Platelet Rich Plasma and Adipose-Derived Mesenchymal Stem Cells Mitigate Methotrexate-Induced Nephrotoxicity in Rat via Nrf2/Pparγ/HO-1 and NF-Κb/Keap1/Caspase-3 Signaling Pathways: Oxidative Stress and Apoptosis Interplay
Abstract
:1. Introduction
2. Materials and Methods
2.1. Methotrexate
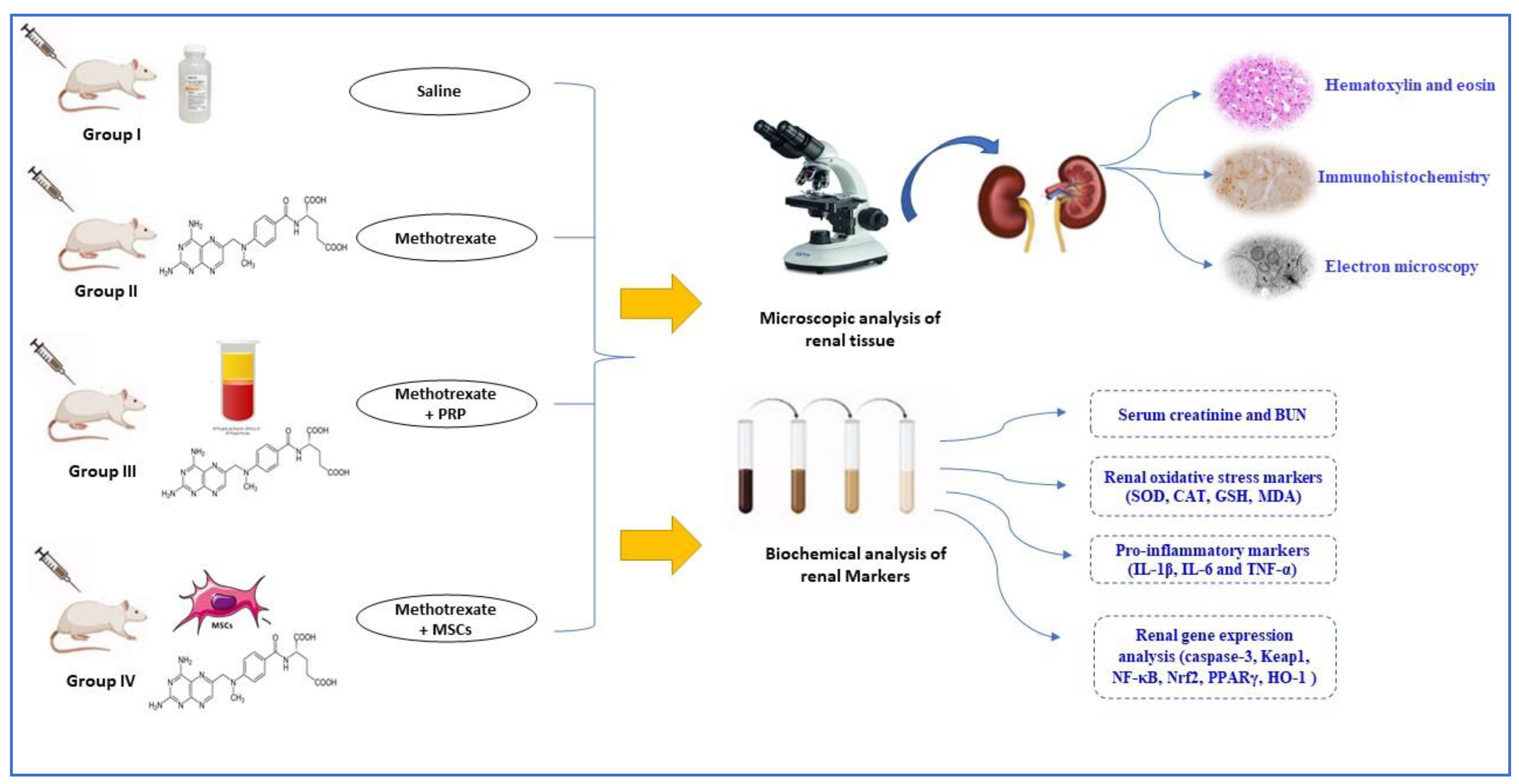
2.2. Experimental Design
- Group I control-group rats received PBS equivalent to the amount of other injections.
