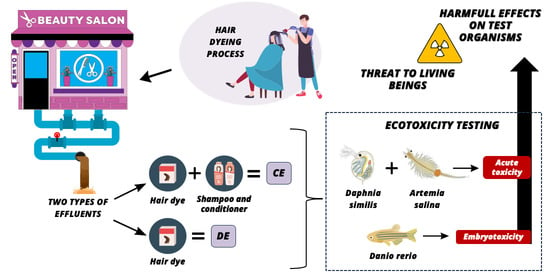
Toxicity of Beauty Salon Effluents Contaminated with Hair Dye on Aquatic Organisms
Abstract
1. Introduction
2. Materials and Methods
2.1. Obtaining, Storing, and Determining Effluent Samples
2.2. Treatments
2.3. Bioassays
2.3.1. Test Method with Artemia salina
2.3.2. Test Method with Daphnia similis
2.3.3. FET Test
Zebrafish Maintenance and Spawning
Fish Embryo Exposure Assays
2.4. Physicochemical Analysis
2.5. Spectrophotometric Analysis
2.6. Statistical Analysis
3. Results
3.1. Artemia salina
3.2. Daphnia similis
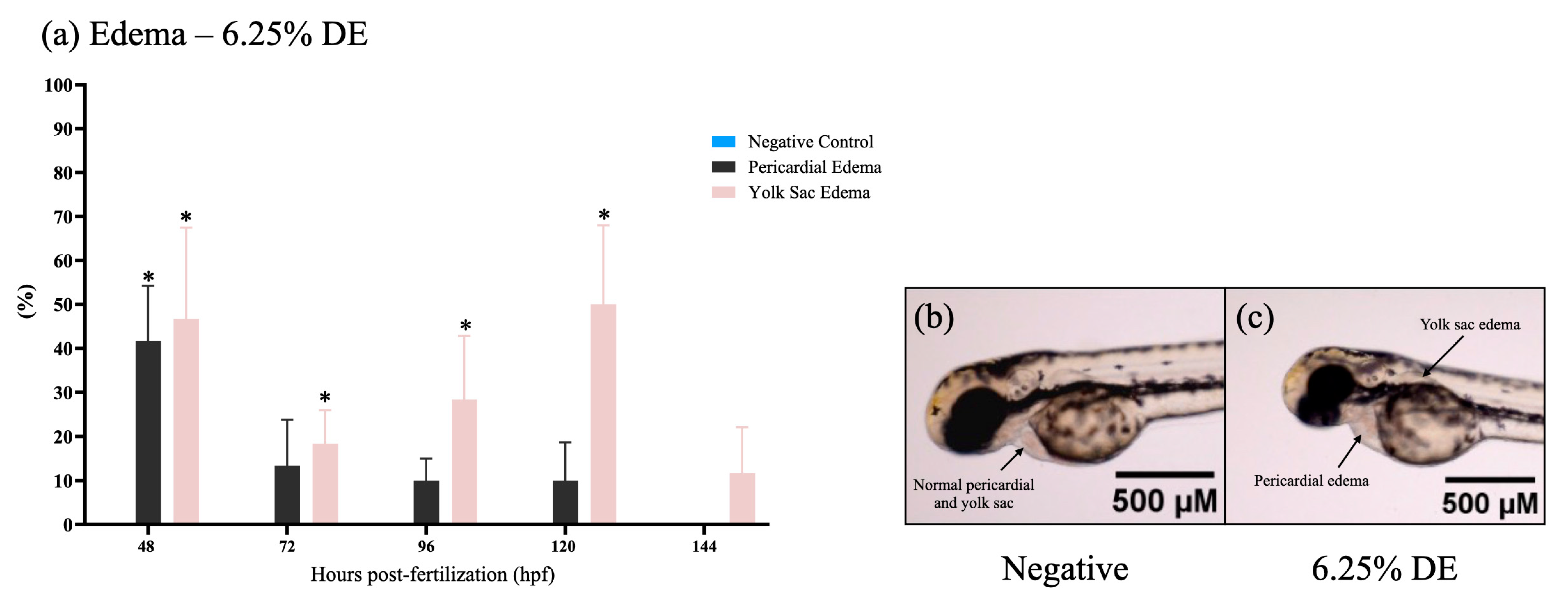
3.3. FET Test
3.4. Physicochemical Analysis
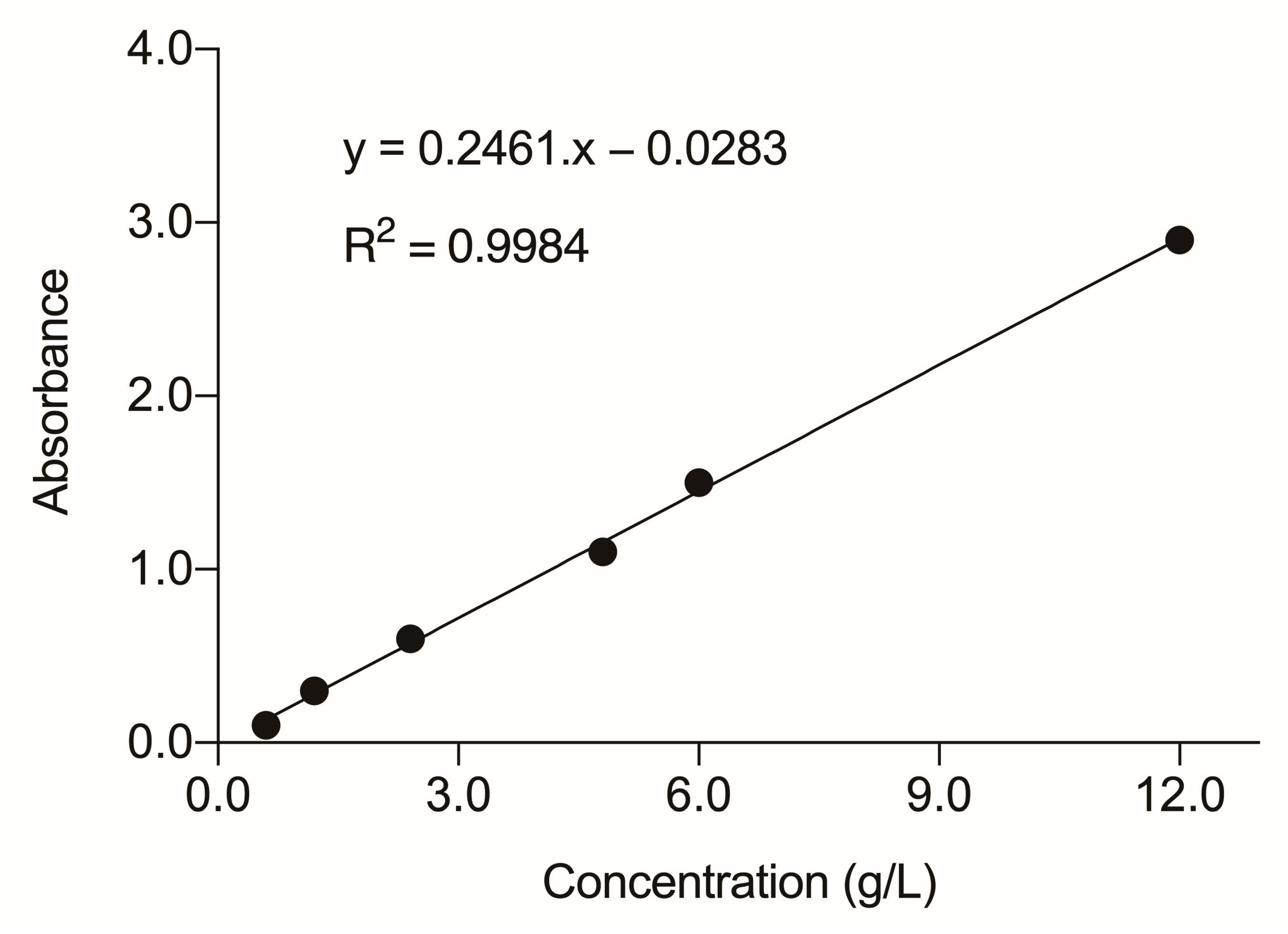
3.5. Spectrophotometric Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sieber, G.; Beisser, D.; Bock, C.; Boenigk, J. Protistan and fungal diversity in soils and freshwater lakes are substantially different. Sci. Rep. 2020, 10, 20025. [Google Scholar] [CrossRef]
- Saxena, A.; Tiwari, A.; Kaushik, R.; Iqbal, H.M.; Parra-Saldívar, R. Diatoms recovery from wastewater: Overview from an ecological and economic perspective. J. Water Process. Eng. 2021, 39, 101705. [Google Scholar] [CrossRef]
- Zhou, Y.; Ashokkumar, V.; Amobonye, A.; Bhattacharjee, G.; Sirohi, R.; Singh, V.; Flora, G.; Kumar, V.; Pillai, S.; Zhang, Z.; et al. Current research trends on cosmetic microplastic pollution and its impacts on the ecosystem: A review. Environ. Pollut. 2023, 320, 121106. [Google Scholar]
- Chowdhury, A.; Naz, A.; Maiti, S.K. Distribution, speciation, and bioaccumulation of potentially toxic elements in the grey mangroves at Indian Sundarbans, in relation to vessel movements. Mar. Environ. Res. 2023, 189, 106042. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Lichtfouse, E.; Liu, G.; Balaram, V.; Ribeiro, A.R.L.; Lu, Z.; Stock, F.; Carmona, E.; Teixeira, M.R.; Picos-Corrales, L.A.; et al. Worldwide cases of water pollution by emerging contaminants: A review. Environ. Chem. Lett. 2022, 20, 2311–2338. [Google Scholar]
- Coronado-Apodaca, K.G.; González-Meza, G.M.; Aguayo-Acosta, A.; Araújo, R.G.; Gonzalez-Gonzalez, R.B.; Oyervides-Muñoz, M.A.; Martínez-Ruiz, M.; Melchor-Martínez, E.M.; Barceló, D.; Parra-Saldívar, R.; et al. Immobilized Enzyme-based Novel Biosensing System for Recognition of Toxic Elements in the Aqueous Environment. Top. Catal. 2023, 66, 606–624. [Google Scholar] [CrossRef]
