Cysteine Attenuates the Impact of Bisphenol A-Induced Oxidative Damage on Growth Performance and Intestinal Function in Piglets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals, Treatments and Management
2.2. Growth Performance and Sample Collection
2.3. Apparent Nutrient Digestibility Analysis
2.4. Serum Biochemical Indexes
2.5. Serum and Jejunal Antioxidant Assessment
2.6. Jejunal Morphological Observation
2.7. Quantitative Real-Time PCR (qRT-PCR) Analysis
2.8. Jejunal Disaccharidase Activity Analysis
2.9. Statistical Analysis
3. Results
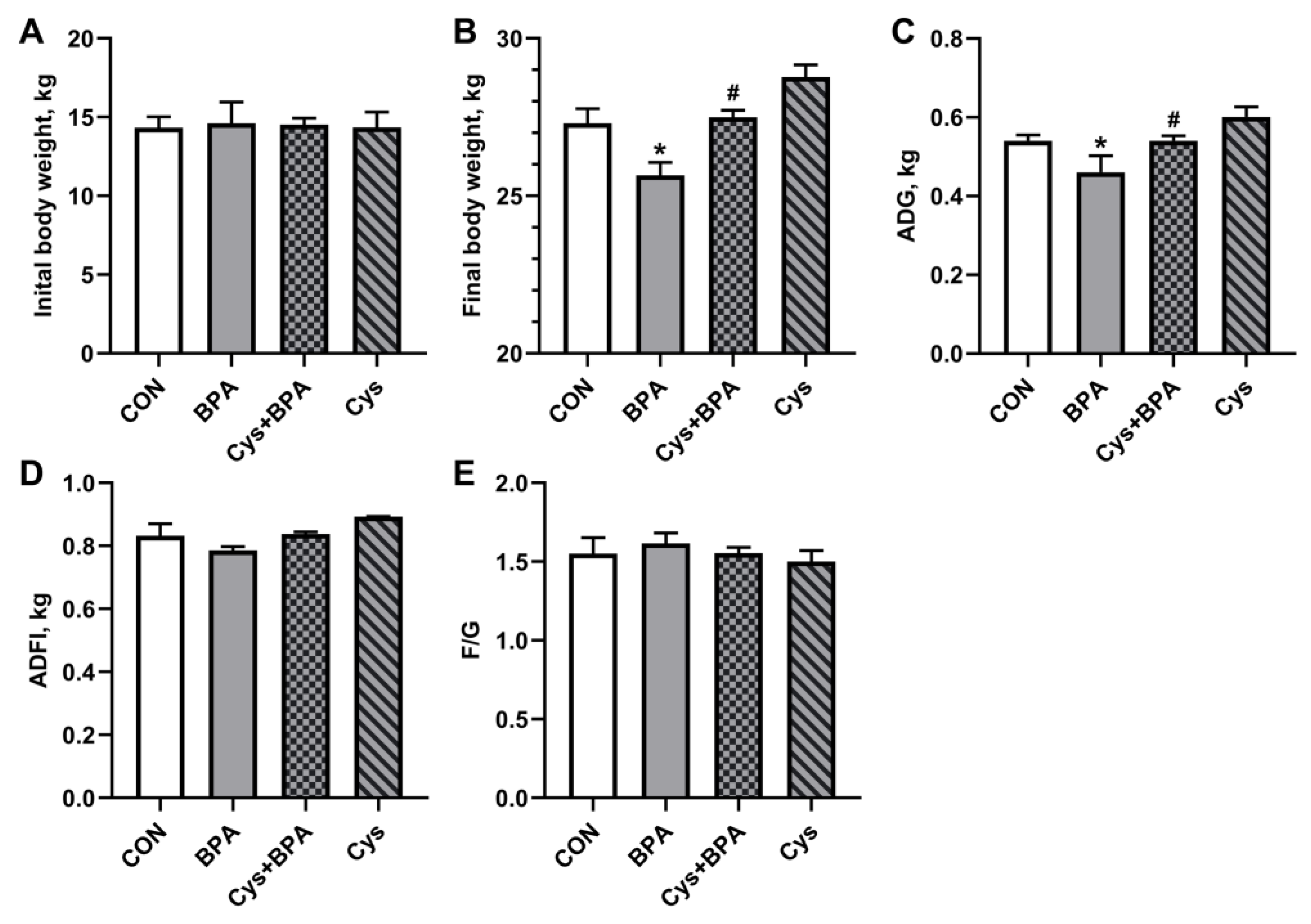
3.1. Piglet Growth Performance
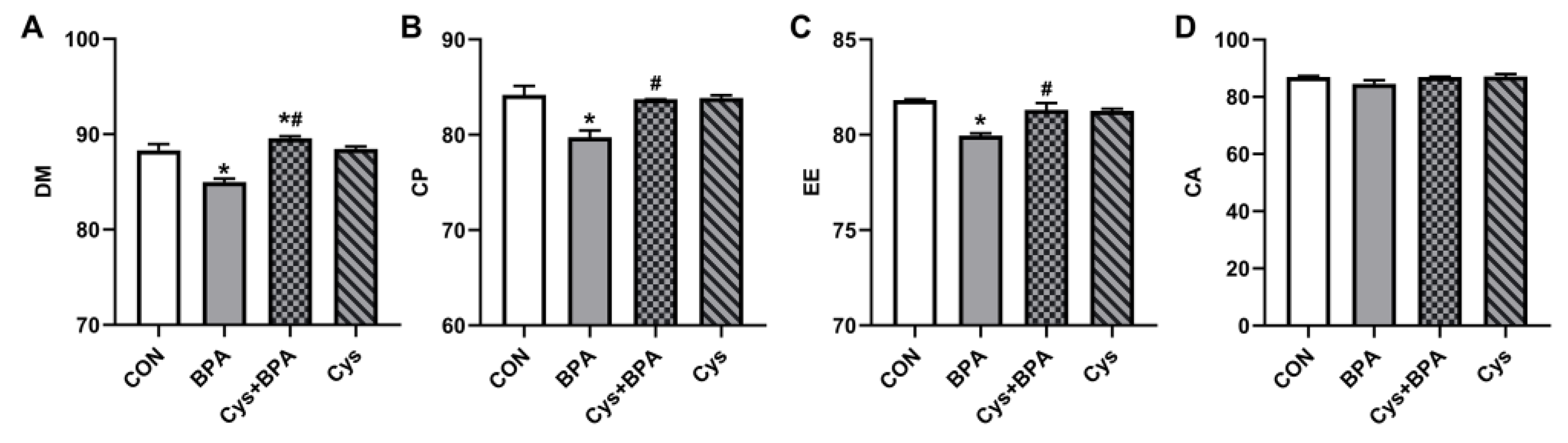
3.2. Apparent Digestibility of Nutrients
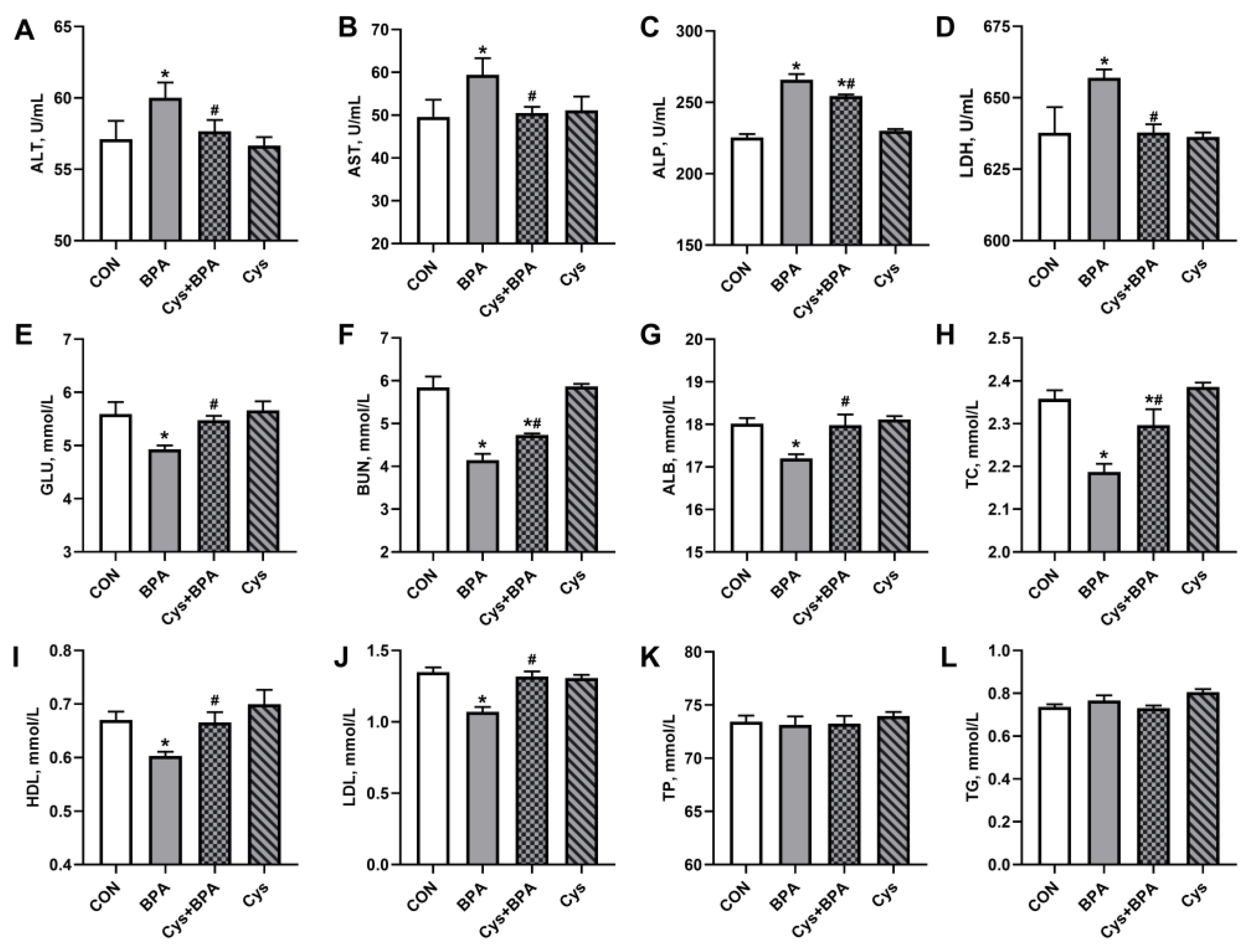
3.3. Serum Biochemistry and Antioxidants
3.4. Intestinal Morphological Examination
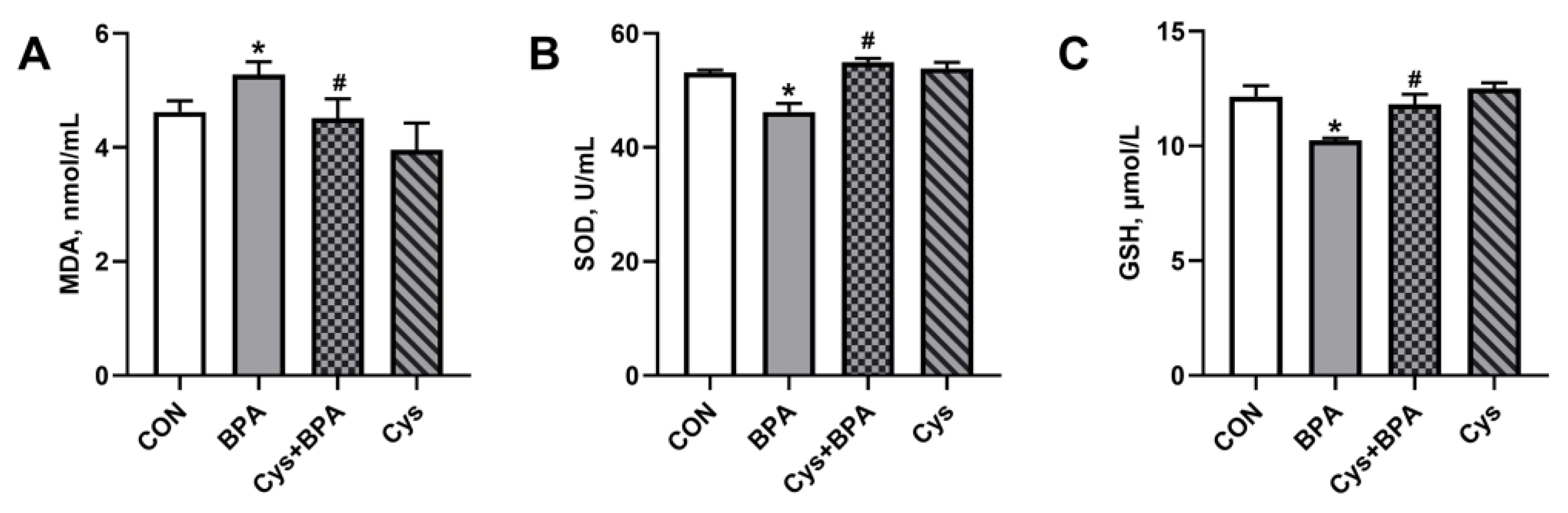
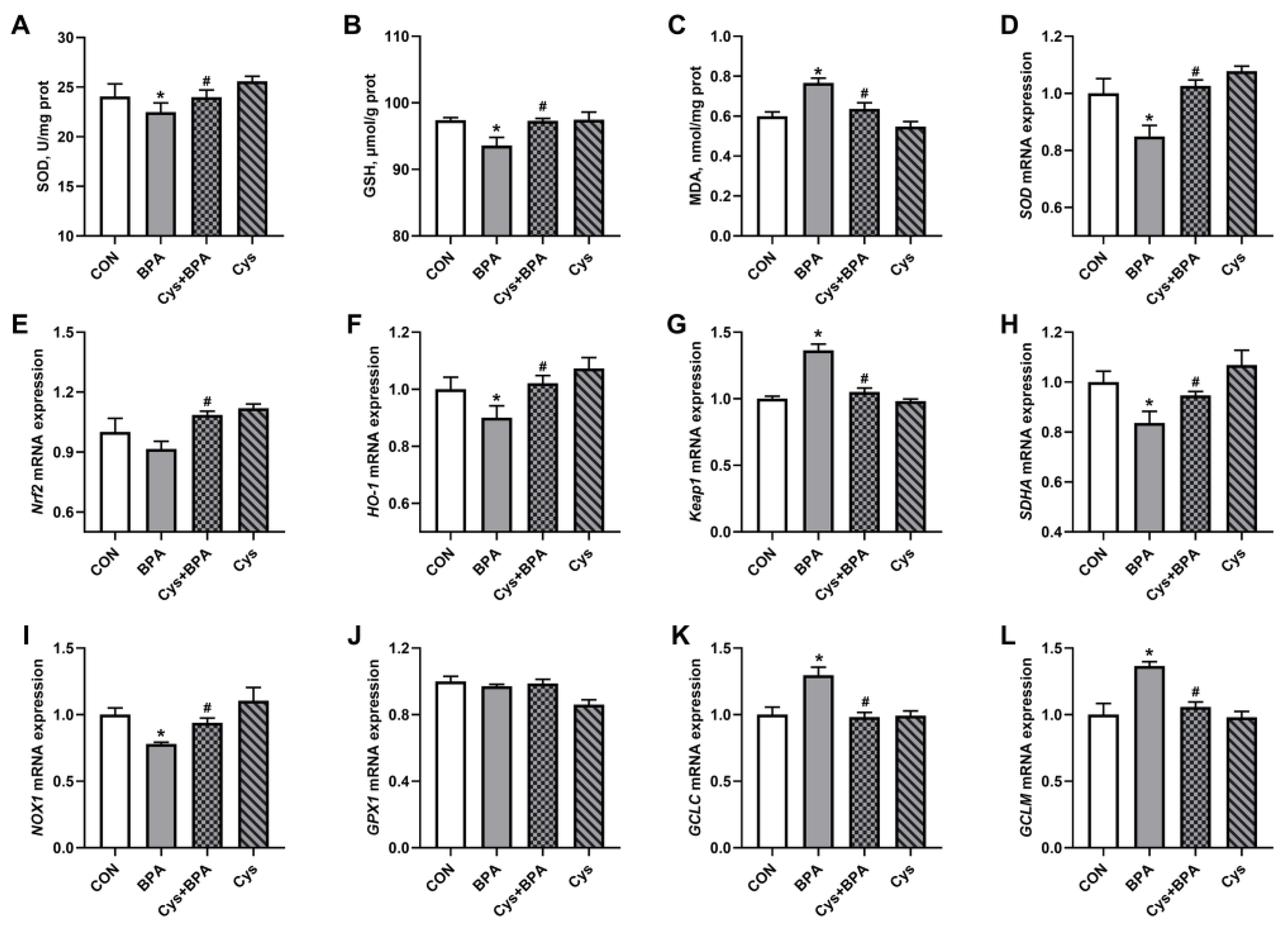
3.5. Jejunal Antioxidants
3.6. Intestinal Barrier Function
3.7. Disaccharidase Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rochester, J.R. Bisphenol A and human health: A review of the literature. Reprod. Toxicol. 2013, 42, 132–155. [Google Scholar]
- Jiao, N.; Xu, D.D.; Qiu, K.; Wang, L.Q.; Wang, L.; Piao, X.S.; Yin, J.D. Restoring mitochondrial function and normalizing ROS-JNK/MAPK pathway exert key roles in glutamine ameliorating bisphenol A-induced intestinal injury. FASEB J. 2020, 34, 7442–7461. [Google Scholar]
- Zhao, Z.; Qu, W.; Wang, K.; Chen, S.; Zhang, L.; Wu, D.; Chen, Z. Bisphenol A inhibits mucin 2 secretion in intestinal goblet cells through mitochondrial dysfunction and oxidative stress. Biomed. Pharmacother. 2019, 111, 901–908. [Google Scholar]
- Bhattacharyya, A.; Chattophadhyay, R.; Mitra, S.; Crowe, M.S. Oxidative stress: An essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar]
