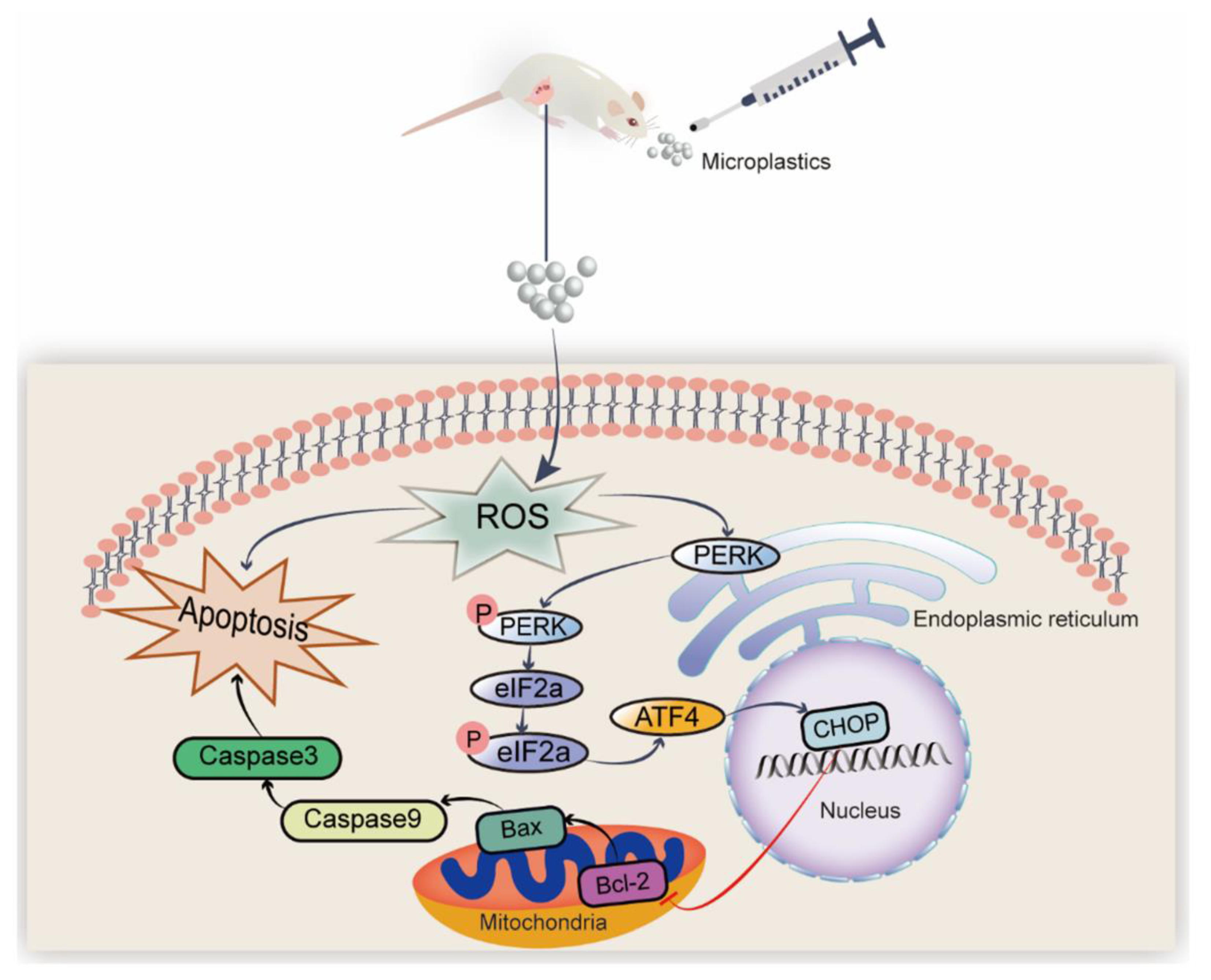
Polystyrene Microplastics Induced Ovarian Toxicity in Juvenile Rats Associated with Oxidative Stress and Activation of the PERK-eIF2α-ATF4-CHOP Signaling Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
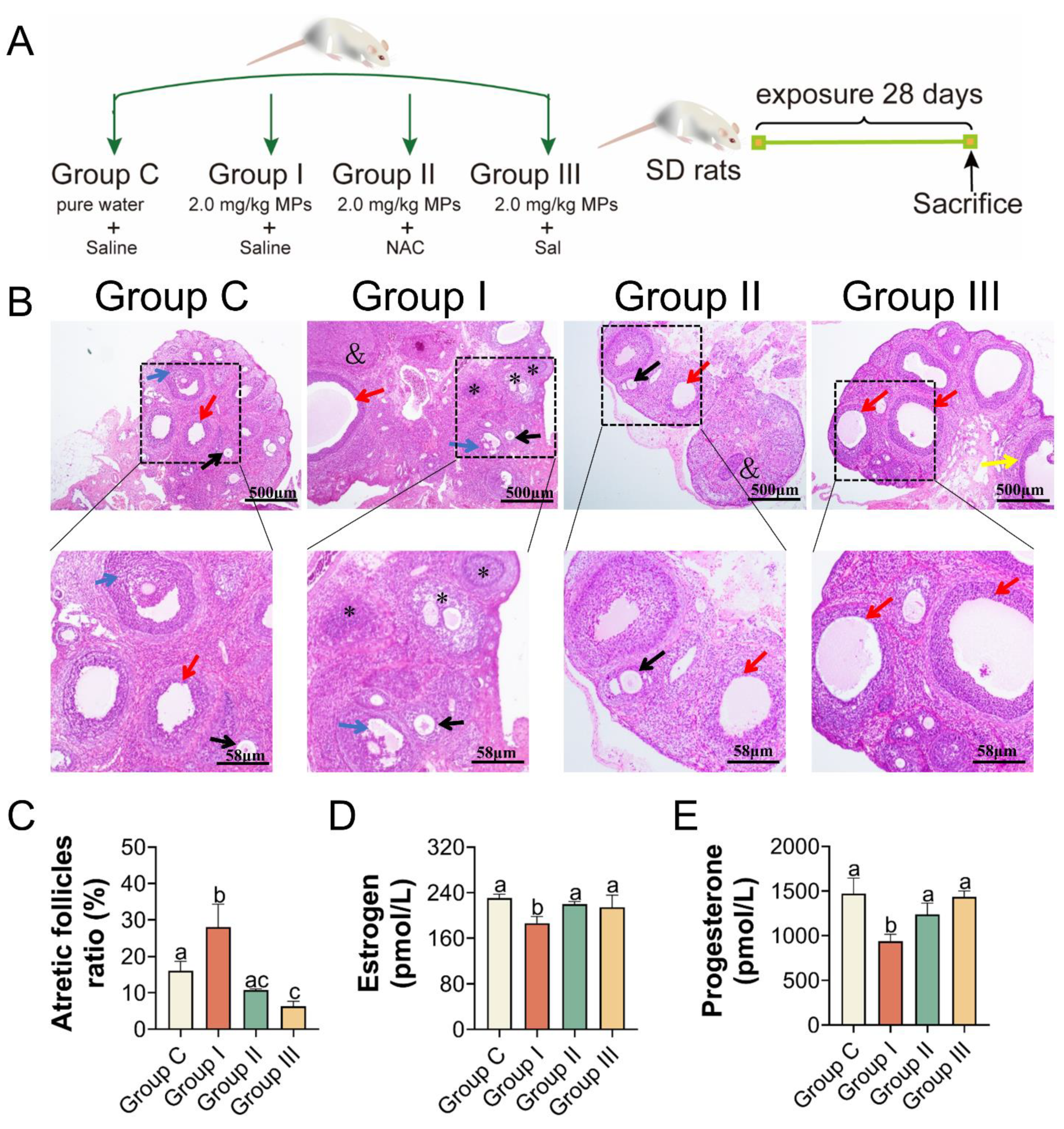
2.2. Animals and Experiment Design
2.3. NAC and Sal Supplementation
2.4. Staining with Hematoxylin and Eosin (H&E) and Histomorphometric Analysis
2.5. Analysis of Sex Hormones and Oxidative Stress
2.6. Quantitative Polymerase Chain Reaction in Real-Time (RT-qPCR)
2.7. Immunohistochemical (IHC) Analysis
2.8. Assay for Immunofluorescence
2.9. Analytical Statistics
3. Results
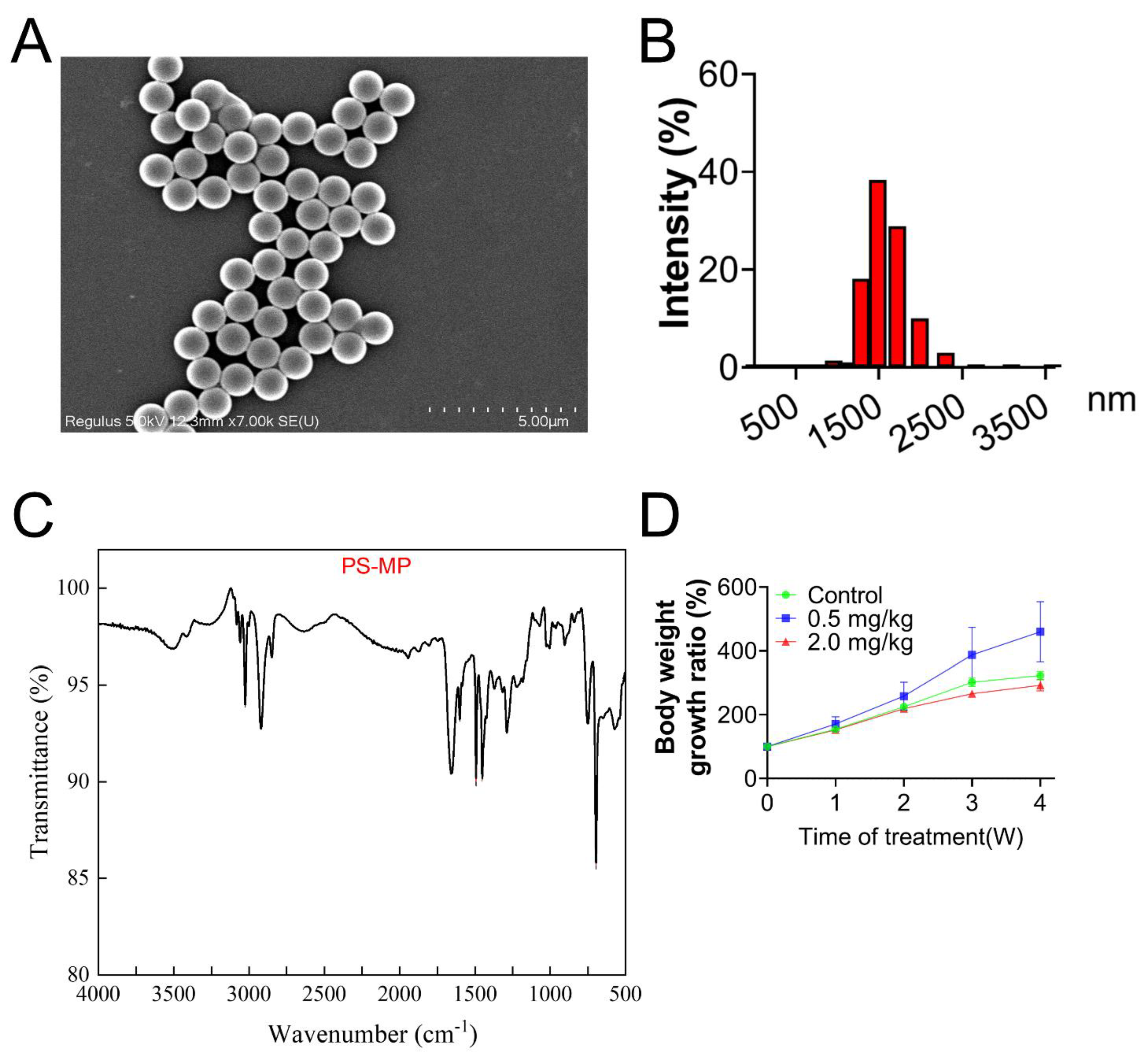
3.1. Characterization of PS-MPs
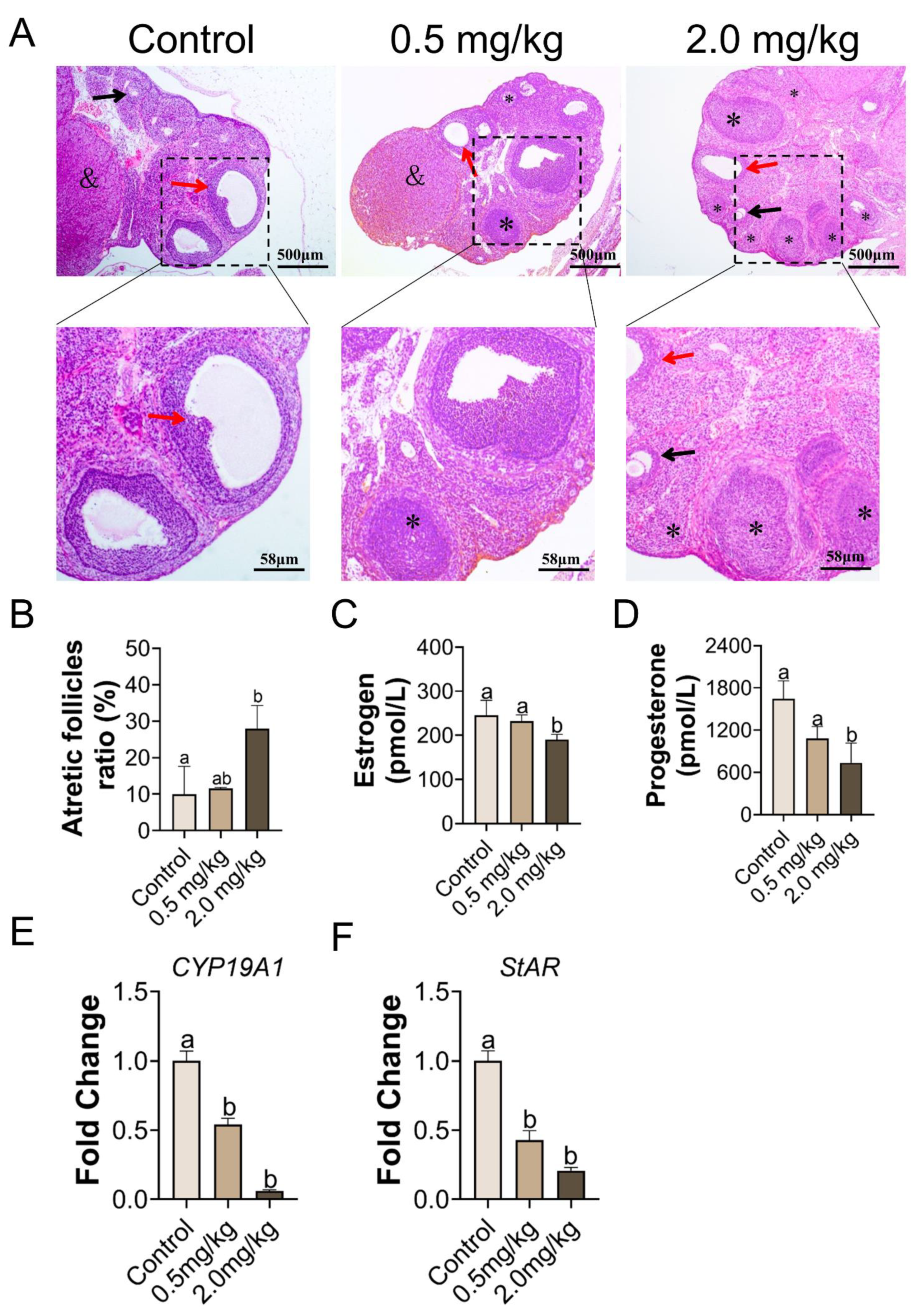
3.2. PS-MPs Exposure Has Negative Impacts on the Development of Follicles
3.3. Exposure to PS-MPs Culminated in Oxidative Response in the Ovary
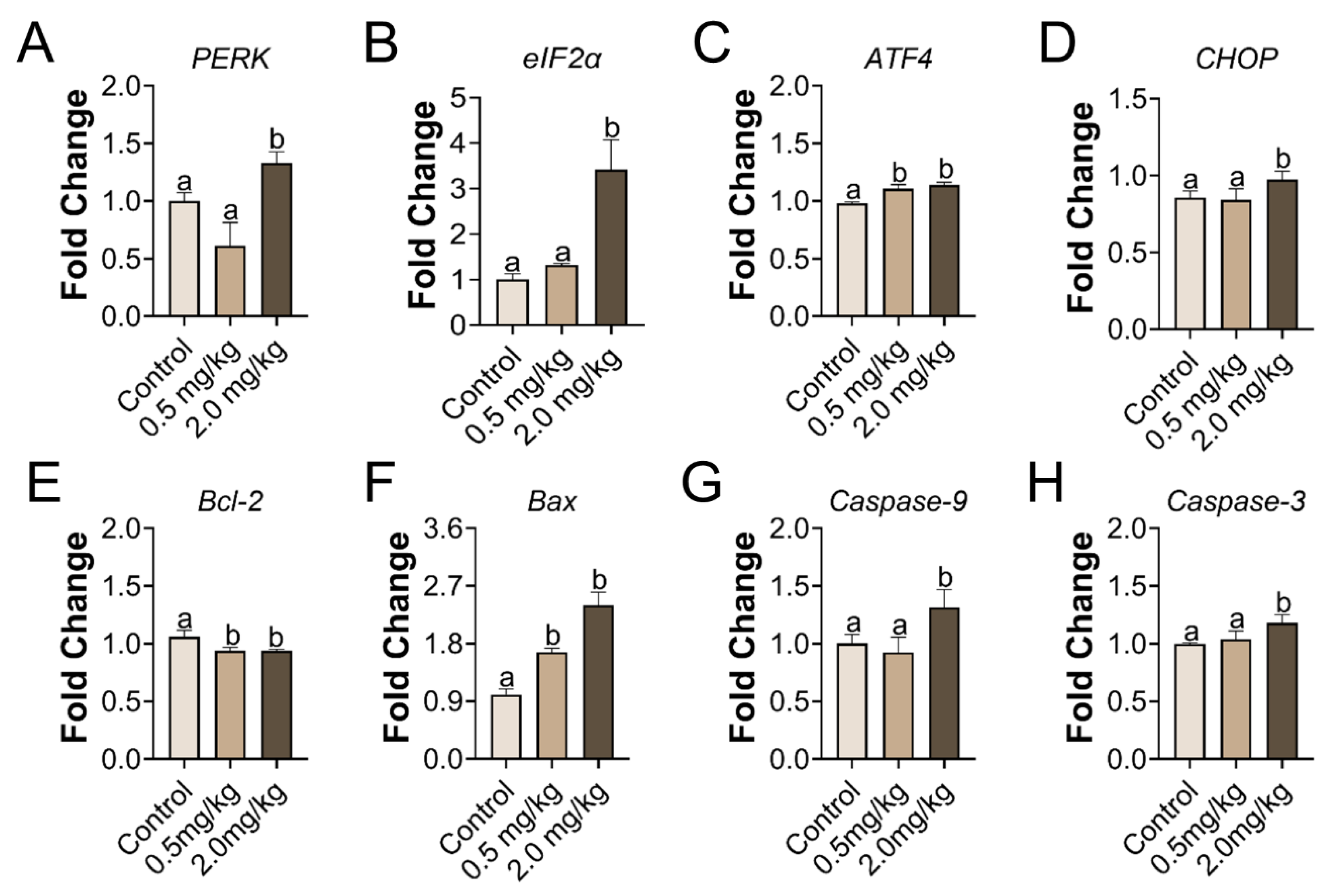
3.4. PS-MPs Consumption Led to ER Stress and Apoptosis in the Ovary
3.5. PS-MPs Exposure Induced Ovarian Apoptosis Closely Associated with Oxidative Stress and ER Stress
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, D.; Ma, Y.; Han, X.; Chen, Y. Systematic toxicity evaluation of polystyrene nanoplastics on mice and molecular mechanism investigation about their internalization into Caco-2 cells. J. Hazard. Mater. 2021, 417, 126092. [Google Scholar] [CrossRef]
- Horton, A.A.; Walton, A.; Spurgeon, D.J.; Lahive, E.; Svendsen, C. Microplastics in freshwater and terrestrial environments: Evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci. Total. Environ. 2017, 586, 127–141. [Google Scholar] [CrossRef] [Green Version]
- Fendall, L.S.; Sewell, M.A. Contributing to marine pollution by washing your face: Microplastics in facial cleansers. Mar. Pollut. Bull. 2009, 58, 1225–1228. [Google Scholar] [CrossRef]
- Jing, J. Polystyrene micro-/nanoplastics induced hematopoietic damages via the crosstalk of gut microbiota, metabolites, and cytokines. Environ. Int. 2022, 161, 107131. [Google Scholar] [CrossRef] [PubMed]
