Mechanisms of Organophosphate Toxicity and the Role of Acetylcholinesterase Inhibition
Abstract
:1. Introduction
2. Cholinergic Mechanisms of OP Toxicity
2.1. Depression of Respiration
2.2. Induction of Seizures and Status Epilepticus
2.3. Seizures and Status Epilepticus as the Primary Mediators of OP Neurotoxicity
3. Mechanisms of OP Toxicity That Do Not Depend on Status Epilepticus or AChE Inhibition
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Footnotes
References
- Aroniadou-Anderjaska, V.; Apland, J.P.; Figueiredo, T.H.; de Araujo Furtado, M.; Braga, M.F. Acetylcholinesterase inhibitors (nerve agents) as weapons of mass destruction: History, mechanisms of action, and medical countermeasures. Neuropharmacology 2020, 181, 108298. [Google Scholar] [CrossRef] [PubMed]
- Sirin, G.S.; Zhang, Y. How is acetylcholinesterase phosphonylated by soman? An ab initio QM/MM molecular dynamics study. J. Phys. Chem. A. 2014, 118, 9132–9139. [Google Scholar] [CrossRef] [PubMed]
- Breer, H.; Hanke, W.; Benke, D.; Tareilus, E.; Krieger, J. Nicotinic acetylcholine receptors in the nervous system of insects. In Molecular Biology of Neuroreceptors and Ion Channels; Maelicke, A., Ed.; NATO ASI Series (Series H: Cell Biology); Springer: Berlin/Heidelberg, Germany, 1989; Volume 32, pp. 55–68. [Google Scholar]
- Thany, S.H.; Tricoire-Leignel, H. Emerging Pharmacological Properties of Cholinergic Synaptic Transmission: Comparison between Mammalian and Insect Synaptic and Extrasynaptic Nicotinic Receptors. Curr. Neuropharmacol. 2011, 9, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Casida, J.E.; Durkin, K.A. Anticholinesterase insecticide retrospective. Chem. Biol. Interact. 2013, 203, 221–225. [Google Scholar] [CrossRef]
- Carey, J.L.; Dunn, C.; Gaspari, R.J. Central respiratory failure during acute organophosphate poisoning. Respir. Physiol. Neurobiol. 2013, 189, 403–410. [Google Scholar] [CrossRef]
- Shao, X.M.; Feldman, J.L. Acetylcholine modulates respiratory pattern: Effects mediated by M3-like receptors in preBötzinger complex inspiratory neurons. J. Neurophysiol. 2000, 83, 1243–1252. [Google Scholar] [CrossRef]
- Lai, J.; Shao, X.M.; Pan, R.W.; Dy, E.; Huang, C.H.; Feldman, J.L. RT-PCR reveals muscarinic acetylcholine receptor mRNA in the pre-Bötzinger complex. Am. J. Physiol. Lung Cell Mol. Physiol. 2001, 281, L1420–L1424. [Google Scholar] [CrossRef]
- Shao, X.M.; Feldman, J.L. Cholinergic neurotransmission in the preBötzinger Complex modulates excitability of inspiratory neurons and regulates respiratory rhythm. Neuroscience 2005, 130, 1069–1081. [Google Scholar] [CrossRef]
- Zheng, F.; Nixdorf-Bergweiler, B.E.; Edelmann, E.; van Brederode, J.F.M.; Alzheimer, C. Muscarinic Modulation of Morphologically Identified Glycinergic Neurons in the Mouse PreBötzinger Complex. Front. Cell Neurosci. 2020, 13, 562. [Google Scholar] [CrossRef]
- Hulse, E.J.; Davies, J.O.; Simpson, A.J.; Sciuto, A.M.; Eddleston, M. Respiratory complications of organophosphorus nerve agent and insecticide poisoning. Implications for respiratory and critical care. Am. J. Respir. Crit. Care Med. 2014, 190, 1342–1354. [Google Scholar] [CrossRef]
- Shao, X.M.; Feldman, J.L. Central cholinergic regulation of respiration: Nicotinic receptors. Acta Pharmacol. Sin. 2009, 30, 761–770. [Google Scholar] [CrossRef]
