Hypoglycemic Potential of Carica papaya in Liver Is Mediated through IRS-2/PI3K/SREBP-1c/GLUT2 Signaling in High-Fat-Diet-Induced Type-2 Diabetic Male Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Collection of C. papaya Leaves
2.3. Animals
2.4. T2DM Induction
2.5. Experimental Design
- Group 1:
- Control rats
- Group 2:
- T2DM-induced rats
- Group 3:
- T2DM rats treated with ethanolic leaf extract of C. papaya (600 mg/kg bwt for 45 days)
- Group 4:
- Metformin-treated T2DM rats (50 mg/kg bwt for 45 days)
- Group 5:
- Control rats with ethanolic leaf extract of C. papaya (600 mg/kg bwt for 45 days).
2.6. Liver and Renal Function Markers
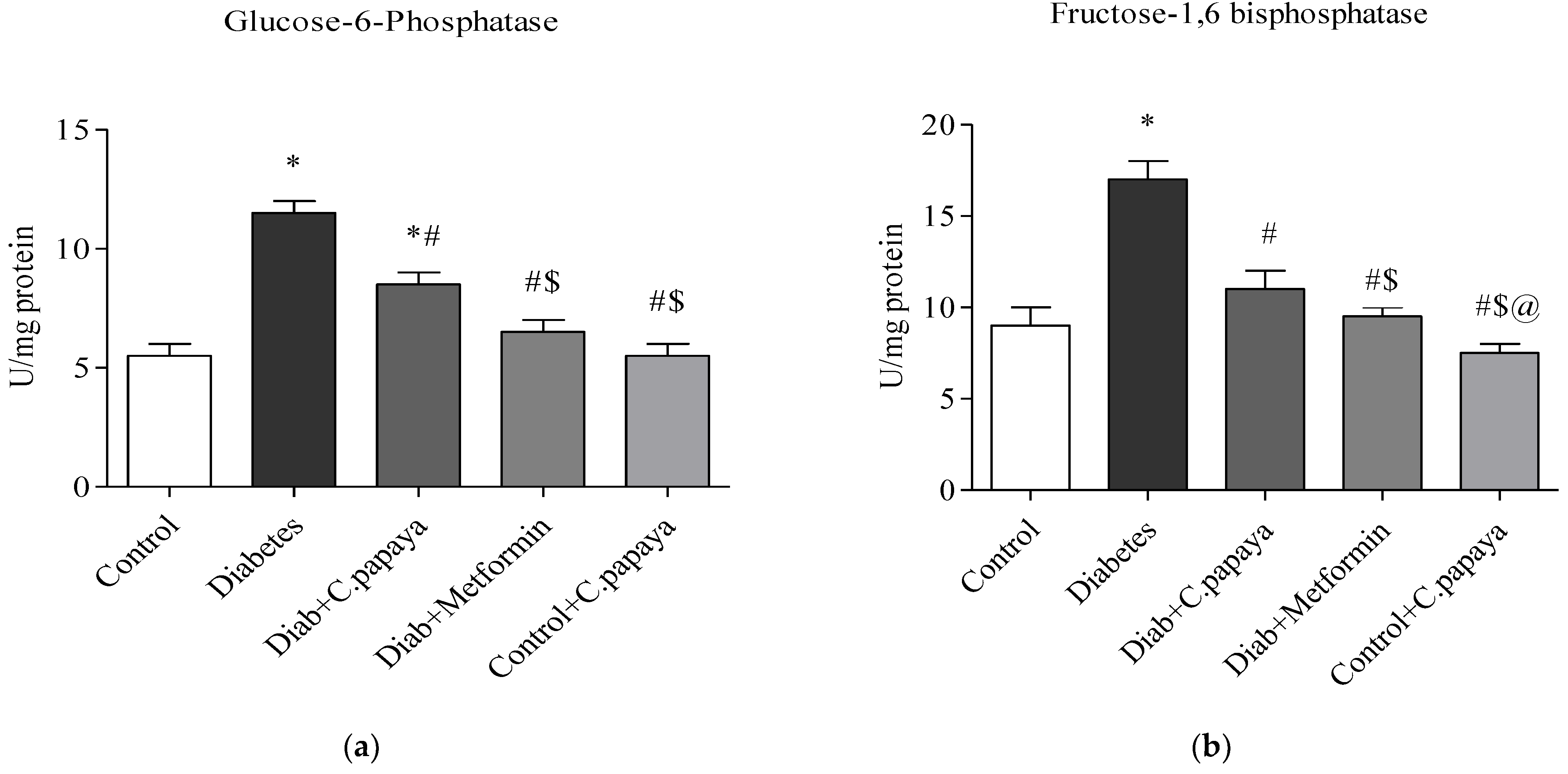
2.7. Gluconeogenic Enzymes
2.7.1. Assay for Glucose-6-Phosphatase
2.7.2. Assay for Fructose-1,6 Bisphosphatase
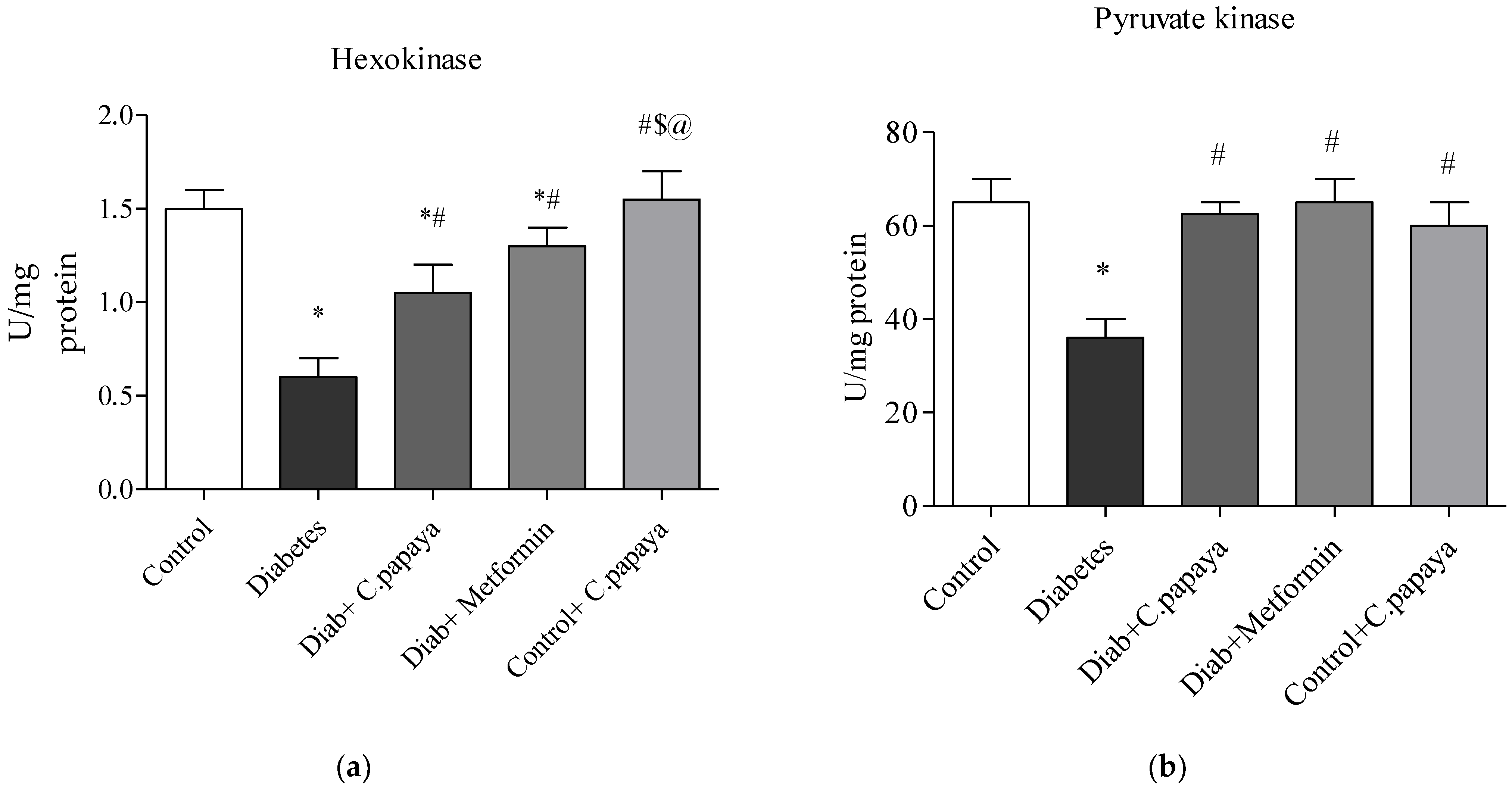
2.8. Determination of Glycolytic Enzymes
2.9. Glycogen Level
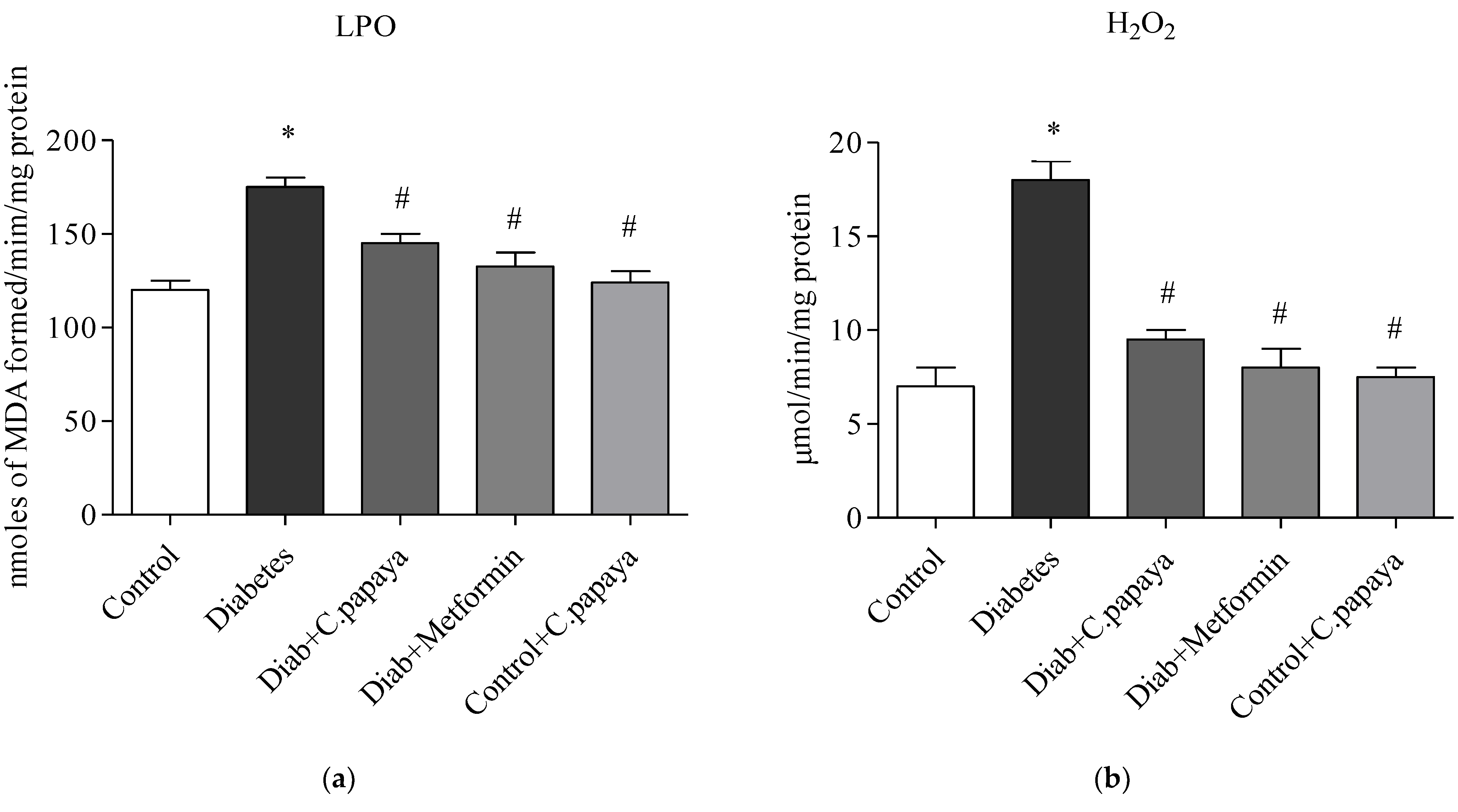
2.10. Oxidative Stress Markers
2.11. Enzymatic Antioxidants
2.12. Total RNA, cDNA Synthesis, and Real-Time PCR
2.13. Histopathology
2.14. Immunohistochemical Analysis
2.15. Statistical Analysis
2.16. Molecular Docking
2.16.1. Ligand Molecule Preparation
2.16.2. Protein Macromolecule Preparation
2.16.3. Ligand–Protein Docking
3. Results
3.1. Efficacy of C. papaya on Liver and Renal Function Markers
3.2. Impact of C. papaya on Gluconeogenic Enzymes and Glycolytic Enzymes
3.3. Outcome of C. papaya on Hepatic Glycogen Level
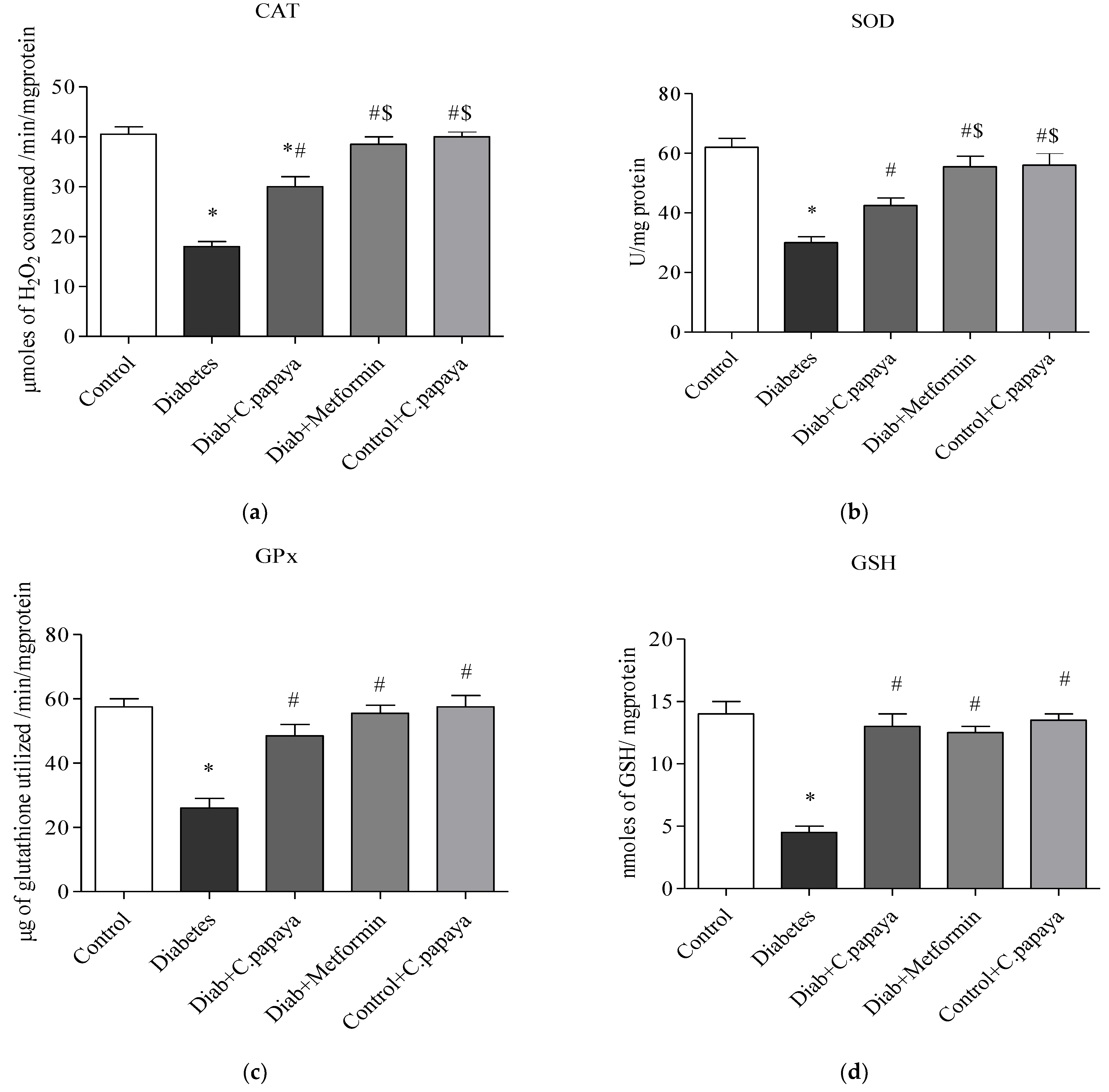
3.4. Efficacy of C. papaya on Oxidative Stress Markers
3.5. Impact of C. papaya on Enzymatic Antioxidants
3.6. Impact of C. papaya on mRNA Expression of IRS-2, PI3K, SREBP-1c and GLUT-2 in Liver
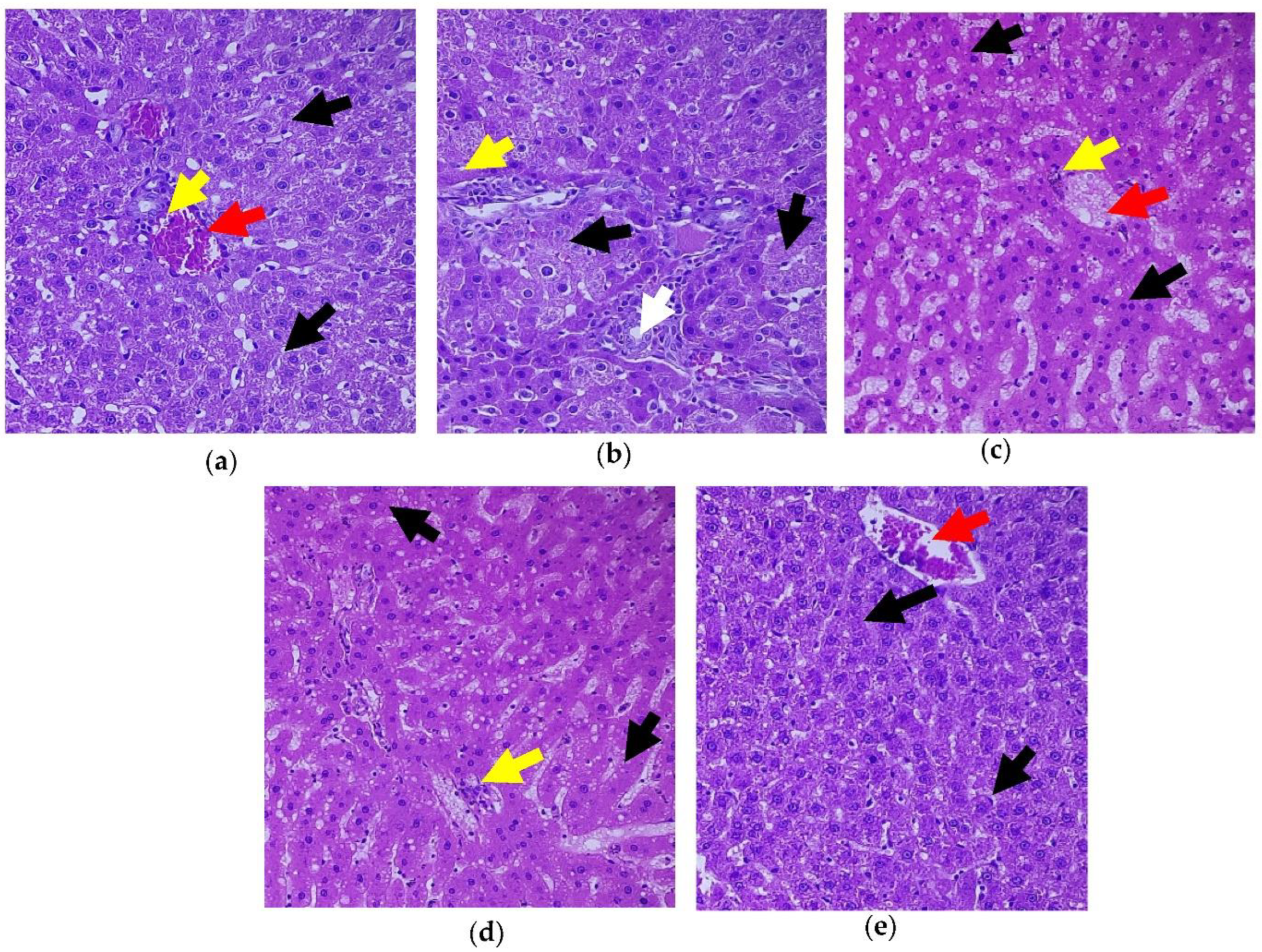
3.7. Role of C. papaya on the Liver Tissue’s Histopathological Changes
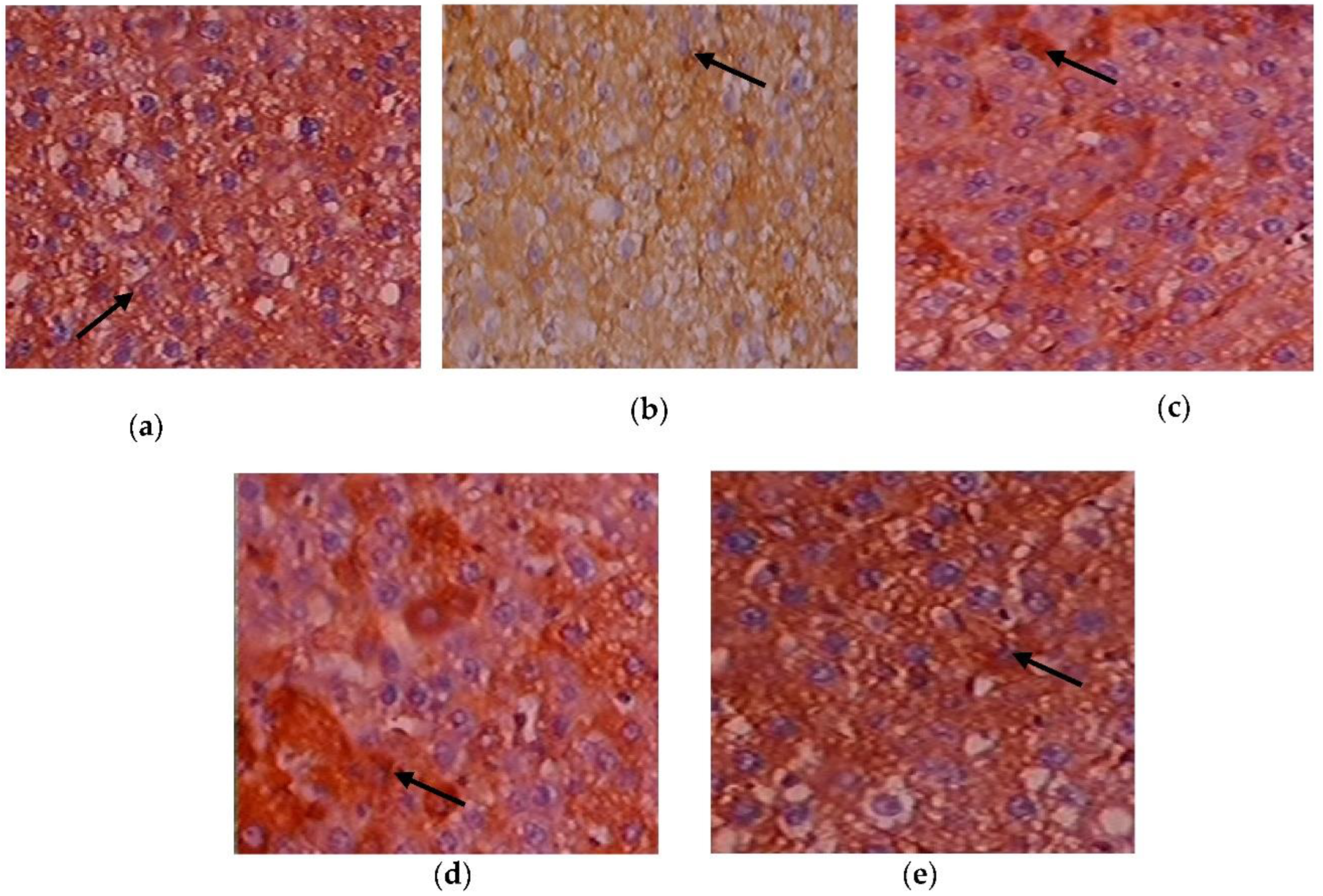
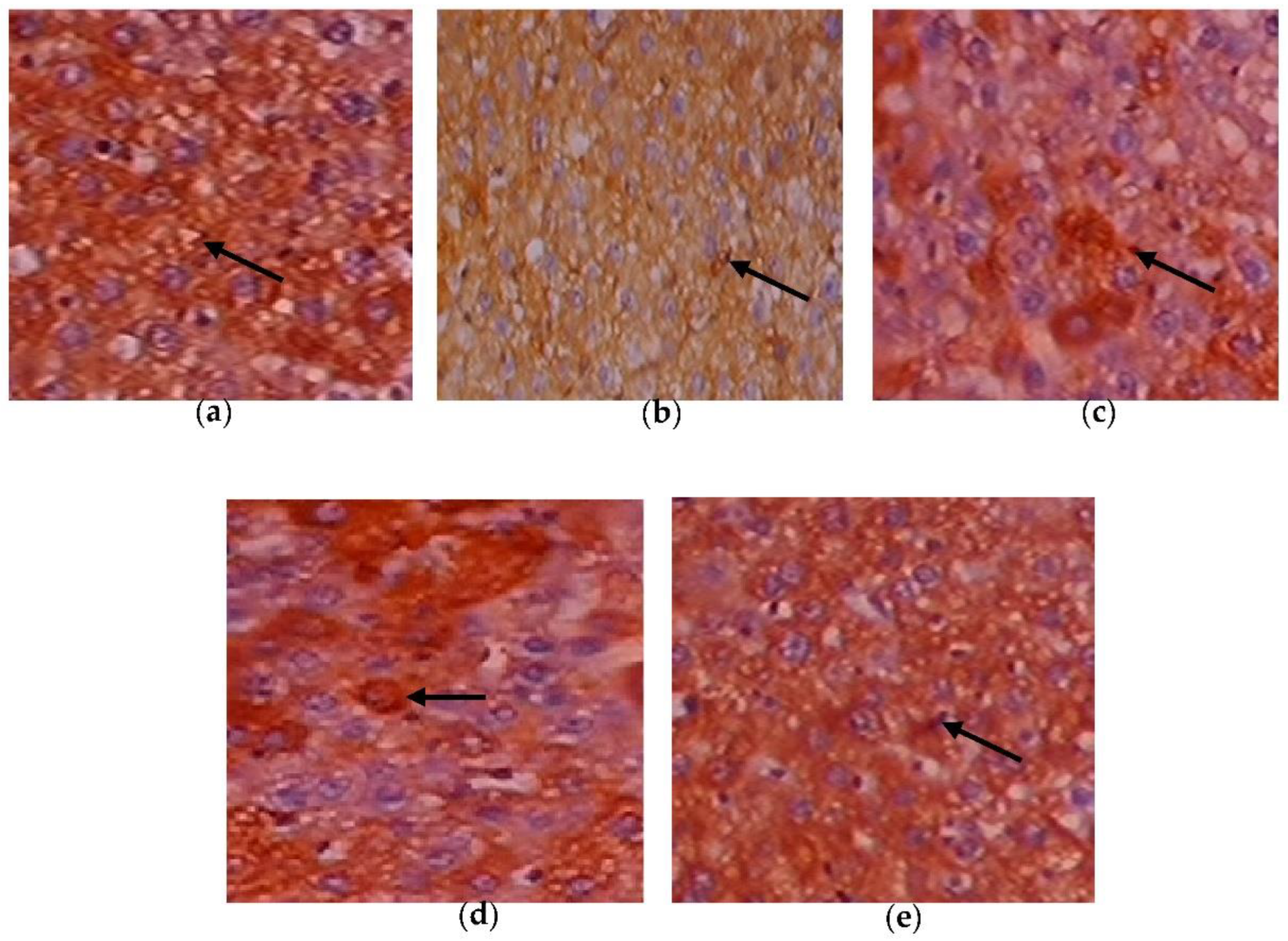
3.8. Efficacy of C. papaya on the Immunohistochemistry Alterations in Liver Tissue
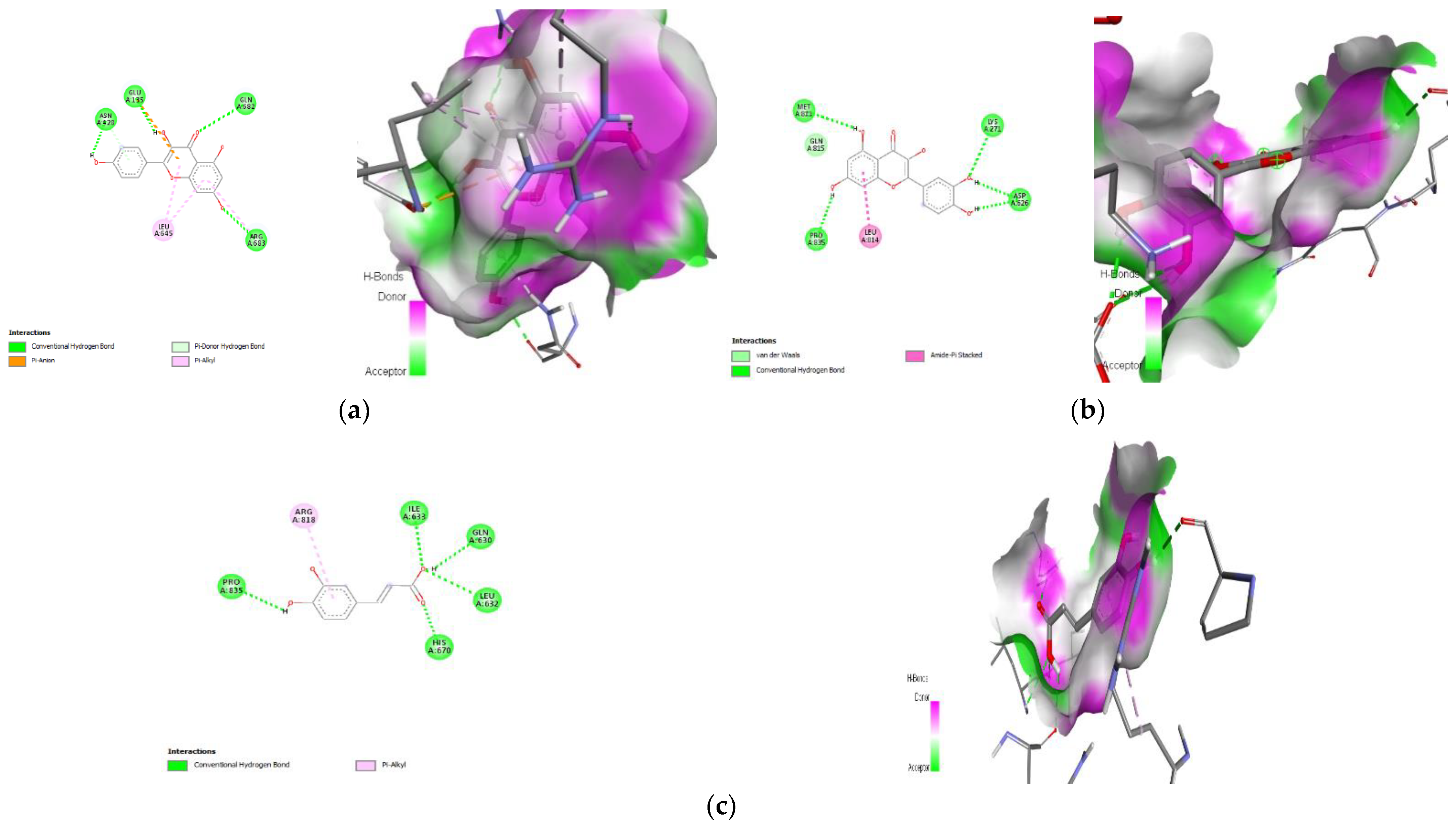
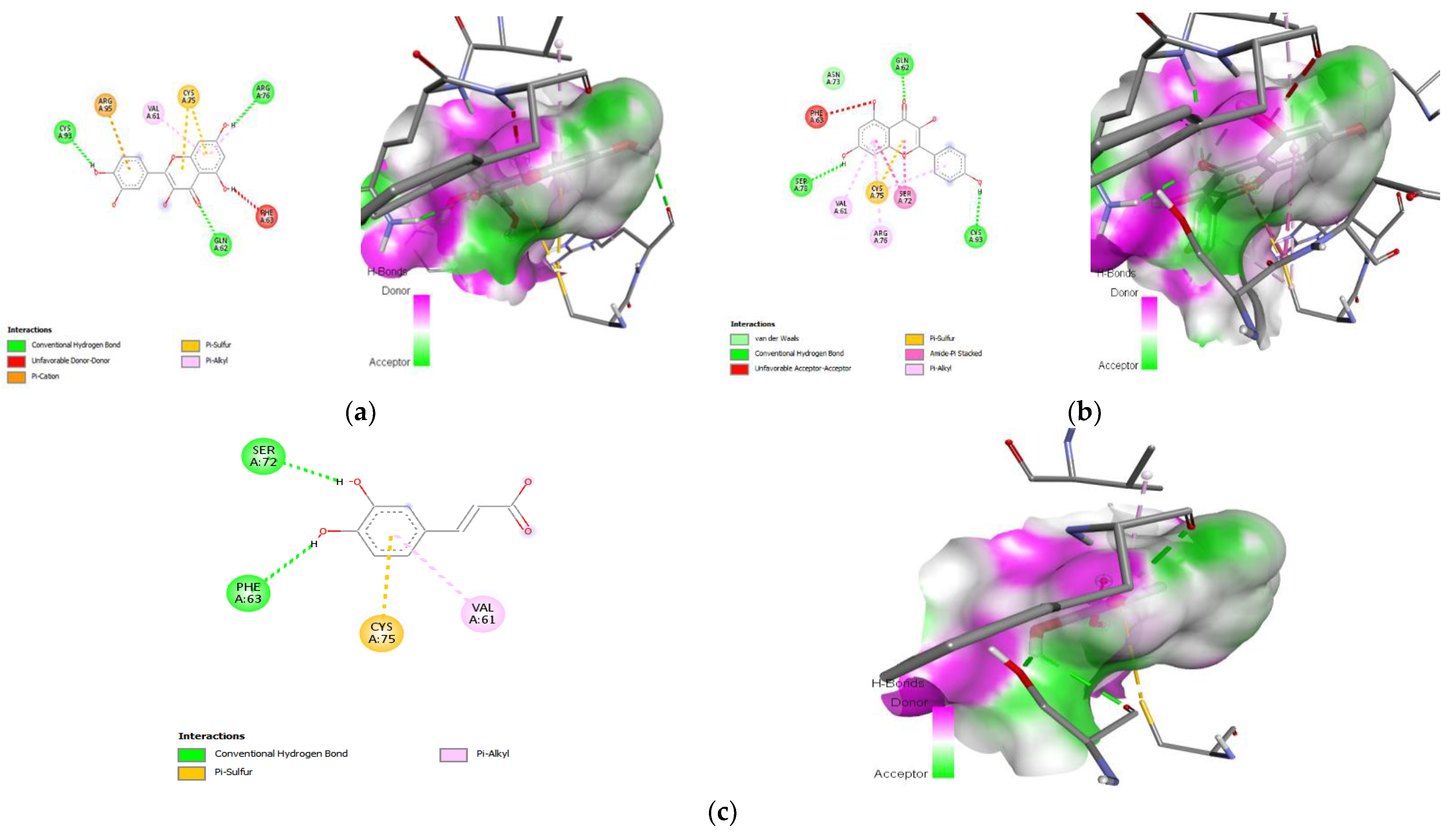
3.9. Molecular Docking
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; Fernandes, J.D.R.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Bekele, B.B.; Negash, S.; Bogale, B.; Tesfaye, M.; Getachew, D.; Weldekidan, F.; Balcha, B. Effect of diabetes self-management education (DSME) on glycated hemoglobin (HbA1c) level among patients with T2DM: Systematic review and meta-analysis of randomized controlled trials. Diabetes Metab. Syndr. 2021, 15, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Czech, M.P. Insulin action and resistance in obesity and type 2 diabetes. Nat. Med. 2017, 23, 804–814. [Google Scholar] [CrossRef] [PubMed]
- Watt, M.J.; Miotto, P.M.; De Nardo, W.; Montgomery, M.K. The Liver as an Endocrine Organ-Linking NAFLD and Insulin Resistance. Endocr. Rev. 2019, 40, 1367–1393. [Google Scholar] [CrossRef] [PubMed]
- Tanase, D.M.; Gosav, E.M.; Costea, C.F.; Ciocoiu, M.; Lacatusu, C.M.; Maranduca, M.A.; Ouatu, A.; Floria, M. The Intricate Relationship between Type 2 Diabetes Mellitus (T2DM), Insulin Resistance (IR), and Nonalcoholic Fatty Liver Disease (NAFLD). J. Diabetes Res. 2020, 2020, 3920196. [Google Scholar] [CrossRef]
- Roden, M.; Petersen, K.; Shulman, G. Insulin resistance in type 2 diabetes. In Textbook of Diabetes, 5th ed.; Holt, R., Cockram, C., Flyvbjerg, A., Goldstein, B., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 174–186. [Google Scholar]
