Synthesis of Mixed-Phase TiO2–ZrO2 Nanocomposite for Photocatalytic Wastewater Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents Used
2.2. Synthesis of TiO2, ZrO2, and Mixed Phase TiO2–ZrO2 Nanocomposite
2.2.1. Synthesis of TiO2
2.2.2. Synthesis of ZrO2
2.2.3. Synthesis of Mixed-Phase TiO2–ZrO2 Nanocomposite
3. Characterization Technique
4. Results and Discussion
4.1. XRD
4.2. TEM
4.3. XPS
4.4. FTIR
4.5. Determination of Band Gap Using the Kubelka–Munk Function
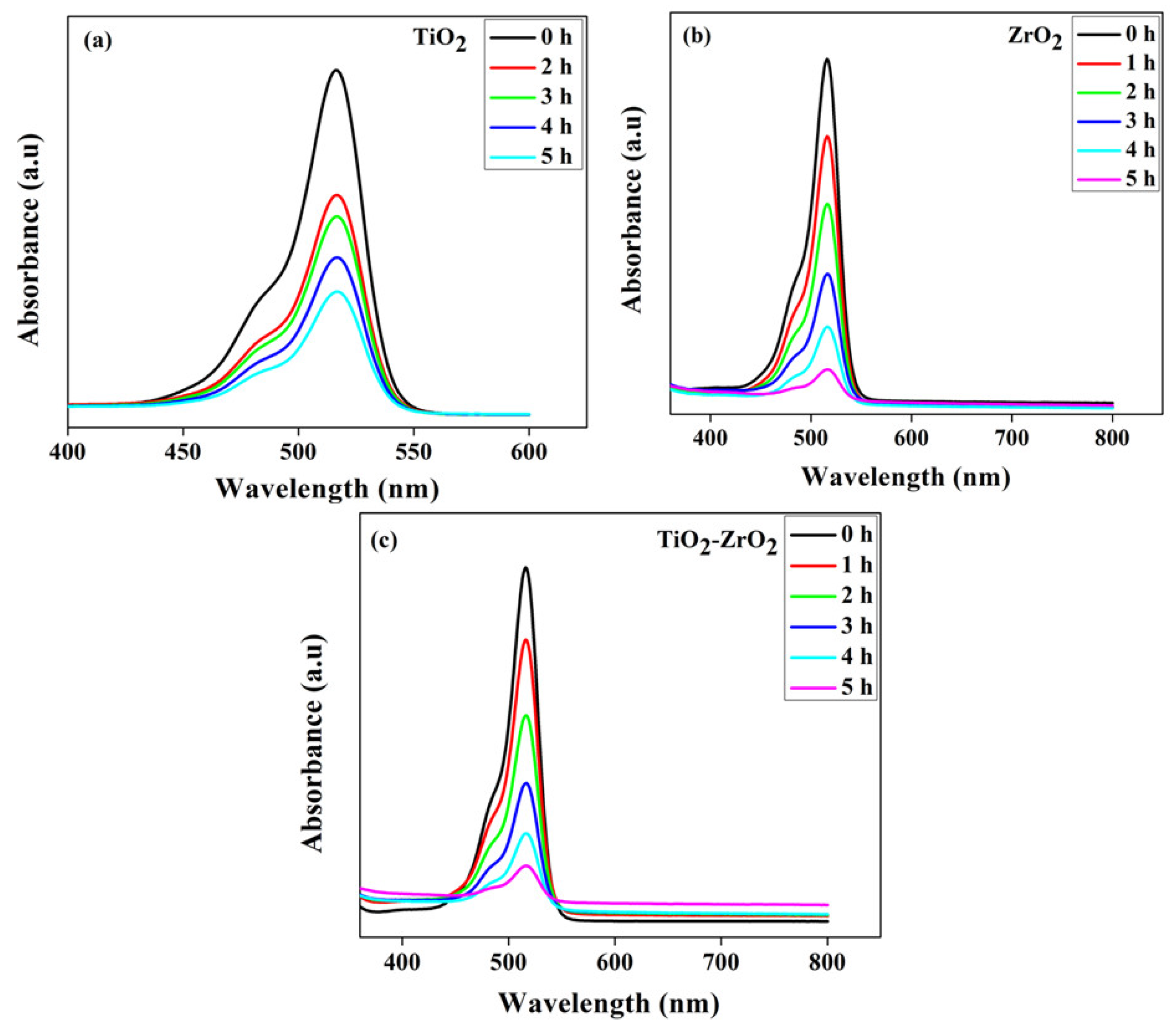
4.6. Photocatalytic Activity
4.7. Kinetic Study for the Degradation of Dye
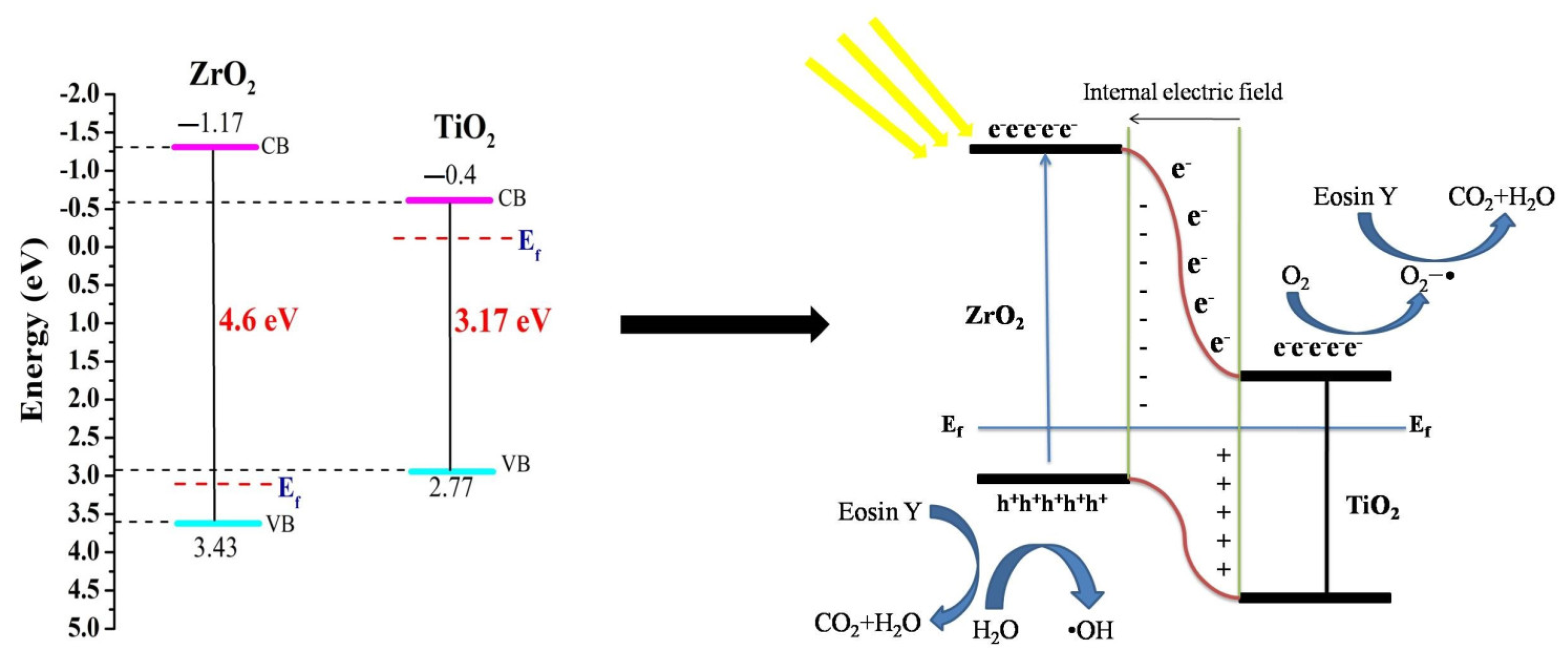
4.8. Proposed Model
5. Conclusions
6. Future Work
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Basahel, S.N.; Ali, T.T.; Mokhtar, M.; Narasimharao, K. Influence of crystal structure of nanosized ZrO2 on photocatalytic degradation of methyl orange. Nanoscale Res. Lett. 2015, 10, 73. [Google Scholar] [CrossRef] [Green Version]
- Ren, G.; Han, H.; Wang, Y.; Liu, S.; Zhao, J.; Meng, X.; Li, Z. Recent Advances of Photocatalytic Application in Water Treatment: A Review. Nanomaterials 2021, 11, 1804. [Google Scholar] [CrossRef]
- Cesa, F.S.; Turra, A.; Baruque-Ramos, J. Synthetic fibers as microplastics in the marine environment: A review from textile perspective with a focus on domestic washings. Sci. Total Environ. 2017, 598, 1116–1129. [Google Scholar] [CrossRef]
- Katheresan, V.; Kansedo, J.; Lau, J. Efficiency of various recent wastewater dye removal methods: A review. J. Environ. Chem. Eng. 2018, 6, 4676–4697. [Google Scholar] [CrossRef]
- Adel, A.M.; El-Wahab, Z.H.A.; Ibrahim, A.A.; Al-Shemy, M.T. Characterization of microcrystalline cellulose prepared from lignocellulosic materials. Part I. Acid catalyzed hydrolysis. Bioresour. Technol. 2010, 101, 4446–4455. [Google Scholar] [CrossRef]
- El-Mekkawi, D.M.; Galal, H.R.; Abd El Wahab, R.M.; Mohamed, W.A.A. Photocatalytic activity evaluation of TiO2 nanoparticles based on COD analyses for water treatment applications: A standardization attempt. Int. J. Environ. Sci. Technol. 2016, 13, 1077–1088. [Google Scholar] [CrossRef] [Green Version]
