Enhancing the Toxicity of Cypermethrin and Spinosad against Spodoptera littoralis (Lepidoptera: Noctuidae) by Inhibition of Detoxification Enzymes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Compounds
2.2. Insect
2.3. Bioassays
2.4. Synergism Assays
2.5. Enzyme Inhibition Assays
2.5.1. Tissue Homogenate Preparation
2.5.2. Carboxylesterase (CarE) Activity
2.5.3. Glutathione S-Transferases (GSTs) Activity
2.5.4. Cytochrome P450 Monooxygenase Activity
2.6. Statistical Analysis
3. Results
3.1. Insecticidal Activity of Tested Insecticides and Enzyme Inhibitors against the Fourth Larval Instars of S. littoralis
3.2. Effects of Tested Insecticides and Enzyme Inhibitors on Carboxylesterases, Glutathione S-Transferases, and Cytochrome P450 Monooxygenase Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ismail, S.M. Synergistic Efficacy of Plant Essential Oils with Cypermethrin and Chlorpyrifos Against Spodoptera littoralis, Field Populations in Egypt. Int. J. Adv. Biol. Biomed. Res. 2021, 9, 128–137. [Google Scholar] [CrossRef]
- Hatem, A.E.-S.; Aldebis, H.K.; Osuna, E.V. Effects of the Spodoptera littoralis granulovirus on the development and reproduction of cotton leafworm S. littoralis. Biol. Control 2011, 59, 192–199. [Google Scholar] [CrossRef]
- Ismail, S.; Abdel-Galil, F.A.; Hafez, S.; Abu El-Ghiet, U.M. Influence of Some Insecticides on The Incidence of Common Lepidopterous Insect-Pests in Cotton Field. Egypt. Acad. J. Biol. Sci. F Toxicol. Pest Control 2020, 12, 23–30. [Google Scholar] [CrossRef] [Green Version]
- Mokbel, E.; Fouad, E.A.; El-Sherif, S.A. Resistance monitoring of cotton leaf worm, Spodoptera littoralis (Boisduval) (Lepidoptera: Noctuidae) against certain alternative insecticides of four different field populations in Egypt. J. Biol. Chem. Environ. Sci. 2019, 14, 319–333. [Google Scholar]
- Elghar, G.E.A.; Elbermawy, Z.A.; Yousef, A.G.; Elhady, H.K.A. Monitoring and Characterization of Insecticide Resistance in the Cotton Leafworm, Spodoptera littoral is (Boisd.) (Lepidoptera: Noctuidae). J. Asia-Pac. Èntomol. 2005, 8, 397–410. [Google Scholar] [CrossRef]
- Moustafa, M.A.M.; Fouad, E.A.; Abdel-Mobdy, Y.; Hamow, K.; Mikó, Z.; Molnár, B.P.; Fónagy, A. Toxicity and sublethal effects of chlorantraniliprole and indoxacarb on Spodoptera littoralis (Lepidoptera: Noctuidae). Appl. Èntomol. Zool. 2021, 56, 115–124. [Google Scholar] [CrossRef]
