Analyzing the Impact of Diesel Exhaust Particles on Lung Fibrosis Using Dual PCR Array and Proteomics: YWHAZ Signaling
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Diesel Exhaust Particulate Matter
2.2. NHBE Cell Culture and Stimulation with DEPs
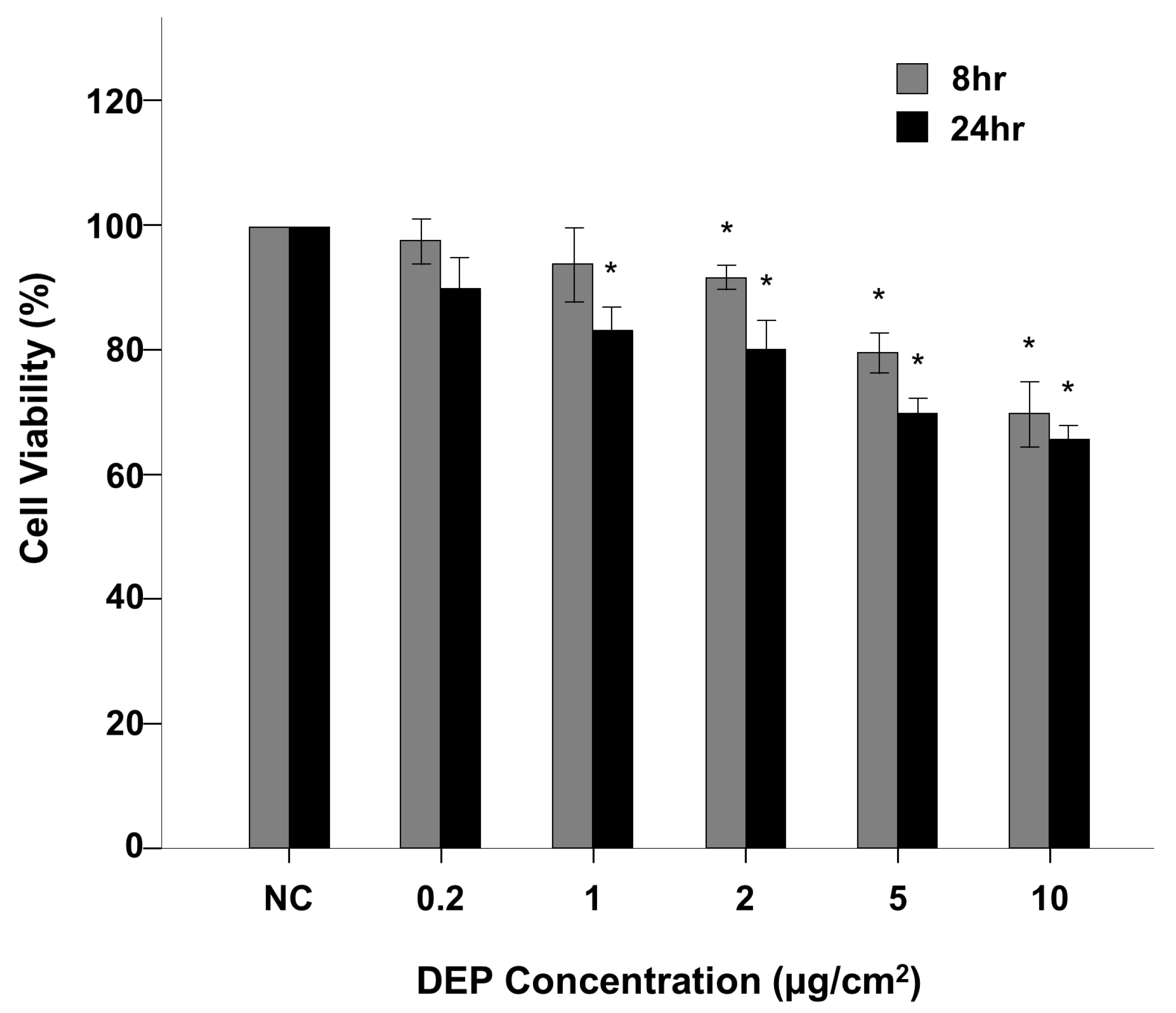
2.3. NHBE Cell Viability Assays
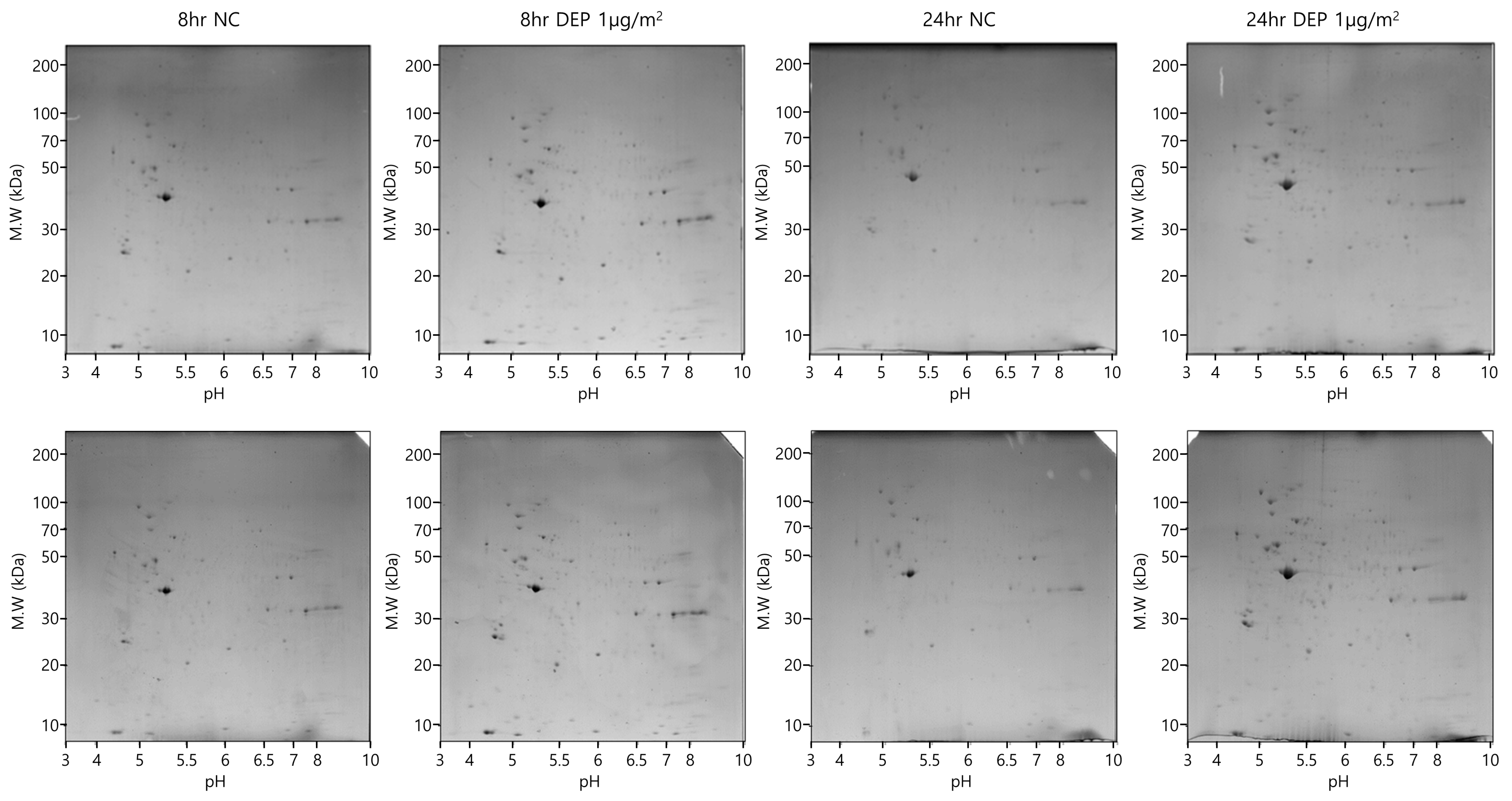
2.4. Two-Dimensional (2D) Electrophoresis and Image Analysis in NHBE Cells
2.5. Protein Identification via LC-MS/MS Analysis
2.6. Database Search and Protein Identification
2.7. miRNA Extraction and cDNA Synthesis
2.8. Total RNA Extraction and Polymerase Chain Reaction (PCR)
2.9. Profiling of PCR Array
2.10. miRNA Target Prediction and Bioinformatics Data Analysis
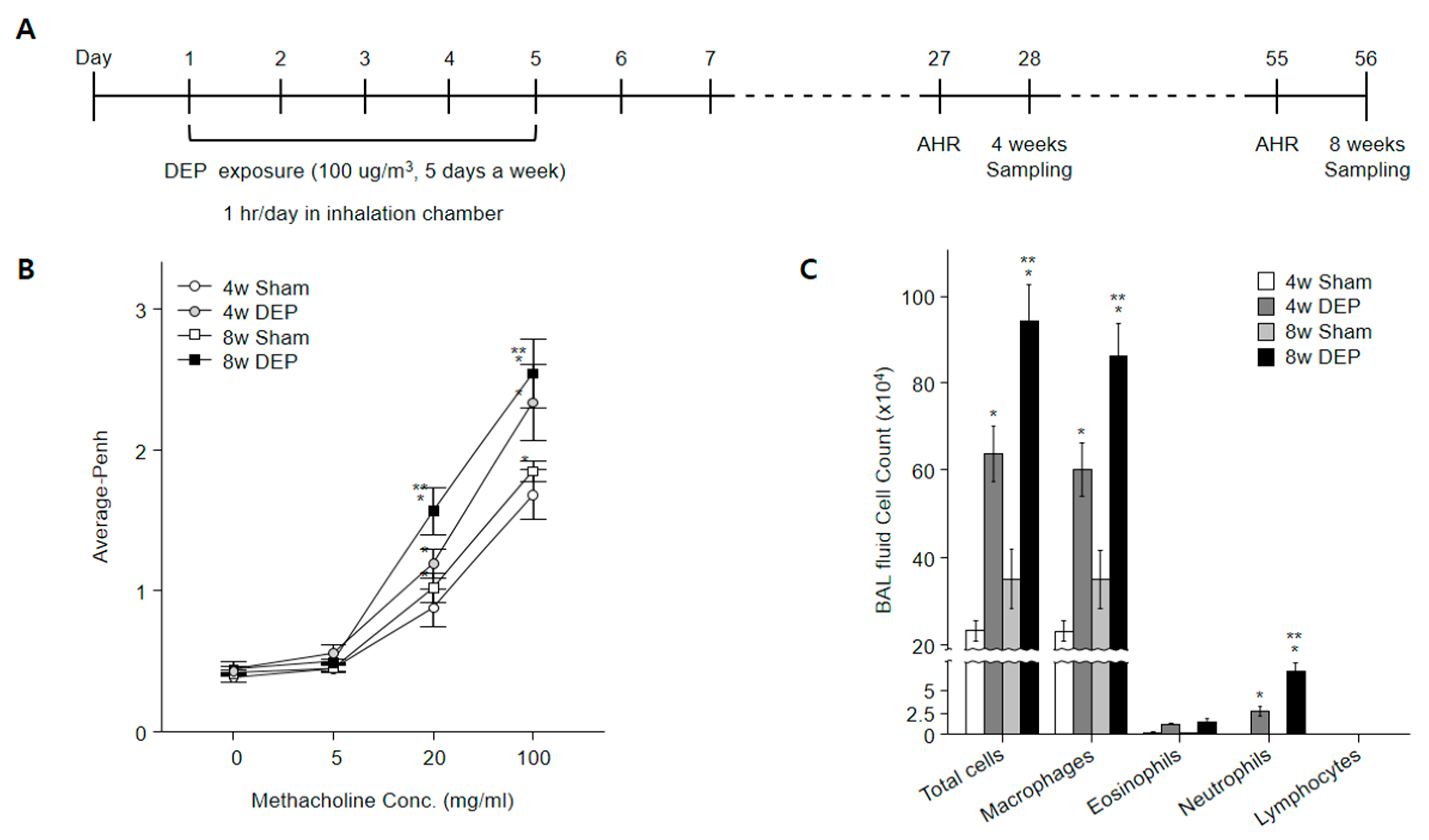
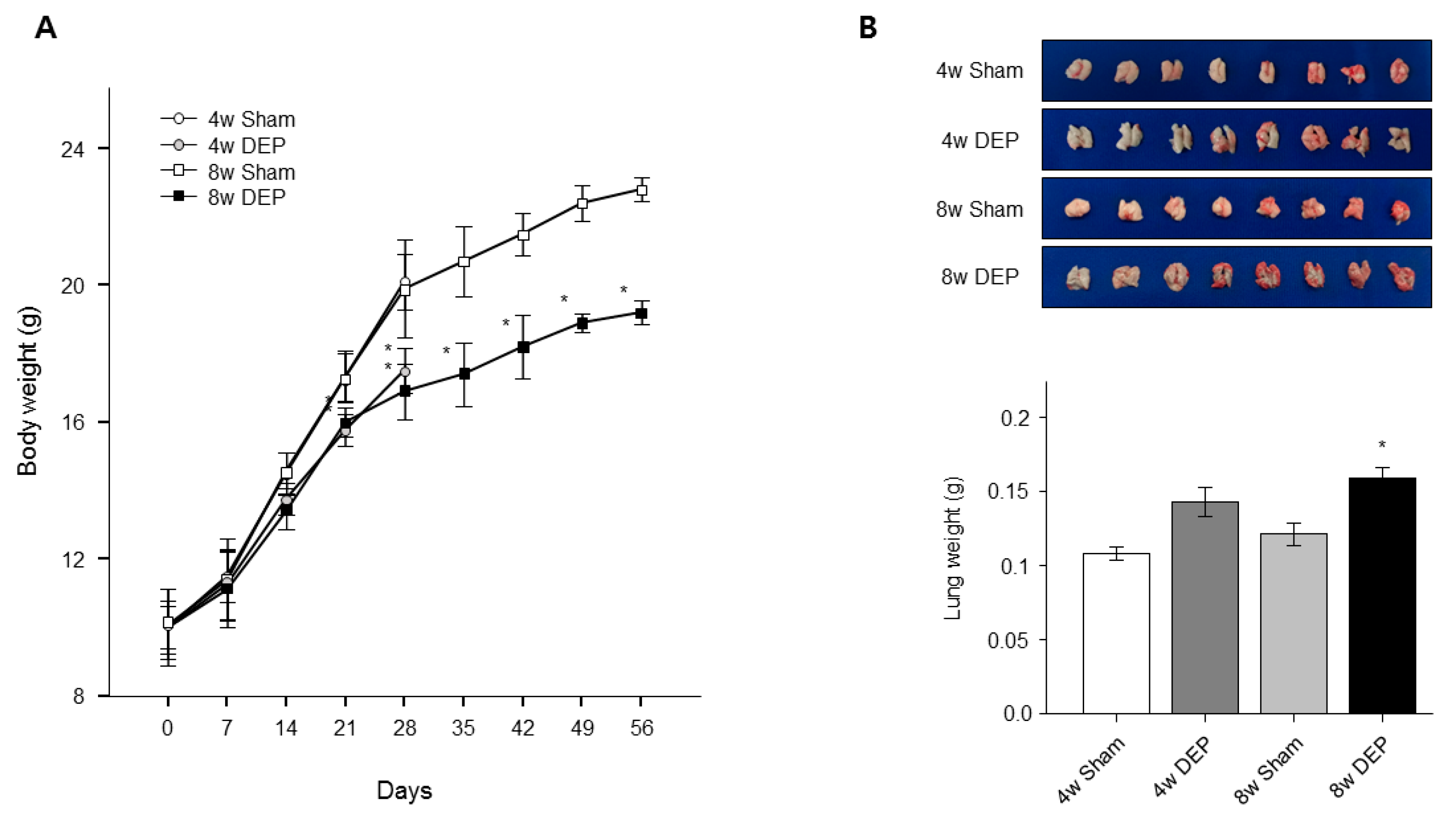
2.11. Design of In Vivo Experiment
2.12. Determination of Airway Responsiveness to Methacholine
2.13. Bronchoalveolar Lavage Fluid Morphology Analysis
2.14. Preparation of Lung Tissues for Histology
2.15. Western Blotting
2.16. Immunohistochemistry
2.17. Masson’s Trichrome Statin
2.18. Statistical Analysis
3. Results
3.1. NHBE Cell Viability
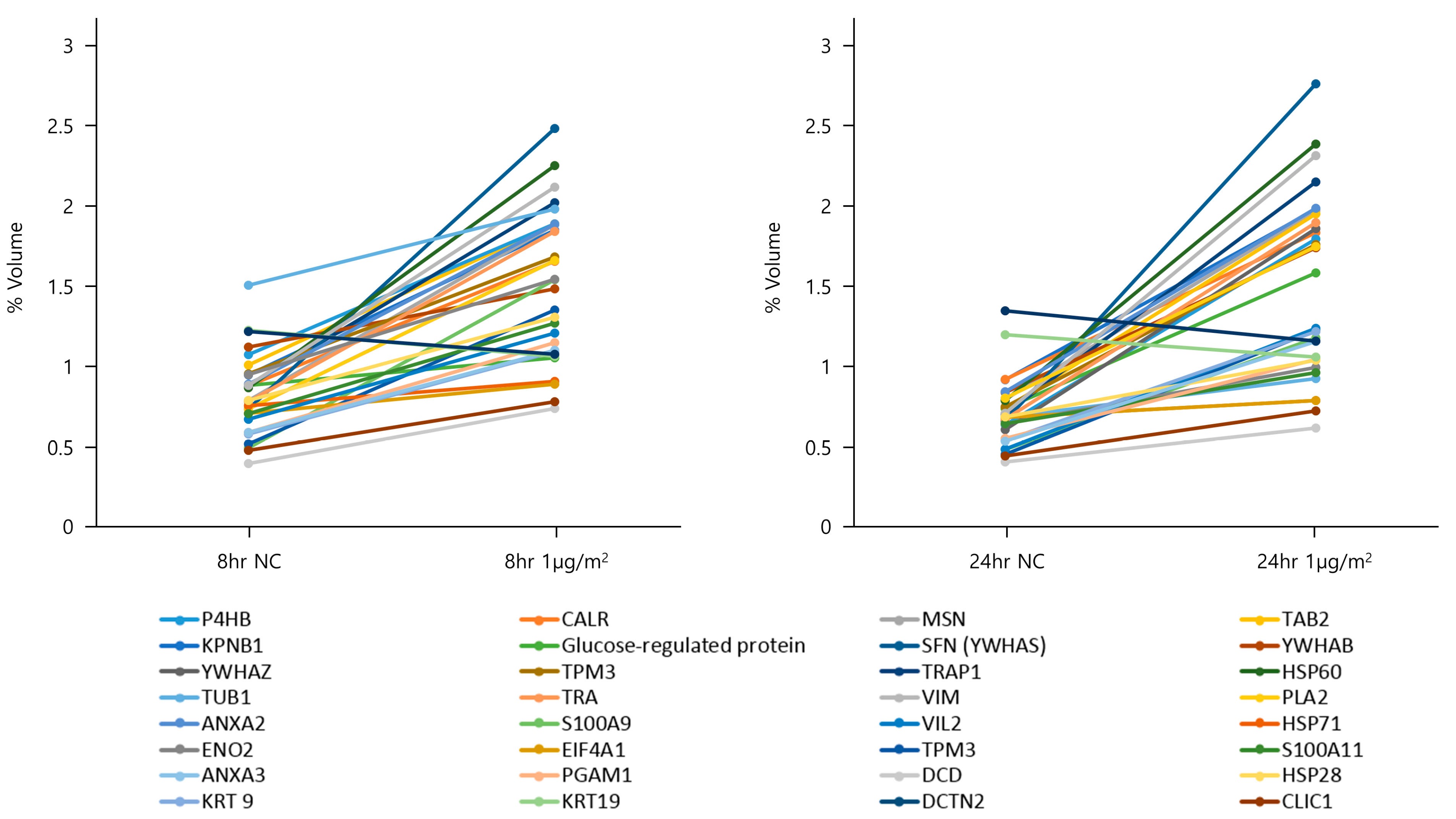
3.2. Two-Dimensional PAGE and LC-MS/MS in NHBE Cells
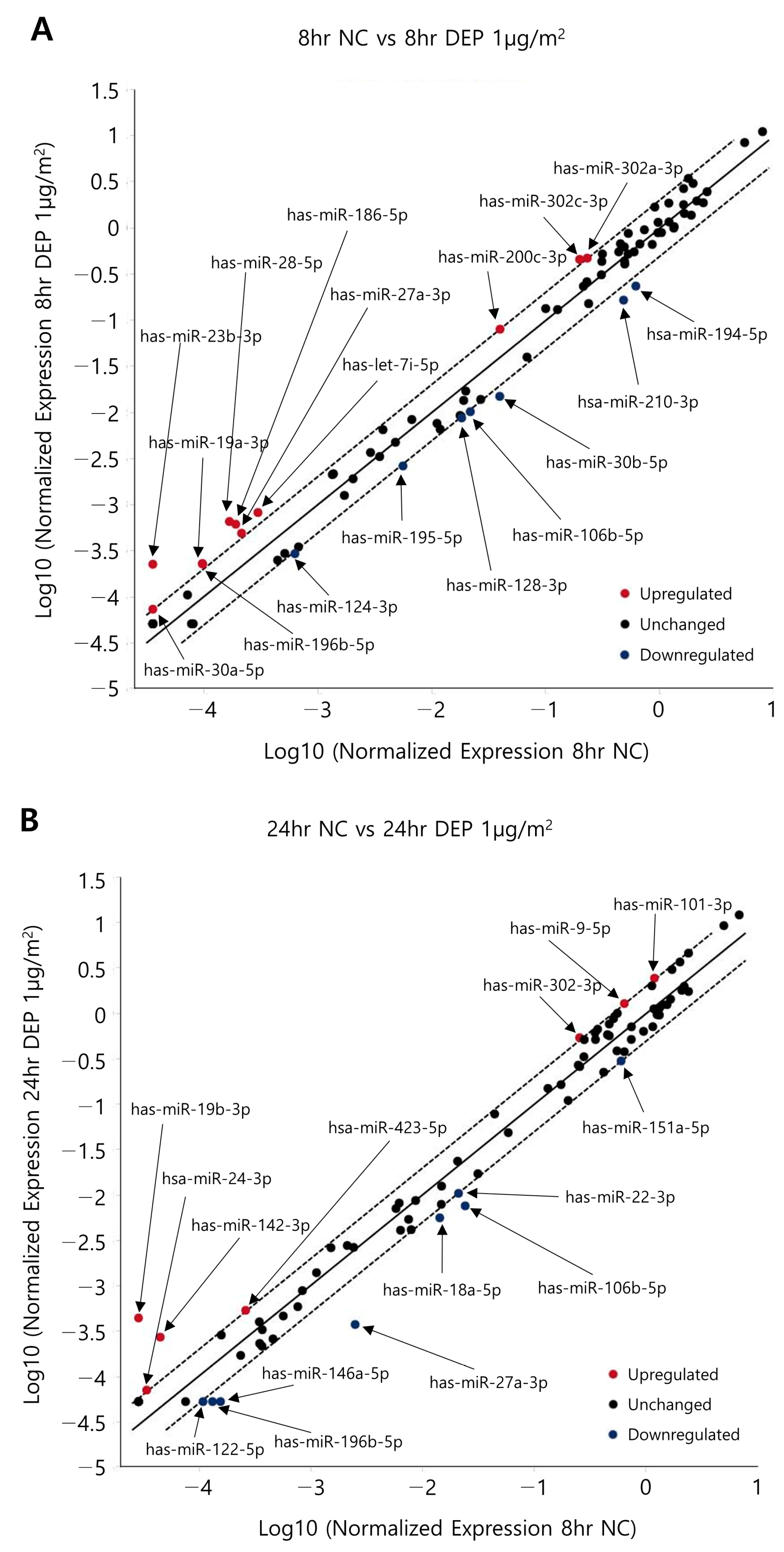
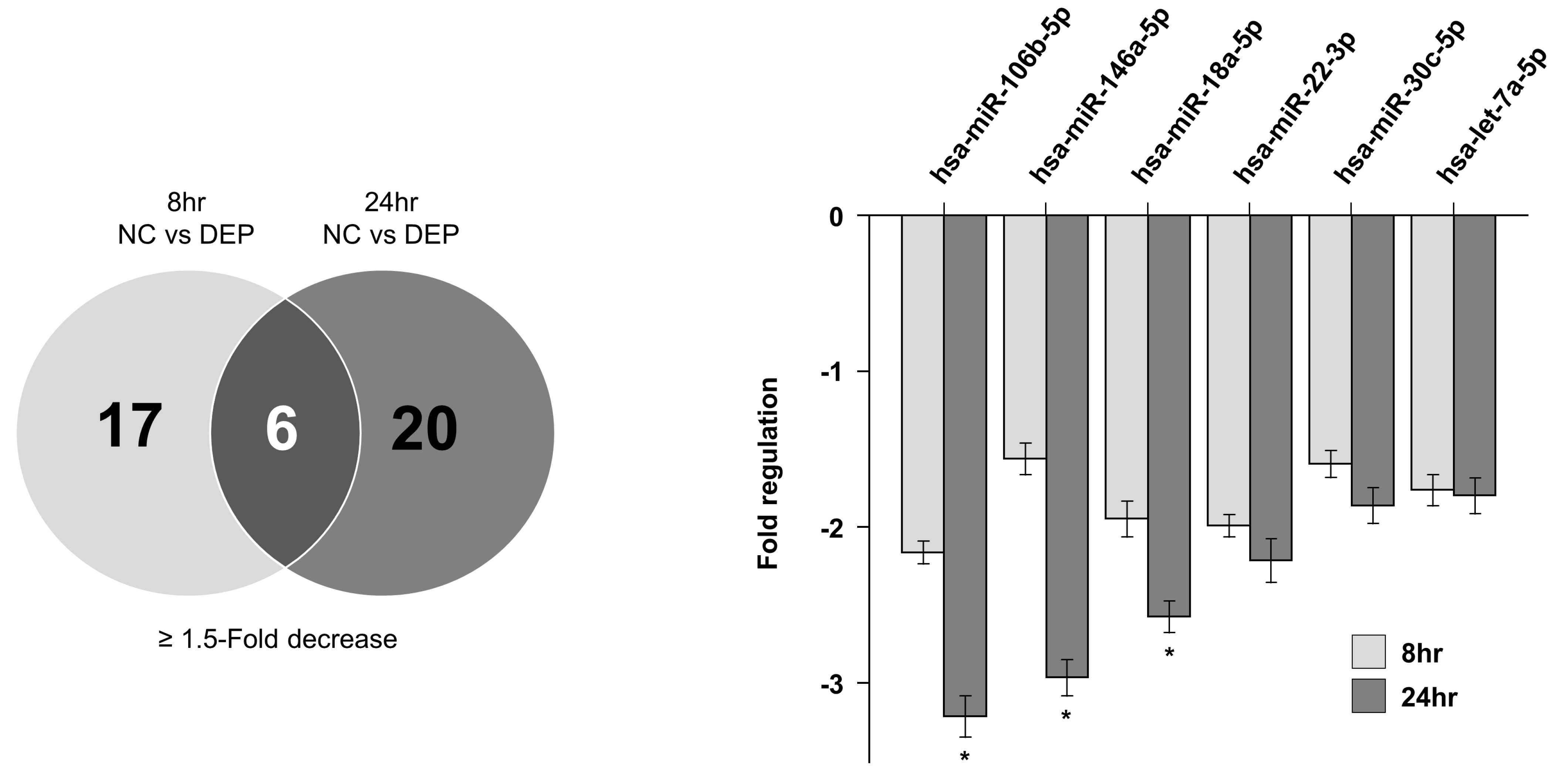
3.3. miRNA Expression Levels in NHBE Cells
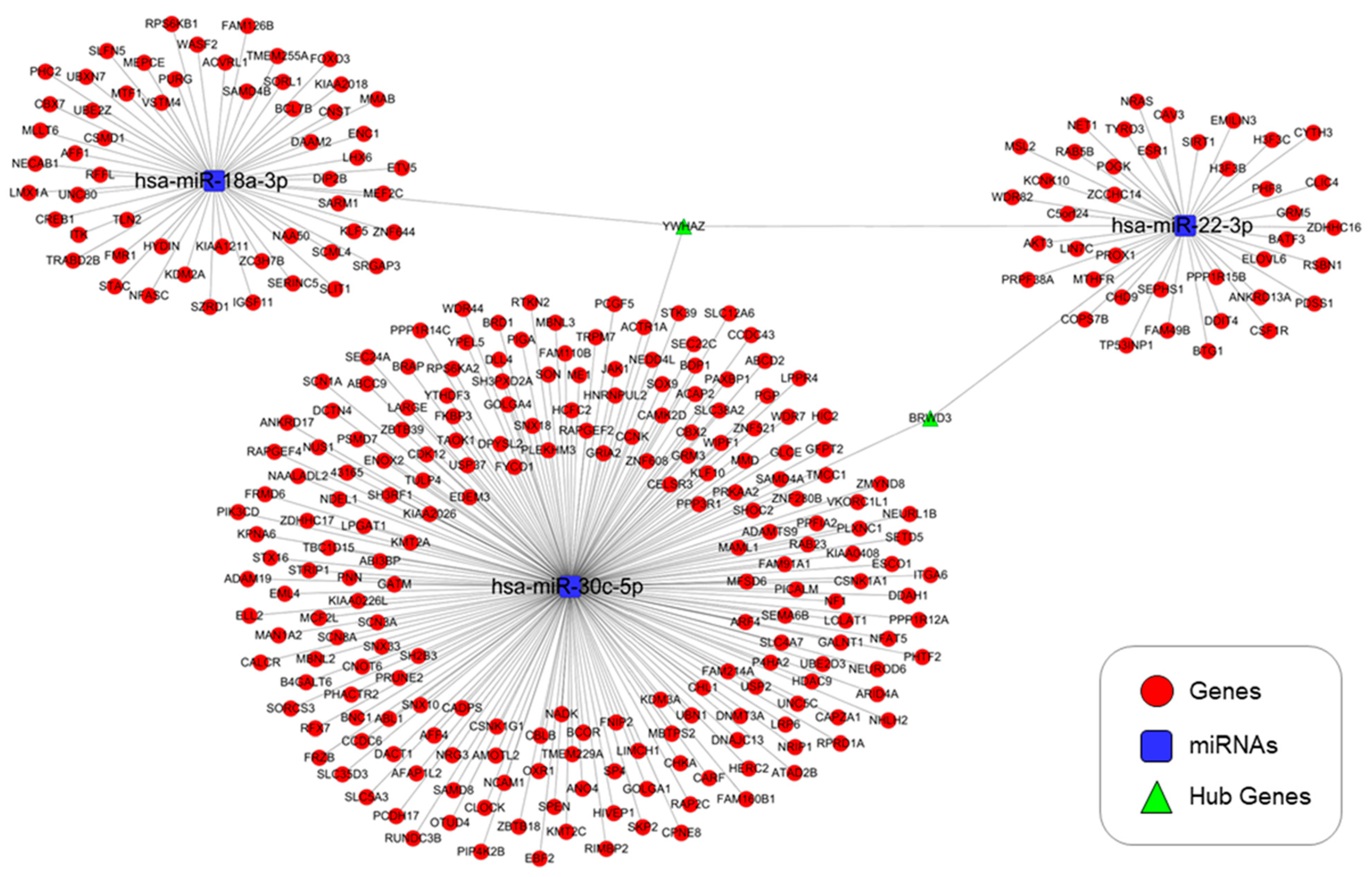
3.4. Predicted Targets of miRNAs of Interest
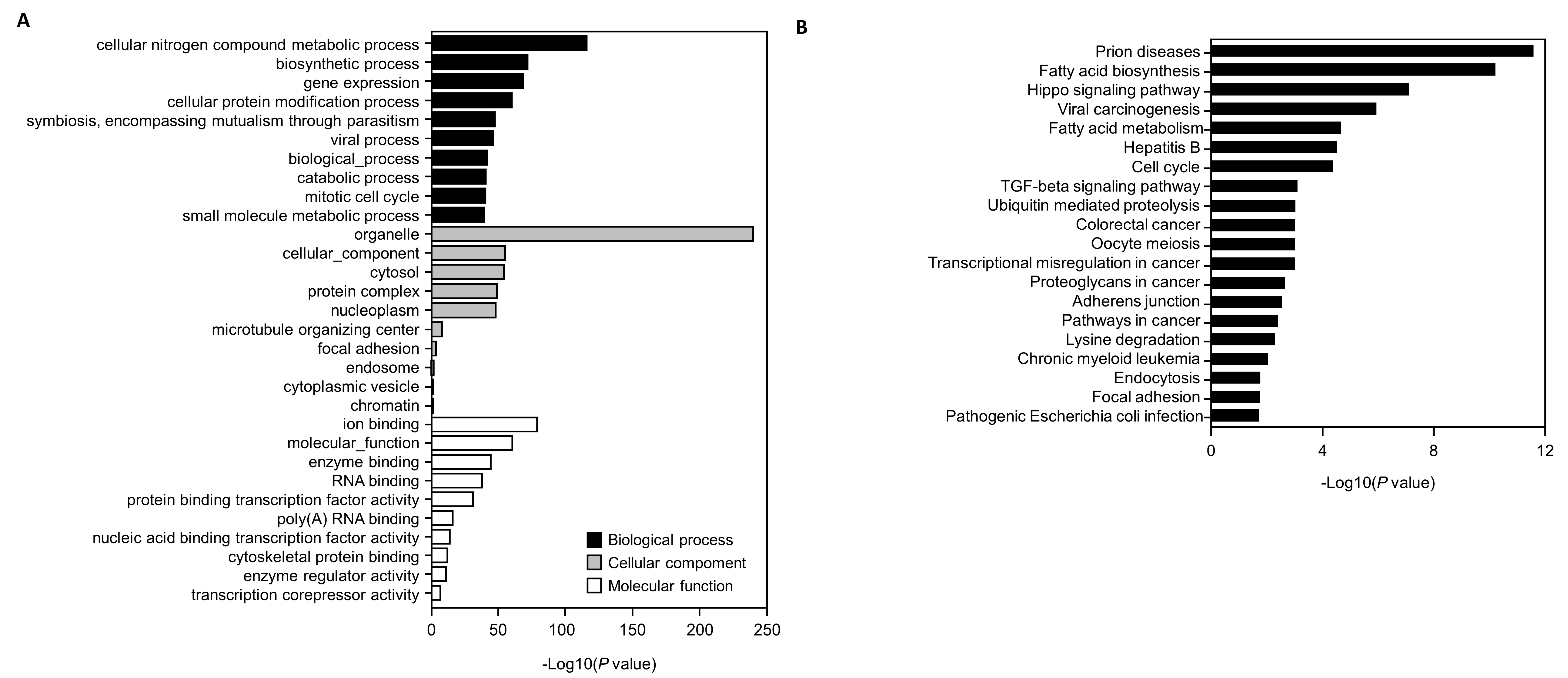
3.5. Genes Targeted by the Selected miRNAs and GO and KEGG Pathway Analyses
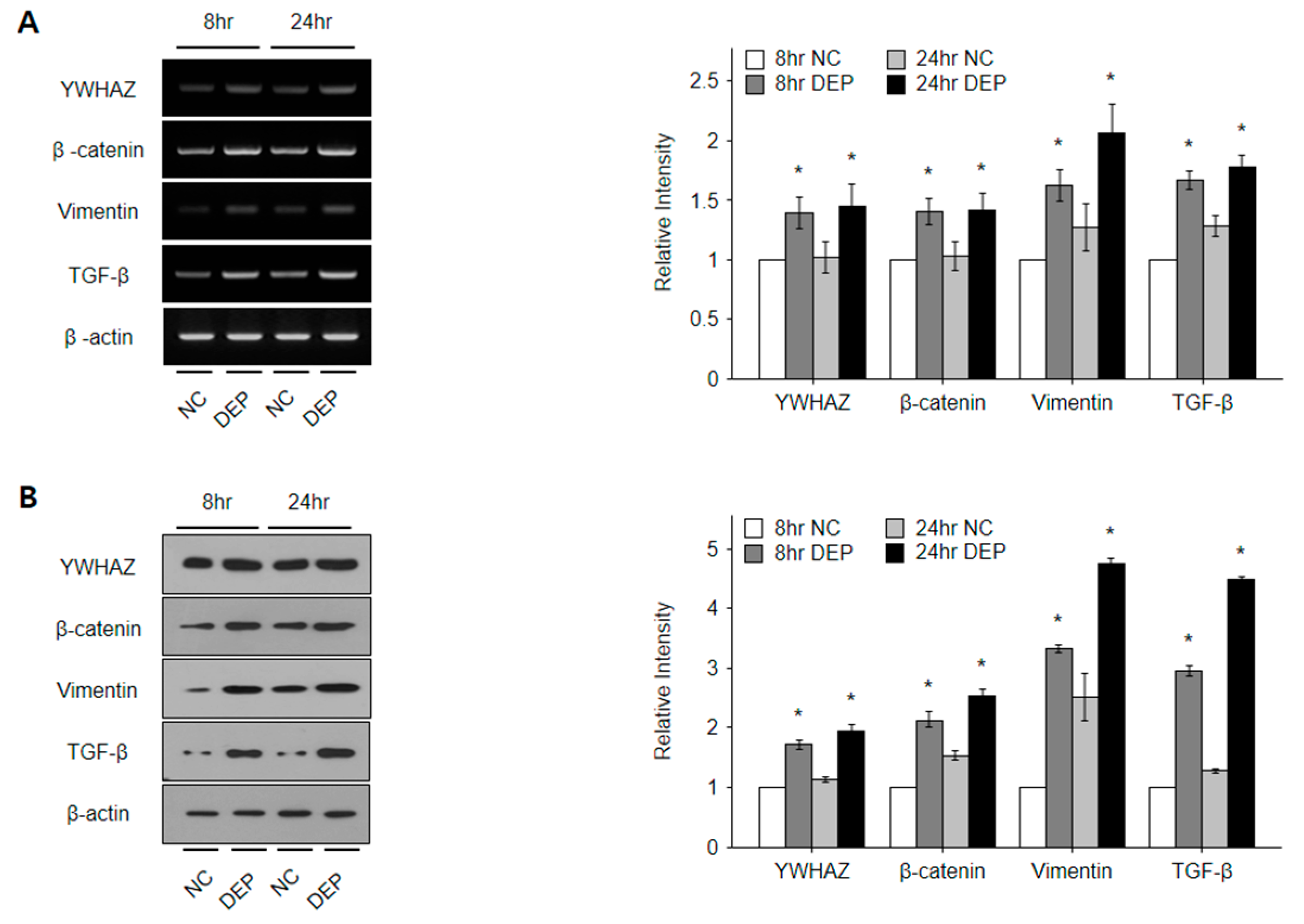
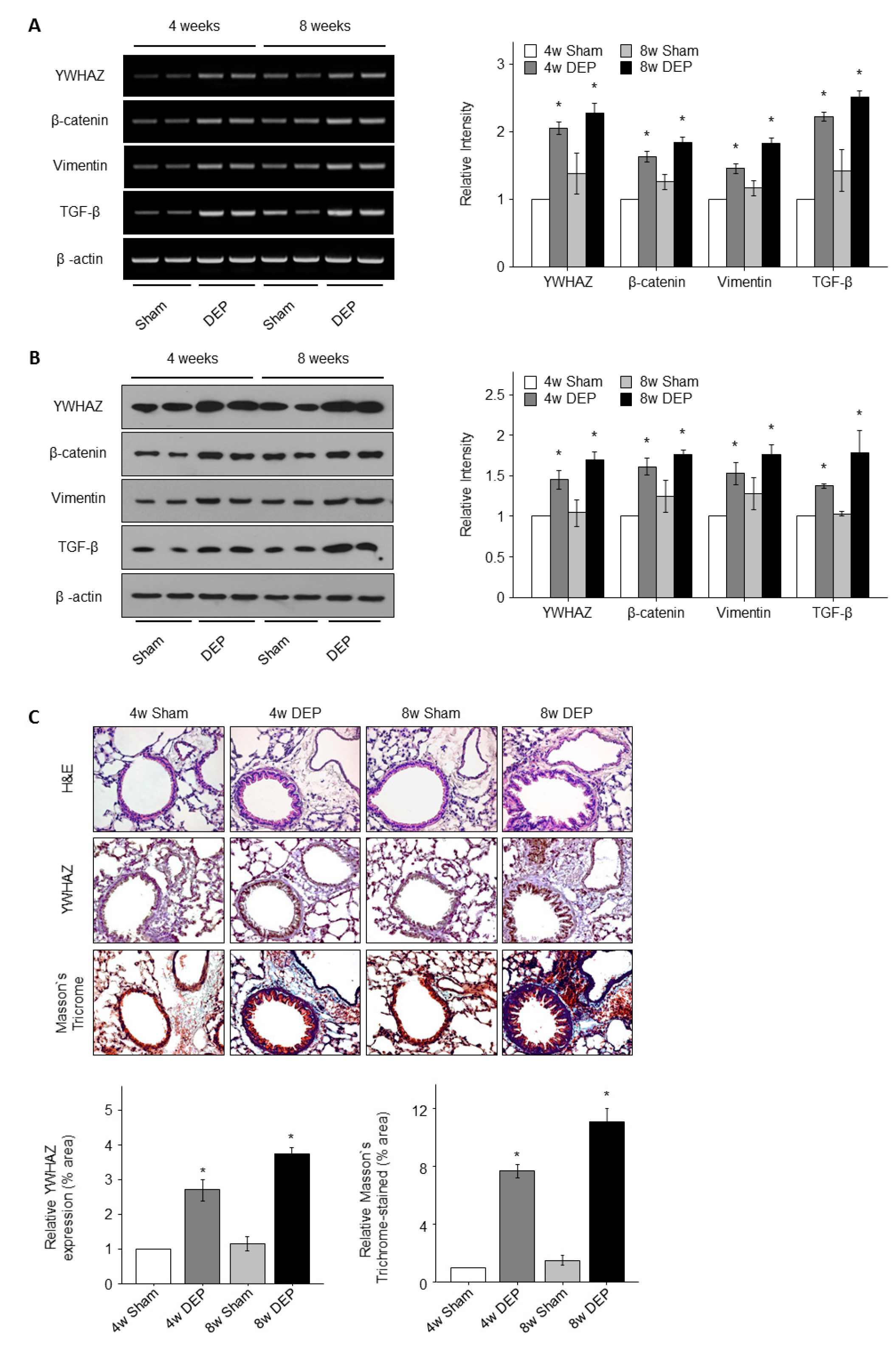
3.6. DEPs Triggered YWHAZ, β-Catenin, Vimentin, and TGF-β mRNA and Protein Expression in NHBE Cells
3.7. Expression of YWHAZ, β-Catenin, Vimentin, and TGF-β in DEP-Exposed Mice
3.8. Increased Lung Fibrosis Following DEP Inhalation
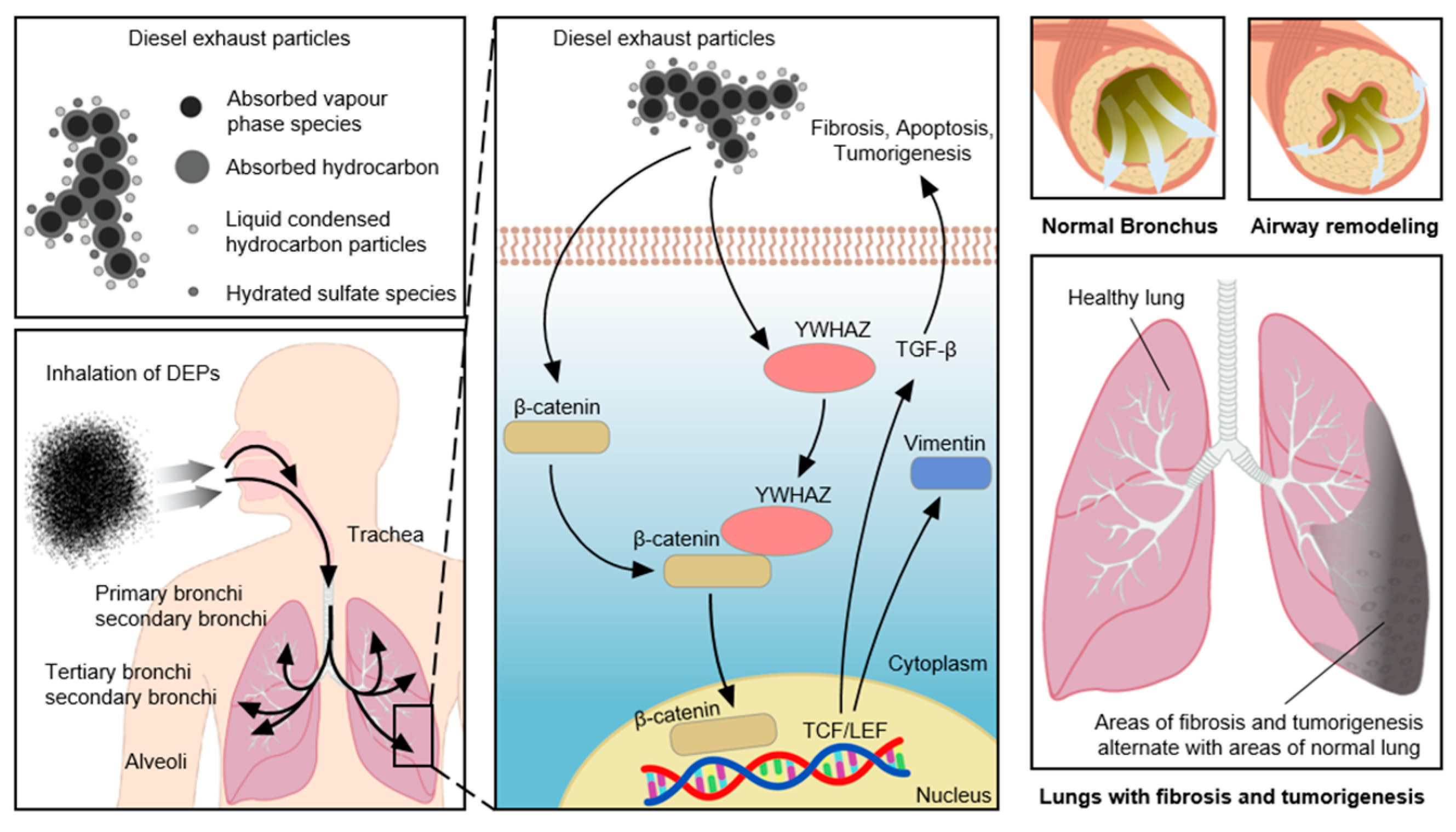
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, B.; Kan, H. Air pollution and population health: A global challenge. Environ. Health Prev. 2008, 13, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Kelly, F.J.; Fussell, J.C. Air pollution and airway disease. Clin. Exp. Allergy 2011, 41, 1059–1071. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.M.; Grigg, J. Diesel, children and respiratory disease. BMJ Paediatr. Open 2018, 2, e000210. [Google Scholar] [CrossRef]
- Ji, H.; Biagini Myers, J.M.; Brandt, E.B.; Brokamp, C.; Ryan, P.H.; Khurana Hershey, G.K. Air pollution, epigenetics, and asthma. Allergy Asthma Clin. Immunol. 2016, 12, 51. [Google Scholar] [CrossRef] [PubMed]
- Phalen, R.F. The Particulate Air Pollution Controversy. Nonlinearity Biol. Toxicol. Med. 2004, 2, 259–292. [Google Scholar] [CrossRef] [PubMed]
- Hao, M.; Comier, S.; Wang, M.; Lee, J.J.; Nel, A. Diesel exhaust particles exert acute effects on airway inflammation and function in murine allergen provocation models. J. Allergy Clin. Immunol. 2003, 112, 905–914. [Google Scholar] [CrossRef]
