Silver Nanoparticles Decorated with Curcumin Enhance the Efficacy of Metformin in Diabetic Rats via Suppression of Hepatotoxicity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Methods
2.1.1. Animal Studies
- Group I:
- The control group without any treatment
- Group II:
- The diabetic group (a single dose of streptozocin at 50 mg/kg) without any treatment
- Group III:
- Normal rats treated with silver nanoparticles decorated with curcumin (AgNPs-Cur) at 5 mg/kg.
- Group IV:
- Diabetic rats treated with eight doses of metformin (200 mg/kg) [29].
- Group V:
- Diabetic rats treated with eight doses of metformin with AgNPs-Cur (5 mg/kg)
- Group VI:
- Diabetic rats treated with eight doses of metformin with AgNPs-Cur (10 mg/kg)
2.1.2. Preparation of Silver Nanoparticles Modified with Curcumin
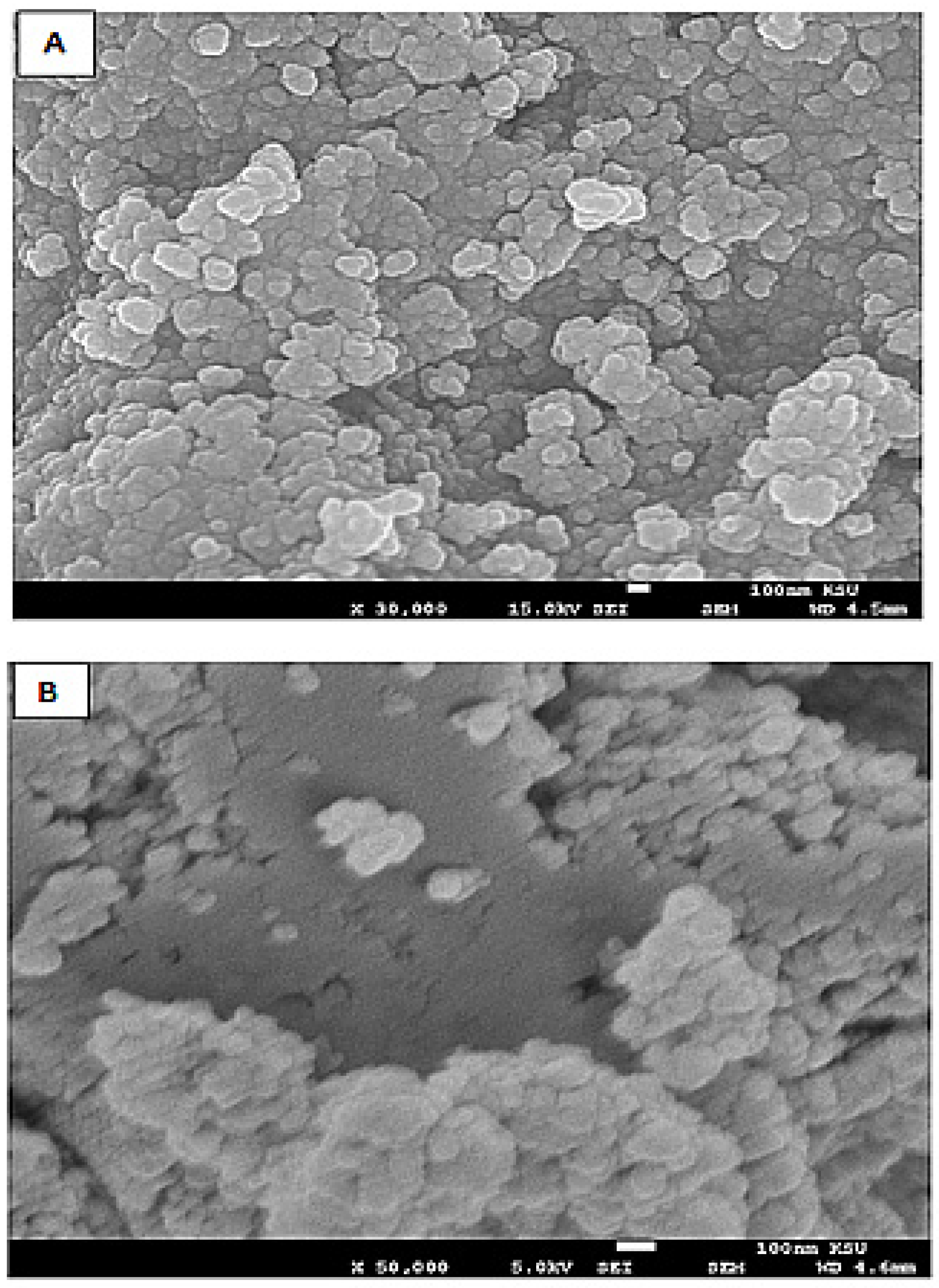
Characteristics of the Silver Nanoparticles-Embedded Curcumin
2.1.3. Preparation of Biological Samples
2.1.4. Glucose Test for the Evaluation of Diabetes
2.1.5. Measurement of Glucose Level
2.1.6. Assessment of Lipid Profiling
2.1.7. Measurement of Redox Parameters
2.1.8. Analysis of Liver Function Markers
2.1.9. Histopathological Analysis
2.1.10. Comet Assay
2.2. Statistical Analysis
3. Results
3.1. Effect on Fasting Glucose Level
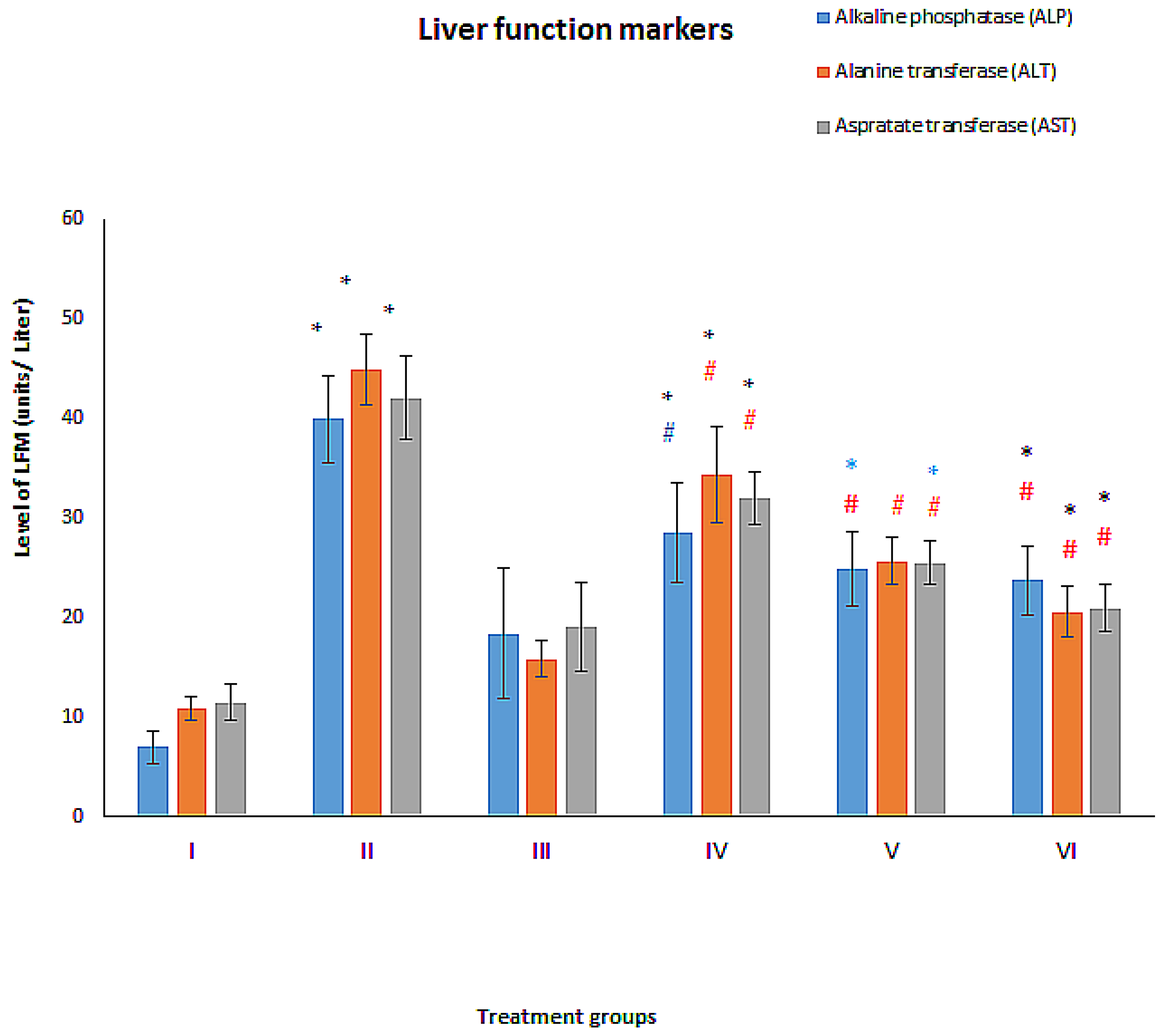
3.2. Effect on Liver Function Markers
3.2.1. ALT
3.2.2. AST
3.2.3. ALP
3.3. Effect on Hepatotoxicity Markers
3.3.1. Total Bilirubin
3.3.2. Gamma-Glutamyl Transferase (GGT)
3.3.3. Glutathione–S-Transferase (GST)
3.3.4. Lactate Dehydrogenase (LDH)
3.4. Effect on Oxidative Stress Markers
3.4.1. Reduced Glutathione (GSH)
3.4.2. Total Malondialdehyde (MDA)
3.4.3. Catalase
3.5. Effect on Lipid Profile
3.5.1. Cholesterol
3.5.2. High-Density Lipids (HDL)
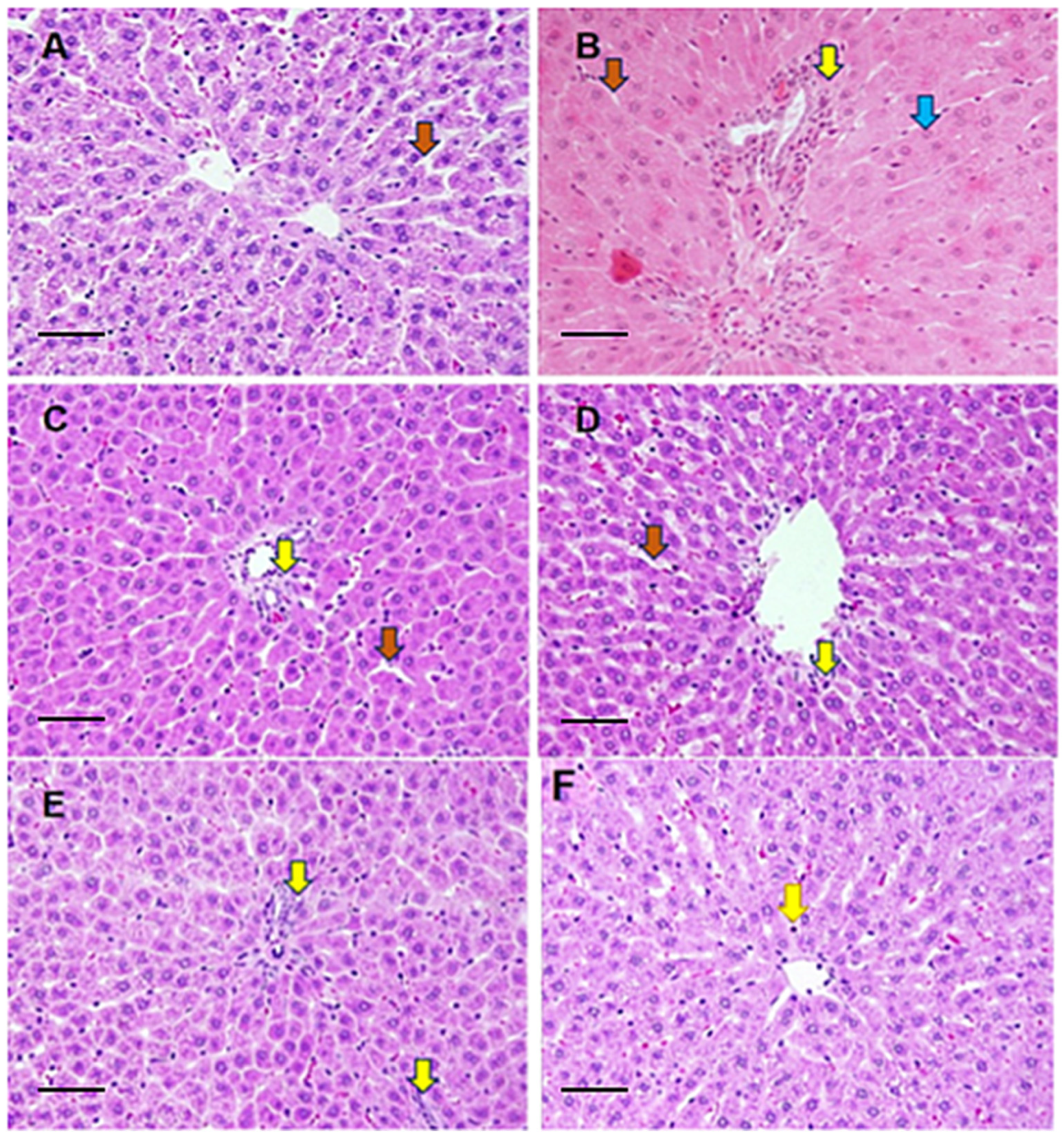
3.6. Histological Evaluation
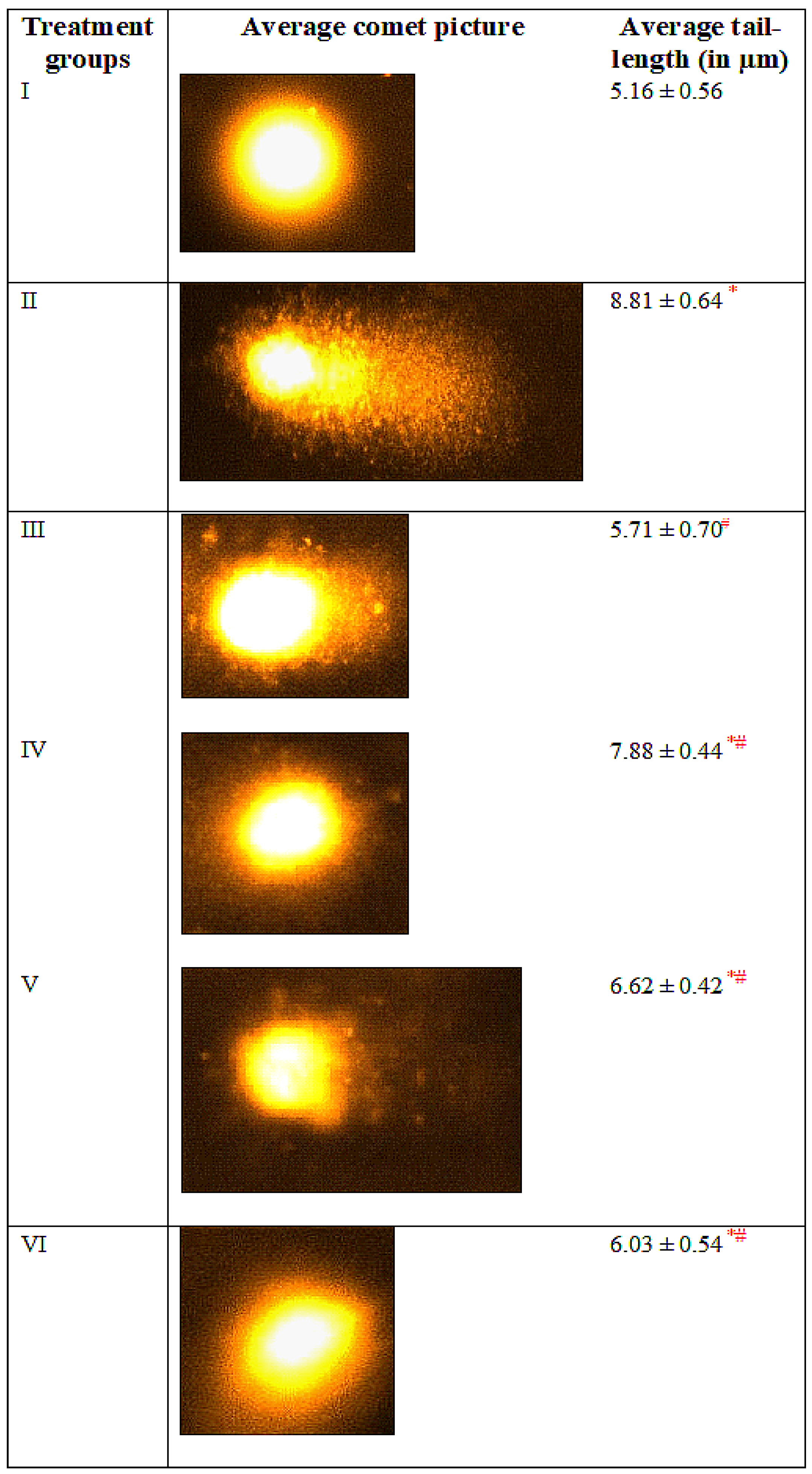
3.7. Comet Assay
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Bellou, V.; Belbasis, L.; Tzoulaki, I.; Evangelou, E. Risk factors for type 2 diabetes mellitus: An exposure-wide umbrella review of meta-analyses. PLoS ONE 2018, 13, e0194127. [Google Scholar] [CrossRef] [PubMed]