- Rui, L. Energy metabolism in the liver. Compr. Physiol. 2014, 4, 177–197. [Google Scholar]
- Wilcox, G. Insulin and insulin resistance. Clin. Biochem. Rev. 2005, 26, 19–39. [Google Scholar]
- Wree, A.; Schlattjan, M.; Bechmann, L.P.; Claudel, T.; Sowa, J.P.; Stojakovic, T.; Scharnagl, H.; Köfeler, H.; Baba, H.A.; Gerken, G.; et al. Adipocyte cell size, free fatty acids and apolipoproteins are associated with non-alcoholic liver injury progression in severely obese patients. Metabolism 2014, 63, 1542–1552. [Google Scholar] [CrossRef]
- Yang, Y.; Li, W.; Li, Y.; Wang, Q.; Gao, L.; Zhao, J. Dietary Lycium barbarum polysaccharide induces Nrf2/ARE pathway and ameliorates insulin resistance induced by high-fat via activation of PI3K/AKT signaling. Oxid. Med. Cell. Longev. 2014, 2014, 145641. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.Y.; Shi, C.X.; Gao, R.; Sun, H.J.; Xiong, X.Q.; Ding, L.; Chen, Q.; Li, Y.H.; Wang, J.J.; Kang, Y.M.; et al. Irisin inhibits hepatic gluconeogenesis and increases glycogen synthesis via the PI3K/Akt pathway in type 2 diabetic mice and hepatocytes. Clin. Sci. 2015, 129, 839–850. [Google Scholar] [CrossRef]
- Fan, Y.; Xiong, W.; Li, J.; Hu, A.; He, Z.; Zhang, J.; Zhou, G.; Yin, Q. Mechanism of TangGanJian on nonalcoholic fatty liver disease with type 2 diabetes mellitus. Pharm. Biol. 2018, 56, 567–572. [Google Scholar] [CrossRef] [Green Version]
- Tziomalos, K.; Athyros, V.G.; Karagiannis, A. Non-alcoholic fatty liver disease in type 2 diabetes: Pathogenesis and treatment options. Curr. Vasc. Pharmacol. 2012, 10, 162–172. [Google Scholar] [CrossRef]
- Huang, W.; Metlakunta, A.; Dedousis, N.; Zhang, P.; Sipula, I.; Dube, J.J.; Scott, D.K.; O’Doherty, R.M. Depletion of liver Kupffer cells prevents the development of diet-induced hepatic steatosis and insulin resistance. Diabetes 2010, 59, 347–357. [Google Scholar] [CrossRef] [Green Version]
- Odegaard, J.I.; Ricardo-Gonzalez, R.R.; Red Eagle, A.; Vats, D.; Morel, C.R.; Goforth, M.H.; Subramanian, V.; Mukundan, L.; Ferrante, A.W.; Chawla, A. Alternative M2 activation of Kupffer cells by PPAR delta ameliorates obesity-induced insulin resistance. Cell Metab. 2008, 7, 496–507. [Google Scholar] [CrossRef] [Green Version]
- Tilg, H.; Moschen, A.R.; Roden, M. NAFLD and diabetes mellitus. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 32–42. [Google Scholar] [CrossRef]
- Tseng, P.S.; Ande, C.; Moremen, K.W.; Crich, D. Influence of Side Chain Conformation on the Activity of Glycosidase Inhibitors. Angew. Chem. 2023, 135, e202217809. [Google Scholar] [CrossRef]
- Rajasekaran, P.; Ande, C.; Vankar, Y.D. Synthesis of (5, 6 & 6, 6)-oxa-oxa annulated sugars as glycosidase inhibitors from 2-formyl galactal using iodocyclization as a key step. Arkivoc 2022, 2022, 5–23. [Google Scholar]
- Adetayo, M.O.; Adetayo, A.M.; Adetunji, O.A.; Coker-Osiwoga, T.F.; Mordi, A.C. Hypoglycemic, Hypolipidemic and Hepatoprotective Activities of Ripe and Unripe Carica papaya Methanol Extracts in Streptozotocin-Induced Diabetic Male Rats. Trop. J. Nat. Prod. 2021, 5, 1673–1676. [Google Scholar]
- Mohd Abd Razak, M.R.; Norahmad, N.A.; Md Jelas, N.H.; Afzan, A.; Misnan, N.M.; Ripen, A.M.; Thayan, R.; Zainol, M.; Syed Mohamed, A.F. Immunomodulatory activities of Carica papaya L. leaf juice in a non-lethal, symptomatic dengue mouse model. Pathogens 2021, 10, 501. [Google Scholar] [CrossRef]