- Kim, B.-G.; Lee, P.-H.; Lee, S.-H.; Kim, Y.-E.; Shin, M.-Y.; Kang, Y.; Bae, S.-H.; Kim, M.-J.; Rhim, T.; Park, C.-S.; et al. Long-Term Effects of Diesel Exhaust Particles on Airway Inflammation and Remodeling in a Mouse Model. Allergy Asthma Immunol. Res. 2016, 8, 246–256. [Google Scholar] [CrossRef]
- Chin, J.Y.; Batterman, S.A.; Northrop, W.F.; Bohac, S.V.; Assanis, D.N. Gaseous and Particulate Emissions from Diesel Engines at Idle and under Load: Comparison of Biodiesel Blend and Ultralow Sulfur Diesel Fuels. Energy Fuels 2012, 26, 6737–6748. [Google Scholar] [CrossRef]
- Takahashi, G.; Tanaka, H.; Wakahara, K.; Nasu, R.; Hashimoto, M.; Miyoshi, K.; Takano, H.; Yamashita, H.; Inagaki, N.; Nagai, H. Effect of diesel exhaust particles on house dust mite–induced airway eosinophilic inflammation and remodeling in mice. J. Pharmacol. Sci. 2010, 112, 192–202. [Google Scholar] [CrossRef]
- Yoda, Y.; Takagi, H.; Wakamatsu, J.; Ito, T.; Nakatsubo, R.; Horie, Y.; Hiraki, T.; Shima, M. Acute effects of air pollutants on pulmonary function among students: A panel study in an isolated island. Environ. Health Prev. Med. 2017, 22, 33. [Google Scholar] [CrossRef]
- Andersen, Z.J.; Bønnelykke, K.; Hvidberg, M.; Jensen, S.S.; Ketzel, M.; Loft, S.; Sørensen, M.; Tjønneland, A.; Overvad, K.; Raaschou-Nielsen, O. Chronic obstructive pulmonary disease and long- term exposure to traffic-related air pollution: A cohort study. Am. J. Respir. Crit. Care Med. 2011, 183, 455–461. [Google Scholar] [CrossRef]
- Yin, Y.; Mu, C.; Wang, J.; Wang, Y.; Hu, W.; Zhu, W.; Yu, X.; Hao, W.; Zheng, Y.; Li, Q.; et al. CXCL17 Attenuates Diesel Exhaust Emissions Exposure-Induced Lung Damage by Regulating Macrophage Function. Toxics 2023, 11, 646. [Google Scholar] [CrossRef] [PubMed]
- Harlan, R.; Zhang, H. Targeted proteomics: A bridge between discovery and validation. Expert Rev. Proteom. 2014, 11, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Wouters, B.G. Proteomics: Methodologies and applications in oncology. Semin. Radiat. Oncol. 2008, 18, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Hammond, S.M. An overview of microRNAs. Adv. Drug Deliv. Rev. 2015, 87, 3–14. [Google Scholar] [CrossRef]
- Ladomery, M.R.; Maddocks, D.G.; Wilson, I.D. MicroRNAs: Their discovery, biogenesis, function and potential use as biomarkers in non-invasive prenatal diagnostics. Int. J. Mol. Epidemiol. Genet. 2011, 2, 253–260. [Google Scholar]
- Macfarlane, L.A.; Murphy, P.R. MicroRNA: Biogenesis, Function and Role in Cancer. Curr. Genom. 2010, 11, 537–561. [Google Scholar] [CrossRef]
- Jørgensen, C.; Linding, R. Simplistic pathways or complex networks? Curr. Opin. Genet. Dev. 2010, 20, 15–22. [Google Scholar] [CrossRef]
- Martinez, N.J.; Ow, M.C.; Barrasa, M.I.; Hammell, M.; Sequerra, R.; Doucette-Stamm, L.; Roth, F.P.; Ambros, V.R.; Walhout, A.J.M. A C. elegans genome-scale microRNA network contains composite feedback motifs with high flux capacity. Genes Dev. 2008, 22, 2535–2549. [Google Scholar] [CrossRef]