- Al-Rubeaan, K.; Al Derwish, M.; Ouizi, S.; Youssef, A.M.; Subhani, S.N.; Ibrahim, H.M.; Alamri, B.N. Diabetic foot complications and their risk factors from a large retrospective cohort study. PLoS ONE 2015, 10, e0124446. [Google Scholar] [CrossRef] [PubMed]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of type 2 diabetes mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef] [PubMed]
- Falavigna, M.; Klitgaard, M.; Steene, E.; Flaten, G.E. Mimicking regional and fasted/fed state conditions in the intestine with the mucus-PVPA in vitro model: The impact of pH and simulated intestinal fluids on drug permeability. Eur. J. Pharm. Sci. 2019, 132, 44–54. [Google Scholar] [CrossRef]
- Wooley, A.C.; Kerr, J.L. Monitoring patients on metformin: Recent changes and rationales. J. Pharm. Technol. 2018, 34, 28–36. [Google Scholar] [CrossRef]
- Jose, P.; Sundar, K.; Anjali, C.; Ravindran, A. Metformin-loaded BSA nanoparticles in cancer therapy: A new perspective for an old antidiabetic drug. Cell Biochem. Biophys. 2015, 71, 627–636. [Google Scholar] [CrossRef]
- Maruthur, N.M.; Tseng, E.; Hutfless, S.; Wilson, L.M.; Suarez-Cuervo, C.; Berger, Z.; Chu, Y.; Iyoha, E.; Segal, J.B.; Bolen, S. Diabetes medications as monotherapy or metformin-based combination therapy for type 2 diabetes: A systematic review and meta-analysis. Ann. Intern. Med. 2016, 164, 740–751. [Google Scholar] [CrossRef]
- Zhang, K.; Yang, W.; Dai, H.; Deng, Z. Cardiovascular risk following metformin treatment in patients with type 2 diabetes mellitus: Results from meta-analysis. Diabetes Res. Clin. Pract. 2020, 160, 108001. [Google Scholar] [CrossRef]
- Wang, Y.-W.; He, S.-J.; Feng, X.; Cheng, J.; Luo, Y.-T.; Tian, L.; Huang, Q. Metformin: A review of its potential indications. Drug Des. Dev. Ther. 2017, 11, 2421. [Google Scholar] [CrossRef]