- Chequer, F.M.D.; Oliveira, G.A.R.D.; Ferraz, E.R.A.; Cardoso, J.C.; Zanoni, M.V.B.; Oliveira, D.P.D. Textile Dyes: Dyeing Process and Environmental Impact. Eco-Friendly Text. Dye. Finish. 2013, 6, 151–176. [Google Scholar]
- Mishra, R.; Bera, S.; Chatterjee, R.; Banerjee, S.; Bhattacharya, S.; Biswas, A.; Shreyasi Mallick, S.; Roy, S. A review on Z/S—Scheme heterojunction for photocatalytic applications based on metal halide perovskite materials. Appl. Surf. Sci. Adv. 2022, 9, 100241. [Google Scholar] [CrossRef]
- Das, S.; Deka, T.; Ningthoukhangjam, P.; Chowdhury, A.; Nair, R.G. A critical review on prospects and challenges of metal-oxide embedded g-C3N4-based direct Z-scheme photocatalysts for water splitting and environmental remediation. Appl. Surf. Sci. Adv. 2022, 11, 100273. [Google Scholar] [CrossRef]
- Belessiotis, G.V.; Falara, P.P.; Ibrahim, I.; Kontos, A.G. Magnetic Metal Oxide-Based Photocatalysts with Integrated Silver for Water Treatment. Materials 2022, 15, 4629. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Kuriakose, S.; Satpati, B.; Mohapatra, S. Enhanced photocatalytic activity of Co doped ZnO nanodisks and nanorods prepared by a facile wet chemical method. Phys. Chem. Chem. Phys. 2014, 16, 12741–12749. [Google Scholar] [CrossRef]
- Kuriakose, S.; Choudhary, V.; Satpati, B.; Mohapatra, S. Facile synthesis of Ag–ZnO hybrid nanospindles for highly efficient photocatalytic degradation of methyl orange. Phys. Chem. Chem. Phys. 2014, 16, 17560–17568. [Google Scholar] [CrossRef]
- Cheng, C.; Amini, A.; Zhu, C.; Xu, Z.; Song, H.; Wang, N. Enhanced photocatalytic performance of TiO2-ZnO hybrid nanostructures. Sci. Rep. 2014, 4, 4181. [Google Scholar] [CrossRef] [Green Version]
- Ren, L.; Li, Y.; Hou, J.; Zhao, X.; Pan, C. Preparation and Enhanced Photocatalytic Activity of TiO2 Nanocrystals with Internal Pores. ACS Appl. Mater. Interfaces 2014, 6, 1608–1615. [Google Scholar] [CrossRef]
- Navio, J.A.; Hidalgo, M.C.; Colon, G.; Botta, S.G.; Litter, M.I. Preparation and Physicochemical Properties of ZrO2 and Fe/ZrO2 Prepared by a Sol−Gel Technique. Langmuir 2000, 17, 202–210. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Parveen, T.; Umar, K.; Ibrahim, M.N. Role of Nanomaterials in the Treatment of Wastewater: A Review. Water 2020, 12, 495. [Google Scholar] [CrossRef] [Green Version]