- Ochoa, E.L.; Chattopadhyay, A.; McNamee, M.G. Desensitization of the nicotinic acetylcholine receptor: Molecular mechanisms and effect of modulators. Cell Mol. Neurobiol. 1989, 9, 141–178. [Google Scholar] [CrossRef] [PubMed]
- Quick, M.W.; Lester, R.A. Desensitization of neuronal nicotinic receptors. J. Neurobiol. 2002, 53, 457–478. [Google Scholar] [CrossRef] [PubMed]
- Paradiso, K.G.; Steinbach, J.H. Nicotine is highly effective at producing desensitization of rat α4β2 neuronal nicotinic receptors. J. Physiol. 2003, 553, 857–871. [Google Scholar] [CrossRef] [PubMed]
- Stewart, W.C.; Anderson, E.A. Effect of a cholinesterase inhibitor when injected into the medulla of the rabbit. J. Pharmacol. Exp. Ther. 1968, 162, 309–318. [Google Scholar] [PubMed]
- Houze, P.; Pronzola, L.; Kayouka, M.; Villa, A.; Debray, M.; Baud, F.J. Ventilatory effects of low-dose paraoxon result from central muscarinic effects. Toxicol. Appl. Pharmacol. 2008, 233, 186–192. [Google Scholar] [CrossRef]
- Bird, S.B.; Gaspari, R.J.; Dickson, E.W. Early death due to severe organophosphate poisoning is a centrally mediated process. Acad. Emerg. Med. 2003, 10, 295–298. [Google Scholar] [CrossRef]
- Okumura, T.; Takasu, N.; Ishimatsu, S.; Miyanoki, S.; Mitsuhashi, A.; Kumada, K.; Tanaka, K.; Hinohara, S. Report on 640 victims of the Tokyo subway sarin attack. Ann. Emerg. Med. 1996, 28, 129–135. [Google Scholar] [CrossRef]
- Peng, X.; Perkins, M.W.; Simons, J.; Witriol, A.M.; Rodriguez, A.M.; Benjamin, B.M.; Devorak, J.; Sciuto, A.M. Acute pulmonary toxicity following inhalation exposure to aerosolized VX in anesthetized rats. Inhal. Toxicol. 2014, 26, 371–379. [Google Scholar] [CrossRef]
- Figueiredo, T.H.; Apland, J.P.; Braga, M.F.M.; Marini, A.M. Acute and long-term consequences of exposure to organophosphate nerve agents in humans. Epilepsia 2018, 59, 92–99. [Google Scholar] [CrossRef]
- Blum, A.S. Respiratory physiology of seizures. J. Clin. Neurophysiol. 2009, 26, 309–315. [Google Scholar] [CrossRef]
- Dlouhy, B.J.; Gehlbach, B.K.; Kreple, C.J.; Kawasaki, H.; Oya, H.; Buzza, C.; Granner, M.A.; Welsh, M.J.; Howard, M.A.; Wemmie, J.A.; et al. Breathing inhibited when seizures spread to the amygdala and upon amygdala stimulation. J. Neurosci. 2015, 35, 10281–10289. [Google Scholar] [CrossRef] [PubMed]
- Šimić, G.; Tkalčić, M.; Vukić, V.; Mulc, D.; Španić, E.; Šagud, M.; Bordonau, F.E.; Vukšić, M.; R Hof, P. Understanding Emotions: Origins and Roles of the Amygdala. Biomolecules 2021, 11, 823. [Google Scholar] [CrossRef] [PubMed]
- Aroniadou-Anderjaska, V.; Fritsch, B.; Qashu, F.; Braga, M.F. Pathology and pathophysiology of the amygdala in epileptogenesis and epilepsy. Epilepsy Res. 2008, 78, 102–116. [Google Scholar] [CrossRef] [PubMed]
- McDonough, J.H., Jr.; McLeod, C.G., Jr.; Nipwoda, M.T. Direct microinjection of soman or VX into the amygdala produces repetitive limbic convulsions and neuropathology. Brain Res. 1987, 435, 123–137. [Google Scholar] [CrossRef]
- Prager, E.M.; Aroniadou-Anderjaska, V.; Almeida-Suhett, C.P.; Figueiredo, T.H.; Apland, J.P.; Braga, M.F. Acetylcholinesterase inhibition in the basolateral amygdala plays a key role in the induction of status epilepticus after soman exposure. Neurotoxicology 2013, 38, 84–90. [Google Scholar] [CrossRef]