- Chen, J.L.; Yu, B.; Chen, D.W.; Huang, Z.Q.; Mao, X.B.; Zheng, P.; Yu, J.; Luo, J.Q.; He, J. Chlorogenic acid improves intestinal barrier functions by suppressing mucosa inflammation and improving antioxidant capacity in weaned pigs. J. Nutr. Biochem. 2018, 59, 84–92. [Google Scholar]
- Hao, Z.; Li, Z.; Huo, J.J.; Li, J.; Liu, F.; Yin, P. Effects of Chinese wolfberry and Astragalus extract on the antioxidant capacity of Tibetan pig liver. PLoS ONE 2021, 16, e0245749. [Google Scholar]
- Mou, D.L.; Wang, J.; Liu, H.; Chen, Y.L.; Che, L.Q.; Fang, Z.F.; Xu, S.Y.; Lin, Y.; Feng, B.; Li, J.; et al. Maternal methyl donor supplementation during gestation counteracts bisphenol A-induced oxidative stress in sows and offspring. Nutrition 2018, 45, 76–84. [Google Scholar]
- Liu, H.; Wang, J.; Mou, D.L.; Che, L.Q.; Fang, Z.F.; Feng, B.; Lin, Y.; Xu, S.Y.; Li, J.; Wu, D. Maternal Methyl Donor Supplementation during Gestation Counteracts the Bisphenol A-Induced Impairment of Intestinal Morphology, Disaccharidase Activity, and Nutrient Transporters Gene Expression in Newborn and Weaning Pigs. Nutrients 2017, 9, 423. [Google Scholar]
- Yin, J.; Ren, W.K.; Yang, G.; Duan, J.L.; Huang, X.G.; Fang, R.J.; Li, C.Y.; Li, T.J.; Yin, Y.L.; Hou, Y.Q.; et al. L-Cysteine metabolism and its nutritional implications. Mol. Nutr. Food Res. 2016, 60, 134–146. [Google Scholar]
- Bauchart-Thevret, C.; Cottrell, J.; Stoll, B.; Burrin, D.G. First-pass splanchnic metabolism of dietary cysteine in weanling pigs. J. Anim. Sci. 2022, 12, 4093–4099. [Google Scholar]
- Chen, L.X.; Li, P.; Wang, J.J.; Li, X.L.; Gao, H.J.; Yin, Y.L.; Hou, Y.Q.; Wu, G.Y. Catabolism of nutritionally essential amino acids in developing porcine enterocytes. Amino Acids 2009, 37, 143–152. [Google Scholar]
- Windmueller, H.G. Glutamine utilization by the small intestine. Adv. Enzymol. Relat. Areas Mol. Biol. 1982, 53, 201. [Google Scholar]
