Physiological Toxicity and Antioxidant Mechanism of Photoaging Microplastics on Pisum sativum L. Seedlings
Abstract
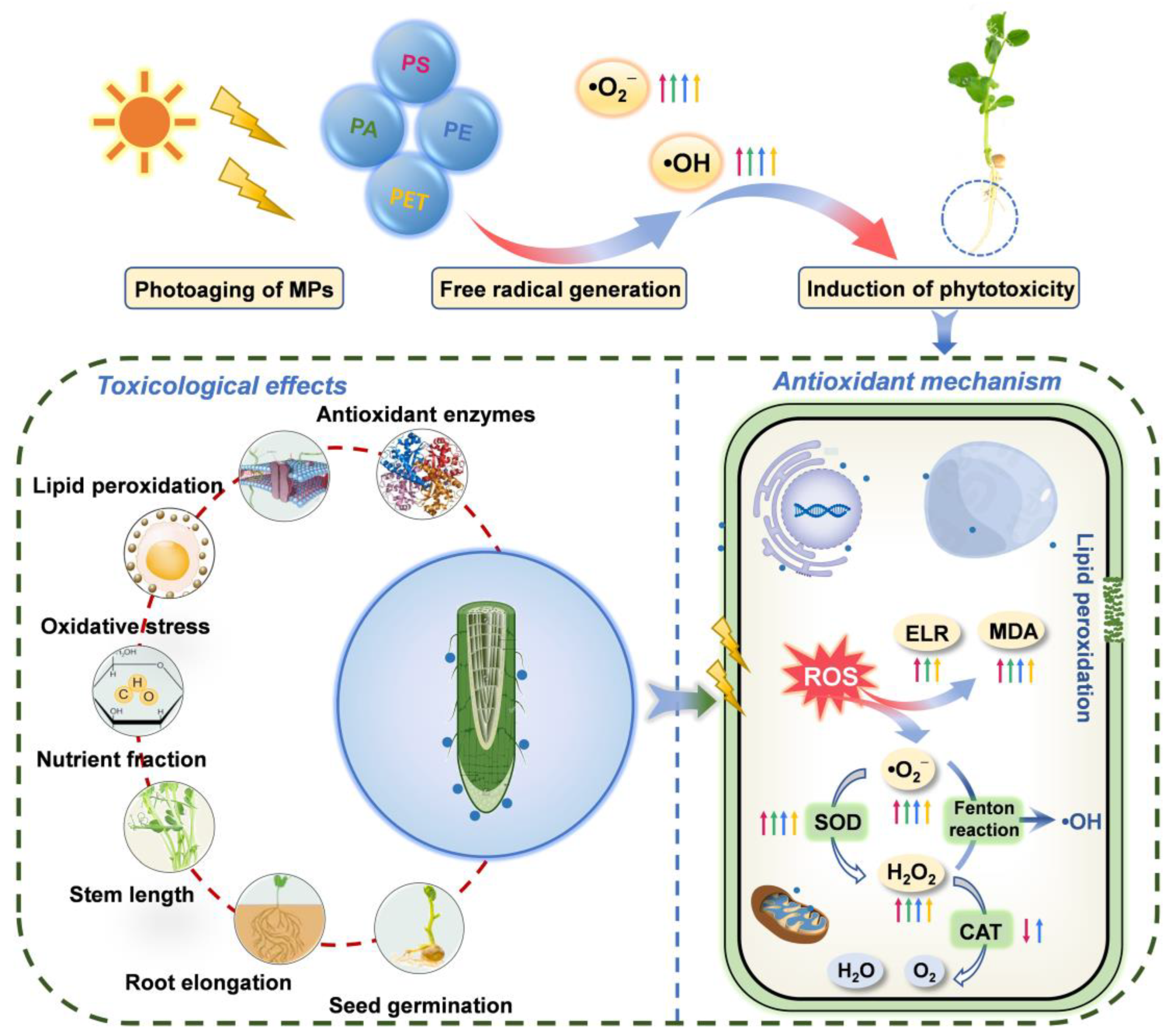
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Light Aging Irradiation Test
2.3. Superoxide Anions and Hydroxyl Radicals in MP Suspensions
2.4. Identification of MPs by Fourier Transform Infrared Microscopy
2.5. Seedling Culture and Treatments
2.6. Physiological and Biochemical Analysis of Seedlings
2.6.1. Soluble Sugar Content Detection
2.6.2. Lipid Peroxidation Testing
2.6.3. Detection of Reactive Oxygen Content
2.6.4. Antioxidant Enzyme Activity Assay
2.7. Data Analysis
3. Results and Discussion
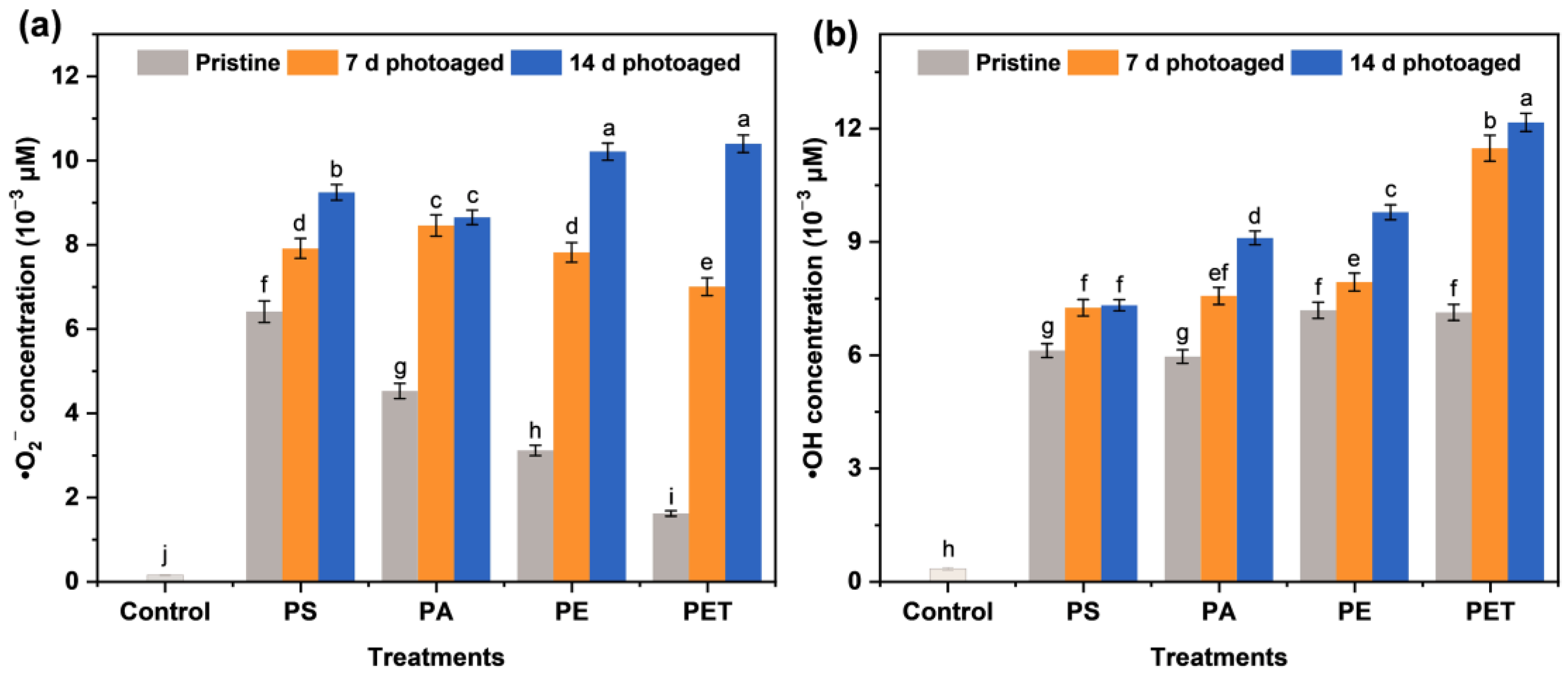
3.1. Free Radical Generation in Photoaging MP Suspensions
3.2. The FTIR Analysis of Photoaging MPs
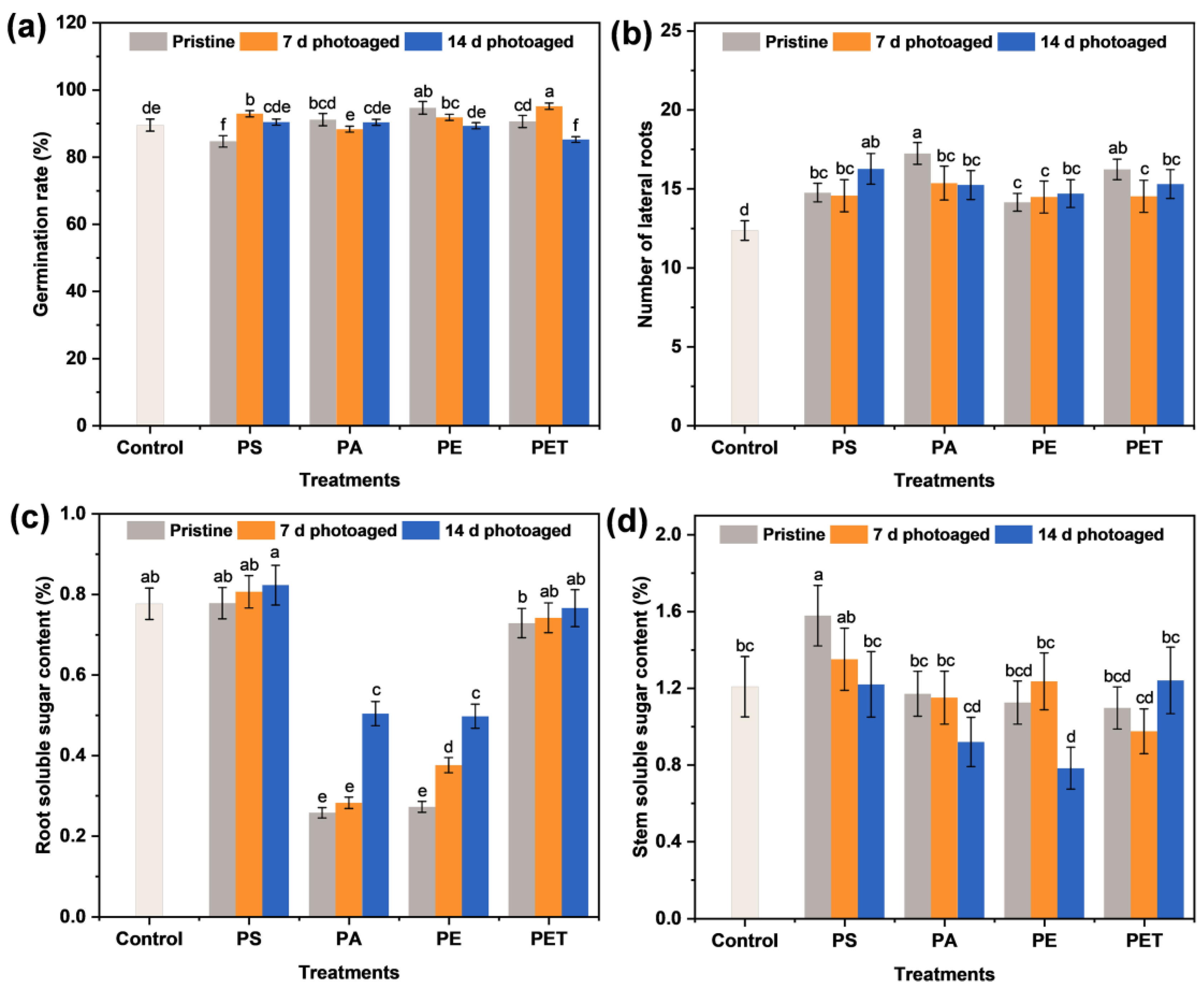
3.3. Seedling Germination and Physiological Status
3.4. Nutrient Transport of Soluble Sugars in Roots and Stems
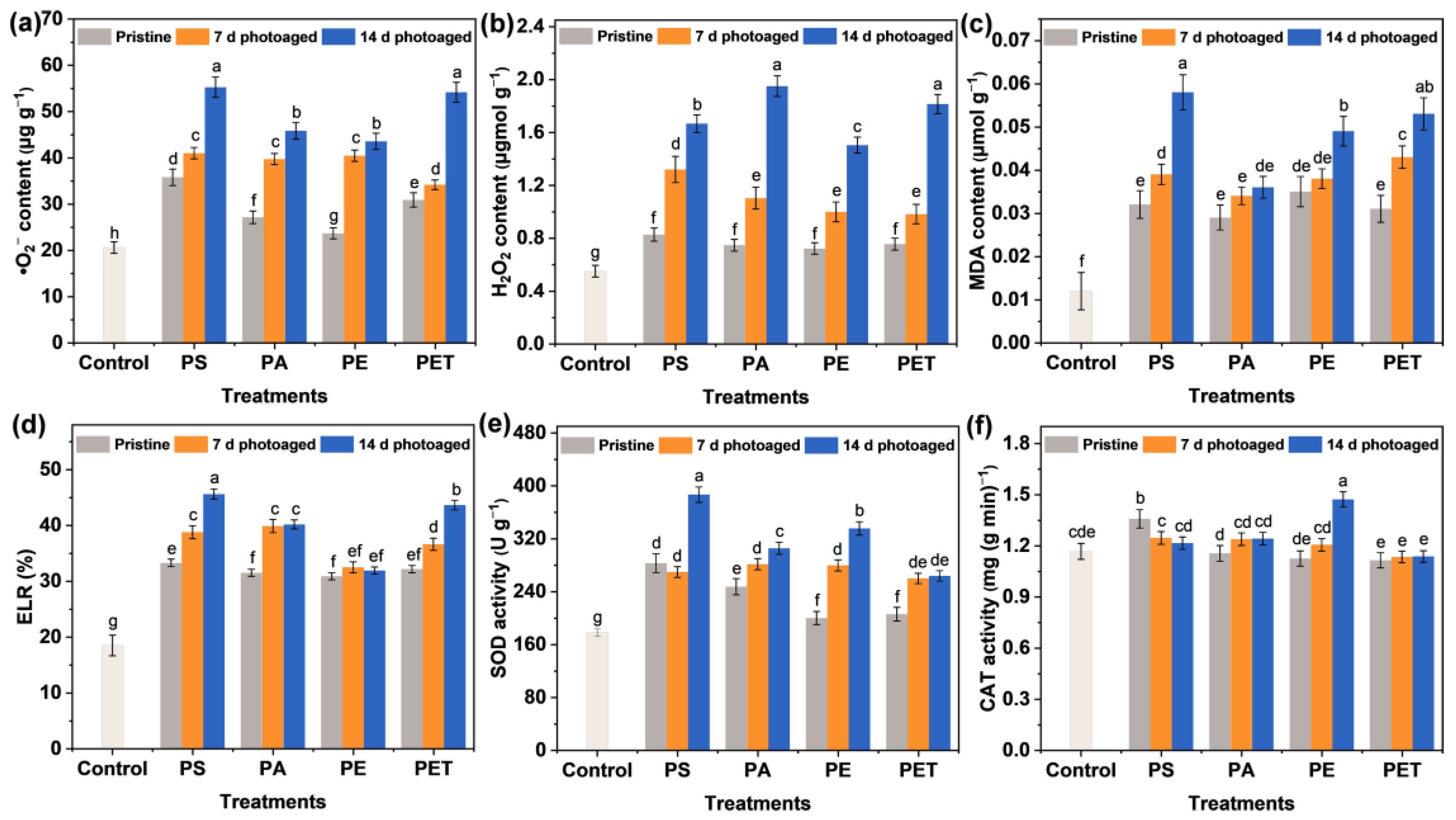
3.5. Oxidative Stress Induced by Photoaging MPs
3.6. Activation and Regulation of the Antioxidant System
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, C.; Zhang, B.; Gu, C.; Shen, C.; Yin, S.; Aamir, M.; Li, F. Are we underestimating the sources of microplastic pollution in terrestrial environment? J. Hazard. Mater. 2020, 400, 123228. [Google Scholar] [CrossRef] [PubMed]
- Akdogan, Z.; Guven, B. Microplastics in the environment: A critical review of current understanding and identification of future research needs. Environ. Pollut. 2019, 254, 113011. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Huang, J.; Zheng, Y.; Yang, Y.; Zhang, Y.; He, F.; Chen, H.; Quan, G.; Yan, J.; Li, T.; et al. Environmental occurrences, fate, and impacts of microplastics. Ecotox. Environ. Safe 2019, 184, 109612. [Google Scholar] [CrossRef]