- Lallement, G.; Carpentier, P.; Collet, A.; Pernot-Marino, I.; Baubichon, D.; Sentenac-Roumanou, H.; Blanchet, G. Involvement of glutamatergic system of amygdala in generalized seizures induced by soman: Comparison with the hippocampus. C. R. Acad. Sci. III 1991, 313, 421–426. [Google Scholar]
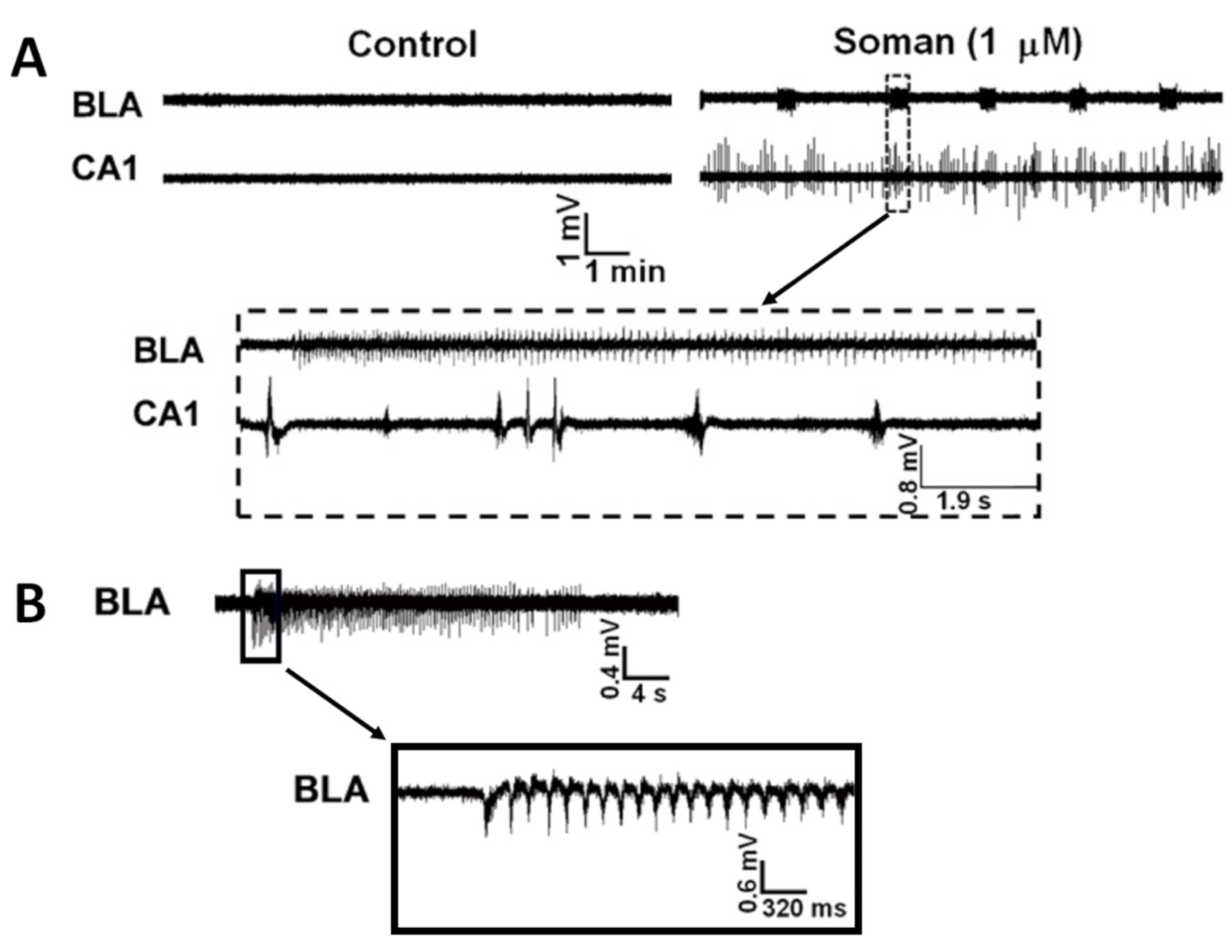
- Apland, J.P.; Aroniadou-Anderjaska, V.; Braga, M.F.M. Soman induces ictogenesis in the amygdala and interictal activity in the hippocampus that are blocked by a GluR5 kainate receptor antagonist in vitro. Neuroscience 2009, 159, 380–389. [Google Scholar] [CrossRef]
- Myhrer, T.; Enger, S.; Aas, P. Roles of perirhinal and posterior piriform cortices in control and generation of seizures: A microinfusion study in rats exposed to soman. Neurotoxicology 2010, 31, 147–153. [Google Scholar] [CrossRef]
- Skovira, J.W.; McDonough, J.H.; Shih, T.M. Protection against sarin-induced seizures in rats by direct brain microinjection of, scopolamine, midazolam or MK-801. J. Mol. Neurosci. 2010, 40, 56–62. [Google Scholar] [CrossRef]
- Picciotto, M.R.; Higley, M.J.; Mineur, Y.S. Acetylcholine as a neuromodulator: Cholinergic signaling shapes nervous system function and behavior. Neuron 2012, 76, 116–129. [Google Scholar] [CrossRef]
- Kruse, A.C.; Kobilka, B.K.; Gautam, D.; Sexton, P.M.; Christopoulos, A.; Wess, J. Muscarinic acetylcholine receptors: Novel opportunities for drug development. Nat. Rev. Drug. Discov. 2014, 13, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Dani, J.A. Neuronal nicotinic acetylcholine receptor structure and function and response to nicotine. Int. Rev. Neurobiol. 2015, 124, 3–19. [Google Scholar] [PubMed]
- Thomsen, M.; Sørensen, G.; Dencker, D. Physiological roles of CNS muscarinic receptors gained from knockout mice. Neuropharmacology 2018, 136, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Colangelo, C.; Shichkova, P.; Keller, D.; Markram, H.; Ramaswamy, S. Cellular, Synaptic and Network Effects of Acetylcholine in the Neocortex. Front. Neural Circuits 2019, 13, 24. [Google Scholar] [CrossRef]
- Lallement, G.; Dorandeu, F.; Filliat, P.; Carpentier, P.; Baille, V.; Blanchet, G. Medical management of organophosphate-induced seizures. J. Physiol. Paris 1998, 92, 369–373. [Google Scholar] [CrossRef]
- Shih, T.M.; McDonough, J.H., Jr.; Koplovitz, I. Anticonvulsants for soman-induced seizure activity. J. Biomed. Sci. 1999, 6, 86–96. [Google Scholar] [CrossRef]
- McDonough, J.H., Jr.; Zoeffel, L.D.; McMonagle, J.; Copeland, T.L.; Smith, C.D.; Shih, T.M. Anticonvulsant treatment of nerve agent seizures: Anticholinergics versus diazepam in soman-intoxicated guinea pigs. Epilepsy Res. 2000, 38, 1–14. [Google Scholar] [CrossRef]
- McDonough, J.H., Jr.; Shih, T.M. Neuropharmacological mechanisms of nerve agent-induced seizure and neuropathology. Neurosci. Biobehav. Rev. 1997, 21, 559–579. [Google Scholar] [CrossRef]
- Miller, S.L.; Aroniadou-Anderjaska, V.; Pidoplichko, V.I.; Figueiredo, T.H.; Apland, J.P.; Krishnan, J.K.; Braga, M.F. The M1 muscarinic receptor antagonist VU0255035 delays the development of status epilepticus after organophosphate exposure and prevents hyperexcitability in the basolateral amygdala. J. Pharmacol. Exp. Ther. 2017, 360, 23–32. [Google Scholar] [CrossRef]
