Microplastics in the Surface Water and Gastrointestinal Tract of Salmo trutta from the Mahodand Lake, Kalam Swat in Pakistan
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Area
2.2. Sampling and Laboratory Treatment
2.3. Detection of MPs
2.4. Data Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parker, L. We made plastic we depend on it. Natl. Geogr. 2018, 16, 2. [Google Scholar]
- Hidalgo-Ruz, V.; Gutow, L.; Thompson, R.C.; Thiel, M. Microplastics in the Marine Environment: A Review of the Methods Used for Identification and Quantification. Environ. Sci. Technol. 2012, 46, 3060–3075. [Google Scholar] [CrossRef] [PubMed]
- Boucher, J.; Friot, D. Primary Microplastics in the Oceans: A Global Evaluation of Sources; IUCN: Gland, Switzerland, 2017; p. 43. [Google Scholar]
- Thompson, R.C.; Moore, C.J.; Vom Saal, F.S.; Swan, S.H. Plastics, the environment and human health: Current consensus and future trends. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 2153–2166. [Google Scholar] [CrossRef] [PubMed]
- Barnes, D.K.A.; Galgani, F.; Thompson, R.C.; Barlaz, M. Accumulation and fragmentation of plastic debris in global environments. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 1985–1998. [Google Scholar] [CrossRef] [Green Version]
- Hopewell, J.; Dvorak, R.; Kosior, E. Plastics recycling: Challenges and opportunities. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2115–2126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yonkos, L.T.; Friedel, E.A.; Perez-Reyes, A.C.; Ghosal, S.; Arthur, C.D. Microplastics in Four Estuarine Rivers in the Chesapeake Bay, U.S.A. Environ. Sci. Technol. 2014, 48, 14195–14202. [Google Scholar] [CrossRef]
- GESAMP. Source Fate and Effects of Microplastics in the Marine Environment: A Global Assessment; Kershaw, P.J., Ed.; No. 90; Joint Group of Experts on the Scientific Aspects of Marine Environmental Protection Rep Stud–GESAMP: London, UK, 2015; p. 96. [Google Scholar]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef]
- OSPAR Commission. Marine Litter Regional Action Plan; Publication Number: 643/215; OSPAR: London, UK, 2014; p. 3. ISBN 978-1-906840-85-0. [Google Scholar]
- Fendall, L.S.; Sewell, M.A. Contributing to marine pollution by washing your face: Microplastics in facial cleansers. Mar. Pollut. Bull. 2009, 58, 1225–1228. [Google Scholar] [CrossRef]
- A Cooper, D.; Corcoran, P.L. Effects of mechanical and chemical processes on the degradation of plastic beach debris on the island of Kauai, Hawaii. Mar. Pollut. Bull. 2010, 60, 650–654. [Google Scholar] [CrossRef]