- Revell, L.E.; Kuma, P.; Le Ru, E.C.; Somerville, W.R.C.; Gaw, S. Direct radiative effects of airborne microplastics. Nature 2021, 598, 462–467. [Google Scholar] [CrossRef]
- Horton, A.A.; Walton, A.; Spurgeon, D.J.; Lahive, E.; Svendsen, C. Microplastics in freshwater and terrestrial environments: Evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci. Total Environ. 2017, 586, 127–141. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Cui, Q.; Chen, L.; Zhu, X.; Zhao, S.; Duan, C.; Zhang, X.; Song, D.; Fang, L. A critical review of microplastics in the soil-plant system: Distribution, uptake, phytotoxicity and prevention. J. Hazard. Mater. 2022, 424, 127750. [Google Scholar] [CrossRef] [PubMed]
- Koelmans, A.A.; Mohamed Nor, N.H.; Hermsen, E.; Kooi, M.; Mintenig, S.M.; De France, J. Microplastics in freshwaters and drinking water: Critical review and assessment of data quality. Water Res. 2019, 155, 410–422. [Google Scholar] [CrossRef]
- Li, C.; Busquets, R.; Campos, L.C. Assessment of microplastics in freshwater systems: A review. Sci. Total Environ. 2020, 707, 135578. [Google Scholar] [CrossRef]
- Zhou, B.; Wang, J.; Zhang, H.; Shi, H.; Fei, Y.; Huang, S.; Tong, Y.; Wen, D.; Luo, Y.; Barcelo, D. Microplastics in agricultural soils on the coastal plain of Hangzhou Bay, east China: Multiple sources other than plastic mulching film. J. Hazard. Mater. 2020, 388, 121814. [Google Scholar] [CrossRef]