- El-Aal, E.M.A.; Shahen, M.; Sayed, S.; Kesba, H.; Ansari, M.J.; El-Ashry, R.M.; Aioub, A.A.; Salma, A.S.; Eldeeb, A.M. In Vivo and In Vitro management of Meloidogyne incognita (Tylenchida: Heteroderidae) using rhizosphere bacteria, Pseudomonas spp. and Serratia spp. compared with oxamyl. Saudi J. Biol. Sci. 2021, 28, 4876–4883. [Google Scholar] [CrossRef]
- El-Kholy, R.; El-Bamby, M.; El-Tawil, M.; Abouamer, W. Effect of three plant extracts on some biological aspects of cotton leafworm, Spodoptera littoralis (Boisd.). Middle East J. Appl. Sci. 2014, 4, 243–251. [Google Scholar]
- Wanwimolruk, S.; Duangsuwan, W.; Phopin, K.; Boonpangrak, S. Food safety in Thailand 5: The effect of washing pesticide residues found in cabbages and tomatoes. J. Consum. Prot. Food Saf. 2017, 12, 209–221. [Google Scholar] [CrossRef]
- Khazri, A.; Sellami, B.; Dellali, M.; Corcellas, C.; Eljarrat, E.; Barceló, D.; Beyrem, H.; Mahmoudi, E. Diastereomeric and enantiomeric selective accumulation of cypermethrin in the freshwater mussel Unio gibbus and its effects on biochemical parameters. Pestic. Biochem. Physiol. 2016, 129, 83–88. [Google Scholar] [CrossRef]
- Narahashi, T.; Frey, J.; Ginsburg, K.; Roy, M. Sodium and GABA-activated channels as the targets of pyrethroids and cyclodienes. Toxicol. Lett. 1992, 64–65, 429–436. [Google Scholar] [CrossRef]
- Xu, P.; Huang, L. Effects of α-cypermethrin enantiomers on the growth, biochemical parameters and bioaccumulation in Rana nigromaculata tadpoles of the anuran amphibians. Ecotoxicol. Environ. Saf. 2017, 139, 431–438. [Google Scholar] [CrossRef]
- Parsaeyan, E.; Safavi, S.A.; Saber, M.; Poorjavad, N. Effects of emamectin benzoate and cypermethrin on the demography of Trichogramma brassicae Bezdenko. Crop. Prot. 2018, 110, 269–274. [Google Scholar] [CrossRef]
- Copping, L.G.; Menn, J.J. Biopesticides: A review of their action, applications and efficacy. Pest Manag. Sci. Former. Pestic. Sci. 2000, 56, 651–676. [Google Scholar] [CrossRef]
- Pineda, S.; Smagghe, G.; Schneider, M.I.; Del Estal, P.; Viñuela, E.; Martínez, A.M.; Budia, F. Toxicity and Pharmacokinetics of Spinosad and Methoxyfenozide to Spodoptera littoralis (Lepidoptera: Noctuidae). Environ. Èntomol. 2006, 35, 856–864. [Google Scholar] [CrossRef]
