Artificial Intelligence/Machine Learning-Driven Small Molecule Repurposing via Off-Target Prediction and Transcriptomics
Abstract
:1. Introduction
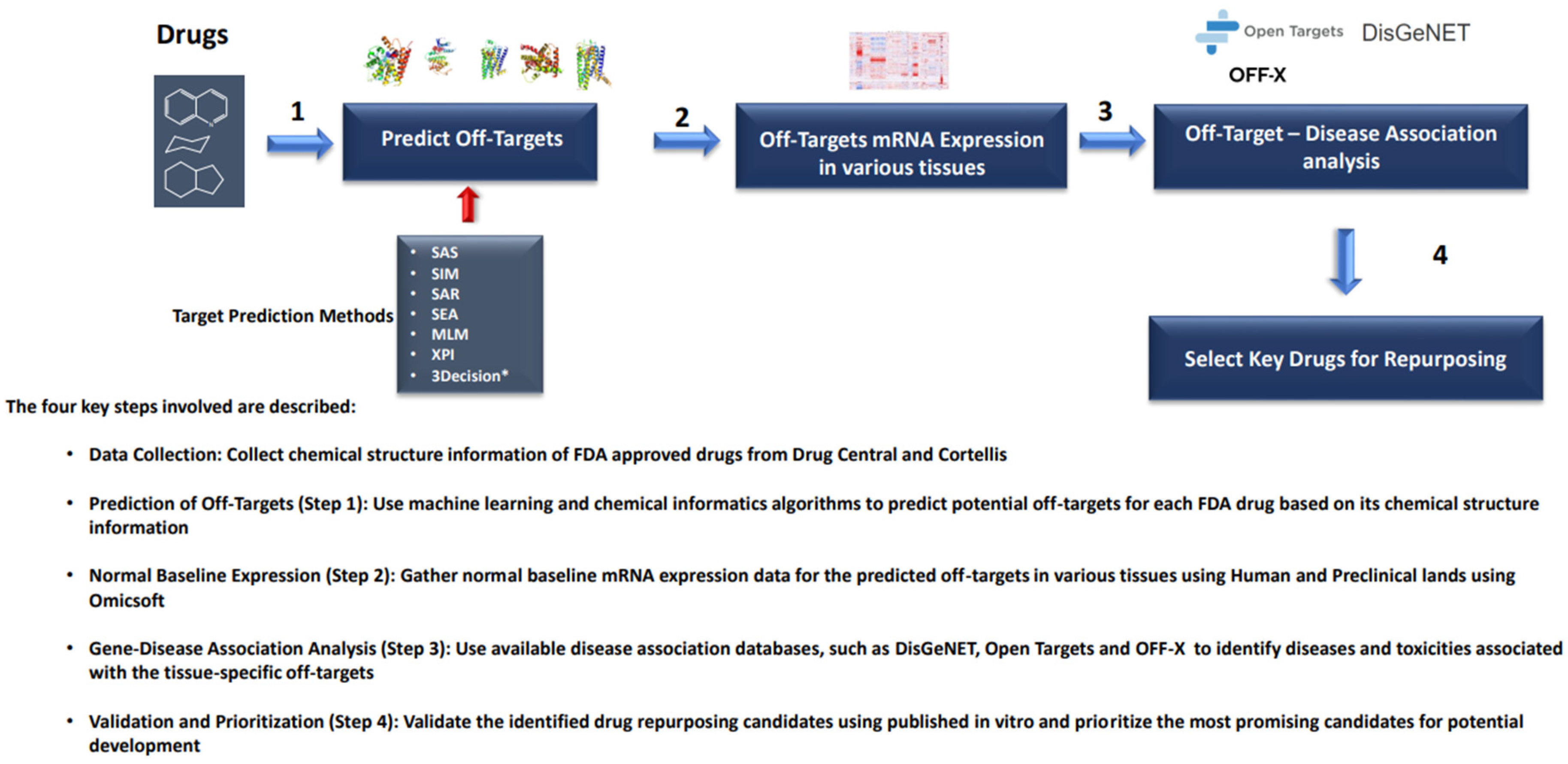
2. Materials and Methods
3. Results
3.1. Chemical Space Assessment in 2677 FDA-approved Drugs: Implications for Discovery and Repurposing
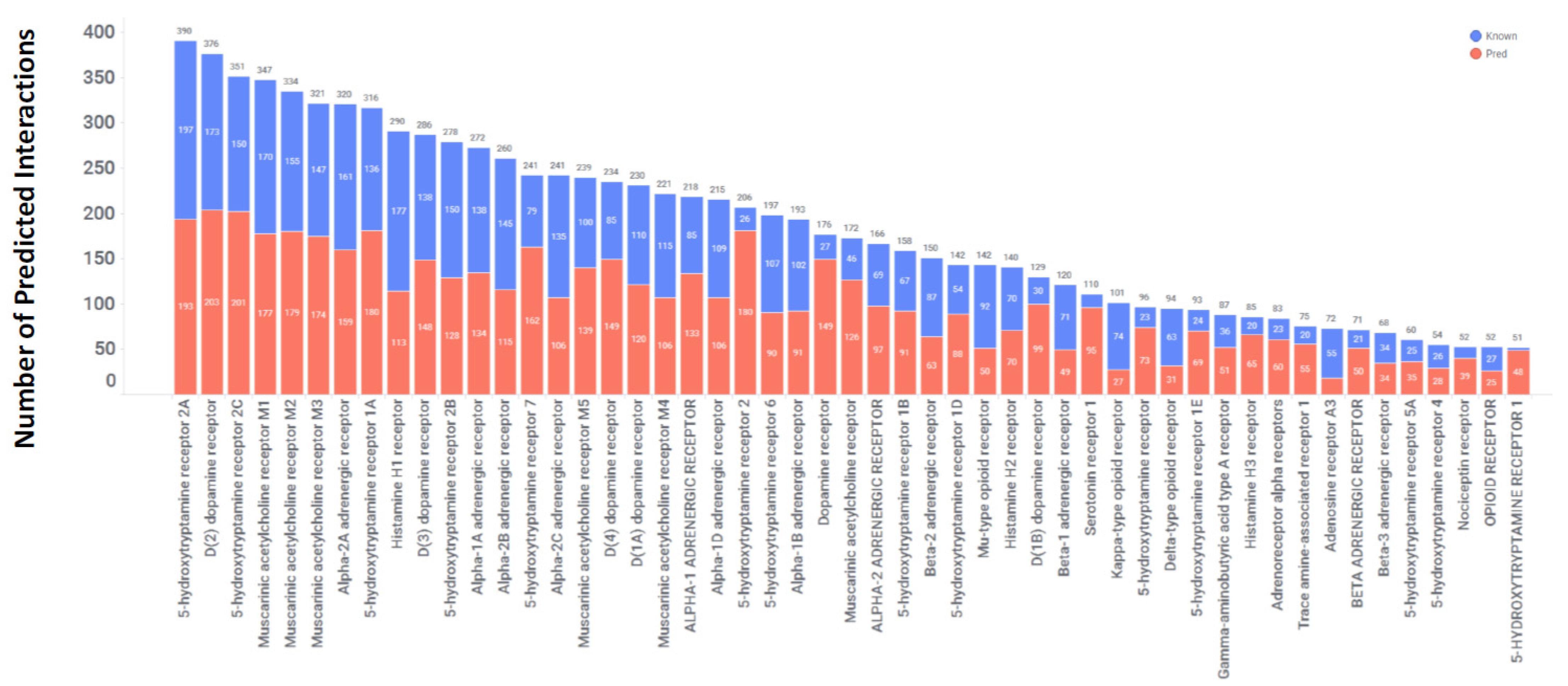
3.2. Predicted Off-Target Interactions in 2766 FDA-approved Drugs: Exploring Potential Repurposing Opportunities
3.3. GPCRs
3.4. Enzymes
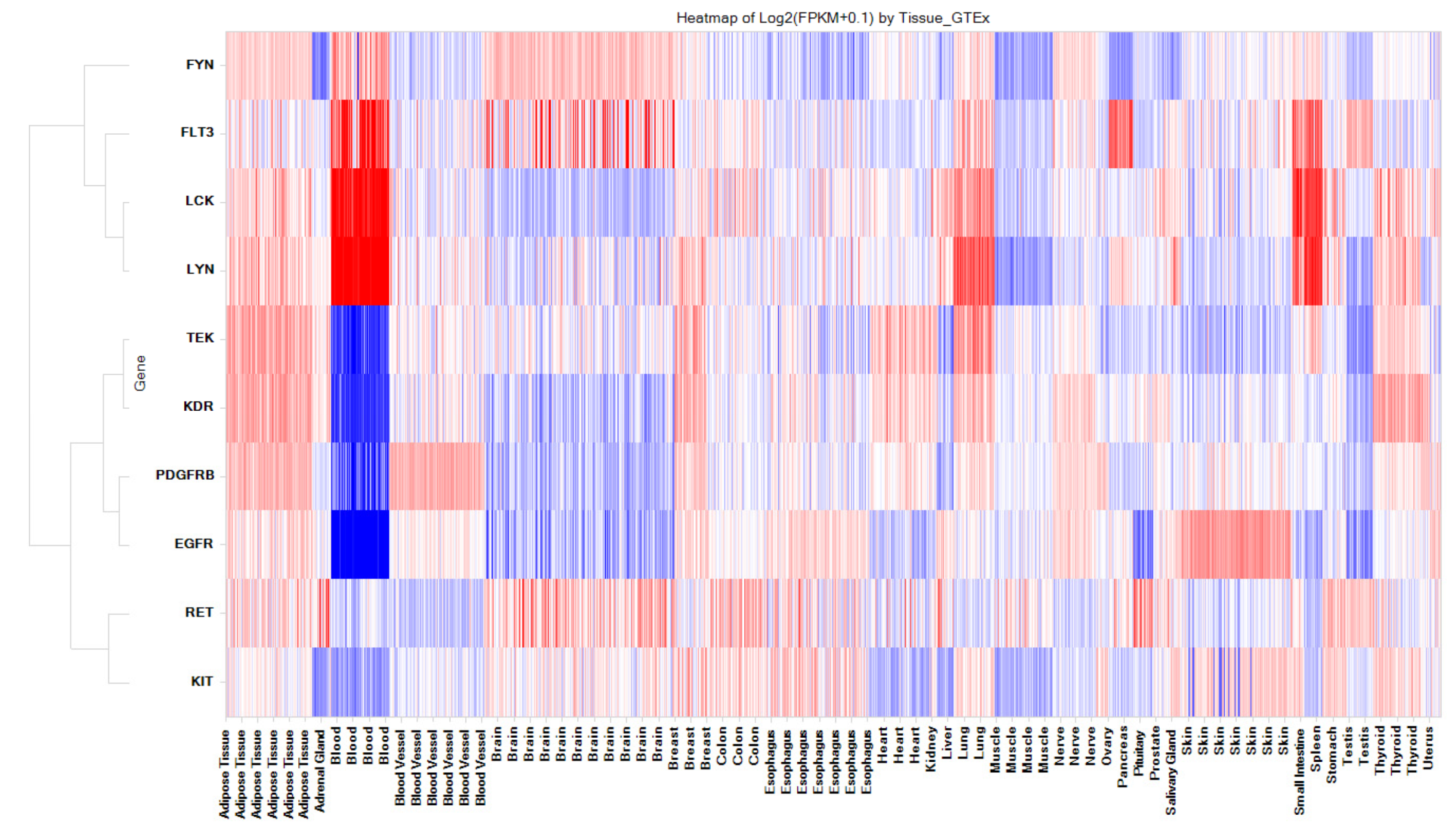
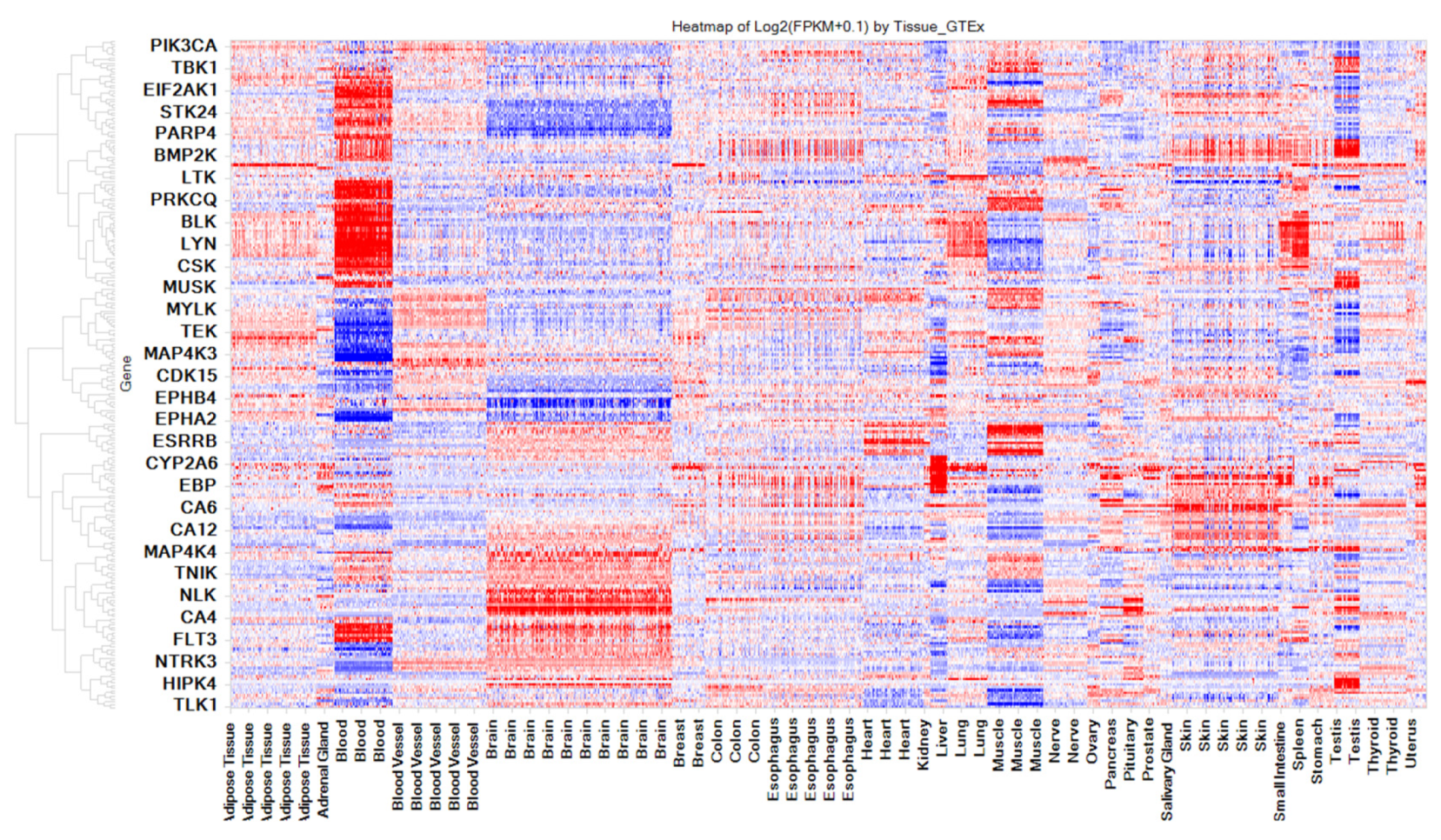
3.5. Kinases
3.6. Ion Channels
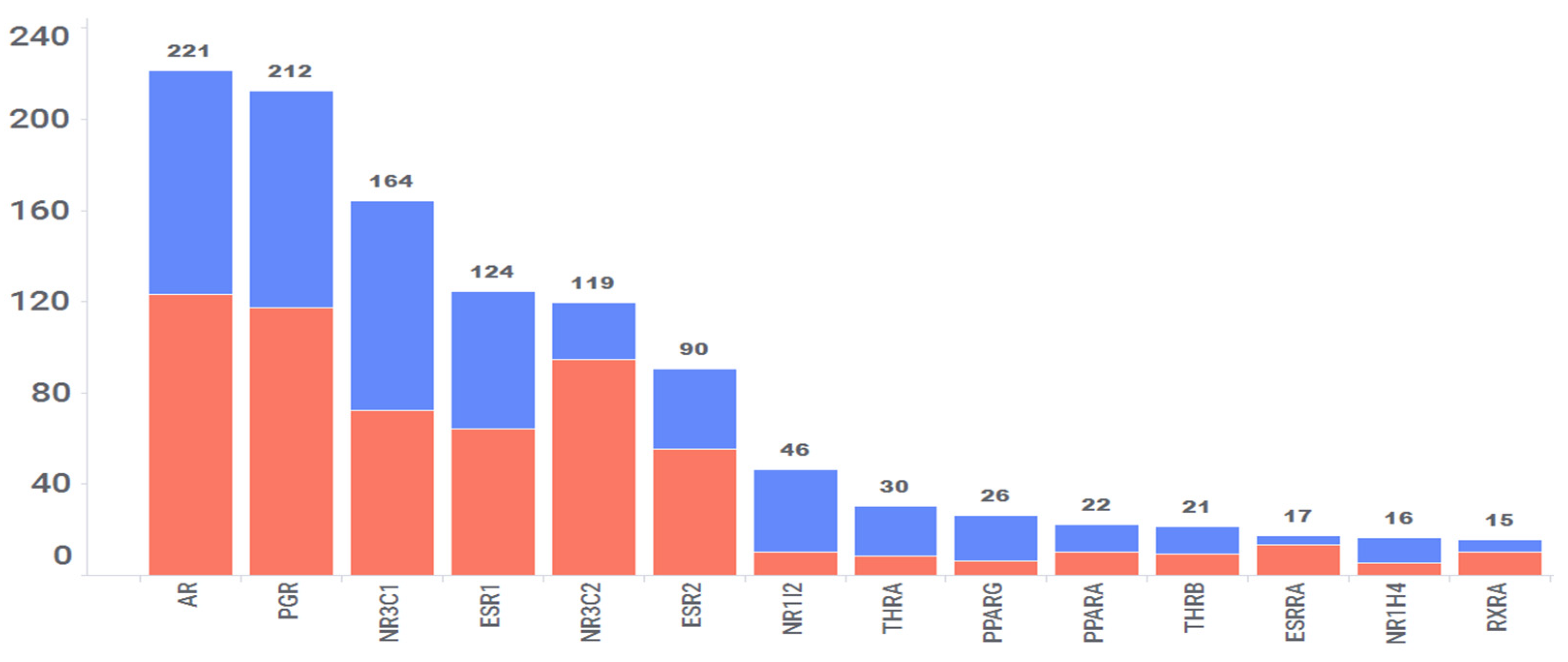
3.7. Nuclear Receptors
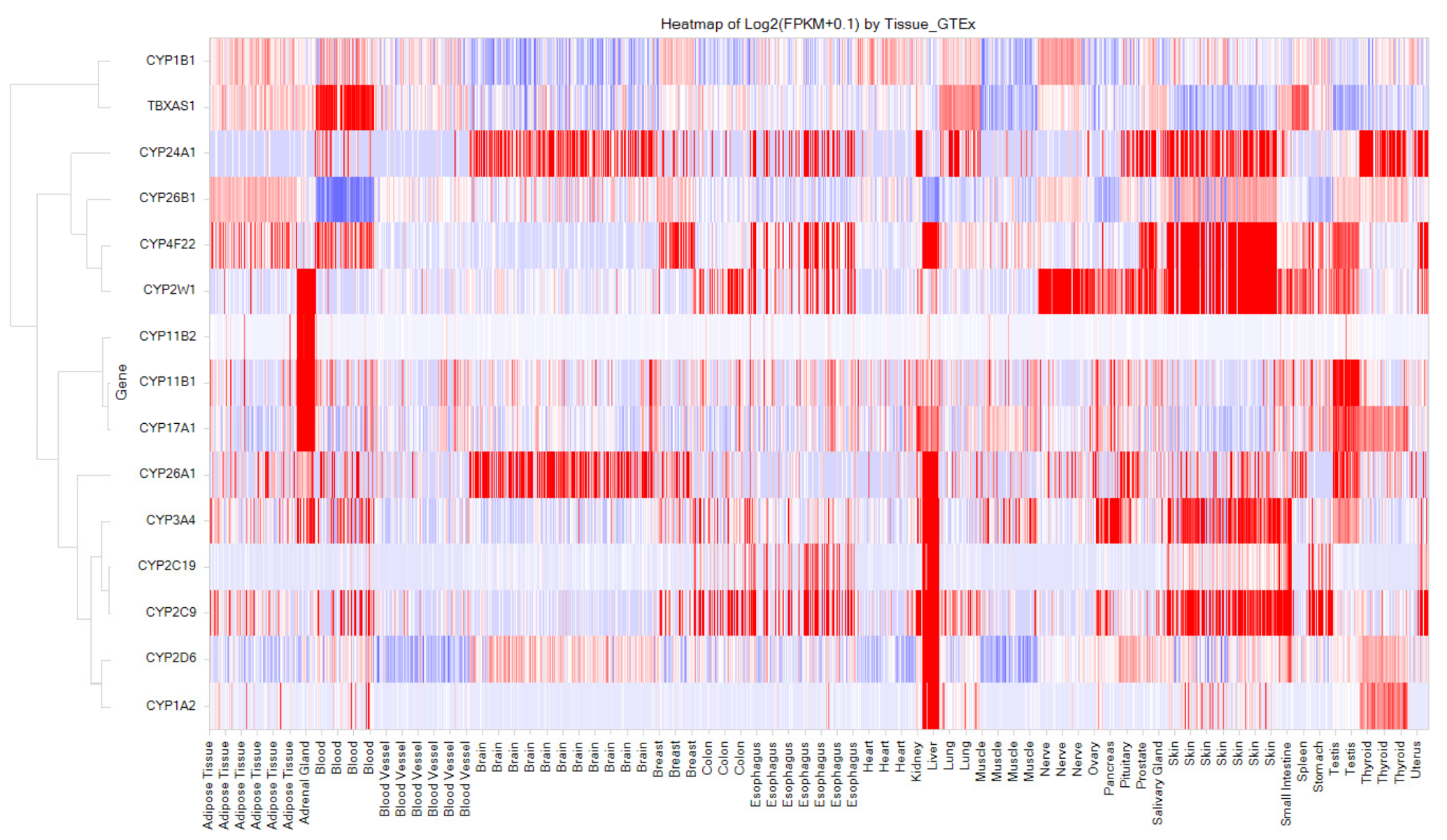
3.8. Cytochromes
3.9. Exploring the Clinically Relevant Off-Target Interactions of 14 Approved Drugs for Repurposing
4. Discussion
4.1. Predicted Interactions and Repurposing Opportunities
4.2. Repurposing GPCR and Kinases
4.3. Repurposing Human Drugs for Animals
4.3.1. The Drug Repurposing Role in the Due Diligence Process
4.3.2. Drug Repurposing in Compound Life Cycle Management
4.3.3. Limitations to Drug Repurposing
4.3.4. Challenges in Drug Repurposing
4.3.5. Failed Attempts
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Holenz, J.; Stoy, P. Advances in Lead Generation. Bioorg. Med. Chem. Lett. 2019, 29, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Drews, J. Drug Discovery: A Historical Perspective. Science 2000, 287, 1960–1964. [Google Scholar] [CrossRef]
- Bleicher, K.H.; Böhm, H.-J.; Müller, K.; Alanine, A.I. Hit and Lead Generation: Beyond High-Throughput Screening. Nat. Rev. Drug Discov. 2003, 2, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Ralston, S. Pre-Development Attrition of Pharmaceuticals: How to Identify the Bad Actors Early. Toxicol. Sci. 2017, 150, 2323. [Google Scholar]
- Reiher, C.A.; Schuman, D.P.; Simmons, N.; Wolkenberg, S.E. Trends in Hit-to-Lead Optimization Following DNA-Encoded Library Screens. ACS Med. Chem. Lett. 2021, 12, 343–350. [Google Scholar] [CrossRef]
- Cerchia, C.; Lavecchia, A. New Avenues in Artificial-Intelligence-Assisted Drug Discovery. Drug Discov. Today 2023, 28, 103516. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.; Kim, T.Y.; Joung, I.; Song, S.O. Industrializing AI/ML during the End-to-End Drug Discovery Process. Curr. Opin. Struct. Biol. 2023, 79, 102528. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Olson, A.J. Using AutoDock for Ligand-Receptor Docking. Curr. Protoc. Bioinform. 2008. [Google Scholar] [CrossRef]
- Moore, H.; Margreitter, C.; Janet, J.P.; Engkvist, O.; de Groot, B.; Gapsys, V. Automated Relative Binding Free Energy Calculations: From SMILES to ΔΔG. Commun. Chem. 2022, 6, 82. [Google Scholar] [CrossRef]