- Airaodion, A.I.; Ogbuagu, E.O.; Ekenjoku, J.A.; Ogbuagu, U.; Okoroukwu, V.N. Antidiabetic effect of ethanolic extract of Carica papaya leaves in alloxan-induced diabetic rats. Am. J. Biomed. 2019, 5, 227–234. [Google Scholar] [CrossRef]
- Deenin, W.; Malakul, W.; Boonsong, T.; Phoungpetchara, I.; Tunsophon, S. Papaya improves non-alcoholic fatty liver disease in obese rats by attenuating oxidative stress, inflammation and lipogenic gene expression. World J. Hepatol. 2021, 13, 315–327. [Google Scholar] [CrossRef]
- Chao, P.C.; Li, Y.; Chang, C.H.; Shieh, J.P.; Cheng, J.T.; Cheng, K.C. Investigation of insulin resistance in the popularly used four rat models of type-2 diabetes. Biomed. Pharmacother. 2018, 101, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Koide, H.; Oda, T. Pathological occurrence of glucose-6-phosphatase in serum in liver diseases. Clin. Chim. Acta 1959, 4, 554–561. [Google Scholar] [PubMed]
- Fiske, C.H.; Subbarow, J. The colorimetric determination of phosphorus. J. Biol. Chem. 1925, 66, 375–400. [Google Scholar] [CrossRef]
- Gancedo, J.M.; Gancedo, C. Fructose-1,6-diphosphatase, phosphofructokinase and glucose-6-phosphate dehydrogenase from fermenting and non fermenting yeasts. Arch. Mikrobiol. 1971, 76, 132–138. [Google Scholar] [CrossRef]
- Brandstrup, N.; Kirk, J.E.; Bruni, C. The hexokinase and phosphoglucoisomerase activities of aortic and pulmonary artery tissue in individuals of various ages. J. Gerontol. 1957, 12, 166–171. [Google Scholar] [CrossRef]
- Valentine, W.N.; Tanaka, K.R. Pyruvate kinase: Clinical aspects. Methods Enzymol. 1966, 9, 468–473. [Google Scholar]
- Hassid, W.Z.; Abraham, S. Determination of glycogen with anthrone reagent. Methods Enzymol. 1975, 3, 34–37. [Google Scholar]
- Kheiripour, N.; Karimi, J.; Khodadadi, I.; Tavilani, H.; Goodarzi, M.T.; Hashemnia, M. Silymarin prevents lipid accumulation in the liver of rats with type 2 diabetes via sirtuin1 and SREBP-1c. J. Basic Clin. Physiol. Pharmacol. 2018, 29, 301–308. [Google Scholar] [CrossRef]
- Xing, L.J.; Zhang, L.; Liu, T.; Hua, Y.Q.; Zheng, P.Y.; Ji, G. Berberine reducing insulin resistance by up-regulating IRS-2 mRNA expression in nonalcoholic fatty liver disease (NAFLD) rat liver. Eur. J. Pharmacol. 2011, 668, 467–471. [Google Scholar] [CrossRef]
- Gandhi, G.R.; Stalin, A.; Balakrishna, K.; Ignacimuthu, S.; Paulraj, M.G.; Vishal, R. Insulin sensitization via partial agonism of PPARγ and glucose uptake through translocation and activation of GLUT4 in PI3K/p-Akt signaling pathway by embelin in type 2 diabetic rats. Biochim. Biophys. Acta 2013, 1830, 2243–2255. [Google Scholar] [CrossRef]
- Narasimhan, A.; Chinnaiyan, M.; Karundevi, B. Ferulic acid regulates hepatic GLUT2 gene expression in high fat and fructose-induced type-2 diabetic adult male rat. Eur. J. Pharmacol. 2015, 761, 391–397. [Google Scholar] [CrossRef]
- Gabe, M. Techniques Histologiques, 6th ed.; Massie e Cie: Paris, France, 1968; p. 1113. [Google Scholar]
- Streba, L.A.; Vere, C.C.; Rogoveanu, I.; Streba, C.T. Nonalcoholic fatty liver disease, metabolic risk factors, and hepatocellular carcinoma: An open question. World J. Gastroenterol. 2015, 21, 4103–4110. [Google Scholar] [CrossRef]
- Loomba, R.; Abraham, M.; Unalp, A.; Wilson, L.; Lavine, J.; Doo, E.; Bass, N.M.; Network, N.S.C.R. Association between diabetes, family history of diabetes, and risk of nonalcoholic steatohepatitis and fibrosis. Hepatology 2012, 56, 943–951. [Google Scholar] [CrossRef] [Green Version]