- Salgado, V. The modes of action of spinosad and other insect control products. Down Earth 1997, 52, 35–43. [Google Scholar]
- Li, Y.; Wei, J.; Fang, J.; Lv, W.; Ji, Y.; Aioub, A.A.; Zhang, J.; Hu, Z. Insecticidal Activity of Four Lignans Isolated from Phryma leptostachya. Molecules 2019, 24, 1976. [Google Scholar] [CrossRef] [Green Version]
- Hilliou, F.; Chertemps, T.; Maïbèche, M.; Le Goff, G. Resistance in the Genus Spodoptera: Key Insect Detoxification Genes. Insects 2021, 12, 544. [Google Scholar] [CrossRef]
- Wang, J.-J.; Wei, D.; Dou, W.; Hu, F.; Liu, W.-F.; Wang, J.-J. Toxicities and Synergistic Effects of Several Insecticides against the Oriental Fruit Fly (Diptera: Tephritidae). J. Econ. Èntomol. 2013, 106, 970–978. [Google Scholar] [CrossRef]
- Feyereisen, R. Molecular biology of insecticide resistance. Toxicol. Lett. 1995, 82–83, 83–90. [Google Scholar] [CrossRef]
- Ffrench-Constant, R. Target site mediated insecticide resistance: What questions remain? Insect Biochem. Mol. Biol. 1999, 29, 397–403. [Google Scholar] [CrossRef]
- Xu, C.; Li, C.Y.-T.; Kong, A.-N.T. Induction of phase I, II and III drug metabolism/transport by xenobiotics. Arch. Pharmacal Res. 2005, 28, 249–268. [Google Scholar] [CrossRef]
- Xiao, L.-F.; Zhang, W.; Jing, T.-X.; Zhang, M.-Y.; Miao, Z.-Q.; Wei, D.-D.; Yuan, G.-R.; Wang, J.-J. Genome-wide identification, phylogenetic analysis, and expression profiles of ATP-binding cassette transporter genes in the oriental fruit fly, Bactrocera dorsalis (Hendel) (Diptera: Tephritidae). Comp. Biochem. Physiol. Part D Genom. Proteom. 2018, 25, 1–8. [Google Scholar] [CrossRef]
- Ju, D.; Mota-Sanchez, D.; Fuentes-Contreras, E.; Zhang, Y.-L.; Wang, X.-Q.; Yang, X.-Q. Insecticide resistance in the Cydia pomonella (L): Global status, mechanisms, and research directions. Pestic. Biochem. Physiol. 2021, 178, 104925. [Google Scholar] [CrossRef]
- Serebrov, V.V.; Gerber, O.N.; Malyarchuk, A.A.; Martemyanov, V.V.; Alekseev, A.A.; Glupov, V.V. Effect of entomopathogenic fungi on detoxification enzyme activity in greater wax moth Galleria mellonella L. (Lepidoptera, Pyralidae) and role of detoxification enzymes in development of insect resistance to entomopathogenic fungi. Biol. Bull. 2006, 33, 581–586. [Google Scholar] [CrossRef]