- Muegge, I.; Hu, Y. Recent Advances in Alchemical Binding Free Energy Calculations for Drug Discovery. ACS Med. Chem. Lett. 2023, 14, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Lobo, S. Is There Enough Focus on Lipophilicity in Drug Discovery? Expert Opin. Drug Discov. 2020, 15, 261–263. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.S. Preclinical Pharmacokinetics: An Approach towards Safer and Efficacious Drugs. Curr. Drug Metab. 2006, 7, 165–182. [Google Scholar] [CrossRef] [PubMed]
- DiMasi, J.A.; Grabowski, H.G.; Hansen, R.W. Innovation in the Pharmaceutical Industry: New Estimates of R&D Costs. J. Health Econ. 2016, 47, 20–33. [Google Scholar] [CrossRef]
- Madsen, E.S.; Wu, Y. Restructuring of the Pharmaceutical Industry. SSRN Electron. J. 2016. [Google Scholar] [CrossRef]
- Schuhmacher, A. Analysis of Pharma R&D Productivity—A New Perspective Needed. Drug Discov. Today 2023, 28, 103726. [Google Scholar]
- Vleet, T.R.V.; Liguori, M.J.; Lynch, J.J.; Rao, M.; Warder, S. Screening Strategies and Methods for Better Off-Target Liability Prediction and Identification of Small-Molecule Pharmaceuticals. SLAS Discov. 2018, 24, 1–24. [Google Scholar] [CrossRef]
- He, H.; Duo, H.; Hao, Y.; Zhang, X.; Zhou, X.; Zeng, Y.; Li, Y.; Li, B. Computational Drug Repurposing by Exploiting Large-Scale Gene Expression Data: Strategy, Methods and Applications. Comput. Biol. Med. 2023, 155, 106671. [Google Scholar] [CrossRef]
- Olgen, S. A Prospective Overview of Drug Repurposing in Drug Discovery and Development. Curr. Med. Chem. 2019, 26, 5338–5339. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, K.K.; Mazumder, R.; Debnath, A.; Patel, M. A Brief Study on Drug Repurposing: New Way of Boosting Drug Discovery. Lett. Drug Des. Discov. 2023, 20, 264–278. [Google Scholar] [CrossRef]
- Kulkarni, V.S.; Alagarsamy, V.; Solomon, V.R.; Jose, P.A.; Murugesan, S. Drug Repurposing: An Effective Tool in Modern Drug Discovery. Russ. J. Bioorg. Chem. 2023, 49, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Sonaye, H.V.; Sheikh, R.Y.; Doifode, C.A. Drug Repurposing: Iron in the Fire for Older Drugs. Biomed. Pharmacother. 2021, 141, 111638. [Google Scholar] [CrossRef] [PubMed]
- Park, K. A Review of Computational Drug Repurposing. Transl. Clin. Pharmacol. 2019, 27, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wu, F.-X.; Li, C.-Z.; Jia, C.-Y.; Su, S.-W.; Hao, G.-F.; Yang, G.-F. ACID: A Free Tool for Drug Repurposing Using Consensus Inverse Docking Strategy. J. Cheminform. 2019, 11, 73. [Google Scholar] [CrossRef]