- Hüttl, R.F.; Bens, O.; Bismuth, C.; Hoechstetter, S.; Frede, H.-G.; Kümpel, H.-J. Introduction: A Critical Appraisal of Major Water Engineering Projects and the Need for Interdisciplinary Approaches. In Society-Water-Technology: A Critical Appraisal of Major Water Engineering Projects; Springer International Publishing: Cham, Switzerland, 2016; pp. 3–9. [Google Scholar] [CrossRef]
- Armstrong, A. Ethical issues in water use and sustainability. Area 2006, 38, 9–15. [Google Scholar] [CrossRef]
- Ford, A.T.; Ågerstrand, M.; Brooks, B.W.; Allen, J.; Bertram, M.G.; Brodin, T.; Dang, Z.; Duquesne, S.; Sahm, R.; Hoffmann, F.; et al. The Role of Behavioral Ecotoxicology in Environmental Protection. Environ. Sci. Technol. 2021, 55, 5620–5628. [Google Scholar] [CrossRef]
- Christie, R.M.; Morel, O.J.X. The Coloration of Human Hair. In The Coloration of Wool and other Keratin Fibres; Lewis, D.M., Rippon, J.A., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2013; pp. 357–391. [Google Scholar] [CrossRef]
- Arman, N.Z.; Salmiati, S.; Aris, A.; Salim, M.R.; Nazifa, T.H.; Muhamad, M.S.; Marpongahtun, M. A Review on Emerging Pollutants in the Water Environment: Existences, Health Effects and Treatment Processes. Water 2021, 13, 3258. [Google Scholar] [CrossRef]
- Yurtsever, M. Glitters as a Source of Primary Microplastics: An Approach to Environmental Responsibility and Ethics. J. Agric. Environ. Ethics 2019, 32, 459–478. [Google Scholar] [CrossRef]
- Bilal, M.; Mehmood, S.; Iqbal, H.M.N. The Beast of Beauty: Environmental and Health Concerns of Toxic Components in Cosmetics. Cosmetics 2020, 7, 13. [Google Scholar] [CrossRef]
- Iqbal, F.; Qureshi, I.Z.; Ali, M. Histopathological changes in the kidney of common carp, Cyprinus carpio, following nitrate exposure. J. Res. 2004, 15, 411–418. [Google Scholar]
- Wang, W.X. Incorporating exposure into aquatic toxicological studies: An imperative. Aquat. Toxicol. 2011, 105, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Pavithra, K.G.; Jaikumar, V. Removal of colorants from wastewater: A review on sources and treatment strategies. J. Ind. Eng. Chem. 2019, 75, 1–19. [Google Scholar] [CrossRef]