- Jiao, N.; Wang, L.; Wang, Y.B.; Xu, D.D.; Zhang, X.; Yin, J.D. Cysteine exerts an essential role in maintaining intestinal integrity and function independent of glutathione. Mol. Nutr. Food Res. 2022, 66, 2100728. [Google Scholar]
- Koo, B.; Choi, J.; Holanda, D.M.; Yang, C.; Nyachoti, C.M. Comparative effects of dietary methionine and cysteine supplementation on redox status and intestinal integrity in immunologically challenged-weaned pigs. Amino Acids 2023, 55, 139–152. [Google Scholar]
- Ni, H.J.; Long, L.N.; Bin, P.; Azad, M.A.K.; Xu, K.; Zhou, X.H.; Ding, X.H.; Liu, G. Maternal cysteine intake influenced oxidative status and lipid-related gut microbiota and plasma metabolomics in male suckling piglets. Anim. Feed Sci. Technol. 2021, 276, 114947. [Google Scholar]
- NRC. Nutrient Requirements of Swine, 11th ed.; National Academies Press: Washington, DC, USA, 2012. [Google Scholar]
- AOAC. Official Methods of Analysis, 19th ed.; Association of Official Agricultural Chemists: Arlington, VA, USA, 2012. [Google Scholar]
- Chen, X.; Ma, X.M.; Yang, C.W.; Jiang, S.Z.; Huang, L.B.; Li, Y.; Zhang, F.; Jiao, N.; Yang, W.R. Low level of dietary organic trace elements improve the eggshell strength, trace element utilization, and intestinal function in late-phase laying hens. Front. Vet. Sci. 2022, 9, 903615. [Google Scholar]
- Ma, Y.; Liu, H.H.; Wu, J.X.; Yuan, L.; Wang, Y.Q.; Du, X.D.; Wang, R.; Marwa, P.W.; Petlulu, P.; Chen, X.H.; et al. The adverse health effects of bisphenol A and related toxicity mechanisms. Environ. Res. 2019, 176, 108575. [Google Scholar]
- Xia, W.; Jiang, Y.; Li, Y.Y.; Wan, Y.J.; Liu, J.; Ma, Y.; Mao, Z.X.; Chang, H.L.; Li, G.Q.; Xu, B.; et al. Early-life exposure to bisphenol a induces liver injury in rats involvement of mitochondria-mediated apoptosis. PLoS ONE 2014, 9, e90443. [Google Scholar]