- Group IV (MTX + MSC): the animals were subjected to MTX-induced nephrotoxicity (as group II) and received 3 × 106 AD-MSCs 2 days after the last dose of MTX and repeated 6 days after.
2.3. Induction of MTX Nephrotoxicity
2.4. Isolation of AD-MSCs
2.5. Flow Cytometric Phenotypic Analysis of the Isolated Cells
2.6. Preparation of PRP
2.7. Administration of PRP and AD-MSCs Transplantation
2.8. Acquisition of Specimen and Histopathological Examination
2.9. Immunohistochemical Staining
2.10. Morphometric Examination of Renal Sections
- Mesangial index in renal cortex stained with PAS X 400. The mesangial matrix area was defined as the PAS–positive area within the tuft area. So, it could be calculated as the ratio of mesangial matrix area divided by the tuft area [29].
- Relative glomerular and interstitial fibrosis area in Masson-stained sections X 400 [36].
- Optical density of Casp-3 and iNOS immunohistochemically stained sections. After subtracting the background noise, six non overlapping fields from each sample were used to calculate the average positive Casp-3 and iNOS immunoactivity [37].
2.11. Transmission Electron Microscopy
2.12. Assessment of Renal Index (Kidney Weight/Body Weight Ratio)
2.13. Biochemical Assay (Urine Collection and Analysis of Serum Creatinine and BUN)
2.14. Assessment of the Oxidative Stress Markers in the Renal Tissue’s Homogenates
2.15. Biochemical Assay of the Pro-Inflammatory Markers
2.16. The Analysis of the Expression of Renal Genes
2.17. Statistical Analyses
3. Results
3.1. Results of Biochemical Assay and Renal Function
3.2. Assessment of Renal Index
3.3. Morphological and Phenotypic Characterization of the Isolated AD-MSCs
3.4. Histopathological Results
3.4.1. Renal Section Stained with H&E
3.4.2. Masson-Trichrome Stained Sections
3.4.3. Periodic Acid Schiff (PAS)-Stained Sections
3.4.4. Immunohistochemically Stained Sections
Expression of Casp-3
Expression of iNOS
3.5. Morphometric Image Analysis Results
3.5.1. Glomerular Mesangial Index
3.5.2. Relative Glomerular Fibrosis
3.5.3. Densitometry of Casp-3 Expression
3.5.4. Densitometry of iNOS Expression
3.6. Ultrastructure Assessment of Renal Tissue
3.7. AD-MSCs and PRP Alleviate Kidney Oxidative Stress in MTX-Induced Renal Damage
3.8. AD-MSCs and PRP Mitigate Renal Inflammatory Response in MTX-Induced Renal Injury
3.9. MTX and PRP Adjust the Expression Levels of Renal Gene in MTX-Intoxicated Rats
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lopez-Olivo, M.; Siddhanamatha, H.; Shea, B.; Tugwell, P.; A Wells, G.; E Suarez-Almazor, M. Methotrexate for treating rheumatoid arthritis. Cochrane Database Syst. Rev. 2014, 2014, CD000957. [Google Scholar] [CrossRef] [PubMed]
- Hazlewood, G.S.; Barnabe, C.; Tomlinson, G.; Marshall, D.; Devoe, D.; Bombardier, C. Methotrexate monotherapy and methotrexate combination therapy with traditional and biologic disease modifying antirheumatic drugs for rheumatoid ar-thritis: Abridged Cochrane systematic review and network meta-analysis. BMJ 2016, 353, i1777. [Google Scholar] [CrossRef] [PubMed]
- Salliot, C.; van der Heijde, D. Long-term safety of methotrexate monotherapy in patients with rheumatoid arthritis: A sys-tematic literature research. Ann. Rheum. Dis. 2009, 68, 1100–1104. [Google Scholar] [CrossRef] [PubMed]