- Lallukka, S.; Yki-Jarvinen, H. Non-alcoholic fatty liver disease and risk of type 2 diabetes. Best Pract. Res. Clin. Endocrinol. Metab. 2016, 30, 385–395. [Google Scholar] [CrossRef] [Green Version]
- Commerford, S.R.; Ferniza, J.B.; Bizeau, M.E.; Thresher, J.S.; Willis, W.T.; Pagliassotti, M.J. Diets enriched in sucrose or fat increase gluconeogenesis and G-6-Pase but not basal glucose production in rats. Am. J. Physiol. Endocrinol. Metab. 2002, 283, E545–E555. [Google Scholar] [CrossRef] [Green Version]
- DeFronzo, R.A. Pathogenesis of type 2 diabetes mellitus. Med. Clin. N. Am. 2004, 88, 787–835. [Google Scholar] [CrossRef]
- Michael, M.D.; Kulkarni, R.N.; Postic, C.; Previs, S.F.; Shulman, G.I.; Magnuson, M.A.; Kahn, C.R. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol. Cell. 2000, 6, 87–97. [Google Scholar] [CrossRef]
- Cheng, Z.; Tseng, Y.; White, M.F. Insulin signaling meets mitochondria in metabolism. Trends. Endocrinol. Metab. 2010, 21, 589–598. [Google Scholar] [CrossRef] [Green Version]
- Hanke, S.; Mann, M. The phosphotyrosine interactome of the insulin receptor family and its substrates IRS-1 and IRS-2. Mol. Cell. Proteom. 2009, 8, 519–534. [Google Scholar] [CrossRef] [Green Version]
- Samuel, V.T.; Shulman, G.I. Mechanisms for insulin resistance: Common threads and missing links. Cell 2012, 148, 852–871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franke, T.F.; Kaplan, D.R.; Cantley, L.C.; Toker, A. Direct regulation of the Akt proto-oncogene product by phosphatidylinositol-3,4-bisphosphate. Science 1997, 275, 665–668. [Google Scholar] [CrossRef] [PubMed]
- Carr, R.M.; Correnti, J. Insulin resistance in clinical and experimental alcoholic liver disease. Ann. N. Y. Acad. Sci. 2015, 1353, 1–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beurel, E.; Grieco, S.F.; Jope, R.S. Glycogen synthase kinase-3 (GSK3): Regulation, actions, and diseases. Pharmacol. Ther. 2015, 148, 114–131. [Google Scholar] [CrossRef] [Green Version]
- Gross, D.N.; Wan, M.; Birnbaum, M.J. The role of FOXO in the regulation of metabolism. Curr. Diabetes Rep. 2009, 9, 208–214. [Google Scholar] [CrossRef]
- Shao, W.; Espenshade, P.J. Expanding roles for SREBP in metabolism. Cell Metab. 2012, 16, 414–419. [Google Scholar] [CrossRef] [Green Version]
- Xuguang, H.; Aofei, T.; Tao, L.; Longyan, Z.; Weijian, B.; Jiao, G. Hesperidin ameliorates insulin resistance by regulating the IRS1-GLUT2 pathway via TLR4 in HepG2 cells. Phytother. Res. 2019, 33, 1697–1705. [Google Scholar] [CrossRef]
- Lotta, L.A.; Gulati, P.; Day, F.R.; Payne, F.; Ongen, H.; Van De Bunt, M.; Gaulton, K.J.; Eicher, J.D.; Sharp, S.J.; Luan, J.A.; et al. Integrative genomic analysis implicates limited peripheral adipose storage capacity in the pathogenesis of human insulin resistance. Nat. Genet. 2017, 49, 17–26. [Google Scholar] [CrossRef]
- Agius, L. Glucokinase and molecular aspects of liver glycogen metabolism. Biochem. J. 2008, 414, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Lu, M.; Wan, M.; Leavens, K.F.; Chu, Q.; Monks, B.R.; Fernandez, S.; Ahima, R.S.; Ueki, K.; Kahn, C.R.; Birnbaum, M.J. Insulin regulates liver metabolism in vivo in the absence of hepatic Akt and Foxo1. Nat. Med. 2012, 18, 388–395. [Google Scholar] [CrossRef]