- Sheffler, D.J.; Williams, R.; Bridges, T.M.; Xiang, Z.; Kane, A.S.; Byun, N.E.; Jadhav, S.; Mock, M.M.; Zheng, F.; Lewis, L.M.; et al. A novel selective muscarinic acetylcholine receptor subtype 1 antagonist reduces seizures without impairing hippocampus dependent learning. Mol. Pharmacol. 2009, 76, 356–368. [Google Scholar] [CrossRef] [PubMed]
- Fisahn, A.; Yamada, M.; Duttaroy, A.; Gan, J.W.; Deng, C.X.; McBain, C.J.; Wess, J. Muscarinic induction of hippocampal gamma oscillations requires coupling of the M1 receptor to two mixed cation currents. Neuron 2002, 33, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Williamson, J.; Singh, T.; Kapur, J. Neurobiology of organophosphate-induced seizures. Epilepsy Behav. 2019, 101, 106426. [Google Scholar] [CrossRef] [PubMed]
- Aroniadou-Anderjaska, V.; Figueiredo, T.H.; Apland, J.P.; Prager, E.M.; Pidoplichko, V.I.; Miller, S.L.; Braga, M.F. Long-term neuropathological and behavioral impairments after exposure to nerve agents. Ann. N. Y. Acad. Sci. 2016, 1374, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, T.H.; Aroniadou-Anderjaska, V.; Apland, J.P.; Rossetti, K.; Braga, M.F. Delayed tezampanel and caramiphen treatment but not midazolam protects against long-term neuropathology after soman exposure. Exp. Biol. Med. 2023, 248, 612–623. [Google Scholar] [CrossRef]
- de Araujo Furtado, M.; Aroniadou-Anderjaska, V.; Figueiredo, T.H.; Pidoplichko, V.I.; Apland, J.P.; Rossetti, K.; Braga, M.F.M. Preventing Long-Term Brain Damage by Nerve Agent-Induced Status Epilepticus in Rat Models Applicable to Infants: Significant Neuroprotection by Tezampanel Combined with Caramiphen but not by Midazolam Treatment. J. Pharmacol. Exp. Ther. 2023, in press.
- de Araujo Furtado, M.; Lumley, L.A.; Robison, C.; Tong, L.C.; Lichtenstein, S.; Yourick, D.L. Spontaneous recurrent seizures after status epilepticus induced by soman in Sprague-Dawley rats. Epilepsia 2010, 51, 1503–1510. [Google Scholar] [CrossRef]
- Chapman, S.; Yaakov, G.; Egoz, I.; Rabinovitz, I.; Raveh, L.; Kadar, T.; Gilat, E.; Grauer, E. Sarin-induced brain damage in rats is attenuated by delayed administration of midazolam. Neurotoxicology 2015, 49, 132–138. [Google Scholar] [CrossRef]
- Gilat, E.; Kadar, T.; Levy, A.; Rabinovitz, I.; Cohen, G.; Kapon, Y.; Sahar, R.; Brandeis, R. Anticonvulsant treatment of sarin-induced seizures with nasal midazolam: An electrographic, behavioral, and histological study in freely moving rats. Toxicol. Appl. Pharmacol. 2005, 209, 74–85. [Google Scholar] [CrossRef]
- Apland, J.P.; Aroniadou-Anderjaska, V.; Figueiredo, T.H.; de Araujo Furtado, M.; Braga, M.F.M. Full protection against soman-induced seizures and brain damage by LY293558 and caramiphen combination treatment in adult rats. Neurotox. Res. 2018, 34, 511–524. [Google Scholar] [CrossRef]
- Figueiredo, T.H.; Aroniadou-Anderjaska, V.; Pidoplichko, V.I.; Apland, J.P.; Braga, M.F.M. Antiseizure and Neuroprotective Efficacy of Midazolam in Comparison with Tezampanel (LY293558) against Soman-Induced Status Epilepticus. Toxics 2022, 10, 409. [Google Scholar] [CrossRef]
- Shih, T.M.; Duniho, S.M.; McDonough, J.H. Control of nerve agent-induced seizures is critical for neuroprotection and survival. Toxicol. Appl. Pharmacol. 2003, 188, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, T.N.; Barkovich, A.J.; Bollen, A.W.; Hart, A.P.; Ferriero, D.M. Childhood status epilepticus and excitotoxic neuronal injury. Pediatr. Neurol. 2007, 36, 253–257. [Google Scholar] [CrossRef]