- Hamed, H.S.; Abdel-Tawwab, M. Ameliorative effect of propolis supplementation on alleviating bisphenol-A toxicity: Growth performance, biochemical variables, and oxidative stress biomarkers of Nile tilapia, Oreochromis niloticus (L.) fingerlings. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2017, 202, 63–69. [Google Scholar]
- Wang, K.; Kong, X.F.; Azad, M.A.K.; Zhu, Q.; Xiong, L.; Zheng, Y.Z.; Hu, Z.L.; Yin, Y.L.; He, Q.H. Maternal Probiotic or Synbiotic Supplementation Modulates Jejunal and Colonic Antioxidant Capacity, Mitochondrial Function, and Microbial Abundance in Bama Mini-piglets. Oxid. Med. Cell. Longev. 2021, 2021, 6618874. [Google Scholar]
- Pirinccioglu, A.G.; Gökalp, D.; Pirinccioglu, M.; Kizil, G.; Kizil, M. Malondialdehyde (MDA) and protein carbonyl (PCO) levels as biomarkers of oxidative stress in subjects with familial hypercholesterolemia. Clin. Biochem. 2010, 43, 1220–1224. [Google Scholar]
- Wang, J.; Xiao, Y.X.; Li, J.J.; Qi, M.; Tan, B. Serum biochemical parameters and amino acids metabolism are altered in piglets by early-weaning and proline and putrescine supplementations. Anim. Nutr. 2021, 7, 334–345. [Google Scholar]
- Rushworth, G.F.; Megson, I.L. Existing and potential therapeutic uses for N-acetylcysteine: The need for conversion to intracellular glutathione for antioxidant benefits. Pharmacol. Ther. 2014, 141, 150–159. [Google Scholar]
- Zhao, Y.W.; Niu, Y.; He, J.T.; Zhang, L.L.; Wang, C.; Wang, T. Dietary dihydroartemisinin supplementation attenuates hepatic oxidative damage of weaned piglets with intrauterine growth retardation through the Nrf2/ARE signaling pathway. Animals 2019, 9, 1144. [Google Scholar]
- Katsuoka, F.; Otsuki, A.; Takahashi, M.; Ito, S.; Yamamoto, M. Direct and specific functional evaluation of the Nrf2 and mafG heterodimer by introducing a tethered dimer into small Maf-deficient cells. Mol. Cell. Biol. 2019, 39, e00273-19. [Google Scholar]