- Di, M.; Liu, X.; Wang, W.; Wang, J. Manuscript prepared for submission to environmental toxicology and pharmacology pollution in drinking water source areas: Microplastics in the Danjiangkou Reservoir, China. Environ. Toxicol. Pharmacol. 2019, 65, 82–89. [Google Scholar] [CrossRef] [PubMed]
- de Souza Machado, A.A.; Lau, C.W.; Kloas, W.; Bergmann, J.; Bachelier, J.B.; Faltin, E.; Becker, R.; Gorlich, A.S.; Rillig, M.C. Microplastics can changesoil properties and affect plant performance. Environ. Sci. Technol. 2019, 53, 6044–6052. [Google Scholar] [CrossRef] [Green Version]
- Pignattelli, S.; Broccoli, A.; Renzi, M. Physiological responses of garden cress (Lepidium sativum) to different types of microplastics. Sci. Total Environ. 2020, 727, 138609. [Google Scholar] [CrossRef]
- Qi, Y.; Yang, X.; Pelaez, A.M.; Huerta Lwanga, E.; Beriot, N.; Gertsen, H.; Garbeva, P.; Geissen, V. Macro- and micro- plastics in soil-plant system: Effects of plastic mulch film residues on wheat (Triticum aestivum) growth. Sci. Total Environ. 2018, 645, 1048–1056. [Google Scholar] [CrossRef]
- Shi, R.; Liu, W.; Lian, Y.; Wang, Q.; Zeb, A.; Tang, J. Phytotoxicity of polystyrene, polyethylene and polypropylene microplastics on tomato (Lycopersicon esculentum L.). J. Environ. Manage. 2022, 317, 115441. [Google Scholar] [CrossRef]
- Bhagat, K.; Barrios, A.C.; Rajwade, K.; Kumar, A.; Oswald, J.; Apul, O.; Perreault, F. Aging of microplastics increases their adsorption affinity towards organic contaminants. Chemosphere 2022, 298, 134238. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.; Zhang, Q.; Fan, Z.; Qi, H.; Wang, Z.; Peng, L. Aged microplastics polyvinyl chloride interact with copper and cause oxidative stress towards microalgae Chlorella vulgaris. Aquat. Toxicol. 2019, 216, 105319. [Google Scholar] [CrossRef]