- Hanslik, L. Microplastics in Limnic Ecosystems-Investigation of Biological Fate and Effects of Microplastic Particles and Associated Contaminants in Zebrafish (Danio rerio). Ph. D. Thesis, Combined Faculty of Natural Sciences and Mathematics Heidelberg University, Heidelberg, Germany, 2020. [Google Scholar]
- Napper, I.E.; Bakir, A.; Rowland, S.J.; Thompson, R.C. Characterization quantity and sorptive properties of microplastics ex-tracted from cosmetics. Mar. Pollut. Bull. 2015, 99, 178–185. [Google Scholar] [CrossRef] [Green Version]
- Driedger, A.G.; Dürr, H.H.; Mitchell, K.; Van Cappellen, P. Plastic debris in the Laurentian Great Lakes: A review. J. Great Lakes Res. 2015, 41, 9–19. [Google Scholar] [CrossRef]
- Dris, R.; Gasperi, J.; Saad, M.; Mirande, C.; Tassin, B. Synthetic fibres in atmospheric fallout: A source of microplastics in the environment? Mar. Pollut. Bull. 2016, 104, 290–293. [Google Scholar] [CrossRef] [PubMed]
- Khalid, N.; Aqeel, M.; Noman, A.; Khan, S.M.; Akhter, N. Interactions and effects of microplastics with heavy metals in aquatic and terrestrial environments. Environ. Pollut. 2021, 290, 118104. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Ali, H.; Hassan, H.U.; Khan, S.U.; Ghafar, R.; Akram, W.; Ahmad, H.; Mushtaq, S.; Jafari, H.; Yaqoob, H.; et al. Cadmium (Cd) influences calcium (Ca) levels in the skeleton of a freshwater fish Channa gachua. Braz. J. Biol. 2022, 84, e264336. [Google Scholar] [CrossRef]
- Bilal, M.; Ali, H.; Ahmad, A.; Khan, F.A.; Bashir, K. Effects of heavy metal Cd on essential metal Zn levels in freshwater fish Channa gachua. Int. J. Aquat. Sci. 2021, 12, 687–698. [Google Scholar]
- Ortega, D.R.; Esquivel, D.G.; Ayala, T.B.; Pineda, B.; Manzo, S.G.; Quino, J.M.; Mora, P.C.; de la Cruz, V.P. Cognitive Impairment Induced by Lead Exposure during Lifespan: Mechanisms of Lead Neurotoxicity. Toxics 2021, 9, 23. [Google Scholar] [CrossRef]
- Abidin, Z.U.; Hassan, H.U.; Masood, Z.; Rafique, N.; Paray, B.A.; Gabol, K.; Shah, M.I.A.; Gulnaz, A.; Ullah, A.; Zulfiqar, T.; et al. Effect of dietary supple-mentation of Neem, Azadirachta indica leaf extracts on enhancing the growth performance, chemical composition and survival of rainbow trout, Oncorhynchus mykiss. Saudi J. Biol. Sci. 2022, 29, 3075–3081. [Google Scholar] [CrossRef]
- Hassan, H.U.; Razzaq, W.; Masood, Z. Elemental composition of three-spot swimming crab Portunus sanguinolentus (Herbst, 1783) shell from the coasts of Sindh and Balochistan, Pakistan. Environ. Sci. Pollut. Res. 2021, 29, 25679–25684. [Google Scholar] [CrossRef]