- Rao, M.S.; Gupta, R.; Liguori, M.J.; Hu, M.; Huang, X.; Mantena, S.R.; Mittelstadt, S.W.; Blomme, E.A.G.; Vleet, T.R.V. Novel Computational Approach to Predict Off-Target Interactions for Small Molecules. Front. Big Data 2019, 2, 25. [Google Scholar] [CrossRef]
- Willett, P. Similarity Methods in Chemoinformatics. Annu. Rev. Inf. Sci. Technol. 2009, 43, 1–117. [Google Scholar] [CrossRef]
- Gregori-Puigjane, E.; Mestres, J. A Ligand-Based Approach to Mining the Chemogenomic Space of Drugs. Comb. Chem. High Throughput Screen. 2008, 11, 669–676. [Google Scholar] [CrossRef]
- Vidal, D.; Mestres, J. In Silico Receptorome Screening of Antipsychotic Drugs. Mol. Inform. 2010, 29, 543–551. [Google Scholar] [CrossRef]
- Keiser, M.J.; Setola, V.; Irwin, J.J.; Laggner, C.; Abbas, A.; Hufeisen, S.J.; Jensen, N.H.; Kuijer, M.B.; Matos, R.C.; Tran, T.B.; et al. Predicting New Molecular Targets for Known Drugs. Nature 2009, 462, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, F.; Matter, H.; Hessler, G.; Czich, A. Predictive in Silico Off-Target Profiling in Drug Discovery. Future Med. Chem. 2014, 6, 295–317. [Google Scholar] [CrossRef]
- Kotsiantis, S.B.; Zaharakis, I.D.; Pintelas, P.E. Machine Learning: A Review of Classification and Combining Techniques. Artif. Intell. Rev. 2006, 26, 159–190. [Google Scholar] [CrossRef]
- Rao, M.S.; Vleet, T.R.V.; Ciurlionis, R.; Buck, W.R.; Mittelstadt, S.W.; Blomme, E.A.G.; Liguori, M.J. Comparison of RNA-Seq and Microarray Gene Expression Platforms for the Toxicogenomic Evaluation of Liver From Short-Term Rat Toxicity Studies. Front. Genet. 2019, 9, 636. [Google Scholar] [CrossRef] [PubMed]
- Agamah, F.E.; Mazandu, G.K.; Hassan, R.; Bope, C.D.; Thomford, N.E.; Ghansah, A.; Chimusa, E.R. Computational/in Silico Methods in Drug Target and Lead Prediction. Brief. Bioinform. 2019, 21, 1663–1675. [Google Scholar] [CrossRef] [PubMed]
- Avram, S.; Bologa, C.G.; Holmes, J.; Bocci, G.; Wilson, T.B.; Nguyen, D.-T.; Curpan, R.; Halip, L.; Bora, A.; Yang, J.J.; et al. DrugCentral 2021 Supports Drug Discovery and Repositioning. Nucleic Acids Res. 2020, 49, D1160–D1169. [Google Scholar] [CrossRef] [PubMed]
- Piñero, J.; Bravo, À.; Queralt-Rosinach, N.; Gutiérrez-Sacristán, A.; Deu-Pons, J.; Centeno, E.; García-García, J.; Sanz, F.; Furlong, L.I. DisGeNET: A Comprehensive Platform Integrating Information on Human Disease-Associated Genes and Variants. Nucleic Acids Res. 2017, 45, D833–D839. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A. Lead- and Drug-like Compounds: The Rule-of-Five Revolution. Drug Discov. Today 2004, 1, 337–341. [Google Scholar] [CrossRef]
- Bassani, D.; Moro, S. Past, Present, and Future Perspectives on Computer-Aided Drug Design Methodologies. Molecules 2023, 28, 3906. [Google Scholar] [CrossRef] [PubMed]