- Jiang, M.; Qi, Y.; Liu, H.; Chen, Y. The Role of Nanomaterials and Nanotechnologies in Wastewater Treatment: A Bibliometric Analysis. Nanoscale Res. Lett. 2018, 13, 233. [Google Scholar] [CrossRef]
- Amor, C.; Marchão, L.; Lucas, M.S.; Peres, J.A. Application of Advanced Oxidation Processes for the Treatment of Recalcitrant Agro-Industrial Wastewater: A Review. Water 2019, 11, 205. [Google Scholar] [CrossRef] [Green Version]
- Polisetti, S.; Deshpande, P.A.; Madras, G. Photocatalytic Activity of Combustion Synthesized ZrO2 and ZrO2–TiO2 Mixed Oxides. Ind. Eng. Chem. Res. 2011, 50, 12915–12924. [Google Scholar] [CrossRef]
- Basavegowda, N.; Somu, P.; Shabbirahmed, A.M.; Asma Musfra Shabbirahmed, A.M.; Gomez, L.A.; Thathapud, J.J. Bimetallic p-ZnO/n-CuO nanocomposite synthesized using Aegle marmelos leaf extract exhibits excellent visible-light-driven photocatalytic removal of 4-nitroaniline and methyl orange. Photochem. Photobiol. Sci. 2022, 21, 1357–1370. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, I.; Belessiotis, G.V.; Arfanis, M.K.; Athanasekou, C.; Philippopoulos, A.I.; Mitsopoulou, C.A.; Romanos, G.E.; Falaras, P. Surfactant Effects on the Synthesis of Redox Bifunctional V2O5 Photocatalysts. Materials 2020, 13, 4665. [Google Scholar] [CrossRef]
- Lai, Y.J.; Lee, D.J. Solid mediator Z-scheme heterojunction photocatalysis for pollutant oxidation in water: Principles and synthesis perspectives. J. Taiwan Inst. Chem. Eng. 2021, 125, 88–114. [Google Scholar] [CrossRef]
- Yuan, Y.; Guo, R.T.; Hong, L.F.; Ji, X.Y.; Lin, Z.d.; Li, Z.S.; Pan, W.G. A review of metal oxide-based Z-scheme heterojunction photocatalysts: Actualities and developments. Mater. Today Energy 2021, 21, 100829. [Google Scholar] [CrossRef]
- Krishnan, P.; Liu, M.; Itty, P.A.; Liu, Z.; Rheinheimer, V.; Zhang, M.H.; Monteiro, P.J.M.; Yu, L.E. Characterization of photocatalytic TiO2 powder under varied environments using near ambient pressure X-ray photoelectron spectroscopy. Sci. Rep. 2017, 7, 43298. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, I.; Belessiotis, G.V.; Elseman, A.M.; Mohamed, M.M.; Ren, Y.; Salama, T.M.; Mohamed, M.B.I. Magnetic TiO2/CoFe2O4 Photocatalysts for Degradation of Organic Dyes and Pharmaceuticals without Oxidants. Nanomaterials 2022, 12, 3290. [Google Scholar] [CrossRef]