- Barker-Haliski, M.; White, H.S. Glutamatergic mechanisms associated with seizures and epilepsy. Cold Spring Harb. Perspect. Med. 2015, 5, 022863. [Google Scholar] [CrossRef]
- Deshpande, L.S.; Carter, D.S.; Blair, R.E.; DeLorenzo, R.J. Development of a prolonged calcium plateau in hippocampal neurons in rats surviving status epilepticus induced by the organophosphate diisopropylfluorophosphate. Toxicol. Sci. 2010, 116, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Fujikawa, D.G. Programmed mechanisms of status epilepticus-induced neuronal necrosis. Epilepsia Open 2023, 8, S25–S34. [Google Scholar] [CrossRef] [PubMed]
- Prathiksha, J.; Narasimhamurthy, R.K.; Dsouza, H.S.; Mumbrekar, K.D. Organophosphate pesticide-induced toxicity through DNA damage and DNA repair mechanisms. Mol. Biol. Rep. 2023, 50, 5465–5479. [Google Scholar] [CrossRef]
- Fink, S.L.; Cookson, B.T. Apoptosis, pyroptosis, and necrosis: Mechanistic description of dead and dying eukaryotic cells. Infect. Immun. 2005, 73, 1907–1916. [Google Scholar] [CrossRef]
- Pinton, P.; Giorgi, C.; Siviero, R.; Zecchini, E.; Rizzuto, R. Calcium and apoptosis: ER-mitochondria Ca2+ transfer in the control of apoptosis. Oncogene 2008, 27, 6407–6418. [Google Scholar] [CrossRef]
- Dingledine, R.; Varvel, N.H.; Dudek, F.E. When and how do seizures kill neurons, and is cell death relevant to epileptogenesis? Adv. Exp. Med. Biol. 2014, 813, 109–122. [Google Scholar]
- Du, K.; He, M.; Zhao, D.; Wang, Y.; Ma, C.; Liang, H.; Wang, W.; Min, D.; Xue, L.; Guo, F. Mechanism of cell death pathways in status epilepticus and related therapeutic agents. Biomed. Pharmacother. 2022, 149, 112875. [Google Scholar] [CrossRef] [PubMed]
- Niquet, J.; Auvin, S.; Archie, M.; Seo, D.W.; Allen, S.; Sankar, R.; Wasterlain, C.W. Status pilepticus triggers caspase-3 activation and necrosis in the immature rat brain. Epilepsia 2007, 48, 1203–1206. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Meraz, M.L.; Wasterlain, C.G.; Rocha, L.; Allen, S.; Niquet, J. Vulnerability of postnatal hippocampal neurons to seizures varies regionally with their maturational stage. Neurobiol. Dis. 2010, 37, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Niquet, J.; Lopez-Meraz, M.L.; Wasterlain, C.G. Programmed Necrosis After Status Epilepticus. In Jasper’s Basic Mechanisms of the Epilepsies, 4th ed.; Noebels, J.L., Avoli, M., Rogawski, M.A., Olsen, R.W., Delgado-Escueta, A.V., Eds.; National Center for Biotechnology Information (US): Bethesda, MD, USA, 2012; pp. 377–386. [Google Scholar]
- Ding, S.; Fellin, T.; Zhu, Y.; Lee, S.Y.; Auberson, Y.P.; Meaney, D.F.; Coulter, D.A.; Carmignoto, G.; Haydon, P.G. Enhanced astrocytic Ca2+ signals contribute to neuronal excitotoxicity after status epilepticus. J. Neurosci. 2007, 27, 10674–10684. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Rodríguez, J.J.; Parpura, V. Calcium signalling in astroglia. Mol. Cell Endocrinol. 2012, 353, 45–56. [Google Scholar] [CrossRef]
- Mahmoud, S.; Gharagozloo, M.; Simard, C.; Gris, D. Astrocytes Maintain Glutamate Homeostasis in the CNS by Controlling the Balance between Glutamate Uptake and Release. Cells 2019, 8, 184. [Google Scholar] [CrossRef]
- Vezzani, A.; Aronica, E.; Mazarati, A.; Pittman, Q.J. Epilepsy and brain inflammation. Exp. Neurol. 2013, 244, 11–21. [Google Scholar] [CrossRef]