- Chao, J.; Cheng, H.-Y.; Chang, M.-L.; Huang, S.-S.; Liao, J.-W.; Cheng, Y.-C.; Peng, W.-H.; Pao, L.-H. Gallic Acid Ameliorated Impaired Lipid Homeostasis in a Mouse Model of High-Fat Diet-and Streptozotocin-Induced NAFLD and Diabetes through Improvement of β-oxidation and Ketogenesis. Front. Pharmacol. 2020, 11, 2469. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. Nanomedicine and the COVID-19 vaccines. Nat. Nanotechnol. 2020, 15, 963. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Kim, B.; Rutka, J.T.; Chan, W.C. Nanoparticle-mediated cellular response is size-dependent. Nat. Nanotechnol. 2008, 3, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Anwar, A.; Soomaroo, A.; Anwar, A.; Siddiqui, R.; Khan, N.A. Metformin-coated silver nanoparticles exhibit anti-acanthamoebic activities against both trophozoite and cyst stages. Exp. Parasitol. 2020, 215, 107915. [Google Scholar] [CrossRef]
- Hassan, I.; Husain, F.M.; Khan, R.A.; Ebaid, H.; Al-Tamimi, J.; Alhazza, I.M.; Aman, S.; Ibrahim, K.E. Ameliorative effect of zinc oxide nanoparticles against potassium bromate-mediated toxicity in Swiss albino rats. Environ. Sci. Pollut. Res. 2019, 26, 9966–9980. [Google Scholar] [CrossRef]
- Souto, E.B.; Souto, S.B.; Campos, J.R.; Severino, P.; Pashirova, T.N.; Zakharova, L.Y.; Silva, A.M.; Durazzo, A.; Lucarini, M.; Izzo, A.A. Nanoparticle delivery systems in the treatment of diabetes complications. Molecules 2019, 24, 4209. [Google Scholar] [CrossRef]
- Chuengsamarn, S.; Rattanamongkolgul, S.; Luechapudiporn, R.; Phisalaphong, C.; Jirawatnotai, S. Curcumin extract for prevention of type 2 diabetes. Diabetes Care 2012, 35, 2121–2127. [Google Scholar] [CrossRef] [PubMed]
- Wojcik, M.; Krawczyk, M.; Wojcik, P.; Cypryk, K.; Wozniak, L.A. Molecular mechanisms underlying curcumin-mediated therapeutic effects in type 2 diabetes and cancer. Oxidative Med. Cell. Longev. 2018, 2018, 9698258. [Google Scholar] [CrossRef]
- Wanninger, S.; Lorenz, V.; Subhan, A.; Edelmann, F.T. Metal complexes of curcumin–synthetic strategies, structures and medicinal applications. Chem. Soc. Rev. 2015, 44, 4986–5002. [Google Scholar] [CrossRef]