- Zhu, K.; Jia, H.; Zhao, S.; Xia, T.; Guo, X.; Wang, T.; Zhu, L. Formation of environmentally persistent free radicals on microplastics under light lrradiation. Environ. Sci. Technol. 2019, 53, 8177–8186. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Chen, H.; Liao, Y.; Ye, Z.; Li, M.; Klobucar, G. Ecotoxicity and genotoxicity of polystyrene microplastics on higher plant Vicia faba. Environ. Pollut. 2019, 250, 831–838. [Google Scholar] [CrossRef]
- Li, Z.; Li, R.; Li, Q.; Zhou, J.; Wang, G. Physiological response of cucumber (Cucumis sativus L.) leaves to polystyrene nanoplastics pollution. Chemosphere 2020, 255, 127041. [Google Scholar] [CrossRef] [PubMed]
- Maity, S.; Chatterjee, A.; Guchhait, R.; De, S.; Pramanick, K. Cytogenotoxic potential of a hazardous material, polystyrene microparticles on Allium cepa L. J. Hazard. Mater. 2020, 385, 121560. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sun, P.; Qu, G.; Jing, J.; Zhang, T.; Shi, H.; Zhao, Y. Insight into the characteristics and sorption behaviors of aged polystyrene microplastics through three type of accelerated oxidation processes. J. Hazard. Mater. 2021, 407, 124836. [Google Scholar] [CrossRef] [PubMed]
- Salam, A.; Khan, A.R.; Liu, L.; Yang, S.; Azhar, W.; Ulhassan, Z.; Zeeshan, M.; Wu, J.; Fan, X.; Gan, Y. Seed priming with zinc oxide nanoparticles downplayed ultrastructural damage and improved photosynthetic apparatus in maize under cobalt stress. J. Hazard. Mater. 2022, 423, 127021. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.