- Pallez, D.; Gardès, J.; Pasquier, C. Prediction of miRNA-disease Associations using an Evolutionary Tuned Latent Semantic Analysis. Sci. Rep. 2017, 7, 10548. [Google Scholar] [CrossRef]
- Lagos-Quintana, M.; Rauhut, R.; Yalcin, A.; Meyer, J.; Lendeckel, W.; Tuschl, T. Identification of tissue-specific microRNAs from mouse. Curr. Biol. 2002, 12, 735–739. [Google Scholar] [CrossRef] [PubMed]
- Jardim, M.J. microRNAs: Implications for air pollution research. Mutat. Res. 2011, 717, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Fossati, S.; Baccarelli, A.; Zanobetti, A.; Hoxha, M.; Vokonas, P.S.; Wright, R.O.; Schwartz, J. Ambient particulate air pollution and microRNAs in elderly men. Epidemiology 2014, 25, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Krauskopf, J.; de Kok, T.M.; Hebels, D.H.; Bergdahl, I.A.; Johansson, A.; Spaeth, F.; Kiviranta, H.; Rantakokko, P.; Kyrtopoulos, S.A.; Kleinjans, J.C. MicroRNA profile for health risk assessment: Environmental exposure to persistent organic pollutants strongly affects the human blood microRNA machinery. Sci. Rep. 2017, 7, 9262. [Google Scholar] [CrossRef]
- Kim, J.; Natarajan, S.; Vaickus, L.J.; Bouchard, J.C.; Beal, D.; Cruikshank, W.W.; Remick, D.G. Diesel exhaust particulates exacerbate asthma-like inflammation by increasing CXC chemokines. Am. J. Pathol. 2011, 179, 2730–2739. [Google Scholar] [CrossRef]
- Lee, P.H.; Kim, B.G.; Lee, S.H.; Leikauf, G.D.; Jang, A.S. Proteomic identification of moesin upon exposure to acrolein. Proteome Sci. 2018, 16, 2. [Google Scholar] [CrossRef]
- Pan, S.; Rush, J.; Peskind, E.R.; Galasko, D.; Chung, K.; Quinn, J.; Jankovic, J.; Leverenz, J.B.; Zabetian, C.; Pan, C.; et al. Application of targeted quantitative proteomics analysis in human cerebrospinal fluid using a liquid chromatography matrix-assisted laser desorption/ionization time-of-flight tandem mass spectrometer (LC MALDI TOF/TOF) platform. J. Proteome Res. 2008, 7, 720–730. [Google Scholar] [CrossRef]
- Jang, K.H.; Yoon, H.N.; Lee, J.; Yi, H.; Park, S.Y.; Lee, S.Y.; Lim, Y.; Lee, H.J.; Cho, J.W.; Paik, Y.K.; et al. Liver disease–associated keratin 8 and 18 mutations modulate keratin acetylation and methylation. FASEB J. 2019, 33, 9030–9043. [Google Scholar] [CrossRef]
- Liu, G.; Ding, M.; Chen, J.; Huang, J.; Wang, H.; Jing, Q.; Shen, B. Computational analysis of microRNA function in heart development. Acta Biochim. Biophys. Sin. 2010, 42, 662–670. [Google Scholar] [CrossRef][Green Version]
- Smoot, M.E.; Ono, K.; Ruscheinski, J.; Wang, P.L.; Ideker, T. Cytoscape 2.8: New features for data integration and network visualization. Bioinformatics 2011, 27, 431–432. [Google Scholar] [CrossRef]
- Dennis, G., Jr.; Sherman, B.T.; Hosack, D.A.; Yang, J.; Gao, W.; Lane, H.C.; Lempicki, R.A. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol. 2003, 4, R60. [Google Scholar] [CrossRef]