- Maifadi, S.; Mhlanga, S.D.; Nxumalo, E.N.; Motsa, M.M.; Kuvarega, A.T. Carbon nanotube embedded ultrafiltration membranes for the treatment of rapid granular multimedia prefiltered beauty hair salon and municipal wastewater. Sep. Purif. Technol. 2021, 267, 118618. [Google Scholar] [CrossRef]
- Freddi, L.A.; Américo-Pinheiro, J.H.P. Ecotoxicidade de Efluentes Para Organismos Aquáticos. Rev. Científica ANAP Bras. 2017, 10, 38–48. [Google Scholar] [CrossRef]
- Zagatto, P.A. Ecotoxicologia Aquática: Princípios e Aplicações. In Proceedings of the 2015: IV Seminário sobre Ecotoxicologia, São José do Barreto, Macaé, RJ, Brazil, 10–12 September 2015. [Google Scholar]
- Rodrigues-Chaparro, T.R.; Cleto- Pires, E.C. Toxicity evaluation as a tool to assess the performance of an anaerobic immobilized biomass reactor. Dyna 2010, 77, 284–291. [Google Scholar]
- De Paiva Magalhães, D.; Da, A.; Filho, S.F. A ecotoxicologia como ferramenta no biomonitoramento de ecossistemas aquáticos a ecotoxicologia como ferramenta no biomonitoramento de ecossistemas aquáticos. Oecol. Bras. 2008, 12, 355–381. [Google Scholar] [CrossRef]
- Martins, T.F.G.; de Ferreira, K.S.; Rojas, I.E.B.; Pompêo, M. Aspectos da Ecotoxicidade em Ambientes Aquáticos; Instituto de Biociências da USP: São Paulo, Brazil, 2022; 274p. [Google Scholar]
- Domingues, D.F.; Bertoletti, E. Seleção, Manutenção e Cultivo de Organismos Aquáticos. In Ecotoxicologia Aquática—Princípios e Aplicações; Editora Rima: São Carlos, Brazil, 2006; pp. 153–184. [Google Scholar]
- ABNT—Associação Brasileira de Normas Técnicas. NBR 16530. Ecotoxicologia Aquática—Toxicidade Aguda—Método de Ensaio Com Artemia sp. (Crustacea, Brachiopoda); ABNT: Rio de Janeiro, Brazil, 2016. [Google Scholar]
- ABNT—Associação Brasileira de Normas Técnicas. NBR 12713. Ecotoxicologia Aquática—Toxicidade Aguda—Método de Ensaio Com Daphnia sp. (Crustacea, Cladocera); ABNT: Rio de Janeiro, Brazil, 2016. [Google Scholar]
- OECD. Guidelines for the Testing of Chemicals, Section 2: Effects on Biotic Systems Test No. 236: Fish Embryo Acute Toxicity (FET) Test; Organization for Economic Cooperation and Development: Paris, France, 2013; Available online: https://www.oecd-ilibrary.org/environment/test-no-236-fish-embryo-acute-toxicity-fet-test_9789264203709-en (accessed on 9 January 2023).