- Kralj, S.; Jukič, M.; Bren, U. Molecular Filters in Medicinal Chemistry. Encyclopedia 2023, 3, 501–511. [Google Scholar] [CrossRef]
- Faller, B.; Ottaviani, G.; Ertl, P.; Berellini, G.; Collis, A. Evolution of the Physicochemical Properties of Marketed Drugs: Can History Foretell the Future? Drug Discov. Today 2011, 16, 976–984. [Google Scholar] [CrossRef]
- Hughes, J.D.; Blagg, J.; Price, D.A.; Bailey, S.; DeCrescenzo, G.A.; Devraj, R.V.; Ellsworth, E.; Fobian, Y.M.; Gibbs, M.E.; Gilles, R.W.; et al. Physiochemical Drug Properties Associated with in Vivo Toxicological Outcomes. Bioorg. Med. Chem. Lett. 2008, 18, 4872–4875. [Google Scholar] [CrossRef]
- Waring, M.J.; Arrowsmith, J.; Leach, A.R.; Leeson, P.D.; Mandrell, S.; Owen, R.M.; Pairaudeau, G.; Pennie, W.D.; Pickett, S.D.; Wang, J.; et al. An Analysis of the Attrition of Drug Candidates from Four Major Pharmaceutical Companies. Nat. Rev. Drug Discov. 2015, 14, 475–486. [Google Scholar] [CrossRef]
- Hauser, A.S.; Attwood, M.M.; Rask-Andersen, M.; Schiöth, H.B.; Gloriam, D.E. Trends in GPCR Drug Discovery: New Agents, Targets and Indications. Nat. Rev. Drug Discov. 2017, 16, 829–842. [Google Scholar] [CrossRef]
- Copeland, R.A. Evaluation of Enzyme Inhibitors in Drug Discovery: A Guide for Medicinal Chemists and Pharmacologists. Methods Biochem. Anal. 2005, 46, 1–265. [Google Scholar] [PubMed]
- Rufer, A.C. Drug Discovery for Enzymes. Drug Discov. Today 2021, 26, 875–886. [Google Scholar] [CrossRef] [PubMed]
- Epstein, F.H.; Ackerman, M.J.; Clapham, D.E. Ion Channels—Basic Science and Clinical Disease. N. Engl. J. Med. 1997, 336, 1575–1586. [Google Scholar] [CrossRef]
- Jentsch, T.J.; Hübner, C.A.; Fuhrmann, J.C. Ion Channels: Function Unravelled by Dysfunction. Nat. Cell Biol. 2004, 6, 1039–1047. [Google Scholar] [CrossRef]
- Imbrici, P.; Liantonio, A.; Camerino, G.M.; Bellis, M.D.; Camerino, C.; Mele, A.; Giustino, A.; Pierno, S.; Luca, A.D.; Tricarico, D.; et al. Therapeutic Approaches to Genetic Ion Channelopathies and Perspectives in Drug Discovery. Front. Pharmacol. 2016, 7, 121. [Google Scholar] [CrossRef]
- Kumar, R.; O’Malley, B.W. Nuclear Receptor Coregulators and Human Diseases; World Scientific Publishing Connecting Great Minds: Singapore, 2008; pp. 1–11. [Google Scholar] [CrossRef]
- Robinson-Rechavi, M.; Carpentier, A.-S.; Duffraisse, M.; Laudet, V. How Many Nuclear Hormone Receptors Are There in the Human Genome? Trends Genet. 2001, 17, 554–556. [Google Scholar] [CrossRef]
- Liston, D.R.; Davis, M. Data from Clinically Relevant Concentrations of Anticancer Drugs: A Guide for Nonclinical Studies; American Association for Cancer Research: Philadelphia, PA, USA, 2023. [Google Scholar] [CrossRef]