- Koelmans, B. A scientific perspective on microplastics in nature and society. SAPEA 2019. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L.; Trasande, L.; Kannan, K. Occurrence of polyethylene terephthalate and polycarbonate microplastics in infant and adult feces. Environ. Sci. Technol. Lett. 2021, 8, 989–994. [Google Scholar] [CrossRef]
- Leslie, H.A.; van Velzen, M.J.; Brandsma, S.H.; Vethaak, A.D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and quantification of plastic particle pollution in human blood. Environ. Int. 2022, 163, 107199. [Google Scholar] [CrossRef] [PubMed]
- Ragusa, A.; Svelato, A.; Santacroce, C.; Catalano, P.; Notarstefano, V.; Carnevali, O.; Papa, F.; Rongioletti, M.C.A.; Baiocco, F.; Draghi, S.; et al. Plasticenta: First evidence of microplastics in human placenta. Environ. Int. 2021, 146, 106274. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Sarkar, A.; Yadav, O.P.; Achari, G.; Slobodnik, J. Potential human health risks due to environmental exposure to nano- and microplastics and knowledge gaps: A scoping review. Sci. Total. Environ. 2021, 757, 143872. [Google Scholar] [CrossRef]
- Marcelino, R.C.; Cardoso, R.M.; Domingues, E.L.; Gonçalves, R.V.; Lima, G.D.; Novaes, R.D. The emerging risk of microplastics and nanoplastics on the microstructure and function of reproductive organs in mammals: A systematic review of preclinical evidence. Life Sci. 2022, 295, 120404. [Google Scholar] [CrossRef]
- Barouki, R.; Gluckman, P.D.; Grandjean, P.; Hanson, M.; Heindel, J.J. Developmental origins of non-communicable disease: Implications for research and public health. Environ. Health 2012, 11, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sales, V.M.; Ferguson-Smith, A.C.; Patti, M.-E. Epigenetic mechanisms of transmission of metabolic disease across generations. Cell Metab. 2017, 25, 559–571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sripada, K.; Wierzbicka, A.; Abass, K.; Grimalt, J.O.; Erbe, A.; Röllin, H.B.; Weihe, P.; Díaz, G.J.; Singh, R.R.; Visnes, T.; et al. A children’s health perspective on nano- and microplastics. Environ. Health Perspect. 2022, 130, 9086. [Google Scholar] [CrossRef]
- Nor, N.H.M.; Kooi, M.; Diepens, N.J.; Koelmans, A.A. Lifetime accumulation of microplastic in children and adults. Environ. Sci. Technol. 2021, 55, 5084–5096. [Google Scholar] [CrossRef]