- Li, B.; Nasser, M.I.; Masood, M.; Adlat, S.; Huang, Y.; Yang, B.; Luo, C.; Jiang, N. Efficiency of traditional Chinese medicine targeting the Nrf2/HO-1 signaling pathway. Biomed. Pharmacother. 2020, 126, 110074–110084. [Google Scholar]
- Wang, L.X.; Yan, S.L.; Li, J.Z.; Li, Y.L.; Ding, X.Q.; Yin, J.; Xiong, X.; Yin, Y.L.; Yang, H.S. Rapid communication: The relationship of enterocyte proliferation with intestinal morphology and nutrient digestibility in weaning piglets. J. Anim. Sci. 2019, 97, 353–358. [Google Scholar]
- Song, Z.H.; Tong, G.; Xiao, K.; Jiao, L.F.; Ke, Y.L.; Hu, C.H. L-Cysteine protects intestinal integrity, attenuates intestinal inflammation and oxidant stress, and modulates NF-κB and Nrf2 pathways in weaned piglets after LPS challenge. Innate Immun. 2016, 22, 152–161. [Google Scholar]
- Groschwitz, K.R.; Hogan, S.P. Intestinal barrier function: Molecular regulation and disease pathogenesis. J. Allergy Clin. Immunol. 2009, 124, 3–20. [Google Scholar]
- Casas, G.A.; Blavi, L.; Cross, T.-W.L.; Lee, A.H.; Swanson, K.S.; Stein, H.H. Inclusion of the direct-fed microbial Clostridium butyricum in diets for weanling pigs increases growth performance and tends to increase villus height and crypt depth, but does not change intestinal microbial abundance. J. Anim. Sci. 2020, 98, skz372. [Google Scholar]
- Xu, Z.R.; Hu, C.H.; Xia, M.S.; Zhan, X.A.; Wang, M.Q. Effects of dietary fructooligosaccharide on digestive enzyme activities, intestinal microflora and morphology of male broilers. Poult. Sci. 2003, 82, 1030–1036. [Google Scholar]
- Ajayi, O.I.; Smth, O.F.; Oso, A.O.; Oke, O.E. Evaluation of in ovo feeding of low or high mixtures of cysteine and lysine on performance, intestinal morphology and physiological responses of thermal-challenged broiler embryos. Front. Physiol. 2022, 13, 972041. [Google Scholar]