- Rocha, F.R.P.; Teixeira, L.S.G. Estratégias Para Aumento de Sensibilidade em Espectrofotometria UV-VIS. Química Nova 2004, 27, 807–812. [Google Scholar] [CrossRef]
- Holler, F.J.; Skoog, D.A.; Crouch, S.R.; Pasquini, C.J.; Rohwedder, J.J.R. Princípios de Análise Instrumental, 6th ed.; Bookman: Porto Alegre, Brazil, 2009. [Google Scholar]
- Canassa, T.A.; Lamonato, A.L.; Ribeiro, A.V. Utilização da Lei de Lambert-Beer Para Determinação da Concentração de Soluções. J. Exp. Tech. Instrum. 2018, 1, 23. [Google Scholar] [CrossRef]
- Hamilton, M.A.; Russo, R.C.; Thurston, R.V. Trimmed Spearman-Karber Method for Estimating Median Lethal Concentrations in Toxicity Bioassays. Environ. Sci. Technol. 1977, 11, 714–719. [Google Scholar] [CrossRef]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of Embryonic Development of the Zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef] [PubMed]
- CONAMA—Conselho Nacional de Meio Ambiente. Dispõe Sobre a Classificação Dos Corpos de Água e Diretrizes Ambientais Para Seu Enquadramento, Bem Como Estabelece as Condições e Padrões de Lançamentos de Efluentes. Resoluções no 357, de 17 de março de 2005. Available online: https://www.mpf.mp.br/atuacao-tematica/ccr4/dados-da-atuacao/projetos/qualidade-da-agua/legislacao/resolucoes/resolucao-conama-no-357-de-17-de-marco-de-2005/at_download/file (accessed on 29 September 2023).
- CONAMA—Conselho Nacional de Meio Ambiente CONAMA No 430 de 13 de maio de 2011. Diário Oficial da União. Republica Federativa do Brasil, P.E.B.D. 16 mai. 2011, Ed. 2011. Available online: https://conexaoagua.mpf.mp.br/arquivos/legislacao/resolucoes/resolucao-conama-430-2011.pdf (accessed on 29 September 2023).
- EUR-LEX; Council of the European Communities. Council Directive of 21 May 1991 Concerning Urban Waste Water Treatment (91/271/EEC); Official Journal of the European Communities: Luxembourg, 1991. [Google Scholar]
- Osibanjo, O.; Daso, A.P.; Gbadebo, A.M. The impact of industries on surface water quality of River Ona and River Alaro in Oluyole Industrial Estate, Ibadan, Nigeria. Afr. J. Biotechnol. 2011, 10, 696–702. [Google Scholar]
- Omer, N.H. Water Quality Parameters. In Water Quality—Science, Assessments and Policy; Summers, J.K., Ed.; IntechOpen: London, UK, 2019. [Google Scholar]
- ONU—Organização das Nações Unidas. Take Action for the Sustainable Development Goals. Available online: https://www.un.org/sustainabledevelopment/sustainable-development-goals (accessed on 26 July 2023).
- CETESB. Companhia Ambiental do Estado de São Paulo Qualidade Das Águas Interiores No Estado de São Paulo. Apêndice C—Significado Ambiental e Sanitário Das Variáveis de Qualidade Das Águas e Dos Sedimentos e Metodologias Analíticas e de Amostragem 2021. Available online: https://cetesb.sp.gov.br/aguas-interiores/wp-content/uploads/sites/12/2022/11/Apendice-C-Significado-ambiental-e-sanitario-das-variaveis-de-qualidade-das-aguas-e-dos-sedimentos-e-metodologias-analiticas-e-de-amostragem.pdf (accessed on 26 July 2023).
- Maciel, M.A.M.; Dos Reis, S.M.; Ramalho, H.M.M. Águas Potáveis: Padrões de Qualidade, Metodologias Experimentais e Técnicas de Purificação; Amplla Editora: Campina Grande, Brazil, 2022. [Google Scholar]
- Abreu, C.H.M.; Cunha, A.C. Qualidade Da Água e Índice Trófico em Rio de Ecossistema Tropical Sob Impacto Ambiental. Eng. Sanit. Ambient. 2016, 22, 45–56. [Google Scholar] [CrossRef]
- Rodrigues, J.C.; Oliveira, J.T.; Pires, M.J.R.; Bernardes, A.M.H. A Estudo Da Oxidação Fotoquímica em Efluentes Líquidos Gerados em Salões de Beleza. In Simpósio Internacional de Qualidade Ambiental; ABES-RS: Porto Alegre, Brazil, 2016; pp. 1–11. [Google Scholar]
- D’Souza, P.; Rathi, S.K. Shampoo and Conditioners: What a Dermatologist Should Know? Indian J. Dermatol. 2015, 60, 248. [Google Scholar] [CrossRef] [PubMed]
- Ameen, H.A. Spring Water Quality Assessment Using Water Quality Index in Villages of Barwari Bala, Duhok, Kurdistan Region, Iraq. Appl. Water Sci. 2019, 9, 176. [Google Scholar] [CrossRef]
- BRASIL, Ministério da Saúde; Secretaria de Vigilância em Saúde. Vigilância e Controle da Qualidade da Água Para Consumo Humano; Ministério da Saúde: Brasília, Brazil, 2006.