- Park, E.-J.; Han, J.-S.; Seong, E.; Lee, G.-H.; Kim, D.-W.; Son, H.-Y.; Han, H.-Y.; Lee, B.-S. Repeated-oral dose toxicity of polyethylene microplastics and the possible implications on reproduction and development of the next generation. Toxicol. Lett. 2020, 324, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Della Seta, D.; Minder, I.; Belloni, V.; Aloisi, A.M.; Dessì-Fulgheri, F.; Farabollini, F. Pubertal exposure to estrogenic chemicals affects behavior in juvenile and adult male rats. Horm. Behav. 2006, 50, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Amereh, F.; Babaei, M.; Eslami, A.; Fazelipour, S.; Rafiee, M. The emerging risk of exposure to nano(micro)plastics on endocrine disturbance and reproductive toxicity: From a hypothetical scenario to a global public health challenge. Environ. Pollut. 2020, 261, 114158. [Google Scholar] [CrossRef]
- Liu, Z.; Zhuan, Q.; Zhang, L.; Meng, L.; Fu, X.; Hou, Y. Polystyrene microplastics induced female reproductive toxicity in mice. J. Hazard. Mater. 2022, 424, 127629. [Google Scholar] [CrossRef]
- Xie, X.; Deng, T.; Duan, J.; Xie, J.; Yuan, J.; Chen, M. Exposure to polystyrene microplastics causes reproductive toxicity through oxidative stress and activation of the p38 MAPK signaling pathway. Ecotoxicol. Environ. Saf. 2020, 190, 110133. [Google Scholar] [CrossRef]
- Yang, S.; Zhou, F.; Dong, Y.; Ren, F. α-mangostin induces apoptosis in human osteosarcoma cells through ROS-mediated endoplasmic reticulum stress via the WNT pathway. Cell Transplant. 2021, 30, 9636897211035080. [Google Scholar] [CrossRef]
- Gupta, S.; Ghulmiyyah, J.; Sharma, R.; Halabi, J.; Agarwal, A. Power of proteomics in linking oxidative stress and female infertility. Bio. Med. Res. Int. 2014, 2014, 1–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haddadi, A. Exposure to microplastics leads to a defective ovarian function and change in cytoskeleton protein expression in rat. Environ. Sci. Pollut. Res. Int. 2022, 29, 34594–34606. [Google Scholar] [CrossRef] [PubMed]
