UPLC-MS/MS Based Identification and Quantification of a Novel Dual Orexin Receptor Antagonist in Plasma Samples by Validated SWGTOX Guidelines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Instrumentation and Chromatographic Conditions
2.3. Stock Solution, Calibration Standards (CSs) and Quality Controls (QCs) Sample Preparation
2.4. Sample Extraction Procedure
2.5. Assay Validation
2.5.1. LOD and LOQ Determination
2.5.2. Interference Studies
2.5.3. Calibration Model
2.5.4. Carry-Over Effects
2.5.5. Precision and Bias
2.5.6. Matrix Effects
2.5.7. Assay Recovery
2.5.8. Stability
2.6. Pharmacokinetic Study in Rats
3. Results and Discussion
3.1. Mass Spectrometric Condition Optimization
3.2. Chromatographic Condition Optimization
3.3. Optimization of Sample Extraction Procedure
3.4. Method Validation
3.4.1. LOD and LOQ
3.4.2. Interference and Selectivity Studies
3.4.3. Calibration Model
3.4.4. Carry-Over Effects
3.4.5. Precision and Bias
3.4.6. Recovery and Matrix Effects
3.4.7. Stability
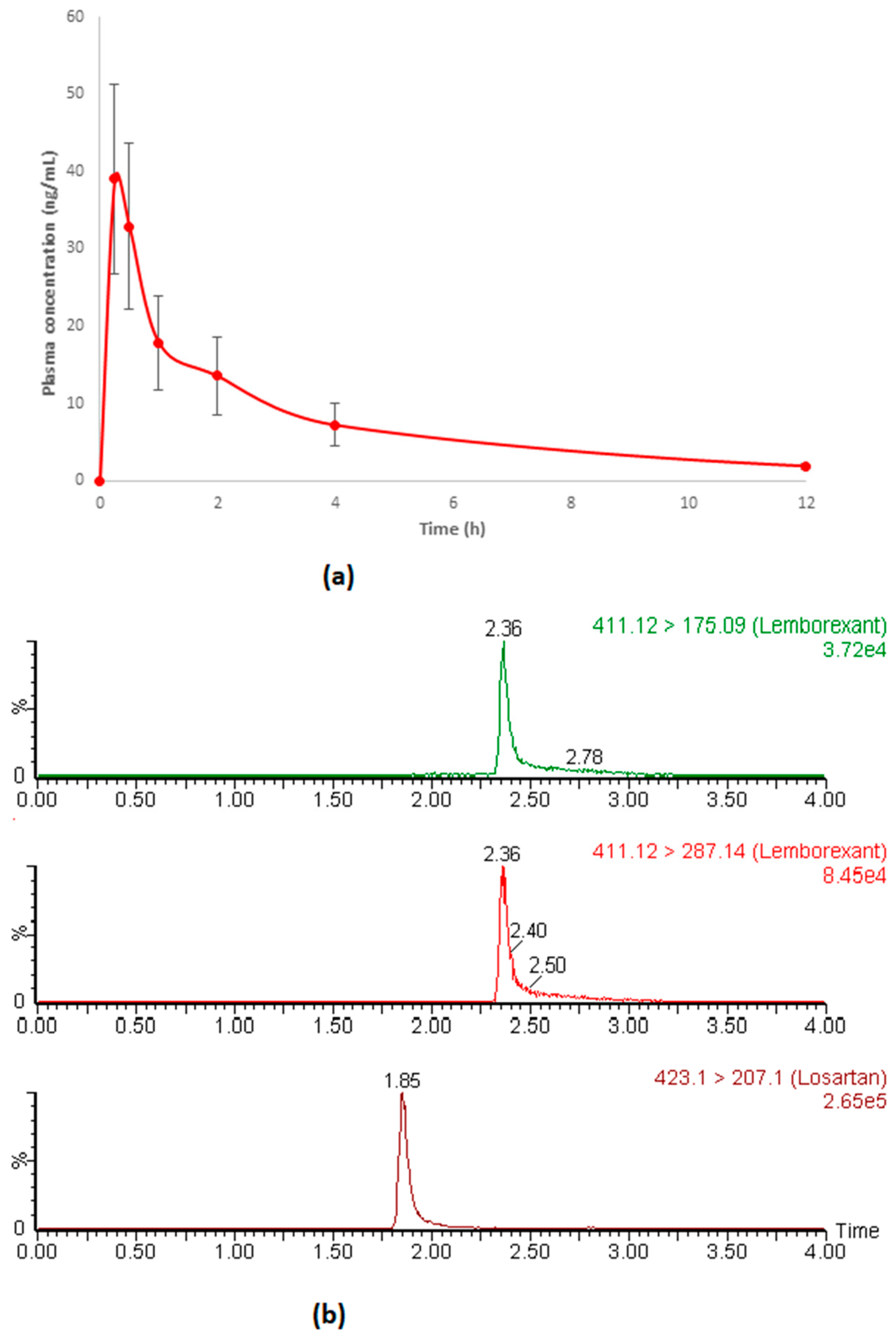
3.5. Application in Pharmacokinetic Study in Rats
3.6. Limitation of the Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chung, K.F.; Yeung, W.F.; Ho, F.Y.; Yung, K.P.; Yu, Y.M.; Kwok, C.W. Cross-cultural and comparative epidemiology of insomnia: The Diagnostic and statistical manual (DSM), International classification of diseases (ICD) and International classification of sleep disorders (ICSD). Sleep Med. 2015, 16, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Asnis, G.M.; Thomas, M.; Henderson, M.A. Pharmacotherapy Treatment Options for Insomnia: A Primer for Clinicians. Int. J. Mol. Sci. 2015, 17, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sateia, M.J.; Buysse, D.J.; Krystal, A.D.; Neubauer, D.N.; Heald, J.L. Clinical Practice Guideline for the Pharmacologic Treatment of Chronic Insomnia in Adults: An American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep Med. 2017, 13, 307–349. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Chanana, P.; Choudhary, S. Emerging role of orexin antagonists in insomnia therapeutics: An update on SORAs and DORAs. Pharmacol. Rep. 2016, 68, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Centre for Drug Evaluation and Research. US FDA Approves Eisai’s DAYVIGOtm (Lemborexant) for the Treatment of Insomnia in Adult Patients. NDA 212028 Multi-Disciplinary Review and Evaluation DAYVIGO (Lemborexant). 2018. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/212028Orig1s000MultidisciplineR.pdf (accessed on 10 December 2022).
- Prescribing Information for DAYVIGO® (Lemborexant) Tablets, for Oral Use, CIV Initial U.S. Approval: 2019. 2019. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/212028s000lbl.pdf (accessed on 10 December 2022).
- Beuckmann, C.T.; Suzuki, M.; Ueno, T.; Nagaoka, K.; Arai, T.; Higashiyama, H. In Vitro and In Silico characterization of lemborexant (E2006), a novel dual orexin receptor antagonist. J. Pharmacol. Exp. Ther. 2017, 362, 287–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moline, M.; Zammit, G.; Yardley, J.; Pinner, K.; Kumar, D.; Perdomo, C.; Cheng, J.Y. Lack of residual morning effects of lemborexant treatment for insomnia: Summary of findings across 9 clinical trials. Postgrad. Med. 2021, 133, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Mayleben, D.; Rosenberg, R.; Pinner, K.; Hussein, Z.; Moline, M. Assessment of morning sleep propensity with lemborexant in adults with insomnia disorder in a randomized, placebo-controlled crossover study. Sleep Adv. 2021, 2, zpab011. [Google Scholar] [CrossRef]
- Murphy, P.; Moline, M.; Mayleben, D.; Rosenberg, R.; Zammit, G.; Pinner, K.; Dhadda, S.; Hong, Q.; Giorgi, L.; Satlin, A. Lemborexant, A Dual Orexin Receptor Antagonist (DORA) for the Treatment of Insomnia Disorder: Results From a Bayesian, Adaptive, Randomized, Double-Blind, Placebo-Controlled Study. J. Clin. Sleep Med. 2017, 13, 1289–1299. [Google Scholar] [CrossRef] [PubMed]