- Galatage, S.T.; Manjappa, A.S.; Waghmode, R.R.; Harale, S.S.; Katkar, R.B.; Desai, S.A.; Chopade, S.S.; Bille, K.S.; Watangi, R.U.; Kalebere, S.N.; et al. Drug Repurposing—Advances, Scopes and Opportunities in Drug Discovery; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Turabi, K.S.; Deshmukh, A.; Paul, S.; Swami, D.; Siddiqui, S.; Kumar, U.; Naikar, S.; Devarajan, S.; Basu, S.; Paul, M.K.; et al. Drug Repurposing—An Emerging Strategy in Cancer Therapeutics. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2022, 395, 1139–1158. [Google Scholar] [CrossRef]
- Cha, Y.; Erez, T.; Reynolds, I.J.; Kumar, D.; Ross, J.; Koytiger, G.; Kusko, R.; Zeskind, B.; Risso, S.; Kagan, E.; et al. Drug Repurposing from the Perspective of Pharmaceutical Companies. Br. J. Pharmacol. 2018, 175, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Roy, V. Repurposing Drugs: An Empowering Approach to Drug Discovery and Development. Drug Res. 2023; Online ahead of print. [Google Scholar] [CrossRef]
- Fetro, C. Connecting Academia and Industry for Innovative Drug Repurposing in Rare Diseases: It Is Worth a Try. Rare Dis. Orphan Drugs J. 2023, 2, 7. [Google Scholar] [CrossRef]
- Mullard, A. Drug Repurposing Programmes Get Lift off. Nat. Rev. Drug Discov. 2012, 11, 505–506. [Google Scholar] [CrossRef]
- Goldstein, I.; Burnett, A.L.; Rosen, R.C.; Park, P.W.; Stecher, V.J. The Serendipitous Story of Sildenafil: An Unexpected Oral Therapy for Erectile Dysfunction. Sex. Med. Rev. 2019, 7, 115–128. [Google Scholar] [CrossRef]
- Sardari, E.; Ebadi, A.; Razzaghi-Asl, N. In Silico Repurposing of CNS Drugs for Multiple Sclerosis. Mult. Scler. Relat. Disord. 2023, 73, 104622. [Google Scholar] [CrossRef]
- Nascimento, A.C.A.; Prudêncio, R.B.C.; Costa, I.G. Computational Methods for Drug Repurposing. Methods Mol. Biol. 2018, 1903, 281–289. [Google Scholar] [CrossRef]
- Halpin-Veszeleiova, K.; Hatfield, S.M. Therapeutic Targeting of Hypoxia-A2-Adenosinergic Pathway in COVID-19 Patients. Physiology 2022, 37, 46–52. [Google Scholar] [CrossRef]
- Zhou, Y.; Huang, Z.; Su, J.; Li, J.; Zhao, S.; Wu, L.; Zhang, J.; He, Y.; Zhang, G.; Tao, J.; et al. Benserazide Is a Novel Inhibitor Targeting PKM2 for Melanoma Treatment. Int. J. Cancer 2020, 147, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-Y.; Choi, J.-H.; Nam, J.-S. Targeting Cancer Stem Cells in Triple-Negative Breast Cancer. Cancers 2019, 11, 965. [Google Scholar] [CrossRef]
- Wang, Q.; Zhong, W.; Deng, L.; Lin, Q.; Lin, Y.; Liu, H.; Xu, L.; Lu, L.; Chen, Y.; Huang, J.; et al. The Expression and Prognostic Value of SUMO1-Activating Enzyme Subunit 1 and Its Potential Mechanism in Triple-Negative Breast Cancer. Front. Cell Dev. Biol. 2021, 9, 729211. [Google Scholar] [CrossRef] [PubMed]