- Sandell, A.; Anderson, M.P.; Alfredsson, Y.; Johansson, M.K.J.; Schnadt, J.; Rensmo, H.; Siegbahn, H.; Uvdal, P. Titanium dioxide thin film growth on silicon(III) by chemical vapor deposition of titanium(IV)isopropoxide. J. Appl. Phys. 2002, 92, 3381–3387. [Google Scholar] [CrossRef]
- Sandell, A.; Andersson, M.P.; Johansson, M.K.J.; Karlsson, P.G.; Alfredsson, Y.; Schadt, J.; Siegbahn, H.; Uvdal, P. Metalorganic chemical vapor deposition of anatase titanium dioxide on Si: Modifying the interface by pre-oxidation. Surf. Sci. 2003, 530, 63–70. [Google Scholar] [CrossRef]
- Stefanov, P.; Shipochka, M.; Stefchev, P.; Raicheva, Z.; Lazarova, V.; Spassov, L. XPS characterization of TiO2 layers deposited on quartzplates. J. Phys. Conf. Ser. 2008, 100, 012039. [Google Scholar] [CrossRef] [Green Version]
- Parmigiani, F.; Deperob, L.E.; Sangaletti, L.; Samoggia, G. An XPS study of yttria-stabilised zirconia single crystals. J. Electron Spectrosc. Relat. Phenom. 1993, 63, 1–10. [Google Scholar] [CrossRef]
- Barreca, D.; Battiston, G.A.; Gerbasi, R.; Tondello, E.; Zanella, P. Zirconium Dioxide Thin Films Characterized by XPS. Surf. Sci. Spectra 2000, 7, 303–309. [Google Scholar] [CrossRef]
- Lackner, P.; Zou, Z.; Mayr, S.; Diebold, U.; Schmid, M. Using photoelectron spectroscopy to observe oxygen spillover to zirconia. Phys. Chem. Chem. Phys. 2019, 21, 17613–17620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrulevičius, M.; Tamulevičius, S.; Gnatyuk, Y.; Vityuk, N.; Smirnova, N.; Eremenko, A. XPS Investigation of TiO2/ZrO2/SiO2 Films Modified with Ag/Au Nanoparticles. Mater. Sci. 2008, 14, 8–14. [Google Scholar]
- Mahy, J.G.; Lambert, S.D.; Tilkin, R.G.; Wolfs, C.; Poelman, D.; Devred, F.; Gaigneau, E.M.; Douven, S. Ambient temperature ZrO2-doped TiO2 crystalline photocatalysts: Highly efficient powders and films for water depollution. Mater. Today Energy 2019, 13, 312–322. [Google Scholar] [CrossRef]
- Bineesh, K.V.; Kim, D.K.; Park, D.W. Synthesis and characterization of zirconium-doped mesoporous nano-crystalline TiO2. Nanoscale 2010, 2, 1222–1228. [Google Scholar] [CrossRef] [PubMed]