- Landry, I.; Hall, N.; Alur, J.; Filippov, G.; Reyderman, L.; Setnik, B.; Henningfield, J.; Moline, M. Acute Cognitive Effects of the Dual Orexin Receptor Antagonist Lemborexant Compared With Suvorexant and Zolpidem in Recreational Sedative Users. J. Clin. Psychopharmacol. 2022, 42, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Landry, I.; Aluri, J.; Nakai, K.; Hall, N.; Miyajima, Y.; Ueno, T.; Dayal, S.; Filippov, G.; Lalovic, B.; Moline, M.; et al. Evaluation of the CYP3A and CYP2B6 Drug-Drug Interaction Potential of Lemborexant. Clin. Pharmacol. Drug Dev. 2021, 10, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Landry, I.; Hall, N.; Aluri, J.; Filippov, G.; Reyderman, L.; Setnik, B.; Henningfield, J.; Moline, M. Abuse Potential of Lemborexant, a Dual Orexin Receptor Antagonist, Compared With Zolpidem and Suvorexant in Recreational Sedative Users. J. Clin. Psychopharmacol. 2022, 42, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Mano, Y.; Ueno, T.; Hotta, K. Establishment of a simultaneous assay for lemborexant, a novel dual orexin receptor antagonist, and its three metabolites, and its application to a clinical protein binding study. J. Pharm. Biomed. Anal. 2020, 187, 113359. [Google Scholar] [CrossRef] [PubMed]
- Scientific Working Group for Forensic Toxicology. Scientific Working Group for Forensic Toxicology (SWGTOX) standard practices for method validation in forensic toxicology. J. Anal. Toxicol. 2013, 37, 452–474. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, M.; Ezzeldin, E.; Al-Rashood, K.A. UPLC-MS/MS assay for identification and quantification of brivaracetam in plasma sample: Application to pharmacokinetic study in rats. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1060, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.; Ezzeldin, E.; Al-Rashood, K.A.; Al-Shdefat, R.; Anwer, M.K. High throughput mu-SPE based elution coupled with UPLC-MS/MS for determination of eluxadoline in plasma sample: Application in pharmacokinetic characterization of PLGA nanoparticle formulations in rats. J. Pharm. Biomed. Anal. 2018, 149, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.; Ezzeldin, E.; Khalil, N.Y.; Alam, P.; Al-Rashood, K.A. UPLC-MS/MS determination of suvorexant in urine by a simplified dispersive liquid-liquid micro-extraction followed by ultrasound assisted back extraction from solidified floating organic droplets. J. Pharm. Biomed. Anal. 2019, 164, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.; Khalil, N.Y.; Ezzeldin, E.; Al-Rashood, K.A. Simultaneous Detection and Quantification of Three Novel Prescription Drugs of Abuse (Suvorexant, Lorcaserin and Brivaracetam) in Human Plasma by UPLC-MS-MS. J. Anal. Toxicol. 2019, 43, 203–211. [Google Scholar] [CrossRef] [PubMed]

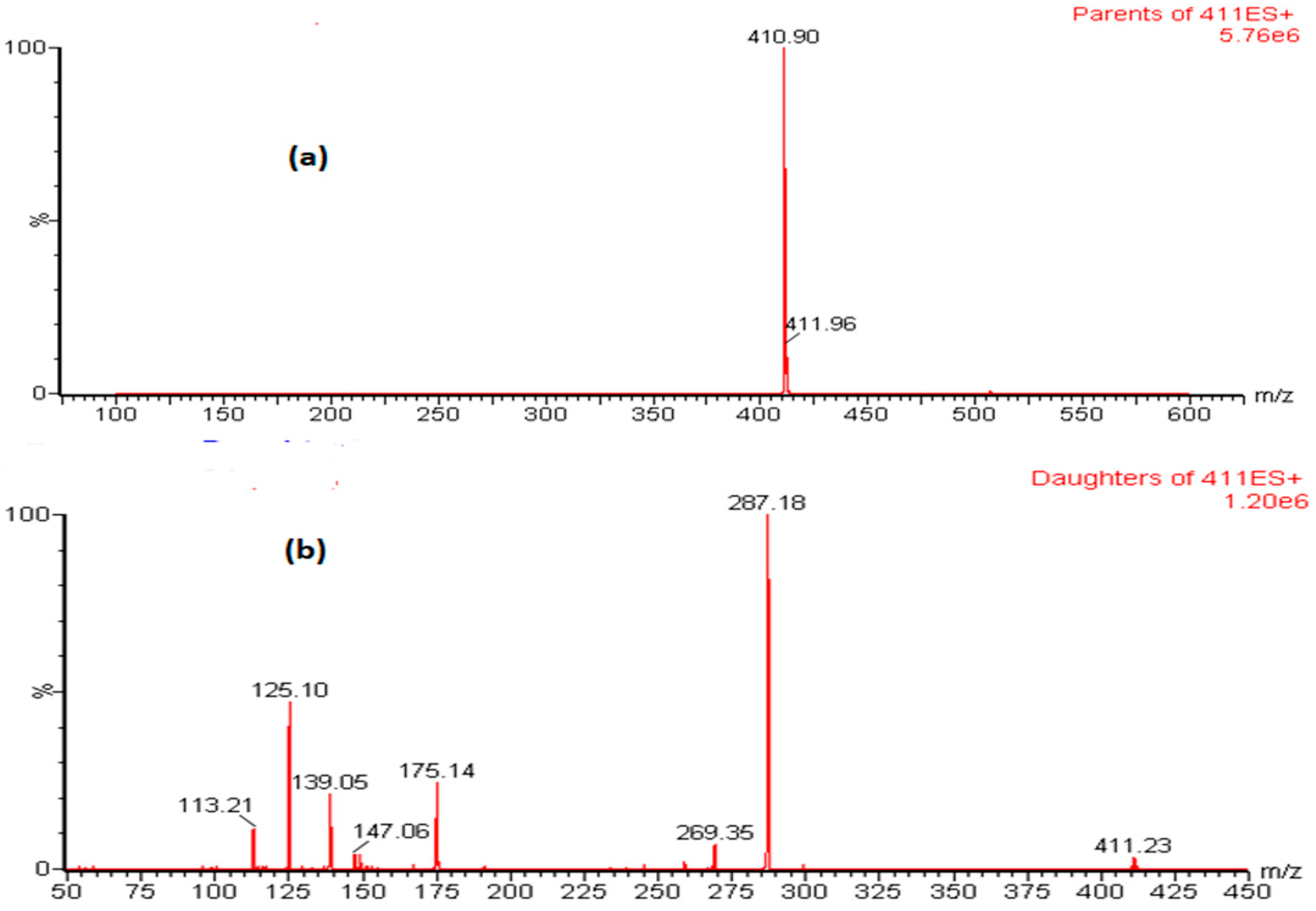

| Compound | tR (min) | Q1 [M+H]+ | CV (V) | Q3 [M+H]+ | CE (eV) | dt (s) |
|---|---|---|---|---|---|---|
| Lemborexant | 2.36 | 411.12 | 26 | 287.14 | 14 | 0.106 |
| 175.09 * | 28 | 0.106 | ||||
| IS | 1.86 | 423.1 | 22 | 207.1 | 20 | 0.106 |
| Time (min) | Flow (mL/min) | Solvent A | Solvent B | Curve |
|---|---|---|---|---|
| initial | 0.3 | 80 | 20 | |
| 0.50 | 0.3 | 20 | 80 | 6 |
| 1.00 | 0.3 | 50 | 50 | 6 |
| 1.50 | 0.3 | 80 | 20 | 6 |
| 4.00 | 0.3 | 80 | 20 | 6 |
| Nominal QC (ng/mL) | Precision (RSD, %) | Bias (RE, %) | ||
|---|---|---|---|---|
| Intra-Day | Inter-Day | Intra-Day | Inter-Day | |
| 1.0 | 11.49 | 9.35 | 6.99 | 10.74 |
| 3.0 | 5.91 | 5.79 | −10.03 | −9.12 |
| 50 | 3.60 | 3.35 | 2.25 | 1.66 |
| 250 | 1.68 | 4.85 | −8.77 | −6.12 |
| Compound | Nominal QC (ng/mL) | Matrix Effects | Extraction Recovery | ||
|---|---|---|---|---|---|
| % Mean | RSD, % | % Mean | RSD, % | ||
| LEM | 3.0 | 106.8 | 2.95 | 79.1 | 6.54 |
| 50 | 93.3 | 5.18 | 67.8 | 3.97 | |
| 500 | 87.4 | 9.65 | 74.7 | 9.49 | |