- Kim, B.G.; Lee, P.H.; Lee, S.H.; Park, M.K.; Jang, A.S. Effect of TiO2 Nanoparticles on Inflammasome-Mediated Airway Inflammation and Responsiveness. Allergy Asthma Immunol. Res. 2017, 9, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.G.; Lee, P.H.; Lee, S.H.; Park, C.S.; Jang, A.S. Impact of ozone on claudins and tight junctions in the lungs. Environ. Toxicol. 2018, 33, 798–806. [Google Scholar] [CrossRef]
- Wichmann, H.E. Diesel exhaust particles. Inhal. Toxicol. 2007, 1, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Nazarenko, Y.; Zhang, L.; Calderon, L.; Lee, K.B.; Garfunkel, E.; Schwander, S.; Tetley, T.D.; Chung, K.F.; Porter, A.E.; et al. Impacts of a nanosized ceria additive on diesel engine emissions of particulate and gaseous pollutants. Environ. Sci. Technol. 2013, 47, 13077–13085. [Google Scholar] [CrossRef]
- Brito, J.M.; Belotti, L.; Toledo, A.C.; Antonangelo, L.; Silva, F.S.; Alvim, D.S.; Andre, P.A.; Saldiva, P.H.N.; Rivero, D.H.R.F. Acute cardiovascular and inflammatory toxicity induced by inhalation of diesel and biodiesel exhaust particles. Toxicol. Sci. 2010, 116, 67–78. [Google Scholar] [CrossRef]
- Atkinson, R.W.; Carey, I.M.; Kent, A.J.; van Staa, T.P.; Anderson, H.R.; Cook, D.G. Long-term exposure to outdoor air pollution and the incidence of chronic obstructive pulmonary disease in a national English cohort. Occup. Environ. Med. 2015, 72, 42–48. [Google Scholar] [CrossRef]
- Fan, T.; Li, R.; Todd, N.W.; Qiu, Q.; Fang, H.B.; Wang, H.; Shen, J.; Zhao, R.Y.; Caraway, N.P.; Katz, R.L. Up-regulation of 14-3-3zeta in lung cancer and its implication as prognostic and therapeutic target. Cancer Res. 2007, 67, 7901–7906. [Google Scholar] [CrossRef]
- Almeida-Silva, M.; Cardoso, J.; Alemão, C.; Santos, S.; Monteiro, A.; Manteigas, V.; Marques-Ramos, A. Impact of Particles on Pulmonary Endothelial Cells. Toxics 2022, 10, 312. [Google Scholar] [CrossRef]
- Saito, A.; Horie, M.; Micke, P.; Nagase, T. The Role of TGF-β Signaling in Lung Cancer Associated with Idiopathic Pulmonary Fibrosis. Int. J. Mol. Sci. 2018, 15, 3611. [Google Scholar] [CrossRef]
- Chilosi, M.; Poletti, V.; Zamò, A.; Lestani, M.; Montagna, L.; Piccoli, P.; Pedron, S.; Bertaso, M.; Scarpa, A.; Murer, B. Aberrant Wnt/beta-catenin pathway activation in idiopathic pulmonary fibrosis. Am. J. Pathol. 2003, 162, 1495–1502. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Li, F.; Luo, M.; Wei, J.; Liu, X. Distinct Roles of Wnt/β-Catenin Signaling in the Pathogenesis of Chronic Obstructive Pulmonary Disease and Idiopathic Pulmonary Fibrosis. Rev. Mediat. Inflamm. 2017, 2017, 3520581. [Google Scholar] [CrossRef] [PubMed]
- Silva-Garcia, O.; Valdez-Alarcon, J.J.; Baizabal-Aguirre, V.M. The Wnt/beta-catenin signaling pathway controls the inflammatory response in infections caused by pathogenic bacteria. Mediat. Inflamm. 2014, 2014, 310183. [Google Scholar] [CrossRef] [PubMed]
- Satelli, A.; Li, S. Vimentin in cancer and its potential as a molecular target for cancer therapy. Cell. Mol. Life Sci. 2011, 68, 3033–3046. [Google Scholar] [CrossRef] [PubMed]