- Mosallanejad, H.; Smagghe, G. Biochemical mechanisms of methoxyfenozide resistance in the cotton leafworm Spodoptera littoralis. Pest Manag. Sci. 2009, 65, 732–736. [Google Scholar] [CrossRef]
- AhMed, M.A.I.; Temerak, S.A.H.; Abdel-Galil, F.-K.; Manna, S.H.M. Susceptibility of field and laboratory strains of Cotton leafworm, Spodoptera littoralis (Boisd.) (Lepidoptera: Noctuidae) to spinosad pesticide under laboratory conditions. Plant Prot. Sci. 2016, 52, 128–133. [Google Scholar] [CrossRef] [Green Version]
- Ismail, S.M. Effect of sublethal doses of some insecticides and their role on detoxication enzymes and protein-content of Spodoptera littoralis (Boisd.) (Lepidoptera: Noctuidae). Bull. Natl. Res. Cent. 2020, 44, 35. [Google Scholar] [CrossRef]
- Kuddus, M. Introduction to food enzymes. In Enzymes in Food Biotechnology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–18. [Google Scholar]
- Bao, H.; Shao, X.; Zhang, Y.; Deng, Y.; Xu, X.; Liu, Z.; Li, Z. Specific synergist for neonicotinoid insecticides: IPPA08, a cis-neonicotinoid compound with a unique oxabridged substructure. J. Agric. Food Chem. 2016, 64, 5148–5155. [Google Scholar] [CrossRef]
- Qie, X.; Lu, W.; Aioub, A.A.; Li, Y.; Wu, W.; Hu, Z. Insight into the detoxification of Haedoxan A and the synergistic effects of Phrymarolin I against Mythimna separata. Ind. Crop. Prod. 2020, 158, 112967. [Google Scholar] [CrossRef]
- Wang, S.-P.; Hu, X.-X.; Meng, Q.-W.; Muhammad, S.A.; Chen, R.-R.; Li, F.; Li, G.-Q. The involvement of several enzymes in methanol detoxification in Drosophila melanogaster adults. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2013, 166, 7–14. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Li, X.; Sun, M.; Wei, Z.; Wang, Y.; Gao, A.; Chen, D.; Zhao, X.; Feng, X. Exploring the Effects of Different Types of Surfactants on Zebrafish Embryos and Larvae. Sci. Rep. 2015, 5, 10107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Xu, Z.; Shi, L.; Shen, G.; He, L. Insecticide resistance monitoring and metabolic mechanism study of the green peach aphid, Myzus persicae (Sulzer) (Hemiptera: Aphididae), in Chongqing, China. Pestic. Biochem. Physiol. 2016, 132, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Pang, S.; You, W.; Duan, L.; Song, X.; Li, X.; Wang, C. Resistance selection and mechanisms of oriental tobacco budworm (Helicoverpa assulta Guenee) to indoxacarb. Pestic. Biochem. Physiol. 2012, 103, 219–223. [Google Scholar] [CrossRef]