- Wree, A.; Kahraman, A.; Gerken, G.; Canbay, A. Obesity affects the liver-the link between adipocytes and hepatocytes. Digestion 2011, 83, 124–133. [Google Scholar] [CrossRef]
- Pandey, S.; Cabot, P.J.; Shaw, P.N.; Hewavitharana, A.K. Anti-inflammatory and immunomodulatory properties of Carica papaya. J. Immunotoxicol. 2016, 13, 590–602. [Google Scholar] [CrossRef] [Green Version]
- Roy, J.R.; Janaki, C.S.; Jayaraman, S.; Periyasamy, V.; Balaji, T.; Vijayamalathi, M.; Veeraraghavan, V.P. Carica papaya Reduces Muscle Insulin Resistance via IR/GLUT4 Mediated Signaling Mechanisms in High Fat Diet and Streptozotocin-Induced Type-2 Diabetic Rats. Antioxidants 2022, 11, 2081. [Google Scholar] [CrossRef]
- Roy, J.R.; Janaki, C.S.; Jayaraman, S.; Periyasamy, V.; Balaji, T.; Vijayamalathi, M.; Veeraraghavan, V.P. Effect of Carica papaya on IRS-1/Akt Signaling Mechanisms in High-Fat-Diet–Streptozotocin-Induced Type 2 Diabetic Experimental Rats: A Mechanistic Approach. Nutrients 2022, 14, 4181. [Google Scholar] [CrossRef]
- D’Argenio, G.; Mazzone, G.; Ribecco, M.T.; Lembo, V.; Vitaglione, P.; Guarino, M.; Morisco, F.; Napolitano, M.; Fogliano, V.; Caporaso, N. Garlic extract attenuating rat liver fibrosis by inhibiting TGF-β1. Clin. Nutr. 2013, 32, 252–258. [Google Scholar] [CrossRef]
- Abdel-Halim, S.A.; Ibrahim, M.T.; Mohsen, M.M.A.; Abou-Setta, L.M.; Sleem, A.A.; Morsy, F.A.; El-Missiry, M.M. Phytochemical and biological investigation of Carica papaya Linn. Leaves cultivated in Egypt (Family Caricaceae). J. Pharmacogn. Phytochem. 2020, 9, 47–54. [Google Scholar] [CrossRef]
- Albrahim, T.; Alonazi, M. Effect of Blueberry Extract on Liver in Aged Rats. Oxid. Med. Cell. Longev. 2022, 2022, 3490776. [Google Scholar] [CrossRef]
- Park, E.; Hong, K.; Kwon, B.M.; Kim, Y.; Kim, J.H. Jaceosidin Ameliorates Insulin Resistance and Kidney Dysfunction by Enhancing Insulin Receptor Signaling and the Antioxidant Defense System in Type 2 Diabetic Mice. J. Med. Food 2020, 23, 1083–1092. [Google Scholar] [CrossRef]
- Latifi, E.; Mohammadpour, A.A.; Fathi, B.; Nourani, H. Antidiabetic and antihyperlipidemic effects of ethanolic Ferula assa-foetida oleo-gum-resin extract in streptozotocin-induced diabetic wistar rats. Biomed. Pharmacother. 2019, 110, 197–202. [Google Scholar] [CrossRef]
- Cui, X.; Qian, D.W.; Jiang, S.; Shang, E.X.; Zhu, Z.H.; Duan, J.A. Scutellariae Radix and Coptidis Rhizoma Improve Glucose and Lipid Metabolism in T2DM Rats via Regulation of the Metabolic Profiling and MAPK/PI3K/Akt Signaling Pathway. Int. J. Mol. Sci. 2018, 19, 3634. [Google Scholar] [CrossRef] [Green Version]
- Pari, L.; Chandramohan, R. Modulatory effects of naringin on hepatic key enzymes of carbohydrate metabolism in high-fat diet/low-dose streptozotocin-induced diabetes in rats. Gen. Physiol. Biophys. 2017, 36, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Latha, M.; Pari, L. Antihyperglycaemic effect of Cassia auriculata in experimental diabetes and its effects on key metabolic enzymes involved in carbohydrate metabolism. Clin. Exp. Pharmacol. Physiol. 2003, 30, 38–43. [Google Scholar] [CrossRef]
- Sureka, C.; Elango, V.; Al-Ghamdi, S.; Aldossari, K.K.; Alsaidan, M.; Geddawy, A.; Abdelaziz, M.A.; Mohideen, A.P.; Ramesh, T. Ameliorative property of Sesbania grandiflora on carbohydrate metabolic enzymes in the liver and kidney of streptozotocin-induced diabetic rats. Saudi J. Biol. Sci. 2021, 28, 3669–3677. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Meng, Z.; Tian, Y.; Gu, R.; Xu, Z.; Fang, H.; Liu, W.; Huang, W.; Ding, G.; Xiao, W. Hypoglycemic and hypolipidemic effects of total glycosides of Cistanche tubulosa in diet/streptozotocin-induced diabetic rats. J. Ethnopharmacol. 2021, 276, 113991. [Google Scholar] [CrossRef] [PubMed]
