]
- Wang, B.; Jia, H.; Zhang, B.; Wang, J.; Ji, C.; Zhu, X.; Yan, Y.; Yin, L.; Yu, J.; Qian, H. Pre-incubation with hucMSC-exosomes prevents cisplatin-induced nephrotoxicity by activating autophagy. Stem Cell Res. Ther. 2017, 8, 75. [Google Scholar]
- Gao, F.; Zuo, B.; Wang, Y.; Li, S.; Yang, J.; Sun, D. Protective function of exosomes from adipose tissue-derived mesenchymal stem cells in acute kidney injury through SIRT1 pathway. Life Sci. 2020, 255, 117719. [Google Scholar]
- Cho, K.-A.; Ju, S.-Y.; Cho, S.J.; Jung, Y.-J.; Woo, S.-Y.; Seoh, J.-Y.; Han, H.-S.; Ryu, K.-H. Mesenchymal stem cells showed the highest potential for the regeneration of injured liver tissue compared with other subpopulations of the bone marrow. Cell Biol. Int. 2009, 33, 772–777. [Google Scholar]
- Kuo, T.K.; Hung, S.P.; Chuang, C.H.; Chen, C.T.; Shih, Y.R.V.; Fang, S.C.Y.; Yang, V.W.; Lee, O.K. Stem cell therapy for liver disease: Parameters governing the success of using bone marrow mesenchymal stem cells. Gastroenterology 2008, 134, 2111–2121.e2113. [Google Scholar]
- Jiang, W.; Tan, Y.; Cai, M.; Zhao, T.; Mao, F.; Zhang, X.; Xu, W.; Yan, Z.; Qian, H.; Yan, Y. Human Umbilical Cord MSC-Derived Exosomes Suppress the Development of CCl(4)-Induced Liver Injury through Antioxidant Effect. Stem Cells Int. 2018, 2018, 6079642. [Google Scholar] [CrossRef]
- Parekkadan, B.; Van Poll, D.; Suganuma, K.; Carter, E.A.; Berthiaume, F.; Tilles, A.W.; Yarmush, M.L. Mesenchymal stem cell-derived molecules reverse fulminant hepatic failure. PLoS ONE 2007, 2, e941. [Google Scholar]
- Yagi, H.; Parekkadan, B.; Suganuma, K.; Soto-Gutierrez, A.; Tompkins, R.G.; Tilles, A.W.; Yarmush, M.L. Long-term superior performance of a stem cell/hepatocyte device for the treatment of acute liver failure. Tissue Eng. Part A 2009, 15, 3377–3388. [Google Scholar]
- Khalaf, A.A.; Hussein, S.; Tohamy, A.F.; Marouf, S.; Yassa, H.D.; Zaki, A.R.; Bishayee, A. Protective effect of Echinacea purpurea (Immulant) against cisplatin-induced immunotoxicity in rats. Daru J. Fac. Pharm. Tehran Univ. Med. Sci. 2019, 27, 233–241. [Google Scholar] [CrossRef]
- Banerjee, S.; Sinha, K.; Chowdhury, S.; Sil, P.C. Unfolding the mechanism of cisplatin induced pathophysiology in spleen and its amelioration by carnosine. Chem. Biol. Interact. 2018, 279, 159–170. [Google Scholar]
- Milićević, Ž.; Slepčević, V.; Nikolić, D.; Živanović, V.; Milićević, N. Effects of cis-diamminedichloroplatinum II (cisplatin) on the splenic tissue of rats: A histoquantitative study. Exp. Mol. Pathol. 1994, 61, 77–81. [Google Scholar]
- Crăciun, C.; Paşca, C. Structural and ultrastructural data on side effects of cisplatin in spleen, kidney and liver of rats. Acta Met. Meemb 2014, 11, 9–22. [Google Scholar]
- Oruc, E.; Kara, A.; Can, I.; Karadeniz, A.; Simsek, N. Caspase-3 and CD68 Immunoreactivity in Lymphoid Tissues and Haematology of Rats Exposed to Cisplatin and L-carnitine. Kafkas Univ. Vet. Fak. Derg. 2012, 18, 871–878. [Google Scholar]