- Shome, S.; Talukdar, A.D.; Choudhury, M.D.; Bhattacharya, M.K.; Upadhyaya, H. Curcumin as potential therapeutic natural product: A nanobiotechnological perspective. J. Pharm. Pharmacol. 2016, 68, 1481–1500. [Google Scholar] [CrossRef]
- Anand, K.; Tiloke, C.; Naidoo, P.; Chuturgoon, A. Phytonanotherapy for management of diabetes using green synthesis nanoparticles. J. Photochem. Photobiol. B Biol. 2017, 173, 626–639. [Google Scholar] [CrossRef]
- Grama, C.N.; Suryanarayana, P.; Patil, M.A.; Raghu, G.; Balakrishna, N.; Kumar, M.R.; Reddy, G.B. Efficacy of biodegradable curcumin nanoparticles in delaying cataract in diabetic rat model. PLoS ONE 2013, 8, e78217. [Google Scholar] [CrossRef]
- Boarescu, P.-M.; Boarescu, I.; Bocșan, I.C.; Gheban, D.; Bulboacă, A.E.; Nicula, C.; Pop, R.M.; Râjnoveanu, R.-M.; Bolboacă, S.D. Antioxidant and anti-inflammatory effects of curcumin nanoparticles on drug-induced acute myocardial infarction in diabetic rats. Antioxidants 2019, 8, 504. [Google Scholar] [CrossRef]
- Hettiarachchi, S.S.; Dunuweera, S.P.; Dunuweera, A.N.; Rajapakse, R.M.G. Synthesis of Curcumin Nanoparticles from Raw Turmeric Rhizome. ACS Omega 2021, 6, 8246–8252. [Google Scholar] [CrossRef]
- Ganugula, R.; Arora, M.; Dwivedi, S.; Chandrashekar, D.S.; Varambally, S.; Scott, E.M.; Kumar, M.N.V.R. Systemic Anti-Inflammatory Therapy Aided by Curcumin-Laden Double-Headed Nanoparticles Combined with Injectable Long-Acting Insulin in a Rodent Model of Diabetes Eye Disease. ACS Nano 2023, 11, 6857–6874. [Google Scholar] [CrossRef]
- El-Naggar, M.E.; Al-Joufi, F.; Anwar, M.; Attia, M.F.; El-Bana, M.A. Curcumin-loaded PLA-PEG copolymer nanoparticles for treatment of liver inflammation in streptozotocin-induced diabetic rats. Colloids Surf. B Biointerfaces 2019, 177, 389–398. [Google Scholar] [CrossRef]
- Ebaid, H.; Bashandy, S.A.; Abdel-Mageed, A.M.; Al-Tamimi, J.; Hassan, I.; Alhazza, I.M. Folic acid and melatonin mitigate diabetic nephropathy in rats via inhibition of oxidative stress. Nutr. Metab. 2020, 17, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ebaid, H.; Al-Tamimi, J.; Hassan, I.; Habila, M.A.; Rady, A.M.; Alhazza, I.M.; Ahmed, A.M. Effect of Selenium Nanoparticles on Carbon Tetrachloride-Induced Hepatotoxicity in the Swiss Albino Rats. Appl. Sci. 2021, 11, 3044. [Google Scholar] [CrossRef]