- Senthebane, D.A.; Rowe, A.; Thomford, N.E.; Shipanga, H.; Munro, D.; Mazeedi, M.A.M.A.; Almazyadi, H.A.M.; Kallmeyer, K.; Dandara, C.; Pepper, M.S.; et al. The Role of Tumor Microenvironment in Chemoresistance: To Survive, Keep Your Enemies Closer. Int. J. Mol. Sci. 2017, 18, 1586. [Google Scholar] [CrossRef]
- Surolia, R.; Li, F.J.; Wang, Z.; Li, H.; Dsouza, K.; Thomas, V.; Mirov, S.; Pérez-Sala, D.; Athar, M.; Thannickal, V.J.; et al. Vimentin intermediate filament assembly regulates fibroblast invasion in fibrogenic lung injury. JCI Insight 2019, 4, e123253. [Google Scholar] [CrossRef]
- Taka, S.; Tzani-Tzanopoulou, P.; Wanstall, H.; Papadopoulos, N.G. MicroRNAs in Asthma and Respiratory Infections: Identifying Common Pathways. Allergy Asthma Immunol. Res. 2020, 12, 4–23. [Google Scholar] [CrossRef]
| Gene | Primer Sequence (5′→3′) | |
|---|---|---|
| Human | ||
| YWHAZ | F: TGATCCCCAATGCTTCACAAG | R: GCCAAGTAACGGTAGTAATCTCC |
| β-catenin | F: GTGCTATCTGTCTGCTCTAGTA | R: CTTCCTGTTTAGTTGCAGCATC |
| Vimentin | F: AGGAAATGGCTCGTCACCTTCGTGAATA | R: GGAGTGTCGGTTGTTAAGAACTAGAGCT |
| TGF-β | F: TCCTGCTTCTCATGGCCA | R: CCTCAGCTGCACTTGTAG |
| β-Actin | F: TGCTGTCCCTGTATGCCTCT | R: CTTTGATGTCACGCACGATTT |
| Mouse | ||
| YWHAZ | F: GAAAAGTTCTTGATCCCCAATGC | R: TGTGACTGGTCCACAATTCCTT |
| β-catenin | F: ATGGAGCCGGACAGAAAAGC | R: CTTGCCACTCAGGGAAGGA |
| Vimentin | F: CCCTCACCTGTGAAGTGGAT | R: TCCAGCAGCTTCCTGTAGGT |
| TGF-β | F: CACCGGAGAGCCCTGGATA | R: TGTACAGCTGCCGCACACA |
| β-Actin | F: CGGTTCCGATGCCCTGAGGCTCTT | R: CGTCACACTTCATGATGGAATTGA |
| No. | Protein Name | Symbol | Functional Categorization | Accession Number (NCBI) | Amino Acid Sequence | pI/Molecular Mass (Da) | Sequence Coverage (%) | Ratio | |
|---|---|---|---|---|---|---|---|---|---|
| 8 h | 24 h | ||||||||
| 1 | Prolyl 4-hydroxylase subunit beta | P4HB | Multifunctional enzyme | 190384 | K.SNFAEALAAHK.Y | 4.76/57,487 | 28 | +1.94 | +2.14 |
| 2 | Calreticulin | CALR | Calcium-binding protein | 117501 | K.GLQTSQDAR.F | 4.29/48,286 | 2 | +1.87 | +1.98 |
| 3 | Moesin | MSN | Cytoskeleton | 127234 | K.VTAQDVR.K | 6.08/67,894 | 13 | +2.37 | +2.46 |
| 4 | TGF-Beta Activated Kinase 1 (MAP3K7) Binding Protein 2 | TAB2 | TGF-beta signaling | 7677466 | U R.KNQIEIK.L | 8.80/77,027 | 1 | +1.86 | +2.62 |
| 5 | Karyopherin (importin) beta 1 | KPNB1 | Energy-dependent process | 893288 | R.VLANPGNSQVAR.V | 4.68/98,442 | 1 | +1.75 | +2.79 |
| 6 | Glucose-regulated protein | - | Glucose regulation | 6900104 | R.VEIIANDQGNR.I | 5.07/72,404 | 30 | +1.19 | +2.13 |
| 7 | Stratifin (14-3-3 sigma) | SFN (YWHAS) | Regulator of mitotic translation | 398953 | K.SNEEGSEEKGPEVR.E | 4.68/27,873 | 44 | +2.28 | +3.02 |
| 8 | Tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, beta (14-3-3 beta) | YWHAB | Signal transduction | 1345590 | K.LAEQAER.Y | 4.76/28,181 | 15 | +1.32 | +2.06 |
| 9 | Tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein zeta (14-3-3 zeta) | YWHAZ | Signal transduction | 30354619 | K.SVTEQGAELSNEER.N | 6.97/35,546 | 4 | +2.24 | +3.03 |