- Khalil, S.; Awad, A.; Elewa, Y. Antidotal impact of extra virgin olive oil against genotoxicity, cytotoxicity and immunotoxicity induced by hexavalent chromium in rat. Int. J. Vet. Sci. Med. 2013, 1, 65–73. [Google Scholar]
- Wang, Z.; Lin, Y.; Jin, S.; Wei, T.; Zheng, Z.; Chen, W. Bone marrow mesenchymal stem cells improve thymus and spleen function of aging rats through affecting P21/PCNA and suppressing oxidative stress. Aging 2020, 12, 11386–11397. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, S.; Xu, D.; Lee, M.Y.; Zhang, Z.; Lee, E.Y.; Darzynkiewicz, Z. Expression of the p12 subunit of human DNA polymerase δ (Pol δ), CDK inhibitor p21WAF1, Cdt1, cyclin A, PCNA and Ki-67 in relation to DNA replication in individual cells. Cell Cycle 2014, 13, 3529–3540. [Google Scholar]
- Jiang, W.; Xu, J. Immune modulation by mesenchymal stem cells. Cell Prolif. 2020, 53, e12712. [Google Scholar] [CrossRef]
- Fan, X.L.; Zhang, Y.; Li, X.; Fu, Q.L. Mechanisms underlying the protective effects of mesenchymal stem cell-based therapy. Cell. Mol. Life Sci. 2020, 77, 2771–2794. [Google Scholar] [CrossRef]
- Lee, E.N.; Park, J.K.; Lee, J.-R.; Oh, S.-O.; Baek, S.-Y.; Kim, B.-S.; Yoon, S. Characterization of the expression of cytokeratins 5, 8, and 14 in mouse thymic epithelial cells during thymus regeneration following acute thymic involution. Anat. Cell Biol. 2011, 44, 14–24. [Google Scholar]
- Odaka, C.; Loranger, A.; Takizawa, K.; Ouellet, M.; Tremblay, M.J.; Murata, S.; Inoko, A.; Inagaki, M.; Marceau, N. Keratin 8 is required for the maintenance of architectural structure in thymus epithelium. PLoS ONE 2013, 8, e75101. [Google Scholar]
- Abdel-Daim, M.M.; Abdel-Rahman, H.G.; Dessouki, A.A.; El-Far, A.H.; Khodeer, D.M.; Bin-Jumah, M.; Alhader, M.S.; Alkahtani, S.; Aleya, L. Impact of garlic (Allium sativum) oil on cisplatin-induced hepatorenal biochemical and histopathological alterations in rats. Sci. Total Environ. 2020, 710, 136338. [Google Scholar] [CrossRef]
- Curry-McCoy, T.V.; Osna, N.A.; Donohue, T.M., Jr. Modulation of lysozyme function and degradation after nitration with peroxynitrite. Biochim. Et Biophys. Acta (BBA)-Gen. Subj. 2009, 1790, 778–786. [Google Scholar]
- Wozniak, K.; Czechowska, A.; Blasiak, J. Cisplatin-evoked DNA fragmentation in normal and cancer cells and its modulation by free radical scavengers and the tyrosine kinase inhibitor STI571. Chem. Biol. Interact. 2004, 147, 309–318. [Google Scholar] [CrossRef]
- Mueller, S.; Schittenhelm, M.; Honecker, F.; Malenke, E.; Lauber, K.; Wesselborg, S.; Hartmann, J.T.; Bokemeyer, C.; Mayer, F. Cell-cycle progression and response of germ cell tumors to cisplatin in vitro. Int. J. Oncol. 2006, 29, 471–479. [Google Scholar]