- Wright, S.L.; Thompson, R.C.; Galloway, T.S. The physical impacts of microplastics on marine organisms: A review. Environ. Pollut. 2013, 178, 483–492. [Google Scholar] [CrossRef]
- Jabeen, K.; Su, L.; Li, J.; Yang, D.; Tong, C.; Mu, J.; Shi, H. Microplastics and mesoplastics in fish from coastal and fresh waters of China. Environ. Pollut. 2017, 221, 141–149. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, S.; Wang, J.; Wang, Y.; Mu, J.; Wang, P.; Lin, X.; Ma, D. Microplastic pollution in the surface waters of the Bohai Sea, China. Environ. Pollut. 2017, 231, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Siddique, M.A.M.; Uddin, A.; Rahman, S.M.A.; Rahman, M.; Islam, S.; Kibria, G. Microplastics in an anadromous national fish, Hilsa shad Tenualosa ilisha from the Bay of Bengal, Bangladesh. Mar. Pollut. Bull. 2021, 174, 113236. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, C.; Pan, Z.; Sun, D.; Zhou, A.; Xie, S.; Wang, J.; Zou, J. Microplastics in wild freshwater fish of different feeding habits from Beijiang and Pearl River Delta regions, south China. Chemosphere 2020, 258, 127345. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Wang, J.; Xie, S.; Tang, H.; Zhang, C.; Xu, G.; Zhou, A. Characterization and spatial distribution of microplastics in two wild captured economic freshwater fish from north and west rivers of Guangdong province. Ecotoxicol. Environ. Saf. 2020, 207, 111555. [Google Scholar] [CrossRef]
- Pegado, T.; Schmid, K.; Winemiller, K.O.; Chelazzi, D.; Cincinelli, A.; Dei, L.; Giarrizzo, T. First evidence of microplastic ingestion by fishes from the Amazon River estuary. Mar. Pollut. Bull. 2018, 133, 814–821. [Google Scholar] [CrossRef]
- Pazos, R.S.; Maiztegui, T.; Colautti, D.C.; Paracampo, A.H.; Gómez, N. Microplastics in gut contents of coastal freshwater fish from Río de la Plata estuary. Mar. Pollut. Bull. 2017, 122, 85–90. [Google Scholar] [CrossRef]
- Azevedo-Santos, V.M.; Gonçalves, G.R.; Manoel, P.S.; Andrade, M.C.; Lima, F.P.; Pelicice, F.M. Plastic ingestion by fish: A global assessment. Environ. Pollut. 2019, 255, 112994. [Google Scholar] [CrossRef]
- O’Connor, J.D.; Murphy, S.; Lally, H.T.; O’Connor, I.; Nash, R.; O’Sullivan, J.; Bruen, M.; Heerey, L.; Koelmans, A.A.; Cullagh, A.; et al. Microplastics in brown trout (Salmo trutta Linnaeus, 1758) from an Irish riverine system. Environ. Pollut. 2020, 267, 115572. [Google Scholar] [CrossRef]