| 10 | Tropomyosin 3 | TPM3 | Actin-binding protein | 12653955 | R.KIQVLQQQADDAEER.A | 4.75/29,243 | 10 | +1.76 | +2.33 |
| 11 | Tumor necrosis factor type 1 receptor associated protein | TRAP1 | Tumor necrosis | 687237 | R.GVVDSEDIPLNLSR.E | 8.05/80,251 | 1 | +3.33 | +4.14 |
| 12 | 60 kDa heat shock protein | HSP60 | Macromolecular assembly | 129379 | U R.TVIIEQSWGSPK.V | 5.70/61,190 | 21 | +2.57 | +3.00 |
| 13 | Alpha tubulin | TUB1 | Cytoskeleton | 37492 | U K.DVNAAIATIK.T | 5.02/50,822 | 2 | +2.10 | +2.34 |
| 14 | T cell receptor alpha | TRA | T-lymphocyte signaling | 902377536 | K.GITLSVRP.- | 9.45/7206 | 12 | +2.34 | +2.79 |
| 15 | Vimentin | VIM | Cytoskeleton | 340219 | R.SLYASSPGGVYATR.S | 5.03/53,739 | 9 | +2.38 | +3.23 |
| 16 | Phospholipase A2 | PLA2 | Phosphorylation | 189953 | K.SVTEQGAELSNEER.N | 4.73/27,902 | 5 | +2.24 | +2.16 |
| 17 | Annexin A2 | ANXA2 | Phospholipid-binding protein | 113950 | R.DALNIETAIK.T | 7.57/38,808 | 25 | +1.31 | +1.35 |
| 18 | S100 Calcium Binding Protein A9 | S100A9 | Calcium-binding protein | 115444 | K.LGHPDTLNQGEFKELVR.K | 5.71/13,291 | 21 | +3.08 | +2.42 |
| 19 | Cytovillin 2 | VIL2 | Cytoskeleton | 340217 | K.IALLEEAR.R | 5.80/68,235 | 5 | +2.58 | +2.67 |
| 20 | Heat shock cognate 71 kDa protein | HSP71 | Macromolecular assembly | 32467 | R.TTPSYVAFTDTER.L | 5.37/71,086 | 2 | +1.20 | +1.15 |
| 21 | Enolase 2 | ENO2 | Glycolytic enzyme | 119339 | R.IGAEVYHNLK.N | 5.78/49,852 | 46 | +1.62 | +1.54 |
| 22 | Eukaryotic initiation factor 4A-I | EIF4A1 | Eukaryotic initiation | 219403 | K.GYDVIAQAQSGTGK.T | 5.32/46,357 | 8 | +1.25 | +1.15 |
| 23 | Tropomyosin 3 | TPM3 | Actin-binding protein | 12653955 | R.KLVIIEGDLER.T | 4.75/29,243 | 7 | +1.78 | +2.52 |
| 24 | Calgizzarin (S100 calcium binding protein A11) | S100A11 | Calcium-binding protein | 560791 | K.NQKDPGVLDR.M | 6.56/11,849 | 9 | +1.79 | +1.49 |
| 25 | Annexin A3 | ANXA3 | Phospholipid-binding protein | 113954 | R.DYPDFSPSVDAEAIQK.A | 5.63/36,527 | 22 | +1.87 | +2.23 |
| 26 | Phosphoglycerate mutase 1 | PGAM1 | Bisphosphoglycerate mutase activity | 130348 | R.HGESAWNLENR.F | 6.67/28,900 | 15 | +1.93 | +1.88 |
| 27 | Dermcidin | DCD | Proteolysis induction | 20141302 | K.ENAGEDPGLAR.Q | 6.08/11,391 | 10 | +1.84 | +1.51 |
| 28 | 28 kDa heat shock protein | HSP28 | Macromolecular assembly | 433598 | U R.QLSSGVSEIR.H | 5.98/22,826 | 18 | +1.65 | +1.51 |
| 29 | Keratin 9 | KRT 9 | Cytoskeleton | 435476 | R.SGGGGGGGLGSGGSIR.S | 5.19/62,320 | 2 | +1.85 | +2.14 |
| 30 | Keratin 19 | KRT19 | Cytoskeleton | 6729681 | R.QSSATSSFGGLGGGSVR.F | 9.30/12,193 | 37 | −1.15 | −1.13 |
| 31 | Dynactin 2 | DCTN2 | Cell division | 12653855 | K.YADLPGIAR.N | 5.10/44,320 | 11 | −1.13 | −1.16 |
| 32 | Chloride intracellular channel protein 1 | CLIC1 | Chloride ion channels | 12643390 | K.GVTFNVTTVDTK.R | 5.09/27,254 | 23 | −1.62 | −1.63 |
| microRNA | 8 h | 24 h | ||
|---|---|---|---|---|