- Arjumand, W.; Sultana, S. Glycyrrhizic acid: A phytochemical with a protective role against cisplatin-induced genotoxicity and nephrotoxicity. Life Sci. 2011, 89, 422–429. [Google Scholar]
- Topalović, D.; Dekanski, D.; Spremo-Potparević, B.; Pirković, A.; Borozan, S.; Bajić, V.; Stojanović, D.; Giampieri, F.; Gasparrini, M.; Živković, L. Dry olive leaf extract attenuates DNA damage induced by estradiol and diethylstilbestrol in human peripheral blood cells in vitro. Mutat. Res. Genet. Toxicol. Environ. Mutagenesis 2019, 845, 402993. [Google Scholar] [CrossRef]
- Ranieri, M.; Di Mise, A.; Difonzo, G.; Centrone, M.; Venneri, M.; Pellegrino, T.; Russo, A.; Mastrodonato, M.; Caponio, F.; Valenti, G. Green olive leaf extract (OLE) provides cytoprotection in renal cells exposed to low doses of cadmium. PLoS ONE 2019, 14, e0214159. [Google Scholar]
- Bakir, M.; Geyikoglu, F.; Koc, K.; Cerig, S. Therapeutic effects of oleuropein on cisplatin-induced pancreas injury in rats. J. Cancer Res. Ther. 2018, 14, 671–678. [Google Scholar] [CrossRef]
- Gao, S.; Zhao, Z.; Wu, R.; Zeng, Y.; Zhang, Z.; Miao, J.; Yuan, Z. Bone marrow mesenchymal stem cell transplantation improves radiation-induced heart injury through DNA damage repair in rat model. Radiat. Environ. Biophys. 2017, 56, 63–77. [Google Scholar]
















| Gene | Primer Sequence |
|---|---|
| Bcl-2 | Forward: 5′-GGATGACTTCTCTCGTCGCTACCGT-3′ |
| Reverse: 5′-ATCCCTGAAGAGTTCCTCCACCAC-3′ | |
| Bax | Forward: 5′-CCAGGACGCATCCACCAAGAAGC-3′ |
| Reverse: 5′-TGCCACACGGAAGAAGACCTCTCG-3′ | |
| Caspase-3 | Forward: 5′-GCAGCAGCCTCAAATTGTTGACTA-3′ |
| Reverse: 5′-TGCTCCGGCTCAAACCATC-3′ | |
| β-actin | Forward: 5′-TCCTCCTGAGCGCAAGTACTCT-3′ |
| Reverse: 5′-GCTCAGTAACAGTCCGCCTAGAA-3′ |
| Parameters | Control | OLE | Cis | Cis + OLE | Cis + CM-MSC | Cis + MSC |
|---|---|---|---|---|---|---|
| Initial body weight(g) | 206.33 ± 7.66 | 208.00 ± 7.07 | 204.50 ± 5.21 | 205.17 ± 5.23 | 206.67 ± 6.65 | 205.67 ± 5.28 |
| Final body weight (g) | 226.33 ± 8.52 | 232.17 ± 9.35 | 194.17 ± 12.83 ab | 212.50 ± 19.10 c | 218.67 ± 13.78 c | 225.00 ± 10.64 c |
| RBW | 109.83 ± 6.10 | 111.68 ± 4.66 | 94.93 ± 5.46 ab | 103.62 ± 9.39 c | 105.78 ± 4.99 c | 109.51 ± 6.85 c |
| Liver weight (g) | 7.22 ± 0.48 | 7.32 ± 0.40 | 5.72 ± 0.59 ab | 6.37 ± 0.61 c | 6.58 ± 0.40 c | 6.90 ± 0.53 c |
| RLW | 3.20 ± 0.28 | 3.16 ± 0.21 | 2.96 ± 0.43 ab | 3.0 ± 0.11 c | 3.03 ± 0.36 c | 3.07 ± 0.29 c |
| Kidney weight (g) | 0.69 ± 0.08 | 0.68 ± 0.08 | 0.88 ± 0.07 ab | 0.78 ± 0.05 c | 0.74 ± 0.08 c | 0.70 ± 0.10 c |
| RKW | 0.30 ± 0.03 | 0.29 ± 0.04 | 0.45 ± 0.06 ab | 0.37 ± 0.04 c | 0.34 ± 0.05 c | 0.31 ± 0.05 c |
| Parameters | Control | OLE | Cis | Cis + OLE | Cis + CM-MSC | Cis + MSC |
|---|---|---|---|---|---|---|
| Liver | ||||||
| ALT (U/L) | 29.50 ± 3.94 | 29.17 ± 3.60 | 142.17 ± 7.05 ab | 70.67 ± 5.47 abc | 66.33 ± 4.93 c | 48.33 ± 3.83 c |