- Health Canada. Guidelines for Canadian Drinking Water Quality: Guideline Technical Document—Turbidity; Water, Air and Climate Change Bureau, Healthy 105 Environments and Consumer Safety Branch, Health Canada: Ottawa, ON, Canada, 2012.
- Nkansah, M.A.; Opoku, F.; Ephraim, J.H.; Wemegah, D.D.; Tetteh, L.P.M. Characterization of Beauty Salon Wastewater from Kwame Nkrumah University of Science and Technology, Kumasi, Ghana, and Its Surrounding Communities. Environ. Health Insights 2016, 10, EHI-S40360. [Google Scholar] [CrossRef]
- SÃO PAULO Decreto No 10.755, de 22 de Novembro de 1977. Dispõe Sobre o Enquadramento Dos Corpos Receptores Na Classificação Prevista No Decreto No 8.468, de 08 de Setembro de 1976 e Dá Providências Correlatas; Diário Oficial do Estado de São Paulo, Ed.; Caderno Executivo, n. 221: São Paulo, Brazil, 1976. Available online: https://www.al.sp.gov.br/repositorio/legislacao/decreto/1977/decreto-10755-22.11.1977.html (accessed on 29 September 2023).
- Oliveira, G.H.; Pinto, A.L.; Pereira, G.A. Avaliação da eficiência da utilização do oxigênio dissolvido como principal indicador da qualidade das águas superficiais da bacia do córrego bom jardim, Brasilândia/MS. Rev. Geomae 2010, 1, 69–82. [Google Scholar]
- Queiroz, J.F.; Boeira, R.C. Boas Práticas de Manejo Para Manter Concentrações Adequadas de Oxigênio Dissolvido em Viveiros de Piscicultura; Embrapa Meio Ambiente: Jaguariúna, Brazil, 2016; Available online: https://ainfo.cnptia.embrapa.br/digital/bitstream/item/172486/1/2017CT03.pdf (accessed on 29 September 2023).
- CETESB—Companhia Ambiental do Estado de São Paulo. Matéria Orgânica e Nutrientes; São Paulo. 2019. Available online: https://cetesb.sp.gov.br/mortandade-peixes/alteracoes-fisicas-e-quimicas/materiaorganica-e-nutrientes/#targetText=A%20mat%C3%A9ria%20org%C3%A2nica%20sofre%20um,do%20oxig%C3%AAnio%20presente%20no%20meio.&targetText=O%20processo%20de%20enriquecimento%20das,vinhoto%2C%20acarretando%20graves%20problemas%20ambientais (accessed on 14 June 2023).
- Ferreira, A.L.G.; Loureiro, S.; Soares, A.M.V.M. Toxicity prediction of binary combinations of cadmium, carbendazim and low dissolved oxygen on Daphnia magna. Aquat. Toxicol. 2008, 89, 28–39. [Google Scholar] [CrossRef]
- Perdigón-Melón, J.A.; Carbajo, J.B.; Petre, A.L.; Rosal, R.; García-Calvo, E. Coagulation–Fenton Coupled Treatment for Ecotoxicity Reduction in Highly Polluted Industrial Wastewater. J. Hazard. Mater. 2010, 181, 127–132. [Google Scholar] [CrossRef]
- De Melo, E.D.; Mounteer, A.H.; Leão, L.H. de S.; Bahia, R.C.B.; Campos, I.M.F. Toxicity Identification Evaluation of Cosmetics Industry Wastewater. J. Hazard. Mater. 2013, 244, 329–334. [Google Scholar] [CrossRef]
- Knie, J.L.W.; Lopes, E.W.B. Testes Ecotoxicológicos: Métodos, Técnicas e Aplicações; FATMA/GTZ: Florianópolis, Brazil, 2004; 289p. [Google Scholar]
- Personne, E.; Loubet, B.; Herrmann, B.; Mattsson, M.; Schjoerring, J.K.; Nemitz, E.; Sutton, M.A.; Cellier, P. SURFATM-NH3: A Model Combining the Surface Energy Balance and Bi-Directional Exchanges of Ammonia Applied at the Field Scale. Biogeosciences 2009, 6, 1371–1388. [Google Scholar] [CrossRef]
- Dodson, S.L.; Cáceres, C.E.; Rogers, D.C. Cladocera and Other Branchiopoda. In Ecology and Classification of North American Freshwater Invertebrates; Academic Press: Cambridge, MA, USA, 2010; pp. 773–827. [Google Scholar] [CrossRef]
- Versteeg, D.J.; Stalmans, M.; Dyer, S.D.; Janssen, C. Ceriodaphnia and Daphnia: A Comparison of Their Sensitivity to Xenobiotics and Utility as a Test Species. Chemosphere 1997, 34, 869–892. [Google Scholar] [CrossRef]