- Juárez-Rojop, I.E.; Tovilla-Zárate, C.A.; Aguilar-Domínguez, D.E.; Lobato-García, C.E.; Blé-Castillo, J.L.; López-Meraz, L.; Díaz-Zagoya, J.C.; Bermúdez-Ocaña, D.Y. Phytochemical screening and hypoglycemic activity of Carica papaya leaf in streptozotocin-induced diabetic rats. Rev. Bras. Farmacogn. 2014, 24, 341–347. [Google Scholar] [CrossRef] [Green Version]
- Luo, D.; Mu, T.; Sun, H. Sweet potato (Ipomoea batatas L.) leaf polyphenols ameliorate hyperglycemia in type 2 diabetes mellitus mice. Food Funct. 2021, 12, 4117–4131. [Google Scholar] [CrossRef]
- Mu, J.; Xin, G.; Zhang, B.; Wang, Y.; Ning, C.; Meng, X. Beneficial effects of Aronia melanocarpa berry extract on hepatic insulin resistance in type 2 diabetes mellitus rats. J. Food Sci. 2020, 85, 1307–1318. [Google Scholar] [CrossRef]
- Tangvarasittichai, S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J. Diabetes 2015, 6, 456–480. [Google Scholar] [CrossRef]
- Putakala, M.; Gujjala, S.; Nukala, S.; Desireddy, S. Beneficial Effects of Phyllanthus amarus against High Fructose Diet Induced Insulin Resistance and Hepatic Oxidative Stress in Male Wistar Rats. Appl. Biochem. Biotechnol. 2017, 183, 744–764. [Google Scholar] [CrossRef]
- Matough, F.A.; Budin, S.B.; Hamid, Z.A.; Alwahaibi, N.; Mohamed, J. The role of oxidative stress and antioxidants in diabetic complications. Sultan Qaboos Univ. Med. J. 2012, 12, 5–18. [Google Scholar] [CrossRef]
- Nain, P.; Saini, V.; Sharma, S.; Nain, J. Antidiabetic and antioxidant potential of Emblica officinalis Gaertn. leaves extract in streptozotocin-induced type-2 diabetes mellitus (T2DM) rats. J. Ethnopharmacol. 2012, 142, 65–71. [Google Scholar] [CrossRef]
- Withers, D.J.; Gutierrez, J.S.; Towery, H.; Burks, D.J.; Ren, J.M.; Previs, S.; Zhang, Y.; Bernal, D.; Pons, S.; Shulman, G.I.; et al. Disruption of IRS-2 causes type 2 diabetes in mice. Nature 1998, 391, 900–904. [Google Scholar] [CrossRef]
- Lin, G.; Liu, X.; Yan, X.; Liu, D.; Yang, C.; Liu, B.; Huang, Y.; Zhao, C. Role of green macroalgae Enteromorpha prolifera polyphenols in the modulation of gene expression and intestinal microflora profiles in type 2 diabetic mice. Int. J. Mol. Sci. 2018, 20, 25. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Wang, S.; Zeng, Z.; Qin, Y.; Shen, Q.; Li, P. Anti-diabetic effects of Bifidobacterium animalis 01 through improving hepatic insulin sensitivity in type 2 diabetic rat model. J. Funct. Foods 2020, 67, 103843. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, J.; Li, Y.; Sun, L.; Xiao, Y.; Gao, W.; Zhang, Z. Mogroside derivatives exert hypoglycemics effects by decreasing blood glucose level in HepG2 cells and alleviates insulin resistance in T2DM rats. J. Funct. Foods 2019, 63, 103566. [Google Scholar] [CrossRef]
- Pettinelli, P.; Del Pozo, T.; Araya, J.; Rodrigo, R.; Araya, A.V.; Smok, G.; Csendes, A.; Gutierrez, L.; Rojas, J.; Korn, O.; et al. Enhancement in liver SREBP-1c/PPAR-α ratio and steatosis in obese patients: Correlations with insulin resistance and n-3 longchain polyunsaturated fatty acid depletion. Biochim. Biophys. Acta 2009, 1792, 1080–1086. [Google Scholar] [CrossRef] [Green Version]