- Chen, J.L.; Li, F.C.; Yang, W.R.; Jiang, S.Z.; Li, Y. Supplementation with exogenous catalase from penicilliumnotatum in the diet ameliorates lipopolysaccharide-induced intestinal oxidative damage through affecting intestinal antioxidant capacity and microbiota in weaned pigs. Microbiol. Spectr. 2021, 9, e0065421. [Google Scholar]
- Wu, Q.J.; Zhou, Y.M.; Wu, Y.N.; Zhang, L.L.; Wang, T. The effects of natural and modified clinoptilolite on intestinal barrier function and immune response to LPS in broiler chickens. Vet. Immunol. Immunopathol. 2013, 153, 70–76. [Google Scholar]
- Hou, Y.Q.; Wang, L.; Zhang, W.; Yang, Z.G.; Ding, B.Y.; Zhu, H.L.; Liu, Y.L.; Qiu, Y.S.; Yin, Y.L.; Wu, G.Y. Protective effects of N-acetylcysteine on intestinal functions of piglets challenged with lipopolysaccharide. Amino Acids 2012, 43, 1233–1242. [Google Scholar]
- Chen, J.L.; Xie, H.M.; Chen, D.W.; Yu, B.; Mao, X.B.; Zheng, P.; Yu, J.; Luo, Y.H.; Luo, J.Q.; He, J. Chlorogenic acid improves intestinal development via suppressing mucosa inflammation and cell apoptosis in weaned pigs. ACS Omega 2018, 3, 2211–2219. [Google Scholar]
- Liu, Y.T.; Azad, M.A.K.; Zhao, X.C.; Zhu, Q.; Kong, X.F. Dietary crude protein levels alter diarrhea incidence, immunity, and intestinal barrier function of Huanjiang mini-pigs during different growth stages. Front. Immunol. 2022, 13, 908753. [Google Scholar]
- Lee, B.; Moon, K.M.; Kim, C.Y. Tight junction in the intestinal epithelium: Its association with diseases and regulation by phytochemicals. J. Immunol. Res. 2018, 2018, 2645465. [Google Scholar]
- Itallie, C.M.V.; Fanning, A.S.; Bridges, A.; Anderson, J.M. ZO-1 Stabilizes the tight junction solute barrier through coupling to the perijunctional cytoskeleton. Mol. Biol. Cell 2009, 20, 3930–3940. [Google Scholar]
- Jiao, N.; Wu, Z.L.; Ji, Y.; Wang, B.; Dai, Z.L.; Wu, G.Y. L-Glutamate enhances barrier and antioxidative functions in intestinal porcine epithelial cells. J. Nutr. 2015, 145, 2258–2264. [Google Scholar]