- Tsunekawa, S.; Asami, K.; Ito, S.; Yashima, M.; Sugimoto, T. XPS study of the phase transition in pure zirconium oxide Nanocrystallitesappl. Surf. Sci. 2005, 252, 1651–1656. [Google Scholar] [CrossRef]
- Verma, R.; Gangwar, J.; Srivastava, A.V. Multiphase TiO2 nanostructures: A review of efficient synthesis, growth mechanism, probing capabilities, and applications in bio-safety and health. RSC Adv. 2017, 70, 44199–44224. [Google Scholar] [CrossRef] [Green Version]
- Hu, S.; Li, F.; Fan, Z. Preparation of SiO2-Coated TiO2Composite Materials with Enhanced Photocatalytic Activity Under UV Light. Bull. Korean Chem. Soc. 2012, 33, 1895–1899. [Google Scholar] [CrossRef] [Green Version]
- Das, J.; Freitas, F.S.; Evans, I.R.; Nogueira, A.F.; Khushalani, D. A facile nonaqueous route for fabricating titania nanorods and their viability in quasi-solid-state dye-sensitized solar cells. J. Mater. Chem. 2010, 20, 4425. [Google Scholar] [CrossRef] [Green Version]
- Yanqing, Z.; Erwei, S.; Suxian, C.; Wenjun, L.; Xingfang, H. Hydrothermal preparation and characterization of brookite-type TiO2 nanocrystallites. J. Mater. Sci. Lett. 2000, 19, 1445–1448. [Google Scholar] [CrossRef]
- Zhou, S.; Garnweitner, G.; Niederberger, M.; Antonietti, M. Dispersion Behavior of Zirconia Nanocrystals and Their Surface Functionalization with Vinyl Group-Containing Ligands. Langmuir 2007, 23, 9178–9187. [Google Scholar] [CrossRef]
- Khan, M.; Naqvi, A.H.; Ahmad, M. Comparative study of the cytotoxic and genotoxic potentials of zinc oxide and titanium dioxide nanoparticles. Toxicol. Rep. 2015, 2, 765–774. [Google Scholar] [CrossRef] [Green Version]
- Lavín, A.; Sivasamy, R.; Mosquera, E.; Morel, M.J. High proportion ZnO/CuO nanocomposites: Synthesis, structural and optical properties, and their photocatalytic behavior. Surf. Interfaces 2019, 17, 100367. [Google Scholar] [CrossRef]
- Yaacob, N.; Ismail, A.F.; Sean, G.P.; Nazri, N.A.M. Structural and photocatalytic properties of co-doped hybrid ZrO2–TiO2 photocatalysts. SN Appl. Sci. 2019, 1, 252. [Google Scholar] [CrossRef] [Green Version]
- Revathi, V.; Karthik, K. Microwave assisted CdO–ZnO–MgO nanocomposite and its photocatalytic and antibacterial studies. J. Mater. Sci. Mater. Electron. 2018, 29, 18519–18530. [Google Scholar] [CrossRef]