| Fold Change | p Value | Fold Change | p Value | |
| hsa-miR-142-5p | 1.54 | 0.000001 | 2.13 | 0.000002 |
| hsa-miR-9-5p | 1.31 | 0.000012 | 2.06 | 0.000003 |
| hsa-miR-150-5p | 1.69 | 0.000019 | 1.48 | 0.000070 |
| hsa-miR-27b-3p | 1.41 | 0.000026 | 1.85 | 0.000047 |
| hsa-miR-101-3p | 1.91 | 0.000006 | 2.11 | 0.000026 |
| hsa-let-7d-5p | 1.98 | 0.000003 | 1.96 | 0.000017 |
| hsa-miR-103a-3p | 1.58 | 0.000002 | 1.82 | 0.000002 |
| hsa-miR-16-5p | 1.29 | 0.000019 | 1.66 | 0.000010 |
| hsa-miR-26a-5p | −2.46 | 0.000002 | −2.1 | 0.000001 |
| hsa-miR-32-5p | −1.31 | 0.000001 | −1.04 | 0.001032 |
| hsa-miR-26b-5p | 1.57 | 0.000001 | 1.87 | 0.000003 |
| hsa-let-7g-5p | 1.19 | 0.000005 | −1.05 | 0.001569 |
| hsa-miR-30c-5p | −1.98 | 0.000001 | −1.89 | 0.000000 |
| hsa-miR-96-5p | 1.09 | 0.001910 | 1.09 | 0.006373 |
| hsa-miR-185-5p | 1.36 | 0.000143 | 1.13 | 0.012914 |
| hsa-miR-142-3p | −1.91 | 0.000880 | 6.5 | 0.000334 |
| hsa-miR-24-3p | 1.44 | 0.015895 | 2.15 | 0.008468 |
| hsa-miR-155-5p | −1.07 | 0.164969 | 1.1 | 0.140376 |
| hsa-miR-146a-5p | −1.76 | 0.000003 | −2.01 | 0.000001 |
| hsa-miR-425-5p | 1.68 | 0.000012 | 2.03 | 0.000009 |
| hsa-miR-181b-5p | −1.16 | 0.000013 | −1.15 | 0.000344 |
| hsa-miR-302b-3p | −1.31 | 0.000006 | −1.31 | 0.000009 |
| hsa-miR-30b-5p | 1.27 | 0.000023 | 1.21 | 0.000108 |
| hsa-miR-21-5p | 1.51 | 0.000014 | 1.85 | 0.000047 |
| hsa-miR-30e-5p | 1 | 0.605624 | 1.2 | 0.000037 |
| hsa-miR-200c-3p | 2.07 | 0.000086 | 1.8 | 0.000183 |
| hsa-miR-15b-5p | 1.14 | 0.000707 | 1.01 | 0.463162 |
| hsa-miR-223-3p | −1.04 | 0.000557 | −1.17 | 0.000000 |
| hsa-miR-194-5p | −2.74 | 0.000000 | −4.58 | 0.000000 |
| hsa-miR-210-3p | −3.06 | 0.000000 | −3.94 | 0.000000 |
| hsa-miR-15a-5p | 1.6 | 0.001296 | 1.32 | 0.013510 |
| hsa-miR-181a-5p | −1.08 | 0.000326 | −1.17 | 0.000032 |
| hsa-miR-125b-5p | −1.17 | 0.000001 | −1.65 | 0.000000 |
| hsa-miR-99a-5p | 1.41 | 0.000004 | 1.82 | 0.000000 |
| hsa-miR-28-5p | 4.12 | 0.000502 | −1.23 | 0.100464 |
| hsa-miR-320a | 1.54 | 0.000001 | 1.91 | 0.000000 |
| hsa-miR-125a-5p | 1.68 | 0.000002 | 1.82 | 0.000006 |
| hsa-miR-29b-3p | −1.3 | 0.000027 | −1.11 | 0.000585 |
| hsa-miR-29a-3p | 1.02 | 0.176756 | −1.06 | 0.056638 |
| hsa-miR-141-3p | −1.39 | 0.000006 | −1.17 | 0.000139 |
| hsa-miR-19a-3p | 2.44 | 0.000933 | 1.16 | 0.235469 |
| hsa-miR-18a-5p | −2.43 | 0.000007 | −2.83 | 0.000007 |
| hsa-miR-374a-5p | −1.93 | 0.000017 | −2.83 | 0.000010 |
| hsa-miR-423-5p | 1.47 | 0.007195 | 2.12 | 0.006269 |
| hsa-let-7a-5p | −1.78 | 0.000324 | −1.82 | 0.000207 |
| hsa-miR-124-3p | −2.22 | 0.000090 | −1.32 | 0.007744 |
| hsa-miR-92a-3p | −1.35 | 0.000001 | −1.4 | 0.000003 |
| hsa-miR-23a-3p | −1.23 | 0.000000 | −1.73 | 0.000001 |
| hsa-miR-25-3p | 1.1 | 0.000004 | −1.25 | 0.000019 |
| hsa-let-7e-5p | −1 | 0.747350 | −1.45 | 0.000000 |
| hsa-miR-376c-3p | −1.09 | 0.000008 | −1.53 | 0.000001 |
| hsa-miR-126-3p | −2.22 | 0.000007 | −6.87 | 0.000002 |
| hsa-miR-144-3p | −1.09 | 0.000005 | 1.79 | 0.000000 |