- Han, X.; Tao, Y.-L.; Deng, Y.-P.; Yu, J.-W.; Cai, J.; Ren, G.-F.; Sun, Y.-N.; Jiang, G.-J. Metformin ameliorates insulitis in STZ-induced diabetic mice. PeerJ 2017, 5, e3155. [Google Scholar] [PubMed]
- Hassan, I.; Ebaid, H.; Alhazza, I.M.; Al-Tamimi, J. The alleviative effect of vitamin B2 on potassium bromate-induced hepatotoxicity in male rats. BioMed Res. Int. 2020, 2020, 8274261. [Google Scholar] [CrossRef]
- Khodashenas, B.; Ghorbani, H.R. Synthesis of silver nanoparticles with different shapes. Arab. J. Chem. 2019, 12, 1823–1838. [Google Scholar] [CrossRef]
- Alhazza, I.M.; Hassan, I.; Ebaid, H.; Al-Tamimi, J.; Alwasel, S.H. Chemopreventive effect of riboflavin on the potassium bromate–induced renal toxicity in vivo. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020, 393, 2355–2364. [Google Scholar] [CrossRef]
- Hassan, I.; Chibber, S.; Khan, A.A.; Naseem, I. Riboflavin ameliorates cisplatin induced toxicities under photoillumination. PLoS ONE 2012, 7, e36273. [Google Scholar] [CrossRef]
- Jollow, D.; Mitchell, J.; Zampaglione, N.; Gillette, J. Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3, 4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology 1974, 11, 151–169. [Google Scholar] [CrossRef] [PubMed]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1978; Volume 52, pp. 302–310. [Google Scholar]
- Chen, Y.; Shan, X.; Luo, C.; He, Z. Emerging nanoparticulate drug delivery systems of metformin. J. Pharm. Investig. 2020, 50, 219–230. [Google Scholar] [CrossRef]
- Damasceno, D.C.; Netto, A.; Iessi, I.; Gallego, F.; Corvino, S.; Dallaqua, B.; Sinzato, Y.; Bueno, A.; Calderon, I.D.M.P.; Rudge, M.V.C. Streptozotocin-induced diabetes models: Pathophysiological mechanisms and fetal outcomes. BioMed Res. Int. 2014, 2014, 819065. [Google Scholar] [CrossRef]
- Graham, M.L.; Janecek, J.L.; Kittredge, J.A.; Hering, B.J.; Schuurman, H.-J. The streptozotocin-induced diabetic nude mouse model: Differences between animals from different sources. Comp. Med. 2011, 61, 356–360. [Google Scholar]