| AST(U/L) | 30.90 ± 4.06 | 33.17 ± 3.71 | 132.50 ± 3.94 ab | 77.00 ± 4.98 abc | 57.33 ± 4.46 c | 50.67 ± 5.28 c |
| ALP(U/L) | 147.50 ± 5.24 | 153.67 ± 7.94 | 198.67 ± 5.28 ab | 179.83 ± 4.26 abc | 164.17 ± 2.64 c | 154.33 ± 6.28 c |
| TP(g/dL) | 6.62 ± 0.71 | 6.70 ± 0.69 | 4.45 ± 0.46 ab | 5.17 ± 0.26 abc | 5.63 ± 0.32 c | 6.10 ± 0.33 c |
| Albumin (g/dL) | 4.43 ± 0.37 | 4.30 ± 0.41 | 2.83 ± 0.34 ab | 3.10 ± 0.17 abc | 3.37 ± 0.39 c | 3.98 ± 0.22 c |
| Total Bilirubin (mg/dL) | 0.29 ± 0.04 | 0.24 ± 0.03 | 0.84 ± 0.08 ab | 0.56 ± 0.04 abc | 0.47 ± 0.09 c | 0.33 ± 0.05 c |
| Kidney | ||||||
| Urea(mg/dL) | 27.38 ± 3.50 | 26.83 ± 3.66 | 45.17 ± 4.54 ab | 35.50 ± 4.64 abc | 31.33 ± 5.92 c | 29.50 ± 5.24 c |
| Creatinine (mg/dL) | 0.75 ± 0.04 | 0.74 ± 0.04 | 1.04 ± 0.18 ab | 0.85 ± 0.08 abc | 0.81 ± 0.04 c | 0.77 ± 0.08 c |
| Parameters | Control | OLE | Cis | Cis + OLE | Cis + CM-MSC | Cis + MSC |
|---|---|---|---|---|---|---|
| Liver | ||||||
| CAT | 348.00 ± 20.45 | 351.00 ± 20.69 | 210.50 ± 14.52 ab | 295.33 ± 12.48 abc | 298.17 ± 10.28 c | 309.83 ± 8.54 c |
| SOD | 288.17 ± 6.21 | 296.67 ± 3.33 | 227.17 ± 7.36 ab | 258.50 ± 7.89 abc | 264.67 ± 4.80 c | 284.33 ± 5.32 c |
| GSH | 6.51 ± 0.29 | 6.61 ± 0.38 | 4.32 ± 0.20 ab | 5.21 ± 0.48 abc | 5.42 ± 0.26 c | 6.00 ± 0.45 c |
| TAC | 88.33 ± 6.59 | 90.17 ± 5.88 | 37.50 ± 8.89 ab | 65.67 ± 6.53 abc | 77.67 ± 9.52 c | 83.33 ± 8.62 c |
| MDA | 49.00 ± 4.60 | 46.17 ± 4.45 | 86.17 ± 4.79 ab | 65.83 ± 5.74 abc | 62.83 ± 3.19 c | 53.83 ± 3.54 c |
| Renal | ||||||
| CAT | 341.50 ± 17.01 | 343.17 ± 15.16 | 214.00 ± 11.10 ab | 299.00 ± 7.85 abc | 321.67 ± 16.38 c | 338.83 ± 10.87 c |
| SOD | 286.00 ± 6.51 | 283.33 ± 6.15 | 234.83 ± 7.81 ab | 254.67 ± 11.20 abc | 264.33 ± 7.00 c | 281.67 ± 7.92 c |
| GSH | 6.78 ± 0.32 | 6.67 ± 0.49 | 4.18 ± 0.33 ab | 5.17 ± 0.42 abc | 5.65 ± 0.45 c | 6.48 ± 0.34 c |
| TAC | 87.5 ± 6.19 | 85.7 ± 4.18 | 39.8 ± 4.49 ab | 66.5 ± 5.43 abc | 72.8 ± 6.11 c | 82.2 ± 3.76 c |
| MDA | 46.33 ± 5.50 | 47.67 ± 5.01 | 82.67 ± 6.25 ab | 56.50 ± 5.13 abc | 49.50 ± 4.04 c | 48.50 ± 4.55 c |
| Parameters | Control | OLE | Cis | Cis + OLE | Cis + CM-MSC | Cis + MSC |
|---|---|---|---|---|---|---|
| Liver | ||||||
| IL-1β | 11.05 ± 0.34 | 11.08 ± 0.21 | 26.33 ± 0.47 ab | 18.78 ± 0.56 abc | 17.07 ± 0.62 c | 12.72 ± 0.57 c |
| IL-6 | 110.83 ± 2.64 | 112.33 ± 4.23 | 176.50 ± 5.43 ab | 129.17 ± 6.62 abc | 121.50 ± 6.47 c | 118.50 ± 5.05 c |
| TNF-α | 2562.00 ± 22.30 | 2552.00 ± 16.70 | 3457.33 ± 14.47 ab | 2975.67 ± 17.73 abc | 2764.33 ± 33.18 c | 2602.17 ± 22.68 c |
| Renal | ||||||
| IL-1β | 13.77 ± 1.00 | 14.07 ± 0.81 | 30.6 ± 0.75 ab | 20.15 ± 1.01 abc | 18.0 ± 1.11 c | 16.4 ± 0.69 c |
| IL-6 | 99.00 ± 5.06 | 97.7 ± 6.06 | 164.7 ± 6.92 ab | 114.5 ± 8.24 abc | 110.0 ± 7.24 c | 105.2 ± 6.43 c |
| TNF-α | 2365.5 ± 53.63 | 2351.8 ± 45.96 | 3268.5 ± 46.47 ab | 2733.5 ± 30.09 abc | 2526.0 ± 31.55 c | 2410.3 ± 41.37 c |