- Radix, P.; Léonard, M.; Papantoniou, C.; Roman, G.; Saouter, E.; Gallotti-Schmitt, S.; Thiébaud, H.; Vasseur, P. Comparison of Brachionus Calyciflorus 2-d and Microtox® Chronic 22-h Tests with Daphnia magna 21-d Test for the Chronic Toxicity Assessment of Chemicals. Environ. Toxicol. Chem. 1999, 18, 2178–2185. [Google Scholar] [CrossRef] [PubMed]
- Baird, C. Química Ambiental, 2nd ed.; Bookman: Porto Alegre, Brazil, 2002; 622p. [Google Scholar]
- De Melo, E.D.; Mounteer, A.; Reis, E.; Costa, E.; Vilete, A. Screening of Physicochemical Treatment Processes for Reducing Toxicity of Hair Care Products Wastewaters. J. Environ. Manag. 2018, 212, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, J.M.; Bomm, M.D.; Pereira, N.F.G.; Garcez, W.S.; Boaventura, M.A.D. Estudo Fitoquímico de Unonopsis Lindmanii—Annonaceae, Biomonitorado Pelo Ensaio de Toxicidade Sobre a Artemia salina Leach. Química Nova 1998, 21, 557–559. [Google Scholar] [CrossRef]
- Zhu, X.; Zhu, L.; Duan, Z.; Qi, R.; Li, Y.; Lang, Y. Comparative Toxicity of Several Metal Oxide Nanoparticle Aqueous Suspensions to Zebrafish (Danio rerio) Early Developmental Stage. J. Environ. Sci. Health 2008, 43, 278–284. [Google Scholar] [CrossRef]
- Dvorak, P.; Benova, K.; Vitek, J. Alternative Biotest on Artemia franciscana. In Ecotoxicology, 1st ed.; Begum, G., Ed.; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef][Green Version]
- Zhang, Y.; Mu, J.; Han, J.; Gu, X. An Improved Brine Shrimp Larvae Lethality Microwell Test Method. Toxicol. Mech. Methods 2011, 22, 23–30. [Google Scholar] [CrossRef]
- Bueno, A.C.; Piovezan, M. Bioensaio Toxicológico Utilizando Artemia salina: Fatores Envolvidos Em Sua Eficácia. Instituto Federal de Santa Catarina. 2015. Available online: http://docente.ifsc.edu.br/michael.nunes/MaterialDidatico/Analises%20Quimicas/TCC%20II/TCC%202015%202/Ariele.pdf (accessed on 29 September 2023).
- Libralato, G.; Prato, E.; Migliore, L.; Cicero, A.M.; Manfra, L. A Review of Toxicity Testing Protocols and Endpoints with Artemia Spp. Ecol. Indic. 2016, 69, 35–49. [Google Scholar] [CrossRef]
- Ntungwe, N.E.; Domínguez-Martín, E.M.; Roberto, A.; Tavares, J.; Isca, V.M.S.; Pereira, P.; Cebola, M.-J.; Rijo, P. Artemia Species: An Important Tool to Screen General Toxicity Samples. Curr. Pharm. Des. 2020, 26, 2892–2908. [Google Scholar] [CrossRef]
- Vega, A.C.S.; Cruz-Alcalde, A.; Mazón, C.S.; Diaz-Cruz, M.S. Nano-TiO2 Phototoxicity in Fresh and Seawater: Daphnia magna and Artemia Sp. as Proxies. Water 2020, 13, 55. [Google Scholar] [CrossRef]
- Favilla, M.; Macchia, L.; Gallo, A.; Altomare, C. Toxicity assessment of metabolites of fungal biocontrol agents using two different (Artemia salina and Daphnia magna) invertebrate bioassays. Food Chem. Toxicol. 2006, 44, 1922–1931. [Google Scholar] [CrossRef] [PubMed]
- Spence, R.; Gerlach, G.; Lawrence, C.; Smith, C. The Behaviour and Ecology of the Zebrafish, Danio rerio. Biol. Rev. 2008, 83, 13–34. [Google Scholar] [CrossRef] [PubMed]
- D’Costa, A.; Shepherd, I.T. Zebrafish Development and Genetics: Introducing Undergraduates to Developmental Biology and Genetics in a Large Introductory Laboratory Class. Zebrafish 2009, 6, 169–177. [Google Scholar] [CrossRef]
- Aluru, N. Epigenetic Effects of Environmental Chemicals: Insights from Zebrafish. Curr. Opin. Toxicol. 2017, 6, 26–33. [Google Scholar] [CrossRef]
- Kari, G.; Rodeck, U.; Dicker, A.P. Zebrafish: An Emerging Model System for Human Disease and Drug Discovery. Clin. Pharmacol. Ther. 2007, 82, 70–80. [Google Scholar] [CrossRef]