-Y.; Li, Q.; Chen, H.; Liu, J.; Xing, S.-F.; Xu, M.; Yan, Z.; Song, C.; Wang, S.-G. Selenium nanoparticles ameliorate Brassica napus L. cadmium toxicity by inhibiting the respiratory burst and scavenging reactive oxygen species. J. Hazard. Mater. 2021, 417, 125900. [Google Scholar] [CrossRef]
- Zhu, K.; Jia, H.; Sun, Y.; Dai, Y.; Zhang, C.; Guo, X.; Wang, T.; Zhu, L. Enhanced cytotoxicity of photoaged phenol-formaldehyde resins microplastics: Combined effects of environmentally persistent free radicals, reactive oxygen species, and conjugated carbonyls. Environ. Int. 2020, 145, 106137. [Google Scholar] [CrossRef]
- Zhu, K.; Jia, H.; Sun, Y.; Dai, Y.; Zhang, C.; Guo, X.; Wang, T.; Zhu, L. Long-term phototransformation of microplastics under simulated sunlight irradiation in aquatic environments: Roles of reactive oxygen species. Water Res. 2020. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, T.; Tian, L.; Liu, X.; Qi, Z.; Ma, Y.; Ji, R.; Chen, W. Aging significantly affects mobility and contaminant-mobilizing ability of nanoplastics in Saturated Loamy sand. Environ. Sci. Technol. 2019, 53, 5805–5815. [Google Scholar] [CrossRef]
- Larue, C.; Sarret, G.; Castillo-Michel, H.; Pradas Del Real, A.E. A critical review on the impacts of nanoplastics and microplastics on aquatic and terrestrial photosynthetic organisms. Small 2021, 17, e2005834. [Google Scholar] [CrossRef]
- Potters, G.; Pasternak, T.P.; Guisez, Y.; Palme, K.J.; Jansen, M.A. Stress-induced morphogenic responses: Growing out of trouble? Trends Plant Sci. 2007, 12, 98–105. [Google Scholar] [CrossRef]