- Kadowaki, H.; Nishitoh, H. Endoplasmic reticulum quality control by garbage disposal. FEBS J. 2019, 286, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Persaud, I.; Shannahan, J.H.; Raghavendra, A.J.; Alsaleh, N.; Podila, R.; Brown, J.M. Biocorona formation contributes to silver nanoparticle induced endoplasmic reticulum stress. Ecotoxicol. Environ. Saf. 2019, 170, 77–86. [Google Scholar] [CrossRef]
- Chen, B.; Hong, W.; Tang, Y.; Zhao, Y.; Aguilar, Z.P.; Xu, H. Protective effect of the NAC and Sal on zinc oxide nanoparticles-induced reproductive and development toxicity in pregnant mice. Food Chem. Toxicol. 2020, 143, 111552. [Google Scholar] [CrossRef] [PubMed]
- Harada, M.; Takahashi, N.; Azhary, J.M.K.; Kunitomi, C.; Fujii, T.; Osuga, Y. Endoplasmic reticulum stress: A key regulator of the follicular microenvironment in the ovary. Mol. Hum. Reprod. 2021, 27, 88. [Google Scholar] [CrossRef]
- Stoker, T.E.; Gibson, E.K.; Zorrilla, L.M. Triclosan exposure modulates estrogen-dependent responses in the female Wistar rat. Toxicol. Sci. 2010, 117, 45–53. [Google Scholar] [CrossRef]
- Qiao, J.; Chen, R.; Wang, M.; Bai, R.; Cui, X.; Liu, Y.; Wu, C.; Chen, C. Perturbation of gut microbiota plays an important role in micro/nanoplastics-induced gut barrier dysfunction. Nanoscale 2021, 13, 8806–8816. [Google Scholar] [CrossRef]
- Babaei, A.A.; Rafiee, M.; Khodagholi, F.; Ahmadpour, E.; Amereh, F. Nanoplastics-induced oxidative stress, antioxidant defense, and physiological response in exposed Wistar albino rats. Environ. Sci. Pollut. Res. 2022, 29, 11332–11344. [Google Scholar] [CrossRef]
- Senathirajah, K. Estimation of the mass of microplastics ingested—A pivotal first step towards human health risk assessment. J. Hazard. Mater. 2021, 404, 124004. [Google Scholar] [CrossRef]
- Nestorović, N.; Lovren, M.; Sekulić, M.; Negić, N.; Šošić-Jurjević, B.; Filipović, B.; Milošević, V. Chronic somatostatin treatment affects pituitary gonadotrophs, ovaries and onset of puberty in rats. Life Sci. 2004, 74, 1359–1373. [Google Scholar] [CrossRef] [PubMed]