- Lieschke, G.J.; Currie, P.D. Animal Models of Human Disease: Zebrafish Swim into View. Nat. Rev. Genet. 2007, 8, 353–367. [Google Scholar] [CrossRef]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The Zebrafish Reference Genome Sequence and Its Relationship to the Human Genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef]
- Hossain, F.; Perales-Perez, O.J.; Hwang, S.; Román, F. Antimicrobial Nanomaterials as Water Disinfectant: Applications, Limitations and Future Perspectives. Sci. Total Environ. 2014, 466, 1047–1059. [Google Scholar] [CrossRef]
- Moore, M.N. Do Nanoparticles Present Ecotoxicological Risks for the Health of the Aquatic Environment? Environ. Int. 2006, 32, 967–976. [Google Scholar] [CrossRef]
- Vale, G.; Mehennaoui, K.; Cambier, S.; Libralato, G.; Jomini, S.; Domingos, R.F. Manufactured Nanoparticles in the Aquatic Environment-Biochemical Responses on Freshwater Organisms: A Critical Overview. Aquatic. Toxicol. 2016, 170, 162–174. [Google Scholar] [CrossRef]
- Adam, V.; Loyaux-Lawniczak, S.; Labille, J.; Galindo, C.; del Nero, M.; Gangloff, S.; Weber, T.; Quaranta, G. Aggregation Behaviour of TiO2 Nanoparticles in Natural River Water. J. Nanoparticle Res. 2016, 18, 1–11. [Google Scholar] [CrossRef]
- Chen, G. Electrochemical Technologies in Wastewater Treatment. Sep. Purif. Technol. 2004, 38, 11–41. [Google Scholar] [CrossRef]
- Clemente, Z.; Castro, V.L.S.S.; Moura, M.A.M.; Jonsson, C.M.; Fraceto, L.F. Toxicity Assessment of TiO2 Nanoparticles in Zebrafish Embryos under Different Exposure Conditions. Aquatic. Toxicol. 2014, 147, 129–139. [Google Scholar] [CrossRef]
- Bar-Ilan, O.; Louis, K.M.; Yang, S.P.; Pedersen, J.A.; Hamers, R.J.; Peterson, R.E.; Heideman, W. Titanium Dioxide Nanoparticles Produce Phototoxicity in the Developing Zebrafish. Nanotoxicology 2012, 6, 670–679. [Google Scholar] [CrossRef]
- Xiong, D.; Fang, T.; Yu, L.; Sima, X.; Zhu, W. Effects of Nano-Scale TiO2, ZnO and Their Bulk Counterparts on Zebrafish: Acute Toxicity, Oxidative Stress and Oxidative Damage. Sci. Total Environ. 2011, 409, 1444–1452. [Google Scholar] [CrossRef]
- Boyle, D.; Al-Bairuty, G.A.; Henry, T.B.; Handy, R.D. Critical Comparison of Intravenous Injection of TiO2 Nanoparticles with Waterborne and Dietary Exposures Concludes Minimal Environmentally-Relevant Toxicity in Juvenile Rainbow Trout Oncorhynchus Mykiss. Environ. Pollut. 2013, 182, 70–79. [Google Scholar] [CrossRef]
- Ma, H.; Diamond, S.A. Phototoxicity of TiO2 Nanoparticles to Zebrafish (Danio rerio) Is Dependent on Life Stage. Environ. Toxicol. Chem. 2013, 32, 2139–2143. [Google Scholar] [CrossRef]
- Ercal, B.; Gurer-Orhan, B.; Nukhet Aykin-Burns, B. Toxic Metals and Oxidative Stress Part I: Mechanisms Involved in Metal-Induced Oxidative Damage. Curr. Top. Med. Chem. 2005, 1, 529–539. [Google Scholar] [CrossRef]
- Henkel, R.R. Leukocytes and Oxidative Stress: Dilemma for Sperm Function and Male Fertility. Asian J. Androl. 2011, 13, 43. [Google Scholar] [CrossRef]
- Huang, X.; Wang, J.; Liu, J.; Hua, L.; Zhang, D.; Hu, T.; Ge, Z.L. Maternal Periodontal Disease and Risk of Preeclampsia: A Meta-Analysis. J. Huazhong Univ. Sci. Technol.—Med. Sci. 2014, 34, 729–735. [Google Scholar] [CrossRef]
- Dejana, E.; Tournier-Lasserve, E.; Weinstein, B.M. The Control of Vascular Integrity by Endothelial Cell Junctions: Molecular Basis and Pathological Implications. Dev. Cell 2009, 16, 209–221. [Google Scholar] [CrossRef]
- Hallare, A.V.; Schirling, M.; Luckenbach, T.; Köhler, H.R.; Triebskorn, R. Combined Effects of Temperature and Cadmium on Developmental Parameters and Biomarker Responses in Zebrafish (Danio rerio) Embryos. J. Therm. Biol. 2005, 30, 7–17. [Google Scholar] [CrossRef]