- Sorrentino, M.C.; Capozzi, F.; Amitrano, C.; De Tommaso, G.; Arena, C.; Iuliano, M.; Giordano, S.; Spagnuolo, V. Facing metal stress by multiple strategies: Morphophysiological responses of cardoon (Cynara cardunculus L.) grown in hydroponics. Environ. Sci. Pollut. Res. 2021, 28, 37616–37626. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.N.; Lado Ribeiro, A.R.; Silva, A.M.T.; Pereira, M.F.R. Can aged microplastics be transport vectors for organic micropollutants?—Sorption and phytotoxicity tests. Sci. Total Environ. 2022, 850, 158073. [Google Scholar] [CrossRef] [PubMed]
- Krahmer, J.; Ganpudi, A.; Abbas, A.; Romanowski, A.; Halliday, K.J. Phytochrome, carbon sensing, metabolism, and plant growth plasticity. Plant Physiol. 2018, 176, 1039–1048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheikh, F.R.; Jose-Santhi, J.; Kalia, D.; Singh, K.; Singh, R.K. Sugars as the regulators of dormancy and sprouting in geophytes. Ind. Crop. Prod. 2022, 189, 115817. [Google Scholar] [CrossRef]
- Ren, X.; Tang, J.; Liu, X.; Liu, Q. Effects of microplastics on greenhouse gas emissions and the microbial community in fertilized soil. Environ. Pollut. 2020, 256, 113347. [Google Scholar] [CrossRef]
- Hong, J.; Huang, X.; Wang, Z.; Luo, X.; Huang, S.; Zheng, Z. Combined toxic effects of enrofloxacin and microplastics on submerged plants and epiphytic biofilms in high nitrogen and phosphorus waters. Chemosphere 2022, 308, 136099. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Li, R.; Li, F.; Guo, X.; Xu, L.; Gan, L.; Yan, M.; Wang, J. Toxicity of nanoplastics to aquatic organisms: Genotoxicity, cytotoxicity, individual level and beyond individual level. J. Hazard. Mater. 2023, 443, 130266. [Google Scholar] [CrossRef] [PubMed]