- Wu, W.; Tu, Y.; Liu, H.; Zhu, J. Celangulins II, III, and IV: New insecticidal sesquiterpenoids from Celastrus angulatus. J. Nat. Prod. 1992, 55, 1294–1298. [Google Scholar] [CrossRef]
- Abbott, W.S. A Method of Computing the Effectiveness of an Insecticide. J. Econ. Èntomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- van Asperen, K. A study of housefly esterases by means of a sensitive colorimetric method. J. Insect Physiol. 1962, 8, 401–416. [Google Scholar] [CrossRef]
- Booth, G.M.; Connor, J.; Metcalf, R.; Larsen, J. A comparative study of the effects of selective inhibitors on esterase isozymes from the mosquito Anopheles punctipennis. Comp. Biochem. Physiol. Part B Comp. Biochem. 1973, 44, 1185–1195. [Google Scholar] [CrossRef]
- Hansen, L.; Hodgson, E. Biochemical characteristics of insect microsomes: N- and O-demethylation. Biochem. Pharmacol. 1971, 20, 1569–1578. [Google Scholar] [CrossRef]
- Tan, J.; Liu, Z.; Wang, R.; Huang, Z.Y.; Chen, A.C.; Gurevitz, M.; Dong, K. Identification of Amino Acid Residues in the Insect Sodium Channel Critical for Pyrethroid Binding. Mol. Pharmacol. 2004, 67, 513–522. [Google Scholar] [CrossRef] [Green Version]
- Orr, N.; Shaffner, A.J.; Richey, K.; Crouse, G.D. Novel mode of action of spinosad: Receptor binding studies demonstrating lack of interaction with known insecticidal target sites. Pestic. Biochem. Physiol. 2009, 95, 1–5. [Google Scholar] [CrossRef]
- Wang, J.; Hong, H.; Xie, R.; Ji, J.; Guo, K.; Bai, L.; Tang, J.; Yu, H.; Ye, J.; Hu, J. Molecular characterization and functional analysis of daf-8 in the pinewood nematode, Bursaphelenchus xylophilus. J. For. Res. 2021, 33, 689–698. [Google Scholar] [CrossRef]
- El-Sheikh, A. Biological, biochemical and histological effects of spinosad, Bacillus thuringiensis var. kurstaki and cypermethrin on the Cotton leafworm, Spodoptera littoralis (Boisd.). Egypt. Acad. J. biolog. Sci. 2019, 4, 113–124. [Google Scholar] [CrossRef]