- Galimberti, D.; Scarpini, E. Pioglitazone for the Treatment of Alzheimer’s Disease. Expert Opin. Investig. Drugs 2017, 26, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Imfeld, P.; Bodmer, M.; Jick, S.S.; Meier, C.R. Metformin, Other Antidiabetic Drugs, and Risk of Alzheimer’s Disease: A Population-Based Case–Control Study. J. Am. Geriatr. Soc. 2012, 60, 916–921. [Google Scholar] [CrossRef] [PubMed]
- Poor, S.R.; Ettcheto, M.; Cano, A.; Sanchez-Lopez, E.; Manzine, P.R.; Olloquequi, J.; Camins, A.; Javan, M. Metformin a Potential Pharmacological Strategy in Late Onset Alzheimer’s Disease Treatment. Pharmaceuticals 2021, 14, 890. [Google Scholar] [CrossRef] [PubMed]
- Bramante, C.T.; Buse, J.B.; Liebovitz, D.M.; Nicklas, J.M.; Puskarich, M.A.; Cohen, K.; Belani, H.K.; Anderson, B.J.; Huling, J.D.; Tignanelli, C.J.; et al. Outpatient Treatment of COVID-19 and Incidence of Post-COVID-19 Condition over 10 Months (COVID-OUT): A Multicentre, Randomised, Quadruple-Blind, Parallel-Group, Phase 3 Trial. Lancet Infect. Dis. 2023, 23, 1119–1129. [Google Scholar] [CrossRef]
- Huggett, S.B.; Hatfield, J.S.; Walters, J.D.; McGeary, J.E.; Welsh, J.W.; Mackay, T.F.C.; Anholt, R.R.H.; Palmer, R.H.C. Ibrutinib as a Potential Therapeutic for Cocaine Use Disorder. Transl. Psychiatry 2021, 11, 623. [Google Scholar] [CrossRef]
- Chen, W.; Mook, R.A.; Premont, R.T.; Wang, J. Niclosamide: Beyond an Antihelminthic Drug. Cell Signal. 2018, 41, 89–96. [Google Scholar] [CrossRef]
- Singh, S.; Weiss, A.; Goodman, J.; Fisk, M.; Kulkarni, S.; Lu, I.; Gray, J.; Smith, R.; Sommer, M.; Cheriyan, J. Niclosamide—A Promising Treatment for COVID-19. Br. J. Pharmacol. 2022, 179, 3250–3267. [Google Scholar] [CrossRef] [PubMed]
- Štrac, D.Š.; Pivac, N.; Mück-Šeler, D. The Serotonergic System and Cognitive Function. Transl. Neurosci. 2016, 7, 35–49. [Google Scholar] [CrossRef]
- Froestl, W. Novel GABAB Receptor Positive Modulators: A Patent Survey. Expert Opin. Ther. Pat. 2010, 20, 1007–1017. [Google Scholar] [CrossRef]
- Xi, Y.; Zhang, Y.; Zhu, S.; Luo, Y.; Xu, P.; Huang, Z. PPAR-Mediated Toxicology and Applied Pharmacology. Cells 2020, 9, 352. [Google Scholar] [CrossRef]
- Güzel, H.G.; Salim, D.K. Tyrosine Kinase Inhibitor-Induced Immune Hemolytic Anemia; Three Different Drugs in Three Separate Cases. J. Oncol. Pharm. Pract. 2023, 10781552231202530. [Google Scholar] [CrossRef]
- Beltrametti, S.P.; Ianniello, A.; Ricci, C. Chronotherapy with Low-Dose Modified-Release Prednisone for the Management of Rheumatoid Arthritis: A Review. Ther. Clin. Risk Manag. 2016, 12, 1763–1776. [Google Scholar] [CrossRef]