- Siggers, R.H.; Siggers, J.; Boye, M.; Thymann, T.; Mølbak, L.; Leser, T.; Jensen, B.B.; Sangild, P.T. Early administration of probiotics alters bacterial colonization and limits diet-induced gut dysfunction and severity of necrotizing enterocolitis in preterm pigs. J. Nutr. 2008, 138, 1437–1444. [Google Scholar]



| Items | Control | Cys |
|---|---|---|
| Ingredients | ||
| Corn | 69.40 | 69.41 |
| Dehulled soybean meal | 5.30 | 5.30 |
| Extruded soybean | 10.00 | 10.00 |
| Fish meal | 3.50 | 3.50 |
| High-protein dried whey | 3.50 | 3.50 |
| Wheat bran | 2.00 | 2.00 |
| Soybean oil | 1.85 | 1.85 |
| Dicalcium phosphate | 0.80 | 0.80 |
| Limestone | 0.40 | 0.40 |
| Salt | 0.25 | 0.25 |
| L-Lysine·HCl | 0.78 | 0.78 |
| DL-methionine | 0.24 | 0.14 |
| L-tryptophan | 0.08 | 0.08 |
| L-threonine | 0.31 | 0.31 |
| L-cysteine | - | 0.11 |
| L-valine | 0.21 | 0.21 |
| L-leucine | 0.05 | 0.05 |
| L-isoleucine | 0.14 | 0.14 |
| L-histidine·HCl | 0.18 | 0.18 |
| L-phenylalanine | 0.16 | 0.16 |
| Alanine | 0.20 | 0.17 |
| Choline chloride | 0.08 | 0.08 |
| Sugar substitute | 0.01 | 0.01 |
| Fragrance | 0.05 | 0.05 |
| Phytase | 0.01 | 0.01 |
| Premix 1 | 0.50 | 0.50 |
| Total | 100.00 | 100.00 |
| Calculated nutrition levels | ||
| ME (Mcal/kg) | 3.35 | 3.35 |
| Crude protein | 16.81 | 16.81 |
| SID lysine | 1.23 | 1.23 |
| SID methionine | 0.47 | 0.36 |
| SID cysteine | 0.21 | 0.32 |
| SID tryptophan | 0.20 | 0.20 |
| SID threonine | 0.73 | 0.73 |
| Calcium | 0.60 | 0.60 |
| STTD phosphorus | 0.44 | 0.44 |
| Gene | Primer Sequence (5′ to 3′) | Accession No. | Product Size (bp) |
|---|---|---|---|
| CLDN1 | F: GGCATCCTGCTGGGACTAAT R: GCAACTAAGATAGCCAGACCTGAA | NM_001244539.1 | 149 |
| GCLC | F: CGGTGGAGGACAATATGAGGAA R: AGCCTAATCTGGGAAATGAAGTGA | XM_003482164.4 | 99 |
| GCLM | F: GGACAAAACCCAGTTGGAGC R: CAGTTAAATCGGGCGGCATC | XM_001926378.4 | 104 |