- Tassinari, R.; Cubadda, F.; Moracci, G.; Aureli, F.; D’Amato, M.; Valeri, M.; De Berardis, B.; Raggi, A.; Mantovani, A.; Passeri, D.; et al. Oral, short-term exposure to titanium dioxide nanoparticles in Sprague-Dawley rat: Focus on reproductive and endocrine systems and spleen. Nanotoxicology 2014, 8, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Rozpedek, W. The role of the PERK/eIF2α/ATF4/CHOP signaling pathway in tumor progression during endoplasmic reticulum stress. Curr. Mol. Med. 2016, 16, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Tian, M.; Ding, C.; Yu, S. The C/EBP Homologous Protein (CHOP) transcription factor functions in endoplasmic reticulum stress-induced apoptosis and microbial infection. Front. Immunol. 2019, 9, 3083. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Guerrero, A.D.; Huang, L.; Shabier, Z.; Pan, M.; Tan, T.-H.; Wang, J. Caspase-9-induced mitochondrial disruption through cleavage of anti-apoptotic BCL-2 family members. J. Biol. Chem. 2007, 282, 33888–33895. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Zhao, Y.; Liu, Y.; Tang, Y.; Xu, X.; Wang, M.; Tao, X.; Xu, H. Pre-exposure to TiO2-NPs aggravates alcohol-related liver injury by inducing intestinal barrier damage in mice. Toxicol. Sci. 2021, 185, 28–37. [Google Scholar] [CrossRef]
- Tang, Y.; Chen, B.; Hong, W.; Chen, L.; Yao, L.; Zhao, Y.; Aguilar, Z.P.; Xu, H. ZnO nanoparticles induced male reproductive toxicity based on the effects on the endoplasmic reticulum stress signaling pathway. Int. J. Nanomed. 2019, 14, 9563–9576. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.; Chang, Y.; Zhang, T.; Qiao, Y.; Klobučar, G.; Li, M. Toxicological effects of polystyrene microplastics on earthworm (Eisenia fetida). Environ. Pollut. 2019, 259, 113896. [Google Scholar] [CrossRef]
- Wu, B.; Wu, X.; Liu, S.; Wang, Z.; Chen, L. Size-dependent effects of polystyrene microplastics on cytotoxicity and efflux pump inhibition in human Caco-2 cells. Chemosphere 2019, 221, 333–341. [Google Scholar] [CrossRef]
- Song, K. Microparticles and microplastics released from daily use of plastic feeding and water bottles and plastic injectors: Potential risks to infants and children in China. Environ. Sci. Pollut. Res. 2021, 28, 59813–59820. [Google Scholar] [CrossRef]