- Vargas-Sánchez, K.; Mogilevskaya, M.; Rodríguez-Pérez, J.; Rubiano, M.G.; Javela, J.J.; González-Reyes, R.E. Astroglial role in the pathophysiology of status epilepticus: An overview. Oncotarget 2018, 9, 26954–26976. [Google Scholar] [CrossRef]
- Banks, C.N.; Lein, P.J. A review of experimental evidence linking neurotoxic organophosphorus compounds and inflammation. Neurotoxicology 2012, 33, 575–584. [Google Scholar] [CrossRef]
- Pulkrabkova, L.; Svobodova, B.; Konecny, J.; Kobrlova, T.; Muckova, L.; Janousek, J.; Pejchal, J.; Korabecny, J.; Soukup, O. Neurotoxicity evoked by organophosphates and available countermeasures. Arch. Toxicol. 2023, 97, 39–72. [Google Scholar] [CrossRef]
- López-Meraz, M.L.; Álvarez-Croda, D.M. Microglia and status epilepticus in the immature brain. Epilepsia Open 2023, 8, S73–S81. [Google Scholar] [CrossRef] [PubMed]
- Vezzani, A.; French, J.; Bartfai, T.; Baram, T.Z. The role of inflammation in epilepsy. Nat. Rev. Neurol. 2011, 7, 31–40. [Google Scholar] [CrossRef]
- Galic, M.A.; Riazi, K.; Pittman, Q.J. Cytokines and brain excitability. Front. Neuroendocrinol. 2012, 33, 116–125. [Google Scholar] [CrossRef]
- Putra, M.; Sharma, S.; Gage, M.; Gasser, G.; Hinojo-Perez, A.; Olson, A.; Gregory-Flores, A.; Puttachary, S.; Wang, C.; Anantharam, V.; et al. Inducible nitric oxide synthase inhibitor, 1400W, mitigates DFP-induced long-term neurotoxicity in the rat model. Neurobiol. Dis. 2020, 133, 104443. [Google Scholar] [CrossRef]
- Lin, T.K.; Chen, S.D.; Lin, K.J.; Chuang, Y.C. Seizure-Induced Oxidative Stress in Status Epilepticus: Is Antioxidant Beneficial? Antioxidants 2020, 9, 1029. [Google Scholar] [CrossRef] [PubMed]
- Gorter, J.A.; van Vliet, E.A.; Aronica, E. Status epilepticus, blood-brain barrier disruption, inflammation, and epileptogenesis. Epilepsy Behav. 2015, 49, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Mukandala, G.; Tynan, R.; Lanigan, S.; O’Connor, J.J. The Effects of Hypoxia and Inflammation on Synaptic Signaling in the CNS. Brain Sci. 2016, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Sha, S.; Tan, J.; Miao, Y.; Zhang, Q. The Role of Autophagy in Hypoxia-Induced Neuroinflammation. DNA Cell Biol. 2021, 40, 733–739. [Google Scholar] [CrossRef]
- Guignet, M.; Lein, P.J. Neuroinflammation in organophosphate-induced neurotoxicity. Adv. Neurotoxicol. 2019, 3, 35–79. [Google Scholar]
- Tian, D.S.; Peng, J.; Murugan, M.; Feng, L.J.; Liu, J.L.; Eyo, U.B.; Zhou, L.J.; Mogilevsky, R.; Wang, W.; Wu, L.J. Chemokine CCL2-CCR2 Signaling Induces Neuronal Cell Death via STAT3 Activation and IL-1β Production after Status Epilepticus. J. Neurosci. 2017, 37, 7878–7892. [Google Scholar] [CrossRef]
- Wolinski, P.; Ksiazek-Winiarek, D.; Glabinski, A. Cytokines and Neurodegeneration in Epileptogenesis. Brain Sci. 2022, 12, 380. [Google Scholar] [CrossRef] [PubMed]
- Rettenbeck, M.L.; von Rüden, E.L.; Bienas, S.; Carlson, R.; Stein, V.M.; Tipold, A.; Potschka, H. Microglial ROS production in an electrical rat post-status epilepticus model of epileptogenesis. Neurosci. Lett. 2015, 599, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Neher, J.J.; Neniskyte, U.; Brown, G.C. Primary phagocytosis of neurons by inflamed microglia: Potential roles in neurodegeneration. Front. Pharmacol. 2012, 3, 27. [Google Scholar] [CrossRef] [PubMed]