- Shiroshita-Takeshita, A.; Brundel, B.J.J.M.; Lavoie, J.; Nattel, S. Prednisone Prevents Atrial Fibrillation Promotion by Atrial Tachycardia Remodeling in Dogs. Cardiovasc. Res. 2006, 69, 865–875. [Google Scholar] [CrossRef]
- Koromina, M.; Pandi, M.-T.; Patrinos, G.P. Rethinking Drug Repositioning and Development with Artificial Intelligence, Machine Learning, and Omics. OMICS J. Integr. Biol. 2019, 23, 539–548. [Google Scholar] [CrossRef]
- Mulangu, S.; Dodd, L.E.; Davey, R.T.; Mbaya, O.T.; Proschan, M.; Mukadi, D.; Manzo, M.L.; Nzolo, D.; Oloma, A.T.; Ibanda, A.; et al. A Randomized, Controlled Trial of Ebola Virus Disease Therapeutics. N. Engl. J. Med. 2019, 381, 2293–2303. [Google Scholar] [CrossRef] [PubMed]
- Copeland, R.A.; Pompliano, D.L.; Meek, T.D. Drug–Target Residence Time and Its Implications for Lead Optimization. Nat. Rev. Drug Discov. 2006, 5, 730–739. [Google Scholar] [CrossRef] [PubMed]
- Self, W.H.; Semler, M.W.; Leither, L.M.; Casey, J.D.; Angus, D.C.; Brower, R.G.; Chang, S.Y.; Collins, S.P.; Eppensteiner, J.C.; Filbin, M.R.; et al. Effect of Hydroxychloroquine on Clinical Status at 14 Days in Hospitalized Patients With COVID-19: A Randomized Clinical Trial. JAMA 2020, 324, 2165. [Google Scholar] [CrossRef] [PubMed]





| Property | Mean | Median | Minimum | Maximum |
|---|---|---|---|---|
| MW | 348.4 | 325.4 | 60.0 | 994.1 |
| logP | 2.6 | 2.8 | −9.1 | 16.7 |
| logS | −3.8 | −3.7 | −19.6 | 6.1 |
| Caco2 | 981.0 | 383.5 | 0.0 | 9906.0 |
| MDCK | 1033.3 | 315.0 | 0.0 | 10,000.0 |
| Number of Metabolites | 3.8 | 4.0 | 0.0 | 17.0 |
| TPSA | 80.5 | 72.5 | 0.0 | 446.5 |
| HBD | 1.7 | 1.0 | 0.0 | 19.0 |
| HBA | 6.0 | 5.25 | 0.0 | 33.2 |
| Amides | 0.15 | 0.0 | 0.0 | 11.0 |
| Number of rotatable bonds | 5.7 | 5.0 | 0.0 | 37.0 |
| Target Class | Total Predicted Interactions | Predicted (Unconfirmed) | Predicted and In Vitro-Confirmed | % Confirmed Predictions |
|---|---|---|---|---|
| GPCR | 10,650 | 5708 | 4942 | 46 |
| Enzymes | 4081 | 1374 | 2707 | 66 |
| Kinase | 3768 | 688 | 3080 | 81 |
| Nuclear Receptor | 1293 | 684 | 609 | 47 |
| Other Families | 605 | 197 | 408 | 67 |
| Transporter | 1788 | 651 | 1137 | 63 |
| Unclassified | 1057 | 429 | 628 | 59 |
| Cytochrome | 2827 | 36 | 2791 | 98 |
| Ion Channel | 1303 | 322 | 981 | 75 |
| Generic Name | Intended Pharmacological Target (s) | Cmax (μM) | PCmax | Number of Predicted Off-Targets with Measured pIC50 > 6.0 | Key Predicted Off-Target Interactions |
|---|---|---|---|---|---|
| Afatinib | EGFR, HER2, HER4 | 0.0520 | 7.2800 | 11 | EGFR, ERBB4, ERBB2, GAK, BLK, IRAK1, EPHA6, HIPK4, PHKG2, LCK, ABL1 |
| Bosutinib | BCR-Abl, Src | 0.3770 | 6.4200 | >50 | ABL1, MAP4K5, ERBB3, LCK, ABL, GAK, ABL2, FRK, STK35, SRC |