- Pineda, S.; Budia, F.; Schneider, M.I.; Gobbi, A.; Viñuela, E.; Valle, J.; Del Estal, P. Effects of two biorational insecticides, spinosad and methoxyfenozide, on Spodoptera littoralis (Lepidoptera: Noctuidae) under laboratory conditions. J. Econ. Entomol. 2004, 97, 1906–1911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fouad, E.A.; Ahmed, F.S.; Moustafa, M.A.M. Monitoring and biochemical impact of insecticides resistance on field populations of Spodoptera littoralis (Boisd.) (Lepidoptera: Noctuidae) in Egypt. Pol. J. Entomol. 2022, 91, 109–118. [Google Scholar] [CrossRef]
- Ghadamyari, M.M.M.-M.; Talebi, K.; Memarizade, N. The effect of Artemisia annua L. (Asteraceae) essential oil on detoxify enzymes of two-spotted spider mite, Tetranychus urticae Koch (Acari: Tetranychidae). J. Plant Prot. 2012, 26, 29. [Google Scholar]
- Huang, Q.; Deng, Y.; Zhan, T.; He, Y. Synergistic and Antagonistic Effects of Piperonyl Butoxide in Fipronil-Susceptible and Resistant Rice Stem Borrers, Chilo suppressalis. J. Insect Sci. 2010, 10, 182. [Google Scholar] [CrossRef] [Green Version]
- Ng, C.H.; Ng, K.K.S.; Lee, S.L.; Suwa, R.; Lee, C.T.; Tnah, L.H. Growth performance and scale insect infestation of Shorea leprosula in a common garden experimental plot. J. For. Res. 2022, 33, 1–12. [Google Scholar] [CrossRef]
- Liu, N.; Yue, X. Insecticide Resistance and Cross-Resistance in the House Fly (Diptera: Muscidae). J. Econ. Èntomol. 2000, 93, 1269–1275. [Google Scholar] [CrossRef]
- Wang, W.; Mo, J.; Cheng, J.; Zhuang, P.; Tang, Z. Selection and characterization of spinosad resistance in Spodoptera exigua (Hübner) (Lepidoptera: Noctuidae). Pestic. Biochem. Physiol. 2006, 84, 180–187. [Google Scholar] [CrossRef]
- Khan, H.A.A.; Akram, W.; Shad, S.A.; Lee, J.-J. Insecticide mixtures could enhance the toxicity of insecticides in a resistant dairy population of Musca domestica L. PLoS ONE 2013, 8, e60929. [Google Scholar] [CrossRef]
- Sharma, R. Enzyme inhibition: Mechanisms and scope. Enzym. Inhib. Bioappl. 2021, 3–36. [Google Scholar]
- Zibaee, I.; Mahmood, K.; Esmaeily, M.; Bandani, A.R.; Kristensen, M. Organophosphate and pyrethroid resistances in the tomato leaf miner Tuta absoluta (Lepidoptera: Gelechiidae) from Iran. J. Appl. Èntomol. 2017, 142, 181–191. [Google Scholar] [CrossRef]