- Nna, V.U.; Bakar, A.B.A.; Ahmad, A.; Mohamed, M. Diabetes-induced testicular oxidative stress, inflammation, and caspase-dependent apoptosis: The protective role of metformin. Arch. Physiol. Biochem. 2020, 126, 377–388. [Google Scholar] [CrossRef]
- Matsunami, T.; Sato, Y.; Sato, T.; Yukawa, M. Antioxidant status and lipid peroxidation in diabetic rats under hyperbaric oxygen exposure. Physiol. Res. 2010, 59, 97–104. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Feng, B.; Tang, L.; Li, W.; Zheng, X.; Liu, Y.; Peng, Y.; Zheng, G.; He, Q. Golgi phosphoprotein 3 (GOLPH3) promotes hepatocellular carcinoma progression by activating mTOR signaling pathway. BMC Cancer 2018, 18, 661. [Google Scholar] [CrossRef]
- Roxo, D.F.; Arcaro, C.A.; Gutierres, V.O.; Costa, M.C.; Oliveira, J.O.; Lima, T.F.O.; Assis, R.P.; Brunetti, I.L.; Baviera, A.M. Curcumin combined with metformin decreases glycemia and dyslipidemia, and increases paraoxonase activity in diabetic rats. Diabetol. Metab. Syndr. 2019, 11, 33. [Google Scholar] [CrossRef]
- Zhang, T.; Guo, P.; Zhang, Y.; Xiong, H.; Yu, X.; Xu, S.; Wang, X.; He, D.; Jin, X. The antidiabetic drug metformin inhibits the proliferation of bladder cancer cells in vitro and in vivo. Int. J. Mol. Sci. 2013, 14, 24603–24618. [Google Scholar] [CrossRef] [PubMed]
- Thota, R.N.; Dias, C.B.; Abbott, K.A.; Acharya, S.H.; Garg, M.L. Curcumin alleviates postprandial glycaemic response in healthy subjects: A cross-over, randomized controlled study. Sci. Rep. 2018, 8, 13679. [Google Scholar] [CrossRef] [PubMed]
- Das, G.; Patra, J.K.; Basavegowda, N.; Vishnuprasad, C.N.; Shin, H.-S. Comparative study on antidiabetic, cytotoxicity, antioxidant and antibacterial properties of biosynthesized silver nanoparticles using outer peels of two varieties of Ipomoea batatas (L.) Lam. Int. J. Nanomed. 2019, 14, 4741. [Google Scholar] [CrossRef] [PubMed]