- Fayazipour, D.; Deckert, J.; Akbari, G.; Soltani, E.; Chmielowska-Bąk, J. Mitochondria specific antioxidant, mitoTEMPO, modulates Cd uptake and oxidative response of soybean seedlings. Antioxidants 2022, 11, 2099. [Google Scholar] [CrossRef]
- Qureshi, M.K.; Gawroński, P.; Munir, S.; Jindal, S.; Kerchev, P. Hydrogen peroxide-induced stress acclimation in plants. Cell. Mol. Life Sci. 2022, 79, 129. [Google Scholar] [CrossRef]
- Li, S.; Wang, T.; Guo, J.; Dong, Y.; Wang, Z.; Gong, L.; Li, X. Polystyrene microplastics disturb the redox homeostasis, carbohydrate metabolism and phytohormone regulatory network in barley. J. Hazard. Mater. 2021, 415, 125614. [Google Scholar] [CrossRef] [PubMed]
- Khare, S.; Singh, N.B.; Niharika; Singh, A.; Amist, N.; Azim, Z.; Bano, C.; Yadav, V.; Yadav, R.K. Salinity-induced attenuation in secondary metabolites profile and herbicidal potential of Brassica nigra L. on Anagallis arvensis L. J. Plant Growth Regul. 2022, 42, 973–988. [Google Scholar] [CrossRef]
- Duan, J.; Li, Y.; Gao, J.; Cao, R.; Shang, E.; Zhang, W. ROS-mediated photoaging pathways of nano- and micro-plastic particles under UV irradiation. Water Res. 2022, 216, 118320. [Google Scholar] [CrossRef]
- Colzi, I.; Renna, L.; Bianchi, E.; Castellani, M.B.; Coppi, A.; Pignattelli, S.; Loppi, S.; Gonnelli, C. Impact of microplastics on growth, photosynthesis and essential elements in Cucurbita pepo L. J. Hazard. Mater. 2022, 423, 127238. [Google Scholar] [CrossRef] [PubMed]

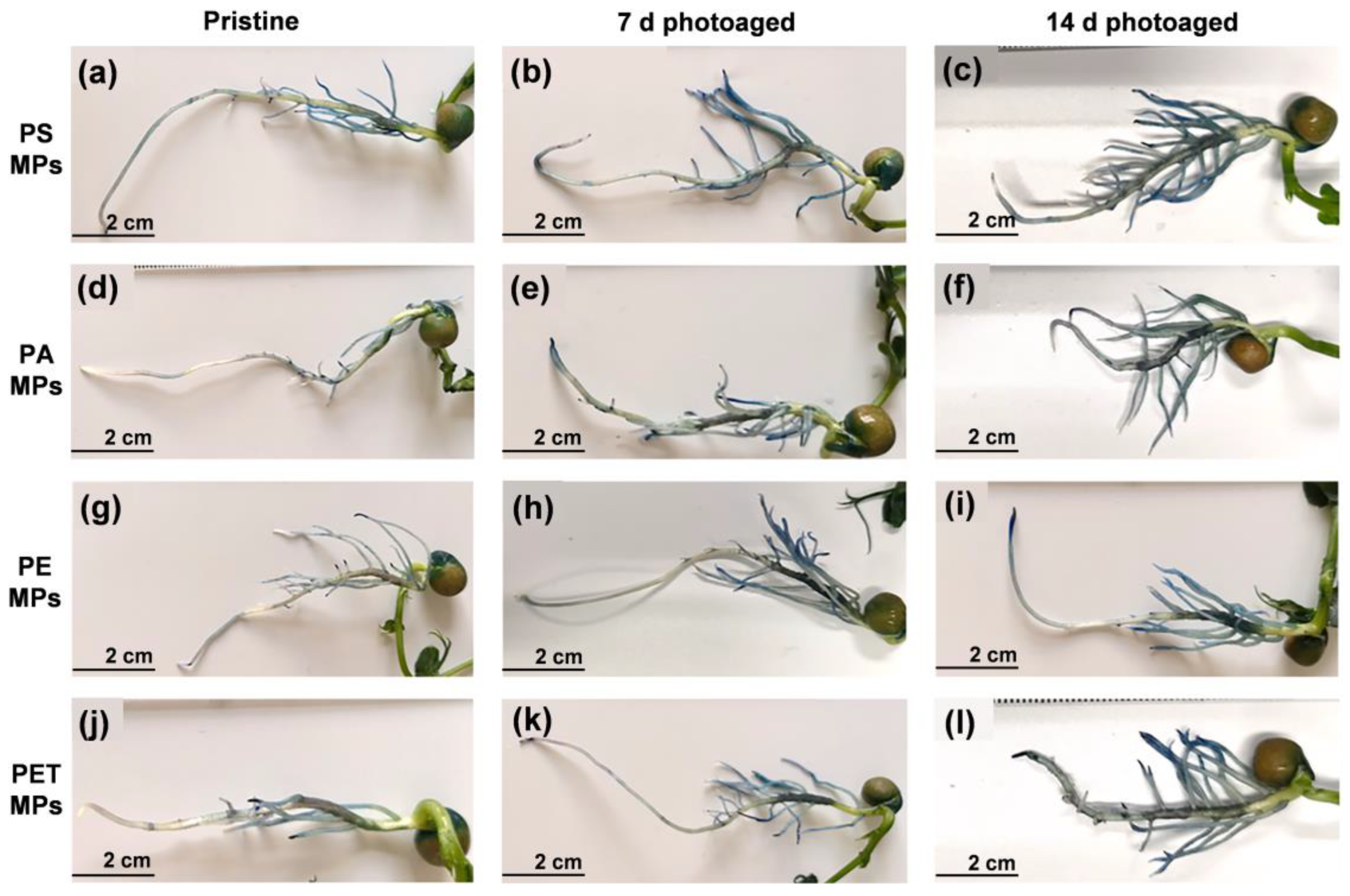
| Plant Elongation | Photoaging Time | Control | PS MPs | PA MPs | PE MPs | PET MPs |