- Karlsson, T.M.; Vethaak, A.D.; Almroth, B.C.; Ariese, F.; van Velzen, M.; Hassellöv, M.; Leslie, H.A. Screening for micro-plastics in sediment, water, marine invertebrates and fish: Method development and microplastic accumulation. Mar. Pollut. Bull. 2017, 122, 403–408. [Google Scholar] [CrossRef]
- Jakubowska, M.; Białowąs, M.; Stankevičiūtė, M.; Chomiczewska, A.; Pažusienė, J.; Jonko-Sobuś, K.; Hallmann, A.; Urban-Malinga, B. Effects of chronic exposure to microplastics of different polymer types on early life stages of sea trout Salmo trutta. Sci. Total Environ. 2020, 740, 139922. [Google Scholar] [CrossRef]
- Schmieg, H.; Burmester, J.K.; Krais, S.; Ruhl, A.S.; Tisler, S.; Zwiener, C.; Köhler, H.-R.; Triebskorn, R. Interacting Effects of Polystyrene Microplastics and the Antidepressant Amitriptyline on Early Life Stages of Brown Trout (Salmo trutta f. fario). Water 2020, 12, 2361. [Google Scholar] [CrossRef]
- Canesi, L.; Ciacci, C.; Bergami, E.; Monopoli, M.; Dawson, K.; Papa, S.; Canonico, B.; Corsi, I. Evidence for immunomodulation and apoptotic processes induced by cationic polystyrene nanoparticles in the hemocytes of the marine bivalve Mytilus. Mar. Environ. Res. 2015, 111, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Romeo, T.; Pietro, B.; Pedà, C.; Consoli, P.; Andaloro, F.; Fossi, M.C. First evidence of presence of plastic debris in stomach of large pelagic fish in the Mediterranean Sea. Mar. Pollut. Bull. 2015, 95, 358–361. [Google Scholar] [CrossRef]
- Schmid, C.; Cozzarini, L.; Zambello, E. Microplastic’s story. Mar. Pollut. Bull. 2020, 162, 111820. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, H.; Chen, J.P. Microplastics in freshwater systems: A review on occurrence, environmental effects, and methods for microplastics detection. Water Res. 2018, 137, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Tunali, M.; Uzoefuna, E.N.; Tunali, M.M.; Yenigun, O. Effect of microplastics and microplastic-metal combinations on growth and chlorophyll-a concentration of Chlorella vulgaris. Sci. Total Environ. 2020, 743, 140–479. [Google Scholar] [CrossRef]
- Pironti, C.; Ricciardi, M.; Motta, O.; Miele, Y.; Proto, A.; Montano, L. Microplastics in the Environment: Intake through the Food Web, Human Exposure and Toxicological Effects. Toxics 2021, 9, 224. [Google Scholar] [CrossRef]
- Bilal, M.; Ali, H.; Ullah, R.; Hussain, I.; Khan, B.A. Overview of Microplastics Threat in Aquatic Animals since 1960 to 2021. IJRAR 2020, 8, 950–964. [Google Scholar]