- Ruttanaphan, T.; Pluempanupat, W.; Aungsirisawat, C.; Boonyarit, P.; Le Goff, G.; Bullangpoti, V. Effect of Plant Essential Oils and Their Major Constituents on Cypermethrin Tolerance Associated Detoxification Enzyme Activities in Spodoptera litura (Lepidoptera: Noctuidae). J. Econ. Èntomol. 2019, 112, 2167–2176. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, M.; Ranganathan, M.; Subramanian, S.M.; Kumarasamy, S.; Kandasamy, S. Toxicity of cypermethrin and enzyme inhibitor synergists in red hairy caterpillar Amsacta albistriga (Lepidoptera: Arctiidae). J. Basic Appl. Zool. 2020, 81, 45. [Google Scholar] [CrossRef]
- Wang, D.; Qiu, X.; Ren, X.; Zhang, W.; Wang, K. Effects of spinosad on Helicoverpa armigera (Lepidoptera: Noctuidae) from China: Tolerance status, synergism and enzymatic responses. Pest Manag. Sci. 2009, 65, 1040–1046. [Google Scholar] [CrossRef]
- Shi, J.; Zhang, L.; Gao, X. Characterisation of spinosad resistance in the housefly Musca domestica (Diptera: Muscidae). Pest Manag. Sci. 2011, 67, 335–340. [Google Scholar] [CrossRef]
- Baker, J.E.; Weaver, D.K.; Throne, J.E.; Zettler, J.L. Resistance to Protectant Insecticides in Two Field Strains of the Stored-Product Insect Parasitoid Bracon hebetor (Hymenoptera: Braconidae). J. Econ. Èntomol. 1995, 88, 512–519. [Google Scholar] [CrossRef]
- Baker, J.E.; Arbogast, R.T. Malathion Resistance in Field Strains of the Warehouse Pirate Bug (Heteroptera: Anthocoridae) and a Prey Species Tribolium castaneum (Coleoptera: Tenebrionidae). J. Econ. Èntomol. 1995, 88, 241–245. [Google Scholar] [CrossRef]
- Wu, G.; Miyata, T.; Kang, C.Y.; Xie, L.H. Insecticide toxicity and synergism by enzyme inhibitors in 18 species of pest insect and natural enemies in crucifer vegetable crops. Pest Manag. Sci. 2007, 63, 500–510. [Google Scholar] [CrossRef]
- Wu, G.; Jiang, S.-R. Effects of enzyme inhibitors on insecticide susceptibility of two parasitoid wasps Pteromalus puparum and Diadromus collaris. Chin. J. Biol. Control 2005, 21, 5. [Google Scholar]
- Ishaaya, I.; Casida, J.E. Pyrethroid Esterase(s) May Contribute to Natural Pyrethroid Tolerance of Larvae of the Common Green Lacewing 1. Environ. Èntomol. 1981, 10, 681–684. [Google Scholar] [CrossRef]
- Plapp, F.W.; Vinson, S.B. Comparative toxicities of some insecticides to the tobacco budworm and its ichneumonid parasite, Campoletis sonorensis. Environ. Entomol. 1977, 6, 381–384. [Google Scholar] [CrossRef]
- Joffe, T. Evaluation of Potential Pyrethrum Synergists on Agriculturally Significant Insect Species. Ph.D. Thesis, University of Tasmania, Hobart, Australia, 2011. [Google Scholar]