|---|---|---|---|---|---|---|
| Roots (cm) | Pristine | 7.16 ± 0.36 def | 8.21 ± 0.33 ab | 7.96 ± 0.26 abcd | 8.57 ± 0.24 a | 8.15 ± 0.43 abc |
| 7 d Photoaged | 7.93 ± 0.40 abcd | 7.80 ± 0.32 abcd | 7.78 ± 0.39 abcde | 7.61 ± 0.35 bcdef | ||
| 14 d Photoaged | 7.38 ± 0.37 cdef | 6.95 ± 0.31 f | 7.35 ± 0.32 cdef | 7.08 ± 0.33 ef | ||
| Stems (cm) | Pristine | 5.42 ± 0.42 bc | 5.34 ± 0.36 c | 5.40 ± 0.38 c | 5.42 ± 0.31 bc | 5.63 ± 0.38 abc |
| 7 d Photoaged | 5.37 ± 0.40 c | 5.29 ± 0.41 c | 5.58 ± 0.42 abc | 6.15 ± 0.41 ab | ||
| 14 d Photoaged | 5.76 ± 0.49 c | 6.17 ± 0.45 a | 5.79 ± 0.52 abc | 6.23 ± 0.48 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, M.; Liu, Y.; Wang, H.; Weng, Y.; Gong, D.; Bai, X. Physiological Toxicity and Antioxidant Mechanism of Photoaging Microplastics on Pisum sativum L. Seedlings. Toxics 2023, 11, 242. https://doi.org/10.3390/toxics11030242
Kang M, Liu Y, Wang H, Weng Y, Gong D, Bai X. Physiological Toxicity and Antioxidant Mechanism of Photoaging Microplastics on Pisum sativum L. Seedlings. Toxics. 2023; 11(3):242. https://doi.org/10.3390/toxics11030242
Chicago/Turabian StyleKang, Mengen, Yi Liu, Haoke Wang, Yuzhu Weng, Dongqing Gong, and Xue Bai. 2023. "Physiological Toxicity and Antioxidant Mechanism of Photoaging Microplastics on Pisum sativum L. Seedlings" Toxics 11, no. 3: 242. https://doi.org/10.3390/toxics11030242
APA StyleKang, M., Liu, Y., Wang, H., Weng, Y., Gong, D., & Bai, X. (2023). Physiological Toxicity and Antioxidant Mechanism of Photoaging Microplastics on Pisum sativum L. Seedlings. Toxics, 11(3), 242. https://doi.org/10.3390/toxics11030242