- Yang, H.; Chen, G.; Wang, J. Microplastics in the Marine Environment: Sources, Fates, Impacts and Microbial Degradation. Toxics 2021, 9, 41. [Google Scholar] [CrossRef]
- Khan, W.; Hassan, H.U.; Gabol, K.; Khan, S.; Gul, Y.; Ahmed, A.E.; Swelum, A.A.; Khooharo, A.; Ahmad, J.; Shafeeq, P.; et al. Biodiversity, distributions and isolation of microplastics pollution in finfish species in the Panjkora River at Lower and Upper Dir districts of Khyber Pakhtunkhwa province of Pakistan. Braz. J. Biol. 2022, 84, e256817. [Google Scholar] [CrossRef]
- Wang, G.; Lu, J.; Li, W.; Ning, J.; Zhou, L.; Tong, Y.; Liu, Z.; Zhou, H.; Xiayihazi, N. Seasonal variation and risk assessment of microplastics in surface water of the Manas River Basin, China. Ecotoxicol. Environ. Saf. 2020, 208, 111477. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.; Qi, K.; Wang, J.; Wang, W.; Wang, Z.; Lu, N.; Qu, J. Microplastics as an emerging anthropogenic vector of trace metals in freshwater: Significance of biofilms and comparison with natural substrates. Water Res. 2020, 184, 116205. [Google Scholar] [CrossRef] [PubMed]
- Kadhum, S.A.; Abed, S.A.; Ewaid, S.H.; Al-Ansari, N.; Naji, A. Microplastic Contamination of Surface Sediment of Euphrates River, Iraq: A Preliminary Study. J. Phys. Conf. Ser. 2020, 1664, 012139. [Google Scholar] [CrossRef]
- Cera, A.; Cesarini, G.; Scalici, M. Microplastics in freshwater: What is the news from the world? Diversity 2020, 12, 276. [Google Scholar] [CrossRef]
- Li, C.; Busquets, R.; Campos, L.C. Assessment of microplastics in freshwater systems: A review. Sci. Total. Environ. 2019, 707, 135578. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Wang, T.; Zhu, L.; Xu, P.; Wang, X.; Gao, L.; Li, D. Analysis of suspended microplastics in the Changjiang Estuary: Implications for riverine plastic load to the ocean. Water Res. 2019, 161, 560–569. [Google Scholar] [CrossRef]
- Zobkov, M.; Esiukova, E. Microplastics in Baltic bottom sediments: Quantification procedures and first results. Mar. Pollut. Bull. 2017, 114, 724–732. [Google Scholar] [CrossRef]
- Irfan, M.; Qadir, A.; Mumtaz, M.; Ahmad, S.R. An unintended challenge of microplastic pollution in the urban surface water system of Lahore, Pakistan. Environ. Sci. Pollut. Res. 2020, 27, 16718–16730. [Google Scholar] [CrossRef]
- Bilal, M.; Qadir, A.; Yaqub, A.; Hassan, H.U.; Irfan, M.; Aslam, M. Microplastics in water, sediments, and fish at Alpine River, originating from the Hindu Kush Mountain, Pakistan: Implications for conservation. Environ. Sci. Pollut. Res. 2022; online ahead of print. [Google Scholar] [CrossRef]