- Segovia-Mendoza, M.; Nava-Castro, K.E.; Palacios-Arreola, M.I.; Garay-Canales, C.; Morales-Montor, J. How microplastic components influence the immune system and impact on children health: Focus on cancer. Birth Defects Res. 2020, 112, 1341–1361. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, S.C.; Korach, K.S. Progesterone action and responses in the αERKO mouse. Steroids 2000, 65, 551–557. [Google Scholar] [CrossRef]
- Kezele, P.; Skinner, M.K. Regulation of ovarian primordial follicle assembly and development by estrogen and progesterone: Endocrine model of follicle assembly. Endocrinology 2003, 144, 3329–3337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, R.; Wang, X.; Yang, L.; Zhang, J.; Wang, N.; Xu, F.; Hou, Y.; Zhang, H.; Zhang, L. Polystyrene microplastics cause granulosa cells apoptosis and fibrosis in ovary through oxidative stress in rats. Toxicology 2021, 449, 152665. [Google Scholar] [CrossRef] [PubMed]
- Hua, D.; Zhou, Y.; Lu, Y.; Zhao, C.; Qiu, W.; Chen, J.; Ju, R. Lipotoxicity impairs granulosa cell function through activated endoplasmic reticulum stress pathway. Reprod. Sci. 2020, 27, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Kunitomi, C.; Harada, M.; Takahashi, N.; Azhary, J.M.K.; Kusamoto, A.; Nose, E.; Oi, N.; Takeuchi, A.; Wada-Hiraike, O.; Hirata, T.; et al. Activation of endoplasmic reticulum stress mediates oxidative stress–induced apoptosis of granulosa cells in ovaries affected by endometrioma. Mol. Hum. Reprod. 2020, 26, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Hou, J. Polystyrene microplastics lead to pyroptosis and apoptosis of ovarian granulosa cells via NLRP3/Caspase-1 signaling pathway in rats. Ecotoxicol. Environ. Saf. 2021, 212, 112012. [Google Scholar] [CrossRef]
- Wei, Z.; Wang, Y.; Wang, S.; Xie, J.; Han, Q.; Chen, M. Comparing the effects of polystyrene microplastics exposure on reproduction and fertility in male and female mice. Toxicology 2022, 465, 153059. [Google Scholar] [CrossRef]
- Ramsperger, A.F.R.M.; Narayana, V.K.B.; Gross, W.; Mohanraj, J.; Thelakkat, M.; Greiner, A.; Schmalz, H.; Kress, H.; Laforsch, C. Environmental exposure enhances the internalization of microplastic particles into cells. Sci. Adv. 2020, 6, eabd1211. [Google Scholar] [CrossRef]