| GPX1 | F: GCAACCAGTTTGGACATCAGGAA R: CGAAGAGCGGGTGAGATTTG | NM_214201.1 | 147 |
| HO-1 | F: AGGCTGAGAATGCCGAGTTC R: TGTGGTACAAGGACGCCATC | NM_001004027.1 | 90 |
| Keap1 | F: GTGTTACTACCCAGAGAGGAATGA R: CCGCAGCATAGATACAGTTGTG | NM_001114671.1 | 104 |
| NOX1 | F: GCATCCCTTTACCCTGACCT R: CTCAATCCTTGGAACTGGCG | XM_021079610.1 | 130 |
| Nrf2 | F: TCAGCACCTTGTACCTTGAAGT R: TTGCCATCTCTTGTTTGCTGC | XM_021075133. | 100 |
| SDHA | F: ATGGAAAACGGGGAGTGTCG R: TTCCGGTAGCGACAACAGTG | XM_021076930.1 | 97 |
| SOD | F: AAGGGGAATTGCTGGAAGCCA R: CGACGGATACAGCGGTCAACT | NM_214127.2 | 81 |
| ZO-1 | F: GCATGATGATCGTCTGTCCTACC R: CCGCCTTCTGTATCTGTGTCTTC | XM_013993251.1 | 108 |
| ZO-2 | F: GCAGAGACAACCCCCACTTT R: CGTTAACCATGACCACCCGA | NM_001206404.1 | 117 |
| ZO-3 | F: CTGTGGTTGTGTCTGACGTGGTAC R: GGCTATCTTGACGCAGGTCTTGAG | XM_005661351.3 | 120 |
| β-Actin | F: CCACGAAACTACCTTCAACTC R: TGATCTCCTTCTGCATCCTGT | NM_001170517.2 | 131 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, P.; Ma, S.; Li, C.; Di, Y.; Liu, Z.; Wang, H.; Li, Y.; Jiang, S.; Yang, W.; Jiao, N. Cysteine Attenuates the Impact of Bisphenol A-Induced Oxidative Damage on Growth Performance and Intestinal Function in Piglets. Toxics 2023, 11, 902. https://doi.org/10.3390/toxics11110902
Qin P, Ma S, Li C, Di Y, Liu Z, Wang H, Li Y, Jiang S, Yang W, Jiao N. Cysteine Attenuates the Impact of Bisphenol A-Induced Oxidative Damage on Growth Performance and Intestinal Function in Piglets. Toxics. 2023; 11(11):902. https://doi.org/10.3390/toxics11110902
Chicago/Turabian StyleQin, Pengxiang, Shangyuan Ma, Changjin Li, Yanjiao Di, Zihao Liu, Huiru Wang, Yang Li, Shuzhen Jiang, Weiren Yang, and Ning Jiao. 2023. "Cysteine Attenuates the Impact of Bisphenol A-Induced Oxidative Damage on Growth Performance and Intestinal Function in Piglets" Toxics 11, no. 11: 902. https://doi.org/10.3390/toxics11110902