| Overall mean | 95.8 | 10.4 | 73.9 | 5.68 | |
| IS | 400 | 86.9 | 6.95 | 80.7 | 7.39 |
| Stability | Nominal Concentration (ng/mL) (n = 6) | Precision (RSD, %) | BIAS (RE, %) |
|---|---|---|---|
| Bench top (8 h) | |||
| 3.0 | 4.80 | 9.22 | |
| 250 | 6.75 | −6.40 | |
| Freeze thaw (3 cycle) | |||
| 3.0 | 8.15 | 12.11 | |
| 250 | −5.56 | −3.93 | |
| Auto-sampler (24 h) | |||
| 3.0 | 6.41 | 2.06 | |
| 250 | 9.07 | 4.87 | |
| 60 days at −80 °C | |||
| 3.0 | 5.13 | −6.78 | |
| 250 | 9.53 | −9.73 |
| Pharmacokinetic Parameters | Unit | Values (mean ± SD) |
|---|---|---|
| Cmax | ng/mL | 39.69 ± 11.69 |
| Tmax | h | 0.25 |
| AUC0−t | ng.h/mL | 109.16 ± 21.06 |
| T1/2 | h | 3.78 ± 1.07 |
| Kel | h | 0.19 ± 0.04 |
| MRT | h−1 | 4.41 ± 1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iqbal, M.; Alshememry, A.; Imam, F.; Kalam, M.A.; Akhtar, A.; Ali, E.A. UPLC-MS/MS Based Identification and Quantification of a Novel Dual Orexin Receptor Antagonist in Plasma Samples by Validated SWGTOX Guidelines. Toxics 2023, 11, 109. https://doi.org/10.3390/toxics11020109
Iqbal M, Alshememry A, Imam F, Kalam MA, Akhtar A, Ali EA. UPLC-MS/MS Based Identification and Quantification of a Novel Dual Orexin Receptor Antagonist in Plasma Samples by Validated SWGTOX Guidelines. Toxics. 2023; 11(2):109. https://doi.org/10.3390/toxics11020109
Chicago/Turabian StyleIqbal, Muzaffar, Abdullah Alshememry, Faisal Imam, Mohd Abul Kalam, Ali Akhtar, and Essam A. Ali. 2023. "UPLC-MS/MS Based Identification and Quantification of a Novel Dual Orexin Receptor Antagonist in Plasma Samples by Validated SWGTOX Guidelines" Toxics 11, no. 2: 109. https://doi.org/10.3390/toxics11020109
APA StyleIqbal, M., Alshememry, A., Imam, F., Kalam, M. A., Akhtar, A., & Ali, E. A. (2023). UPLC-MS/MS Based Identification and Quantification of a Novel Dual Orexin Receptor Antagonist in Plasma Samples by Validated SWGTOX Guidelines. Toxics, 11(2), 109. https://doi.org/10.3390/toxics11020109