- Yanuck, S.F. Microglial Phagocytosis of Neurons: Diminishing Neuronal Loss in Traumatic, Infectious, Inflammatory, and Autoimmune CNS Disorders. Front. Psychiatry 2019, 10, 712. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.Q.; Qian, W.; Suzuki, K.; McNamara, J.O. Formation of complement membrane attack complex in mammalian cerebral cortex evokes seizures and neurodegeneration. J. Neurosci. 2003, 23, 955–960. [Google Scholar] [CrossRef] [PubMed]
- Orsini, F.; De Blasio, D.; Zangari, R.; Zanier, E.R.; De Simoni, M.G. Versatility of the complement system in neuroinflammation, neurodegeneration and brain homeostasis. Front. Cell Neurosci. 2014, 8, 380. [Google Scholar] [CrossRef]
- Ziabska, K.; Ziemka-Nalecz, M.; Pawelec, P.; Sypecka, J.; Zalewska, T. Aberrant Complement System Activation in Neurological Disorders. Int. J. Mol. Sci. 2021, 22, 4675. [Google Scholar] [CrossRef]
- Fabene, P.F.; Merigo, F.; Galiè, M.; Benati, D.; Bernardi, P.; Farace, P.; Nicolato, E.; Marzola, P.; Sbarbati, A. Pilocarpine-induced status epilepticus in rats involves ischemic and excitotoxic mechanisms. PLoS ONE 2007, 2, 1105. [Google Scholar] [CrossRef]
- Millis, R.M.; Archer, P.W.; Whittaker, J.A.; Trouth, C.O. The role of hypoxia in organophosphorus nerve agent intoxication. Neurotoxicology 1988, 9, 273–285. [Google Scholar]
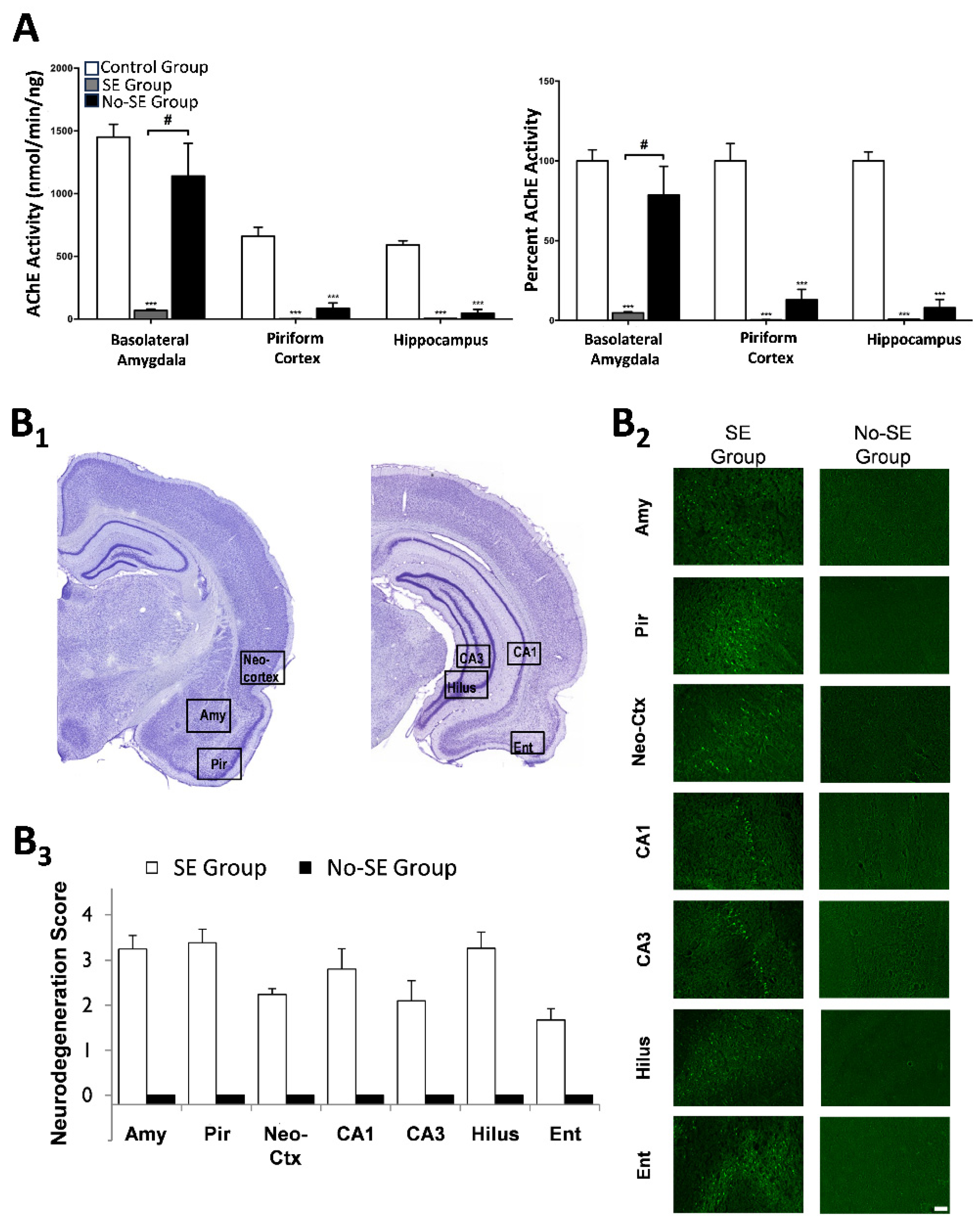
- Prager, E.M.; Aroniadou-Anderjaska, V.; Almeida-Suhett, C.P.; Figueiredo, T.H.; Apland, J.P.; Rossetti, F.; Olsen, C.H.; Braga, M.F. The recovery of acetylcholinesterase activity and the progression of neuropathological and pathophysiological alterations in the rat basolateral amygdala after soman-induced status epilepticus: Relation to anxiety-like behavior. Neuropharmacology 2014, 81, 64–74. [Google Scholar] [CrossRef]
- Sengupta, P. The laboratory rat: Relating its age with human’s. Int. J. Prev. Med. 2013, 4, 624–630. [Google Scholar] [PubMed]
- Agoston, D.V. How to Translate Time? The Temporal Aspect of Human and Rodent Biology. Front. Neurol. 2017, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Karalliedde, L.; Baker, D.; Marrs, T.C. Organophosphate-induced intermediate syndrome: Aetiology and relationships with myopathy. Toxicol. Rev. 2006, 25, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Rosas-Ballina, M.; Tracey, K.J. Cholinergic control of inflammation. J. Intern. Med. 2009, 265, 663–679. [Google Scholar] [CrossRef]
- Astiz, M.; Diz-Chaves, Y.; Garcia-Segura, L.M. Sub-chronic exposure to the insecticide dimethoate induces a proinflammatory status and enhances the neuroinflammatory response to bacterial lypopolysaccharide in the hippocampus and striatum of male mice. Toxicol. Appl. Pharmacol. 2013, 272, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Pearson, J.N.; Patel, M. The role of oxidative stress in organophosphate and nerve agent toxicity. Ann. N.Y. Acad. Sci. 2016, 1378, 17–24. [Google Scholar] [CrossRef]
- Farkhondeh, T.; Mehrpour, O.; Forouzanfar, F.; Roshanravan, B.; Samarghandian, S. Oxidative stress and mitochondrial dysfunction in organophosphate pesticide-induced neurotoxicity and its amelioration: A review. Environ. Sci. Pollut. Res. Int. 2020, 27, 24799–24814. [Google Scholar] [CrossRef]
- Ranjbar, A.; Pasalar, P.; Abdollahi, M. Induction of oxidative stress and acetylcholinesterase inhibition in organophosphorous pesticide manufacturing workers. Hum. Exp. Toxicol. 2002, 21, 179–182. [Google Scholar] [CrossRef]