- Im, S.S.; Kang, S.Y.; Kim, S.Y.; Kim, H.I.; Kim, J.W.; Kim, K.S.; Ahn, Y.H. Glucose-stimulated upregulation of GLUT2 gene is mediated by sterol response element–binding protein-1c in the hepatocytes. Diabetes 2005, 54, 1684–1691. [Google Scholar] [CrossRef] [Green Version]
- Mostafa, D.G.; Khaleel, E.F.; Abdel-Aleem, G.A. Inhibition of the hepatic glucose output is responsible for the hypoglycemic effect of Crataegus aronia against type 2 diabetes mellitus in rats. Arch. Biol. Sci. 2018, 70, 277–287. [Google Scholar] [CrossRef]
- D’Argenio, G.; Amoruso, D.C.; Mazzone, G.; Vitaglione, P.; Romano, A.; Ribecco, M.T.; D’Armiento, M.R.; Mezza, E.; Morisco, F.; Fogliano, V.; et al. Garlic extract prevents CCl(4)-induced liver fibrosis in rats: The role of tissue transglutaminase. Dig. Liver Dis. 2010, 42, 571–577. [Google Scholar] [CrossRef]
- Meena, B.; Ezhilan, R.A.; Rajesh, R.; Hussain, K.S.; Ganesan, B.; Anandan, R. Antihepatotoxic potential of Sargassum polycystum (Phaeophyceae) on antioxidant defense status in D-galactosamine-induced hepatitis in rats. Afr. J. Biochem. Res. 2008, 2, 51–55. [Google Scholar]
- Baffy, G. Kupffer cells in non-alcoholic fatty liver disease: The emerging view. J. Hepatol. 2009, 51, 212–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brancaccio, M.; D’Argenio, G.; Lembo, V.; Palumbo, A.; Castellano, I. Antifibrotic effect of marine ovothiol in an in vivo model of liver fibrosis. Oxid. Med. Cell. Longev. 2018, 2018, 5045734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Motshakeri, M.; Ebrahimi, M.; Goh, Y.M.; Othman, H.H.; Hair-Bejo, M.; Mohamed, S. Effects of brown seaweed (Sargassum polycystum) extracts on kidney, liver, and pancreas of type 2 diabetic rat model. Evid. Based Complement. Altern. Med. 2014, 2014, 379407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J.; Wu, M.; Zhou, H.; Cheng, L.; Wei, X.; Wang, Y. Liubao brick tea activates the PI3K-Akt signaling pathway to lower blood glucose, metabolic disorders and insulin resistance via altering the intestinal flora. Food Res. Int. 2021, 148, 110594. [Google Scholar] [CrossRef]
- Mathur, R.; Dutta, S.; Velpandian, T.; Mathur, S.R. Psidium guajava Linn. leaf extract affects hepatic glucose transporter-2 to attenuate early onset of insulin resistance consequent to high fructose intake: An experimental study. Pharmacogn. Res. 2015, 7, 166–175. [Google Scholar] [CrossRef] [Green Version]
- Nair, J.; Velpandian, T.; Das, U.S.; Sharma, P.; Nag, T.; Mathur, S.R.; Mathur, R. Molecular and metabolic markers of fructose induced hepatic insulin resistance in developing and adult rats are distinct and Aegle marmelos is an effective modulator. Sci. Rep. 2018, 8, 15950. [Google Scholar] [CrossRef] [Green Version]
- Milito, A.; Brancaccio, M.; Lisurek, M.; Masullo, M.; Palumbo, A.; Castellano, I. Probing the interactions of sulfur-containing histidine compounds with human gamma-glutamyl transpeptidase. Mar. Drugs 2019, 17, 650. [Google Scholar] [CrossRef] [Green Version]

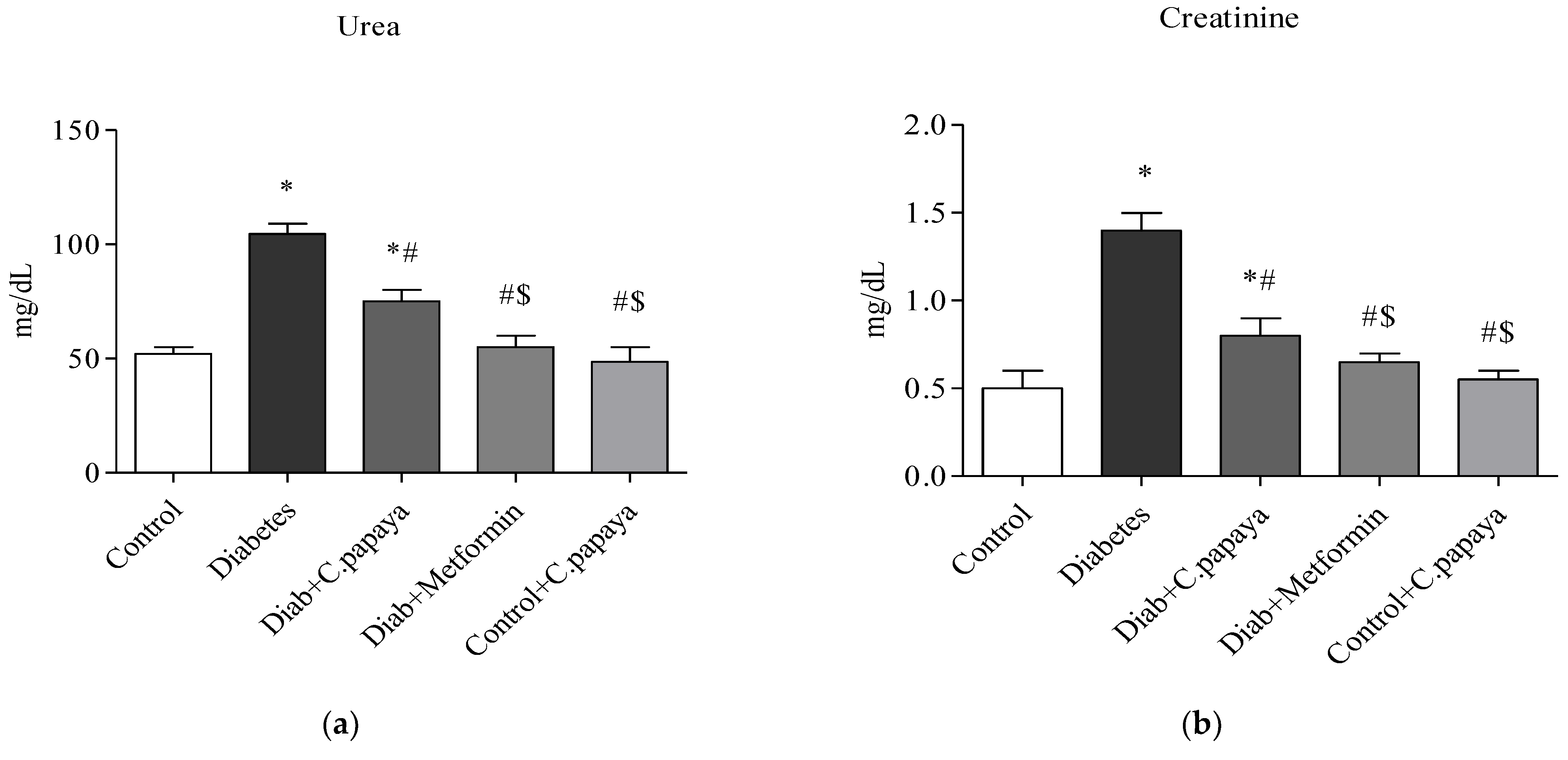





| S.No | Gene Used | Primers’ Sequence | Ref |
|---|---|---|---|
| 1. | Beta actin | Forward CCCGCGAGTACAACCTTCT Reverse CGTCATCCATGGCGAACT | [30] |
| 2. | IRS-2 | Forward CAAGAGTTCCAGCAGTAAC Reverse CAAGAGTTCCAGCAGTAAC | [31] |
| 3. | PI3K | Forward CAAAGCCGAGAACCTATTGC Reverse GGTGGCAGTCTTGT TGATGA | [32] |