- Verma, S.K.; Jha, E.; Panda, P.K.; Mukherjee, M.; Thirumurugan, A.; Makkar, H.; Das, B.; Parashar, S.K.S.; Suar, M. Mechanistic Insight into ROS and Neutral Lipid Alteration Induced Toxicity in the Human Model with Fins (Danio rerio) by Industrially Synthesized Titanium Dioxide Nanoparticles. Toxicol. Res. 2018, 7, 244–257. [Google Scholar] [CrossRef]
- Roberto, M.M.; Christofoletti, C.A.; Roberto, M.M.; Christofoletti, C.A. How to Assess Nanomaterial Toxicity? An Environmental and Human Health Approach. In Nanomaterials—Toxicity, Human Health and Environment; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]

| Treatments | DE | CE |
|---|---|---|
| NC | 0.00% | 0.00% |
| 3.125% | 22.50% | 6.25% |
| 6.250% | 33.75% | 25.00% |
| 12.500% | 41.25% | 28.75% |
| 25.000% | 100.00% | 100.00% |
| 50.000% | 100.00% | 100.00% |
| 100.000% | 100.00% | 100.00% |
| LC50 | 3.874% | 8.327% |
| Treatments | DE | CE |
|---|---|---|
| NC | 0.00% | 0.00% |
| 0.50% | 45.00% | - |
| 1.00% | 90.00% | 0.00% |
| 3.00% | 100.00% | 15.00% |
| 5.00% | - | 100.00% |
| 3.125% | 100.00% | 100.00% |
| 6.25% | 100.00% | 100.00% |
| 12.50% | 100.00% | 100.00% |
| 25.00% | 100.00% | 100.00% |
| 50.00% | 100.00% | 100.00% |
| 100.00% | 100.00% | 100.00% |
| EC50 | 0.54% | 3.43% |
| Stage Kimmel et al. [31] | Exposure Period (hpf) | DE | CE | ||
|---|---|---|---|---|---|
| Test Range (%) | LC50 (%) | Test Range (%) | LC50 (%) | ||
| Pharyngula | 24 | 0.5–12.5 | 7.33 (6.25–8.65) | 3.125–12.5 | 4.59 (3.94–5.36) |
| Early larva | 96 | 0.5–12.5 | 7.73 (6.70–8.97) | 3.125–12.5 | 4.25 (3.67–4.93) |
| Early larva | 144 | 0.5–12.5 | 8.18 (7.13–9.40) | 3.125–12.5 | 4.25 (3.67–4.93) |
| Parameters | Samples | Values Recommended by Brazilian and International Legislation | |||
|---|---|---|---|---|---|
| CONAMA n° 357/2005 | CONAMA n° 430/2011 | Directive 91/271/EEC of 21/05/1991 | |||
| DE | CE | ||||
| Temperature (°C) | 17.89 | 19.46 | <40.0 | <40.0 | --- |
| pH | 6.16 | 5.14 | 5.00 to 9.00 | 5.00 to 9.00 | --- |
| Conductivity (µS/cm) | 391.0 | 204.0 | --- | --- | --- |
| Dissolved oxygen (mg/L) | 2.37 | 5.60 | ≥5.00 | --- | --- |
| TSS (g/L) | 0.254 | 0.126 | ≤0.5 | --- | 35–60 |
| Turbidity (NTU) | 369 | 173 | ≤100 | --- | --- |
| Salinity | 0.01% | 0.00% | ≤0.5% | --- | --- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonçalves, L.C.; Roberto, M.M.; Peixoto, P.V.L.; Viriato, C.; da Silva, A.F.C.; de Oliveira, V.J.A.; Nardi, M.C.C.; Pereira, L.C.; de Angelis, D.d.F.; Marin-Morales, M.A. Toxicity of Beauty Salon Effluents Contaminated with Hair Dye on Aquatic Organisms. Toxics 2023, 11, 911. https://doi.org/10.3390/toxics11110911
Gonçalves LC, Roberto MM, Peixoto PVL, Viriato C, da Silva AFC, de Oliveira VJA, Nardi MCC, Pereira LC, de Angelis DdF, Marin-Morales MA. Toxicity of Beauty Salon Effluents Contaminated with Hair Dye on Aquatic Organisms. Toxics. 2023; 11(11):911. https://doi.org/10.3390/toxics11110911
Chicago/Turabian StyleGonçalves, Letícia C., Matheus M. Roberto, Paloma V. L. Peixoto, Cristina Viriato, Adriana F. C. da Silva, Valdenilson J. A. de Oliveira, Mariza C. C. Nardi, Lilian C. Pereira, Dejanira de F. de Angelis, and Maria A. Marin-Morales. 2023. "Toxicity of Beauty Salon Effluents Contaminated with Hair Dye on Aquatic Organisms" Toxics 11, no. 11: 911. https://doi.org/10.3390/toxics11110911
APA StyleGonçalves, L. C., Roberto, M. M., Peixoto, P. V. L., Viriato, C., da Silva, A. F. C., de Oliveira, V. J. A., Nardi, M. C. C., Pereira, L. C., de Angelis, D. d. F., & Marin-Morales, M. A. (2023). Toxicity of Beauty Salon Effluents Contaminated with Hair Dye on Aquatic Organisms. Toxics, 11(11), 911. https://doi.org/10.3390/toxics11110911