- Saratale, R.G.; Shin, H.S.; Kumar, G.; Benelli, G.; Kim, D.-S.; Saratale, G.D. Exploiting antidiabetic activity of silver nanoparticles synthesized using Punica granatum leaves and anticancer potential against human liver cancer cells (HepG2). Artif. Cells Nanomed. Biotechnol. 2018, 46, 211–222. [Google Scholar] [CrossRef]
- Ganugula, R.; Arora, M.; Jaisamut, P.; Wiwattanapatapee, R.; Jørgensen, H.G.; Venkatpurwar, V.P.; Zhou, B.; Rodrigues Hoffmann, A.; Basu, R.; Guo, S. Nano-curcumin safely prevents streptozotocin-induced inflammation and apoptosis in pancreatic beta cells for effective management of Type 1 diabetes mellitus. Br. J. Pharmacol. 2017, 174, 2074–2084. [Google Scholar] [CrossRef]
- Rena, G.; Hardie, D.G.; Pearson, E.R. The mechanisms of action of metformin. Diabetologia 2017, 60, 1577–1585. [Google Scholar] [CrossRef]
- Wahab, M.; Bhatti, A.; John, P. Evaluation of Antidiabetic Activity of Biogenic Silver Nanoparticles Using Thymus serpyllum on Streptozotocin-Induced Diabetic BALB/c Mice. Polymers 2022, 14, 3138. [Google Scholar] [CrossRef]
- Ebaid, H.; Habila, M.; Hassan, I.; Al-Tamimi, J.; Omar, M.S.; Rady, A.; Alhazza, I.M. Curcumin-containing Silver Nanoparticles Prevent Carbon Tetrachloride-Induced Hepatotoxicity in Mice. Comb. Chem. High Throughput Screen 2021, 24, 1609–1617. [Google Scholar] [CrossRef]
- Li, L.; Li, L.; Zhou, X.; Yu, Y.; Li, Z.; Zuo, D.; Wu, Y. Silver nanoparticles induce protective autophagy via Ca2+/CaMKKβ/AMPK/mTOR pathway in SH-SY5Y cells and rat brains. Nanotoxicology 2019, 13, 369–391. [Google Scholar] [CrossRef]


| Treatment Groups | Gamma Glutamyl Transferase (U/L) | Glutathione-S-Transferase (Units/mg) | Lactate Dehydrogenase (U/L) | Total Bilirubin (µmol/L) |
|---|---|---|---|---|
| Control (Group I) | 78.51 ± 12.41 | 16.42 ±3.32 | 71.04 ± 16.94 | 5.32 ± 0.76 |
| Diabetic group (Group II) | 448.10 ± 64.17 * | 41.53 ± 6.66 * | 210.21 ± 24.22 * | 11.06 ± 0.59 * |
| AgNPs-Curcumin group (Group III) | 146.30 ± 27.61 # | 30.15 ± 2.35 * | 87.53 ± 10.07 # | 7.47 ± 0.90 * |
| Diabetic group + MTF (Group IV) | 373.17 ± 56.41 * | 37.17 ±3.96 * | 146.80± 7.62 *# | 10.70 ± 0.76 *# |