| Celecoxib | COX-2 | 4.600 | 5.3400 | 19 | INSR, CA9, CA12, CA, Ca15, MT-CO2, ACA7, CA2, CA5B, CA6, CA13, NCE103, PTGS2, CA1, CA4, PTGES, CA14, A6YCJ1, CA5A, MAPK14 |
| Ceritinib | ALK | 1.2100 | 5.9200 | 17 | NUAK1, MAP4K4, ACVR1, AXL, PAK4, TYK2, PHKG1, PTK2B, DAPK3, DAPK1, HIPK1, MUSK, TAOK1, RPS6KA4, SRPK3, HIPK4, FRK |
| Erlotinib | EGFR | 3.1500 | 5.5000 | 25 | GAK, MAP3K19, EGFR, SLK, STK10, MAP2K5, RIPK2, LCK, ABL1, BLK, LYN, SLCO2B1, TNNI3K, TNK1, CIT, MKNK1, ULK3, JAK3, DDR1, ERBB4, TIE1, EPHA6, ERBB2, PIP4K2C, ABCB11 |
| Finasteride | 5-Alpha Reductase | 0.1240 | 6.9100 | 4 | SRD5A2, STRD5, SRD5A1, NLRP1 |
| Gefitinib | EGFR | 0.3560 | 6.4500 | 21 | GAK, EGFR, IRAK1, ERBB4, MAP3K19, RIPK2, MKNK1, SIK2, TUBA1A, MKNK2, SBK1, MAP2K5, HIPK4, IRAK4, STK10, CHEK2, ERBB3, ERBB2, LYN, LCK, KDR/VEGFR |
| Hydroxychloroquine | Cathepsin L | 0.3500 | 6.4600 | 8 | MPO, CHRM2, ADRA1D, TLR9, TLR8, TLR7, CRYAB, TLR4 |
| Imiquimod | TLR7R | 0.0056 | 8.2500 | 5 | HRH2, ADRA1D, ADORA2A, HCAR1, TLR |
| Lapatinib | ERBB2, EGFR | 4.1800 | 5.3800 | 11 | EGFR, TUBA1A, ERBB2, PIK3CA, NRAS, BRA, ERBB4, PIK3C2B, KRAS, PI4KB, MAP2K5 |
| Olaparib | PARP | 13.1000 | 4.8800 | 9 | PARP11, PARP2, PAR16, PARP10, PARP1, PARP3, PARP4, RAD51, TNKS |
| Sirolimus | Mammalian target of rapamycin (mTOR) | 0.0160 | 7.7800 | 12 | FKBP1B, FKBP1A, FKBP5, PIK3, ABCB1, MTOR, TEK, NR1I2, EIF4E, PSM, SLCO1B1, ABCB11 |
| Tamoxifen Citrate | Estrogen Receptor | 0.1080 | 6.9700 | 21 | EBPL, SIGMAR1, EPHX2, ESRRG, EBP, ESR2, ESR1, ESRRA, HTR2C, DRD3, KCNH2, TBXAS1, PER1, HTR6, ADRA2A, FYN, CHRM3, CHRM1, ERG, SLC6A2, ERG2 |
| Teniposide | Topoisomerase II | 23.1000 | 4.6400 | 9 | NCOA3, TOP2, NCOA1, AR, ESR1, ESR2, PGR, THRA, THRB |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rao, M.; McDuffie, E.; Sachs, C. Artificial Intelligence/Machine Learning-Driven Small Molecule Repurposing via Off-Target Prediction and Transcriptomics. Toxics 2023, 11, 875. https://doi.org/10.3390/toxics11100875
Rao M, McDuffie E, Sachs C. Artificial Intelligence/Machine Learning-Driven Small Molecule Repurposing via Off-Target Prediction and Transcriptomics. Toxics. 2023; 11(10):875. https://doi.org/10.3390/toxics11100875
Chicago/Turabian StyleRao, Mohan, Eric McDuffie, and Clifford Sachs. 2023. "Artificial Intelligence/Machine Learning-Driven Small Molecule Repurposing via Off-Target Prediction and Transcriptomics" Toxics 11, no. 10: 875. https://doi.org/10.3390/toxics11100875
APA StyleRao, M., McDuffie, E., & Sachs, C. (2023). Artificial Intelligence/Machine Learning-Driven Small Molecule Repurposing via Off-Target Prediction and Transcriptomics. Toxics, 11(10), 875. https://doi.org/10.3390/toxics11100875