- Coppock, R.L.; Cole, M.; Lindeque, P.K.; Queirós, A.M.; Galloway, T.S. A small-scale, portable method for extracting micro-plastics from marine sediments. Environ. Pollut. 2017, 230, 829–837. [Google Scholar] [CrossRef] [Green Version]
- Rahman, S.M.A.; Robin, G.S.; Momotaj, M.; Uddin, J.; Siddique, M.A.M. Occurrence and spatial distribution of microplastics in beach sediments of Cox’s Bazar, Bangladesh. Mar. Pollut. Bull. 2020, 160, 111–587. [Google Scholar] [CrossRef]
- Pellini, G.; Gomiero, A.; Fortibuoni, T.; Ferrà, C.; Grati, F.; Tassetti, A.; Polidori, P.; Fabi, G.; Scarcella, G. Characterization of microplastic litter in the gastrointestinal tract of Solea solea from the Adriatic Sea. Environ. Pollut. 2018, 234, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Mao, R.; Hu, Y.; Zhang, S.; Wu, R.; Guo, X. Microplastics in the surface water of Wuliangsuhai Lake, northern China. Sci. Total. Environ. 2020, 723, 137820. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, L.; Xue, Y.; Gao, Y.; Wang, L.; Peng, M.; Jiang, S.; Zhang, Q. Microplastic pollution characteristic in surface water and freshwater fish of Gehu Lake, China. Environ. Sci. Pollut. Res. 2021, 28, 67203–67213. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Mao, R.F.; Guo, X.; Yang, X.; Zhang, Q.; Yang, C. Microplastics in surface waters and sediments of the Wei River, in the northwest of China. Sci. Total. Environ. 2019, 667, 427–434. [Google Scholar] [CrossRef]
- Malygina, N.; Mitrofanova, E.; Kuryatnikova, N.; Biryukov, R.; Zolotov, D.; Pershin, D.; Chernykh, D. Microplastic Pollution in the Surface Waters from Plain and Mountainous Lakes in Siberia, Russia. Water 2021, 13, 2287. [Google Scholar] [CrossRef]
- Grbić, J.; Helm, P.; Athey, S.; Rochman, C.M. Microplastics entering northwestern Lake Ontario are diverse and linked to urban sources. Water Res. 2020, 174, 115623. [Google Scholar] [CrossRef] [PubMed]
- Devi, S.S.; Sreedevi, A.V.; Kumar, A.B. First report of microplastic ingestion by the alien fish Pirapitinga (Piaractus brachypomus) in the Ramsar site Vembanad Lake, south India. Mar. Pollut. Bull. 2020, 160, 111637. [Google Scholar] [CrossRef]
- Galafassi, S.; Sighicelli, M.; Pusceddu, A.; Bettinetti, R.; Cau, A.; Temperini, M.E.; Gillibert, R.; Ortolani, M.; Pietrelli, L.; Zaupa, S.; et al. Microplastic pollution in perch (Perca fluviatilis, Linnaeus 1758) from Italian south-alpine lakes. Environ. Pollut. 2021, 288, 117782. [Google Scholar] [CrossRef]