- Li, H.; Guo, S.; Cai, L.; Ma, W.; Shi, Z. Lipopolysaccharide and heat stress impair the estradiol biosynthesis in granulosa cells via increase of HSP70 and inhibition of smad3 phosphorylation and nuclear translocation. Cell Signal. 2017, 30, 130–141. [Google Scholar] [CrossRef] [Green Version]
- Zhou, R.; Miao, Y.; Li, Y.; Li, X.; Xi, J.; Zhang, Z. MicroRNA-150 promote apoptosis of ovine ovarian granulosa cells by targeting STAR gene. Theriogenology 2019, 127, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Ijaz, M.U.; Shahzadi, S.; Samad, A.; Ehsan, N.; Ahmed, H.; Tahir, A.; Rehman, H.; Anwar, H. Dose-dependent effect of polystyrene microplastics on the testicular tissues of the male sprague dawley rats. Dose-Response 2021, 19, 15593258211019882. [Google Scholar] [CrossRef] [PubMed]
- Da Broi, M.G.; Jordão-Jr, A.A.; Ferriani, R.A.; Navarro, P.A. Oocyte oxidative DNA damage may be involved in minimal/mild endometriosis-related infertility. Mol. Reprod. Dev. 2018, 85, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Hong, W.; Yang, P.; Tang, Y.; Zhao, Y.; Aguilar, Z.P.; Xu, H. Nano zinc oxide induced fetal mice growth restriction, based on oxide stress and endoplasmic reticulum stress. Nanomaterials 2020, 10, 259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Zhang, X.; Zhang, S.; Huang, H.; Wu, J.; Wang, Y.; Yuan, L.; Liu, C.; Zeng, X.; Cheng, X.; et al. Oxidative stress mediates microcystin-LR-induced endoplasmic reticulum stress and autophagy in KK-1 cells and C57BL/6 mice ovaries. Front. Physiol. 2018, 9, 1058. [Google Scholar] [CrossRef] [Green Version]
- Pan, L.; Yu, D.; Zhang, Y.; Zhu, C.; Yin, Q.; Hu, Y.; Zhang, X.; Yue, R.; Xiong, X. Polystyrene microplastics-triggered mitophagy and oxidative burst via activation of PERK pathway. Sci. Total. Environ. 2021, 781, 146753. [Google Scholar] [CrossRef]
- Wu, L.L. Mitochondrial dysfunction in oocytes of obese mothers: Transmission to offspring and reversal by pharmacological endoplasmic reticulum stress inhibitors. Development 2015, 142, 681–691. [Google Scholar] [CrossRef] [Green Version]



| Gene | Primer | Sequence (5′→3′) |
|---|---|---|
| CYP19A1 | Forward | GGAAATCCACACTGTTGTTGG |
| Reverse | TGAAGTTTTCCACCACTTTCAA | |