| Diabetic group + MTF+ AgNP-Curcumin (5 mg/kg) (Group V) | 276.30 ±65.23 *# | 35.64 ± 2.28 * | 111.30± 1.36 *# | 10.01 ± 0.24 * |
| Diabetic group + MTF+ AgNP-Curcumin (10 mg/kg) (Group VI) | 233.0 ± 35.94 *# | 27.79 ± 2.13 *# | 94.91 ± 6.05 # | 8.78 ± 0.59 *# |
| Treatment Groups | MDA (nmol/mg) | GSH (nmol/mg) | CAT (units/mg) |
|---|---|---|---|
| Control (Group I) | 0.164 ± 0.040 | 0.528 ± 0.077 | 5.662 ± 0.868 |
| Diabetic group (Group II) | 0.512 ± 0.053 * | 0.0413 ± 0.003 | 1.988 ± 0.246 * |
| AgNPs-Curcumin group (Group III) | 0.276 ± 0.016 | 0.354 ± 0.068 *# | 3.943 ± 0.439 * |
| Diabetic group + MTF (Group IV) | 0.384 ± 0.059 *# | 0.240 ± 0.035 * | 2.423 ± 0.332 *# |
| Diabetic group + MTF + AgNP-Curcumin (5 mg/kg) (Group V) | 0.281 ± 0.0192 *# | 0.266 ± 0.029 * # | 3.002 ± 0.347 *# |
| Diabetic group + MTF + AgNP-Curcumin (10 mg/kg) (Group VI) | 0.252 ± 0.038 *# | 0.339 ± 0.007 *# | 3.721 ± 0.150 *# |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, I.; Al-Tamimi, J.; Ebaid, H.; Habila, M.A.; Alhazza, I.M.; Rady, A.M. Silver Nanoparticles Decorated with Curcumin Enhance the Efficacy of Metformin in Diabetic Rats via Suppression of Hepatotoxicity. Toxics 2023, 11, 867. https://doi.org/10.3390/toxics11100867
Hassan I, Al-Tamimi J, Ebaid H, Habila MA, Alhazza IM, Rady AM. Silver Nanoparticles Decorated with Curcumin Enhance the Efficacy of Metformin in Diabetic Rats via Suppression of Hepatotoxicity. Toxics. 2023; 11(10):867. https://doi.org/10.3390/toxics11100867
Chicago/Turabian StyleHassan, Iftekhar, Jameel Al-Tamimi, Hossam Ebaid, Mohamed A. Habila, Ibrahim M. Alhazza, and Ahmed M. Rady. 2023. "Silver Nanoparticles Decorated with Curcumin Enhance the Efficacy of Metformin in Diabetic Rats via Suppression of Hepatotoxicity" Toxics 11, no. 10: 867. https://doi.org/10.3390/toxics11100867
APA StyleHassan, I., Al-Tamimi, J., Ebaid, H., Habila, M. A., Alhazza, I. M., & Rady, A. M. (2023). Silver Nanoparticles Decorated with Curcumin Enhance the Efficacy of Metformin in Diabetic Rats via Suppression of Hepatotoxicity. Toxics, 11(10), 867. https://doi.org/10.3390/toxics11100867