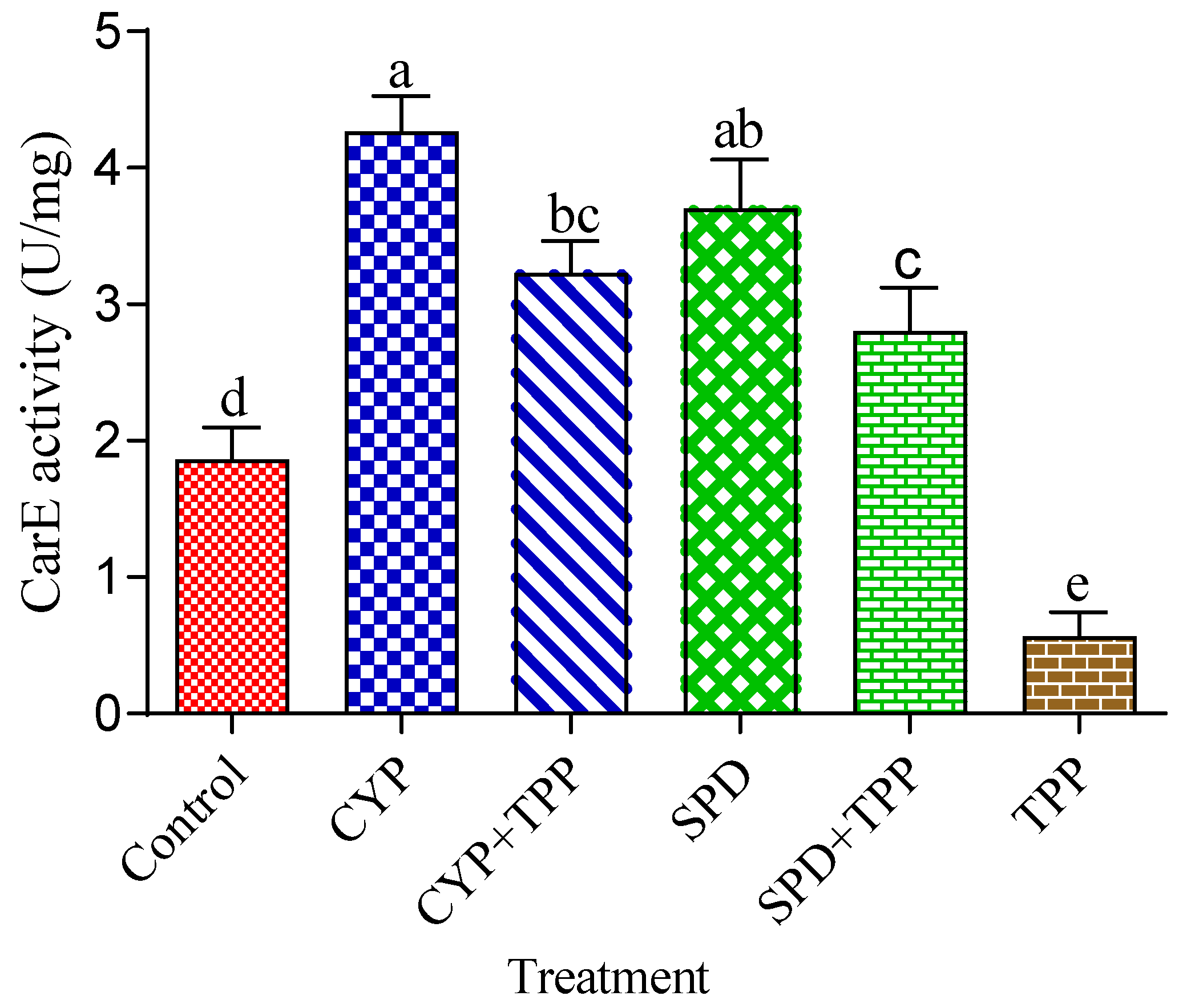

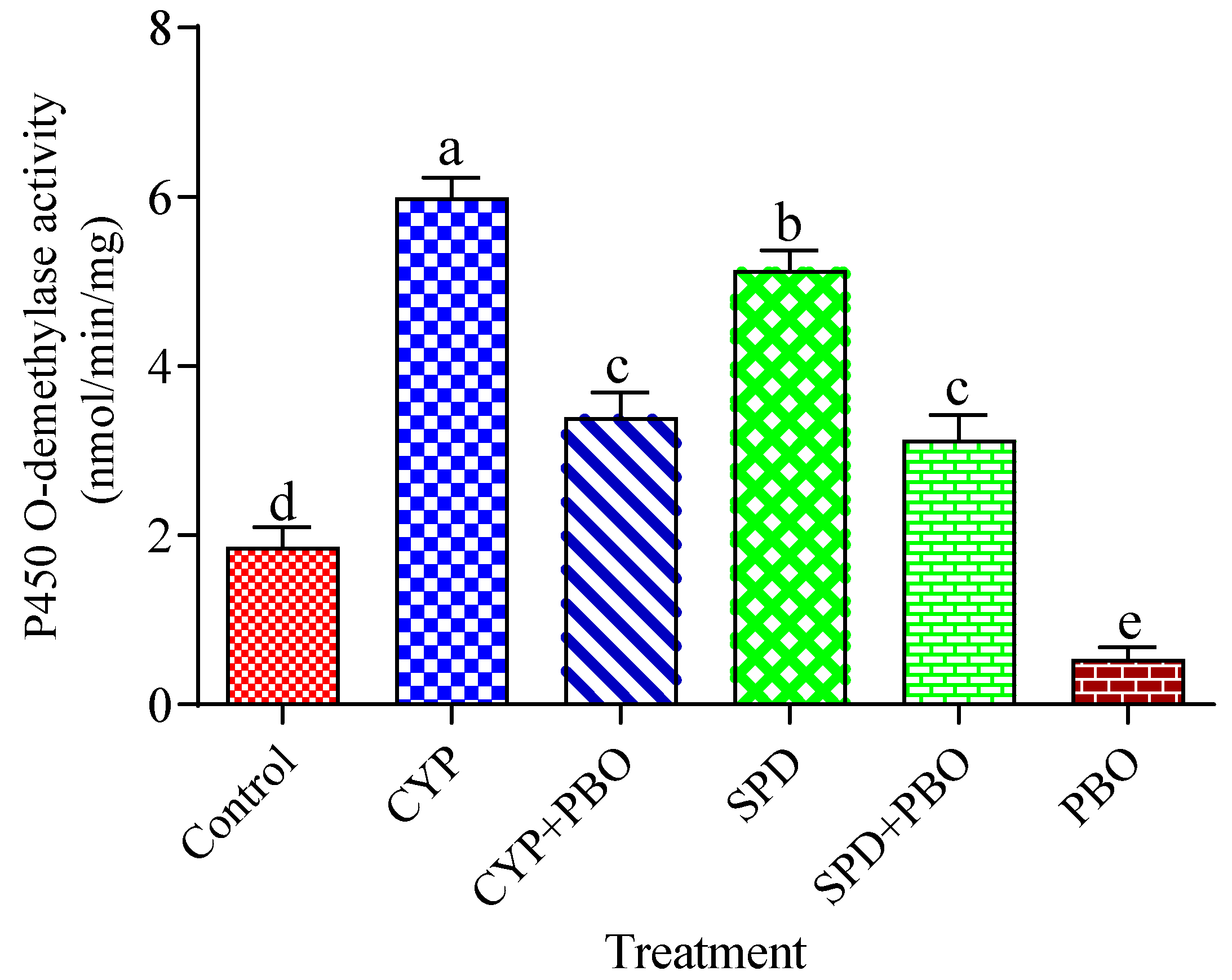
| Compounds | Toxicity Regression Equation | LC50 (μg/mL) | 95% Fiducial Limits (μg/mL) | χ2 | r | df |
|---|---|---|---|---|---|---|
| Cypermethrin | y = 0.0506x + 3.086 | 2.861 | 2.460–2.883 | 0.90 | 0.94 | 4 |
| Spinosad | y = 0.0551x + 2.7615 | 3.273 | 2.904–3.396 | 1.03 | 0.95 | 4 |
| Compounds | Toxicity Regression Equation | LC50 (μg/mL) | 95% Fiducial Limits (μg/mL) | χ2 | r | df |
|---|---|---|---|---|---|---|
| PBO | y = 0.0335x + 3.0098 | 236.2 | 228.7–239.2 | 4.74 | 0.98 | 4 |
| DEM | y = 0.0585x + 0.4371 | 324.5 | 319.1–329.9 | 4.78 | 0.97 | 4 |
| TPP | y = 0.0218x + 3.6632 | 245.8 | 241.9–249.3 | 5.06 | 0.98 | 4 |
| Compounds | Toxicity Regression Equation | LC50 (μg/mL) | 95% Fiducial Limits (μg/mL) | χ2 | r | df | Synergistic Ratio |
|---|---|---|---|---|---|---|---|
| Cypermethrin + PBO | y = 0.0561x + 3.4655 | 1.580 | 1.105–1.809 | 0.38 | 0.94 | 4 | 1.810 |
| Cypermethrin + DEM | y = 0.0503x + 3.3235 | 2.267 | 2.176–2.311 | 0.70 | 0.98 | 4 | 1.262 |
| Cypermethrin + TPP | y = 0.0555x + 3.3675 | 1.962 | 1.877–1.992 | 0.58 | 0.95 | 4 | 1.458 |
| Spinosad + PBO | y = 0.051x + 3.2425 | 2.344 | 2.271–2.395 | 0.72 | 0.96 | 4 | 1.396 |
| Spinsad + DEM | y = 0.0512x + 3.1425 | 2.653 | 2.580–2.717 | 0.84 | 0.95 | 4 | 1.233 |
| Spinosad + TPP | y = 0.0542x + 3.0125 | 2.530 | 2.477–2.583 | 0.80 | 0.97 | 4 | 1.293 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Sayed, M.H.; Ibrahim, M.M.A.; Elsobki, A.E.A.; Aioub, A.A.A. Enhancing the Toxicity of Cypermethrin and Spinosad against Spodoptera littoralis (Lepidoptera: Noctuidae) by Inhibition of Detoxification Enzymes. Toxics 2023, 11, 215. https://doi.org/10.3390/toxics11030215
El-Sayed MH, Ibrahim MMA, Elsobki AEA, Aioub AAA. Enhancing the Toxicity of Cypermethrin and Spinosad against Spodoptera littoralis (Lepidoptera: Noctuidae) by Inhibition of Detoxification Enzymes. Toxics. 2023; 11(3):215. https://doi.org/10.3390/toxics11030215
Chicago/Turabian StyleEl-Sayed, Marwa H., Mohamed M. A. Ibrahim, Ahmed E. A. Elsobki, and Ahmed A. A. Aioub. 2023. "Enhancing the Toxicity of Cypermethrin and Spinosad against Spodoptera littoralis (Lepidoptera: Noctuidae) by Inhibition of Detoxification Enzymes" Toxics 11, no. 3: 215. https://doi.org/10.3390/toxics11030215