- Rohlman, D.S.; Anger, W.K.; Lein, P.J. Correlating neurobehavioral performance with biomarkers of organophosphorous pesticide exposure. Neurotoxicology 2011, 32, 268–276. [Google Scholar] [CrossRef]
- Tsai, Y.H.; Lein, P.J. Mechanisms of organophosphate neurotoxicity. Curr. Opin. Toxicol. 2021, 26, 49–60. [Google Scholar] [CrossRef]
- Phillips, K.F.; Santos, E.; Blair, R.E.; Deshpande, L.S. Targeting Intracellular Calcium Stores Alleviates Neurological Morbidities in a DFP-Based Rat Model of Gulf War Illness. Toxicol. Sci. 2019, 169, 567–578. [Google Scholar] [CrossRef] [PubMed]
- Mense, S.M.; Sengupta, A.; Lan, C.; Zhou, M.; Bentsman, G.; Volsky, D.J.; Whyatt, R.M.; Perera, F.P.; Zhang, L. The common insecticides cyfluthrin and chlorpyrifos alter the expression of a subset of genes with diverse functions in primary human astrocytes. Toxicol. Sci. 2006, 93, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Pope, C.N. Organophosphorus pesticides: Do they all have the same mechanism of toxicity? J. Toxicol. Environ. Health B Crit. Rev. 1999, 2, 161–181. [Google Scholar] [CrossRef] [PubMed]
- Aroniadou-Anderjaska, V.; Figueiredo, T.H.; Apland, J.P.; Braga, M.F. Targeting the glutamatergic system to counteract organophosphate poisoning: A novel therapeutic strategy. Neurobiol. Dis. 2020, 133, 104406. [Google Scholar] [CrossRef] [PubMed]
- Gorecki, L.; Soukup, O.; Korabecny, J. Countermeasures in organophosphorus intoxication: Pitfalls and prospects. Trends Pharmacol. Sci. 2022, 43, 593–606. [Google Scholar] [CrossRef]
- Reddy, D.S.; Abeygunaratne, H.N. Experimental and Clinical Biomarkers for Progressive Evaluation of Neuropathology and Therapeutic Interventions for Acute and Chronic Neurological Disorders. Int. J. Mol. Sci. 2022, 23, 11734. [Google Scholar] [CrossRef]
- Vasanthi, S.S.; Rao, N.S.; Samidurai, M.; Massey, N.; Meyer, C.; Gage, M.; Kharate, M.; Almanza, A.; Wachter, L.; Mafuta, C.; et al. Disease-modifying effects of a glial-targeted inducible nitric oxide synthase inhibitor (1400W) in mixed-sex cohorts of a rat soman (GD) model of epilepsy. J. Neuroinflamm. 2023, 20, 163. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aroniadou-Anderjaska, V.; Figueiredo, T.H.; de Araujo Furtado, M.; Pidoplichko, V.I.; Braga, M.F.M. Mechanisms of Organophosphate Toxicity and the Role of Acetylcholinesterase Inhibition. Toxics 2023, 11, 866. https://doi.org/10.3390/toxics11100866
Aroniadou-Anderjaska V, Figueiredo TH, de Araujo Furtado M, Pidoplichko VI, Braga MFM. Mechanisms of Organophosphate Toxicity and the Role of Acetylcholinesterase Inhibition. Toxics. 2023; 11(10):866. https://doi.org/10.3390/toxics11100866
Chicago/Turabian StyleAroniadou-Anderjaska, Vassiliki, Taiza H. Figueiredo, Marcio de Araujo Furtado, Volodymyr I. Pidoplichko, and Maria F. M. Braga. 2023. "Mechanisms of Organophosphate Toxicity and the Role of Acetylcholinesterase Inhibition" Toxics 11, no. 10: 866. https://doi.org/10.3390/toxics11100866
APA StyleAroniadou-Anderjaska, V., Figueiredo, T. H., de Araujo Furtado, M., Pidoplichko, V. I., & Braga, M. F. M. (2023). Mechanisms of Organophosphate Toxicity and the Role of Acetylcholinesterase Inhibition. Toxics, 11(10), 866. https://doi.org/10.3390/toxics11100866