- Collard, F.; Gasperi, J.; Gabrielsen, G.W.; Tassin, B. Plastic particle ingestion by wild freshwater fish: A critical review. Environ. Sci. Technol 2019, 53, 12974–12988. [Google Scholar] [CrossRef]
- Adeogun, A.O.; Ibor, O.R.; Khan, E.A.; Chukwuka, A.V.; Omogbemi, E.D.; Arukwe, A. Detection and occurrence of microplastics in the stomach of commercial fish species from a municipal water supply lake in southwestern Nigeria. Environ. Sci. Pollut. Res. 2020, 27, 31035–31045. [Google Scholar] [CrossRef]
- Zazouli, M.; Nejati, H.; Hashempour, Y.; Dehbandi, R.; Nam, V.T.; Fakhri, Y. Occurrence of microplastics (MPs) in the gastrointestinal tract of fishes: A global systematic review and meta-analysis and meta-regression. Sci. Total. Environ. 2022, 815, 152743. [Google Scholar] [CrossRef] [PubMed]
- Atici, A.A.; Sepil, A.; Sen, F. High levels of microplastic ingestion by commercial, planktivorous Alburnus tarichi in Lake Van, Turkey. Food Addit. Contam. Part A 2021, 38, 1767–1777. [Google Scholar] [CrossRef]
- Munno, K.; Helm, P.A.; Rochman, C.; George, T.; Jackson, D.A. Microplastic contamination in Great Lakes fish. Conserv. Biol. 2021, 36, e13794. [Google Scholar] [CrossRef] [PubMed]
- Turhan, D.Ö. Evaluation of Microplastics in the Surface Water, Sediment and Fish of Sürgü Dam Reservoir (Malatya) in Turkey. Turk. J. Fish. Aquat. Sci. 2022, 22, 20157. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, T.; Kang, S.; Sillanpää, M. Importance of atmospheric transport for microplastics deposited in remote areas. Environ. Pollut. 2019, 254, 112953. [Google Scholar] [CrossRef] [PubMed]
- Srinivasalu, S.; Natesan, U.; Ayyamperumal, R.; Kalam, N.; Anbalagan, S.; Sujatha, K.; Alagarasan, C. Microplastics as an emerging threat to the freshwater ecosystems of Veeranam lake in South India: A multidimensional approach. Chemosphere 2020, 264, 128502. [Google Scholar]
- Sutton, R.; Mason, S.A.; Stanek, S.K.; Willis-Norton, E.; Wren, I.F.; Box, C. Microplastic contamination in the San Francisco Bay, California, USA. Mar. Pollut. Bull. 2016, 109, 230–235. [Google Scholar] [CrossRef]
- Scherer, C.; Weber, A.; Stock, F.; Vurusic, S.; Egerci, H.; Kochleus, C.; Arendt, N.; Foeldi, C.; Dierkes, G.; Wagner, M.; et al. Comparative assessment of microplastics in water and sediment of a large European river. Sci. Total. Environ. 2020, 738, 139866. [Google Scholar] [CrossRef]
- Allahvaisi, S. Polypropylene in the industry of food packaging. In Polypropylene; Dogan, F., Ed.; IntechOpen: London, UK, 2012; pp. 3–22. [Google Scholar]