| Parameters | Control | OLE | Cis | Cis + OLE | Cis + CM-MSC | Cis + MSC |
|---|---|---|---|---|---|---|
| 8-OH-dG (ng/mL) | 4.15 ± 0.10 | 4.47 ± 0.28 | 17.18 ± 0.75 ab | 9.23 ± 0.67 c | 7.93 ± 0.48 c | 6.02 ± 0.69 c |
| LDH (µ/L) | 2280 ± 51 | 2323 ± 64 | 3912 ± 55 ab | 2620 ± 63 c | 2433 ± 85 c | 2403 ± 78 c |
| Lysozyme activity (µg/mL) | 284.33 ± 4.76 | 289.50 ± 5.32 | 226.83 ± 4.58 ab | 265.17 ± 4.17 c | 269.83 ± 4.62 c | 279.50 ± 6.22 c |
| NO (ng/mL) | 8.55 ± 0.55 | 8.95 ± 0.29 | 19.50 ± 2.43 ab | 13.62 ± 1.23 c | 11.70 ± 1.10 c | 9.63 ± 1.20 c |
| Parameters | Control | OLE | Cis | Cis + OLE | Cis + CM-MSC | Cis + MSC |
|---|---|---|---|---|---|---|
| Mean ± SD | 166.1 ± 24.2 | 170.3 ± 40.4 | 188.4 ± 40.8 * | 173.9 ± 29 *# | 175.2 ± 34 *# | 174.4 ± 32.1 *# |
| Parameters | Control | OLE | Cis | Cis + OLE | Cis + CM-MSC | Cis + MSC |
|---|---|---|---|---|---|---|
| Mean ± SD | 175.8 ± 26.3 | 173.4 ± 27.7 | 149.3 ± 43.4 * | 164.5 ± 30.2 *# | 164.7 ± 37.9 *# | 165.2 ± 32.1 *# |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, M.A.; Khalifa, A.M.; Mohamed, A.A.; Galhom, R.A.; Korayem, H.E.; Abd El-Fadeal, N.M.; Abd-Eltawab Tammam, A.; Khalifa, M.M.; Elserafy, O.S.; Abdel-Karim, R.I. Bone-Marrow-Derived Mesenchymal Stem Cells, Their Conditioned Media, and Olive Leaf Extract Protect against Cisplatin-Induced Toxicity by Alleviating Oxidative Stress, Inflammation, and Apoptosis in Rats. Toxics 2022, 10, 526. https://doi.org/10.3390/toxics10090526
Ibrahim MA, Khalifa AM, Mohamed AA, Galhom RA, Korayem HE, Abd El-Fadeal NM, Abd-Eltawab Tammam A, Khalifa MM, Elserafy OS, Abdel-Karim RI. Bone-Marrow-Derived Mesenchymal Stem Cells, Their Conditioned Media, and Olive Leaf Extract Protect against Cisplatin-Induced Toxicity by Alleviating Oxidative Stress, Inflammation, and Apoptosis in Rats. Toxics. 2022; 10(9):526. https://doi.org/10.3390/toxics10090526
Chicago/Turabian StyleIbrahim, Mahrous A., Athar M. Khalifa, Alaa A. Mohamed, Rania A. Galhom, Horeya E. Korayem, Noha M. Abd El-Fadeal, Ahmed Abd-Eltawab Tammam, Mohamed Mansour Khalifa, Osama S. Elserafy, and Rehab I. Abdel-Karim. 2022. "Bone-Marrow-Derived Mesenchymal Stem Cells, Their Conditioned Media, and Olive Leaf Extract Protect against Cisplatin-Induced Toxicity by Alleviating Oxidative Stress, Inflammation, and Apoptosis in Rats" Toxics 10, no. 9: 526. https://doi.org/10.3390/toxics10090526
APA StyleIbrahim, M. A., Khalifa, A. M., Mohamed, A. A., Galhom, R. A., Korayem, H. E., Abd El-Fadeal, N. M., Abd-Eltawab Tammam, A., Khalifa, M. M., Elserafy, O. S., & Abdel-Karim, R. I. (2022). Bone-Marrow-Derived Mesenchymal Stem Cells, Their Conditioned Media, and Olive Leaf Extract Protect against Cisplatin-Induced Toxicity by Alleviating Oxidative Stress, Inflammation, and Apoptosis in Rats. Toxics, 10(9), 526. https://doi.org/10.3390/toxics10090526