| hsa-miR-424-5p | −1.18 | 0.002114 | 1.15 | 0.013081 |
| hsa-miR-30a-5p | 2.72 | 0.002933 | −1.87 | 0.009875 |
| hsa-miR-23b-3p | 8.6 | 0.001428 | −1.13 | 0.516472 |
| hsa-miR-151a-5p | −1.02 | 0.010708 | −2.06 | 0.000000 |
| hsa-miR-195-5p | −2.21 | 0.000022 | −1 | 0.952589 |
| hsa-miR-143-3p | −1.06 | 0.205299 | −1.96 | 0.000574 |
| hsa-miR-30d-5p | −1.06 | 0.001098 | −1.4 | 0.000020 |
| hsa-miR-191-5p | −1.02 | 0.642426 | 1.23 | 0.012369 |
| hsa-let-7i-5p | 2.86 | 0.000598 | 1.05 | 0.595219 |
| hsa-miR-302a-3p | 2.09 | 0.000032 | 1.88 | 0.000062 |
| hsa-miR-222-3p | −1.38 | 0.001276 | 1.77 | 0.000526 |
| hsa-let-7b-5p | −1.84 | 0.000341 | −4.86 | 0.000134 |
| hsa-miR-19b-3p | 1.44 | 0.015895 | 21.69 | 0.001596 |
| hsa-miR-17-5p | 1.44 | 0.015895 | 1.89 | 0.002513 |
| hsa-miR-93-5p | 1.44 | 0.015895 | 1.89 | 0.002513 |
| hsa-miR-186-5p | 3.36 | 0.000553 | 1.85 | 0.001160 |
| hsa-miR-196b-5p | 2.4 | 0.000872 | −3 | 0.000250 |
| hsa-miR-27a-3p | 2.36 | 0.000702 | −7.22 | 0.000955 |
| hsa-miR-22-3p | −2.08 | 0.000008 | −2.03 | 0.000005 |
| hsa-miR-130a-3p | −1.84 | 0.001210 | −3.62 | 0.000328 |
| hsa-let-7c-5p | −3.18 | 0.000116 | −3.15 | 0.000490 |
| hsa-miR-29c-3p | 1.6 | 0.001198 | 1.25 | 0.006436 |
| hsa-miR-140-3p | 1.28 | 0.006225 | −1.59 | 0.002090 |
| hsa-miR-128-3p | −2.17 | 0.000007 | −2.84 | 0.000001 |
| hsa-let-7f-5p | 1.44 | 0.015895 | 1.89 | 0.002513 |
| hsa-miR-122-5p | −1.49 | 0.000107 | −1.17 | 0.002218 |
| hsa-miR-20a-5p | 1.26 | 0.003875 | −1.42 | 0.000638 |
| hsa-miR-106b-5p | −2.05 | 0.000160 | −1.74 | 0.000260 |
| hsa-miR-7-5p | 1.78 | 0.000549 | 1.33 | 0.010385 |
| hsa-miR-100-5p | −1.13 | 0.000001 | 1.3 | 0.000009 |
| hsa-miR-302c-3p | 2.38 | 0.000029 | 2.19 | 0.000075 |
| Sample | miRNAs Detected | ≥1.5-Fold Increase (n) | ≥1.5-Fold Decrease (n) | ≥2-Fold Increase (n) | ≥2-Fold Decrease (n) |
|---|---|---|---|---|---|
| 8 h NC vs. 8 h DEPs | 84 | 22 | 17 | 11 | 7 |
| 24 h NC vs. 24 h DEPs | 26 | 20 | 7 | 8 | |
| Overlapping miRNAs | 6 | 6 | 1 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, B.-G.; Lee, P.-H.; Hong, J.; Jang, A.-S. Analyzing the Impact of Diesel Exhaust Particles on Lung Fibrosis Using Dual PCR Array and Proteomics: YWHAZ Signaling. Toxics 2023, 11, 859. https://doi.org/10.3390/toxics11100859
Kim B-G, Lee P-H, Hong J, Jang A-S. Analyzing the Impact of Diesel Exhaust Particles on Lung Fibrosis Using Dual PCR Array and Proteomics: YWHAZ Signaling. Toxics. 2023; 11(10):859. https://doi.org/10.3390/toxics11100859
Chicago/Turabian StyleKim, Byeong-Gon, Pureun-Haneul Lee, Jisu Hong, and An-Soo Jang. 2023. "Analyzing the Impact of Diesel Exhaust Particles on Lung Fibrosis Using Dual PCR Array and Proteomics: YWHAZ Signaling" Toxics 11, no. 10: 859. https://doi.org/10.3390/toxics11100859
APA StyleKim, B.-G., Lee, P.-H., Hong, J., & Jang, A.-S. (2023). Analyzing the Impact of Diesel Exhaust Particles on Lung Fibrosis Using Dual PCR Array and Proteomics: YWHAZ Signaling. Toxics, 11(10), 859. https://doi.org/10.3390/toxics11100859