- Keskin, C.S.; Ozdemir, A.; Karabayir, E.; Sengil, I.A. Simultaneous decolorization of ternary dye mixture from aqueous solution by electrocoagulation. Environ. Prot. Eng. 2013, 39, 119–133. [Google Scholar] [CrossRef]
- Thejaswini, V.L.T.; Prabhakaran, D.; Maheswari, M.A. Synthesis of mesoporous worm-like ZrO2–TiO2 monoliths and their photocatalytic applications towards organic dye degradation. J. Photochem. Photobiol. A Chem. 2017, 344, 212–222. [Google Scholar] [CrossRef]
- Wang, F.; Pan, K.; Wei, S.; Ren, Y.; Zhu, H.; Wu, H.; Zhang, Q. Solvothermal preparation and characterization of orderedmesoporous ZrO2/TiO2 composites for photocatalytic degradation of organic dyes. Ceram. Int. 2021, 47, 7632–7641. [Google Scholar] [CrossRef]
- Lee, K.M.; Lai, C.W.; Ngai, K.S.; Juan, J.C. Recent developments of zinc oxide based photocatalyst in water treatment technology: A review. Water Res. 2016, 88, 428–448. [Google Scholar] [CrossRef]
- Xu, A.W.; Gao, Y.; Liu, H.Q. The preparation, characterization, and their photocatalytic activities of rare-earth-doped TiO2 nanoparticles. J. Catal. 2002, 207, 151–157. [Google Scholar] [CrossRef]
- Anwer, H.; Mahmood, A.; Lee, J.; Kim, K.H.; Park, J.W.; Yip, C.K.A. Photocatalysts for degradation of dyes in industrial effluents: Opportunities and challenges. Nano Res. 2019, 12, 955–972. [Google Scholar] [CrossRef]
- Prasanna, V.L.; Rajagopalan, V. A New Synergetic Nanocomposite for Dye Degradation in Dark and Light. Sci. Rep. 2016, 6, 38606. [Google Scholar]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumari, P.; Saha, R.; Saikia, G.; Bhujel, A.; Choudhury, M.G.; Jagdale, P.; Paul, S. Synthesis of Mixed-Phase TiO2–ZrO2 Nanocomposite for Photocatalytic Wastewater Treatment. Toxics 2023, 11, 234. https://doi.org/10.3390/toxics11030234
Kumari P, Saha R, Saikia G, Bhujel A, Choudhury MG, Jagdale P, Paul S. Synthesis of Mixed-Phase TiO2–ZrO2 Nanocomposite for Photocatalytic Wastewater Treatment. Toxics. 2023; 11(3):234. https://doi.org/10.3390/toxics11030234
Chicago/Turabian StyleKumari, Pooja, Rajib Saha, Gaurav Saikia, Aditya Bhujel, Mahua Gupta Choudhury, Pravin Jagdale, and Samrat Paul. 2023. "Synthesis of Mixed-Phase TiO2–ZrO2 Nanocomposite for Photocatalytic Wastewater Treatment" Toxics 11, no. 3: 234. https://doi.org/10.3390/toxics11030234