| GAPDH | Forward | CTCATGACCACAGTCCATGC |
| Reverse | TTCAGCTCTGGGATGACCTT | |
| StAR | Forward | GAAAGCCAGCAGGAGAATGG |
| Reverse | CACCTCCAGTCGGAACACCTT | |
| GSH | Forward | ATCCCACTGCGCTCATGACC |
| Reverse | AGCCAGCCATCACCAAGCC | |
| PERK | Forward | GATACGGCATTTGGCTTGGG |
| Reverse | CCCATGATTCTCGGCATCCA | |
| eIF2α | Forward | AGCAATGGAGAAAATTTGCCTTGA |
| Reverse | TCTGACCAGGAAGGACACCA | |
| CHOP | Forward | GCAGCGACAGAGCCAAAATAA |
| Reverse | CTGCTTTCAGGTGTGGTGGT | |
| BCL-2 | Forward | CTTTGAGTTCGGTGGGGTCA |
| Reverse | CATCCCAGCCTCCGTTATCC | |
| Bax | Forward | CGTCTGCGGGGAGTCAC |
| Reverse | ATCTGTTCAGAGCTGGTGGG | |
| Caspase-3 | Forward | GCGTAAGGAAAGGAGAGGTG |
| Reverse | ACAGACCAGTGCTCACAAGG | |
| Caspase-9 | Forward | GGCCTTCACTTCCTCTCAAG |
| Reverse | GGACACAAGGATGTCACTGG | |
| ATF4 | Forward | AGTCTGCCTTCTCCAGGTGTTC |
| Reverse | GCTGTCTTGTTTTGCTCCATCTT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Guan, J.; Feng, Y.; Liu, S.; Zhao, Y.; Xu, Y.; Xu, H.; Fu, F. Polystyrene Microplastics Induced Ovarian Toxicity in Juvenile Rats Associated with Oxidative Stress and Activation of the PERK-eIF2α-ATF4-CHOP Signaling Pathway. Toxics 2023, 11, 225. https://doi.org/10.3390/toxics11030225
Wang W, Guan J, Feng Y, Liu S, Zhao Y, Xu Y, Xu H, Fu F. Polystyrene Microplastics Induced Ovarian Toxicity in Juvenile Rats Associated with Oxidative Stress and Activation of the PERK-eIF2α-ATF4-CHOP Signaling Pathway. Toxics. 2023; 11(3):225. https://doi.org/10.3390/toxics11030225
Chicago/Turabian StyleWang, Wanzhen, Jiafu Guan, Yueying Feng, Shanji Liu, Yu Zhao, Yuanyuan Xu, Hengyi Xu, and Fen Fu. 2023. "Polystyrene Microplastics Induced Ovarian Toxicity in Juvenile Rats Associated with Oxidative Stress and Activation of the PERK-eIF2α-ATF4-CHOP Signaling Pathway" Toxics 11, no. 3: 225. https://doi.org/10.3390/toxics11030225
APA StyleWang, W., Guan, J., Feng, Y., Liu, S., Zhao, Y., Xu, Y., Xu, H., & Fu, F. (2023). Polystyrene Microplastics Induced Ovarian Toxicity in Juvenile Rats Associated with Oxidative Stress and Activation of the PERK-eIF2α-ATF4-CHOP Signaling Pathway. Toxics, 11(3), 225. https://doi.org/10.3390/toxics11030225