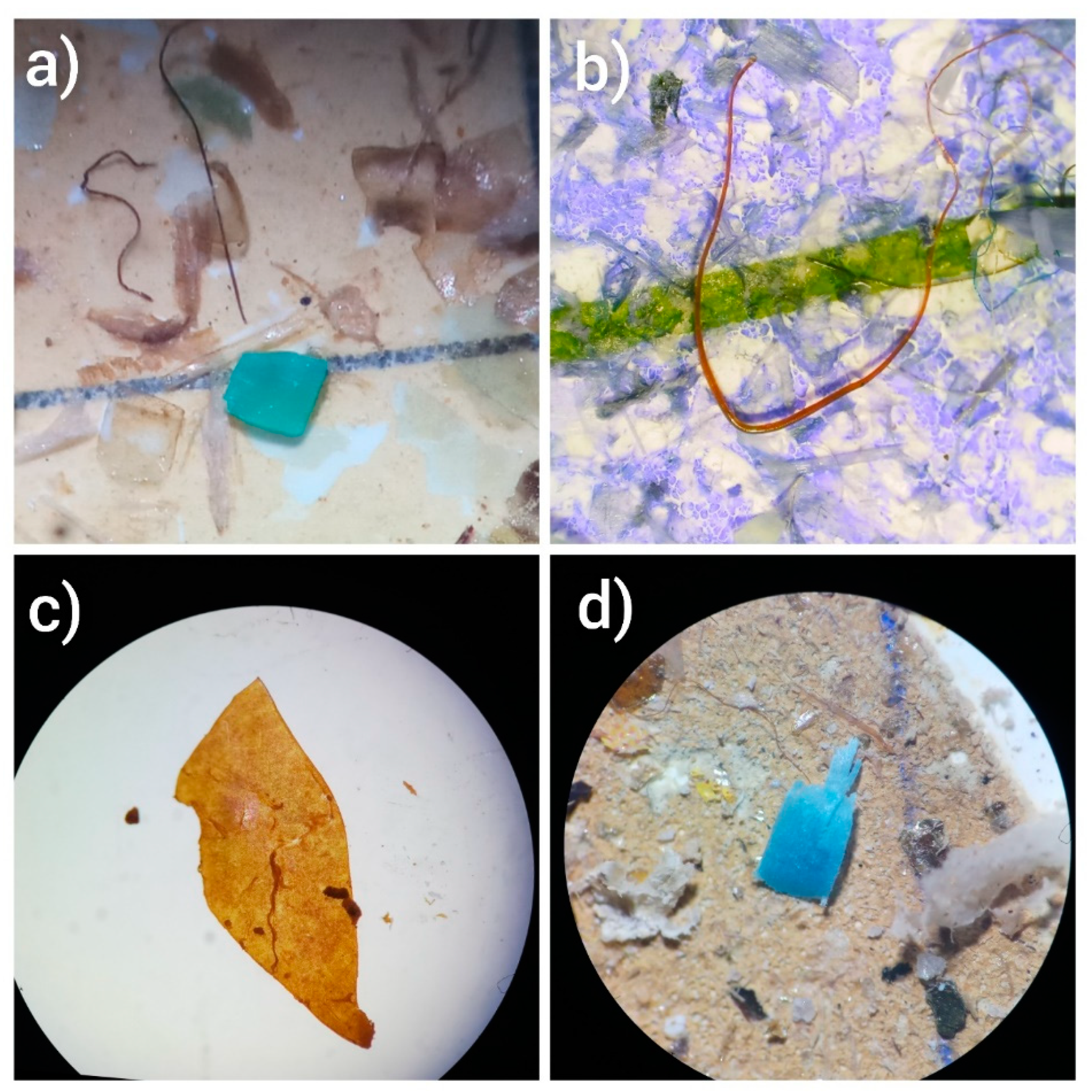

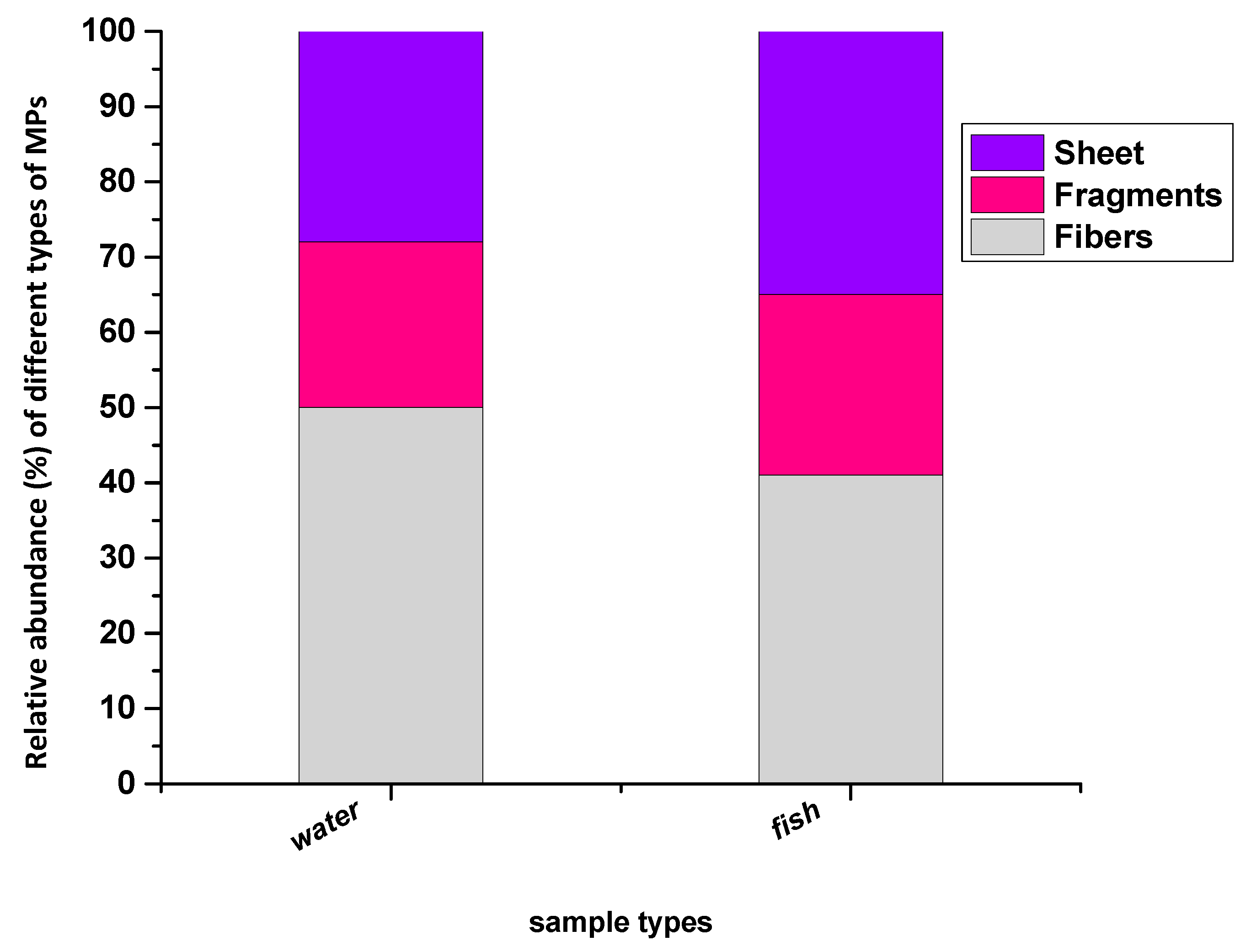

| MPs Detected | Size of MPs | Shapes of MPs | |||||
|---|---|---|---|---|---|---|---|
| 500–300 µm | 300–150 µm | 150–50 µm | Fibers | Sheets | Fragments | ||
| Surface water samples | |||||||
| 1 | 2 | 1 | 0 | 1 | 1 | 1 | 0 |
| 2 | 3 | 2 | 1 | 0 | 3 | 0 | 0 |
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 4 | 3 | 1 | 2 | 0 | 1 | 2 | 0 |
| 5 | 2 | 0 | 2 | 0 | 1 | 0 | 1 |
| 6 | 1 | 0 | 0 | 1 | 1 | 0 | 0 |
| 7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 8 | 3 | 3 | 0 | 0 | 1 | 1 | 1 |
| 9 | 4 | 4 | 0 | 0 | 1 | 2 | 1 |
| 10 | 3 | 2 | 1 | 0 | 1 | 2 | 0 |
| 11 | 3 | 2 | 0 | 1 | 3 | 0 | 0 |
| 12 | 4 | 3 | 0 | 1 | 1 | 1 | 2 |
| 13 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 14 | 5 | 0 | 5 | 0 | 1 | 3 | 1 |
| 15 | 3 | 0 | 0 | 3 | 3 | 0 | 0 |
| 16 | 1 | 0 | 1 | 0 | 1 | 0 | 0 |
| 17 | 2 | 2 | 0 | 0 | 0 | 1 | 1 |
| 18 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 19 | 3 | 3 | 0 | 0 | 0 | 0 | 3 |
| 20 | 4 | 3 | 1 | 0 | 4 | 0 | 0 |
| Fish GIT samples | |||||||
| 1 | 3 | 1 | 2 | 0 | 0 | 2 | 1 |
| 2 | 2 | 1 | 1 | 0 | 1 | 1 | 0 |
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 4 | 1 | 1 | 0 | 0 | 1 | 0 | 0 |
| 5 | 3 | 3 | 0 | 0 | 1 | 1 | 1 |
| 6 | 1 | 0 | 1 | 0 | 1 | 0 | 0 |
| 7 | 1 | 0 | 0 | 1 | 1 | 0 | 0 |
| 8 | 3 | 2 | 0 | 1 | 2 | 1 | 0 |
| 9 | 1 | 0 | 1 | 0 | 0 | 0 | 1 |
| 10 | 2 | 2 | 0 | 0 | 0 | 1 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bilal, M.; Ul Hassan, H.; Siddique, M.A.M.; Khan, W.; Gabol, K.; Ullah, I.; Sultana, S.; Abdali, U.; Mahboob, S.; Khan, M.S.; et al. Microplastics in the Surface Water and Gastrointestinal Tract of Salmo trutta from the Mahodand Lake, Kalam Swat in Pakistan. Toxics 2023, 11, 3. https://doi.org/10.3390/toxics11010003
Bilal M, Ul Hassan H, Siddique MAM, Khan W, Gabol K, Ullah I, Sultana S, Abdali U, Mahboob S, Khan MS, et al. Microplastics in the Surface Water and Gastrointestinal Tract of Salmo trutta from the Mahodand Lake, Kalam Swat in Pakistan. Toxics. 2023; 11(1):3. https://doi.org/10.3390/toxics11010003
Chicago/Turabian StyleBilal, Muhammad, Habib Ul Hassan, Mohammad Abdul Momin Siddique, Wali Khan, Karim Gabol, Imran Ullah, Saira Sultana, Umaiya Abdali, Shahid Mahboob, Muhammad Shahab Khan, and et al. 2023. "Microplastics in the Surface Water and Gastrointestinal Tract of Salmo trutta from the Mahodand Lake, Kalam Swat in Pakistan" Toxics 11, no. 1: 3. https://doi.org/10.3390/toxics11010003