| 4. | SREBP-1c | Forward GGAGCCATGGATTGCACATT Reverse AGGAAGGCTTCCAGAGAGGA | [30] |
| 5. | GLUT-2 | Forward GTCAGAAGACAAGATCACCGGA Reverse AGGTGCATTGATCACACCGA | [33] |
| Sl.No | Name of Compound |
|---|---|
| i. | Transferulic acid |
| ii. | Caffeic acid |
| iii. | Protocatechuic acid |
| iv. | Chlorogenic acid |
| v. | p-coumaric acid |
| vi. | Rutin |
| vii. | Quercetin |
| viii. | Kaempferol |
| S.No | Compound Name | Binding Energy Kcal/mol | Interacting Residues |
|---|---|---|---|
| IRS-2 | |||
| 1. | Quercetin | −6 | ASN-100 (H- bond) ASN-102 (H- bond) ILE-101 (H- bond) LYS-103 (H- bond) ARG-195 (H- bond) |
| 2. | Kaempferol | −5.6 | MET-291 (H-bond) ARG-246 (Pi-Sigma) MET-243 (Pi-Alkyl) LEU-294 (Pi-Alkyl) LYS-295 (Pi-Alkyl) |
| 3. | p-coumaric acid | −4.8 | ILE-101 (Pi donor H- bond) TYR-136 (Van der Waal) |
| PI3K | |||
| 1. | Kaempferol | −7.8 | ARG-683 (H-bond) GLU-135 (H-bond) GLN-682(H-bond) ASN-428 (H-bond) LEU-645 (Pi-Alkyl) |
| 2. | Quercetin | −7.7 | MET-811 (H-bond) LYS-271 (H-bond) ASP-626 (H-bond) PRO-835 (H-bond) |
| 3. | Caffeic acid | −6.6 | ILE-633(H-bond) GLN-630 (H-bond) LEU-632 (H-bond) HIS-670 (H-bond) PRO-835 (H-bond) ARG-818 (Pi-Alkyl) |
| SREBP-1c | |||
| 1. | Quercetin | −7.7 | CYS-93 (H-bond) GLN-62 (H-bond) ARG-76 (H-bond) VAL-61 (Pi-Alkyl) |
| 2. | Kaempferol | −7.6 | GLN-62 (H-bond) CYS-93 (H-bond) SER-78 (H-bond) ARG-76 (Pi-Alkyl) VAL-61 (Pi-Alkyl) |
| 3. | Caffeic acid | −5.5 | SER-72 (H-bond) PHE-63 (H-bond) VAL-61 (Pi-Alkyl) |
| GLUT-2 | |||
| 1. | Quercetin | −8.9 | HIS-192 (H-bond) SER-169 (H-bond) ASN-320 (H-bond) GLN-314 (H-bond) TRP-444 (H-bond) |
| 2. | Kaempferol | −8.3 | GLN-314 (H-bond) ASN-443 (H-bond) ALA-440 (Pi-Alkyl) ILE-28 (Pi- Sigma) |
| 3. | Caffeic acidp-coumaric acid | −6.4 | ASN-447 (H-bond) GLU-412 (H-bond) ASN-320 (H-bond) ASN-447 (H-bond) GLU-412 (H-bond) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roy, J.R.; Janaki, C.S.; Jayaraman, S.; Veeraraghavan, V.P.; Periyasamy, V.; Balaji, T.; Vijayamalathi, M.; Bhuvaneswari, P.; Swetha, P. Hypoglycemic Potential of Carica papaya in Liver Is Mediated through IRS-2/PI3K/SREBP-1c/GLUT2 Signaling in High-Fat-Diet-Induced Type-2 Diabetic Male Rats. Toxics 2023, 11, 240. https://doi.org/10.3390/toxics11030240
Roy JR, Janaki CS, Jayaraman S, Veeraraghavan VP, Periyasamy V, Balaji T, Vijayamalathi M, Bhuvaneswari P, Swetha P. Hypoglycemic Potential of Carica papaya in Liver Is Mediated through IRS-2/PI3K/SREBP-1c/GLUT2 Signaling in High-Fat-Diet-Induced Type-2 Diabetic Male Rats. Toxics. 2023; 11(3):240. https://doi.org/10.3390/toxics11030240
Chicago/Turabian StyleRoy, Jeane Rebecca, Coimbatore Sadagopan Janaki, Selvaraj Jayaraman, Vishnu Priya Veeraraghavan, Vijayalakshmi Periyasamy, Thotakura Balaji, Madhavan Vijayamalathi, Ponnusamy Bhuvaneswari, and Panneerselvam Swetha. 2023. "Hypoglycemic Potential of Carica papaya in Liver Is Mediated through IRS-2/PI3K/SREBP-1c/GLUT2 Signaling in High-Fat-Diet-Induced Type-2 Diabetic Male Rats" Toxics 11, no. 3: 240. https://doi.org/10.3390/toxics11030240
APA StyleRoy, J. R., Janaki, C. S., Jayaraman, S., Veeraraghavan, V. P., Periyasamy, V., Balaji, T., Vijayamalathi, M., Bhuvaneswari, P., & Swetha, P. (2023). Hypoglycemic Potential of Carica papaya in Liver Is Mediated through IRS-2/PI3K/SREBP-1c/GLUT2 Signaling in High-Fat-Diet-Induced Type-2 Diabetic Male Rats. Toxics, 11(3), 240. https://doi.org/10.3390/toxics11030240