- Conway, R.; Low, C.; Coughlan, R.J.; O’Donnell, M.; Carey, J. Risk of liver injury among methotrexate users: A meta-analysis of randomised controlled trials. Semin. Arthritis Rheum. 2015, 45, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.; Ahmed, M.; Conway, R.; Carey, J.J. Risk of infection with methotrexate therapy in inflammatory diseases: A sys-tematic review and meta-analysis. J. Clin. Med. 2018, 8, 15. [Google Scholar] [CrossRef]
- Ramalanjaona, B.; Hevroni, G.; Cham, S.; Page, C.; Salifu, M.O.; McFarlane, S.I. Nephrotoxicity Associated with Low-dose Methotrexate and Outpatient Parenteral Microbial Therapy: A Case Report, Review of the Literature and Pathophysiologic Insights. Am. J. Med. Case Rep. 2020, 8, 400–404. [Google Scholar] [CrossRef]
- Gilani, S.T.A.; Khan, D.A.; Khan, F.A.; Ahmed, M. Adverse effects of low dose methotrexate in rheumatoid arthritis patients. J. Coll. Physicians Surg. Pak. 2012, 22, 101–104. [Google Scholar]
- Koźmiński, P.; Halik, P.K.; Chesori, R.; Gniazdowska, E. Overview of Dual-Acting Drug Methotrexate in Different Neurological Diseases, Autoimmune Pathologies and Cancers. Int. J. Mol. Sci. 2020, 21, 3483. [Google Scholar] [CrossRef]
- Widemann, B.C.; Adamson, P.C. Understanding and Managing Methotrexate Nephrotoxicity. Oncol. 2006, 11, 694–703. [Google Scholar] [CrossRef]
- Widemann, B.C.; Balis, F.; Kim, A.; Boron, M.; Jayaprakash, N.; Shalabi, A.; O’Brien, M.; Eby, M.; Cole, D.E.; Murphy, R.F.; et al. Glucarpidase, Leucovorin, and Thymidine for High-Dose Methotrexate-Induced Renal Dysfunction: Clinical and Pharmacologic Factors Affecting Outcome. J. Clin. Oncol. 2010, 28, 3979–3986. [Google Scholar] [CrossRef]
- Scott, H.; Stefan, S.; Thomas, K.; Suzanne, W. 2013 Annual Meeting of the North American Congress of Clinical Toxicology (NACCT). Clin. Toxicol. 2013, 51, 575–724. [Google Scholar]
- Howard, S.C.; McCormick, J.; Pui, C.-H.; Buddington, R.K.; Harvey, R.D. Preventing and Managing Toxicities of High-Dose Methotrexate. Oncologist 2016, 21, 1471–1482. [Google Scholar] [CrossRef] [PubMed]
- Hassanein, E.H.M.; Shalkami, A.S.; Khalaf, M.M.; Mohamed, W.R.; Hemeida, R.A.M. The impact of Keap1/Nrf2, P(38)MAPK/NF-κB and Bax/Bcl2/caspase-3 signaling pathways in the protective effects of berberine against methotrex-ate-induced nephrotoxicity. Biomed. Pharmacother. 2019, 109, 47–56. [Google Scholar] [CrossRef]
- Alves, R.; Grimalt, R. Randomized placebo-controlled, double-blind, half-head study to assess the efficacy of platelet-rich plasma on the treatment of androgenetic alopecia. Dermatol. Surg. 2016, 42, 491–497. [Google Scholar] [CrossRef]
- Lynch, M.D.; Bashir, S. Applications of platelet-rich plasma in dermatology: A critical appraisal of the literature. J. Dermatol. Treat. 2016, 27, 285–289. [Google Scholar] [CrossRef]
- Alves, R.; Grimalt, R. A Review of Platelet-Rich Plasma: History, Biology, Mechanism of Action, and Classification. Ski. Appendage Disord. 2018, 4, 18–24. [Google Scholar] [CrossRef]
- Marx, R.E. Platelet-Rich Plasma (PRP): What Is PRP and What Is Not PRP? Implant. Dent. 2001, 10, 225–228. [Google Scholar] [CrossRef]
- Eppley, B.L.; Woodell, J.E.; Higgins, J. Platelet Quantification and Growth Factor Analysis from Platelet-Rich Plasma: Implications for Wound Healing. Plast. Reconstr. Surg. 2004, 114, 1502–1508. [Google Scholar] [CrossRef]
- Carlson, N.E.; Roach, R.B., Jr. Platelet-rich plasma: Clinical applications in dentistry. J. Am. Dent. Assoc. 2002, 133, 1383–1386. [Google Scholar] [CrossRef]
- Moghadam, A.; Khozani, T.T.; Mafi, A.; Namavar, M.R.; Dehghani, F. Effects of Platelet-Rich Plasma on Kidney Regeneration in Gentamicin-Induced Nephrotoxicity. J. Korean Med. Sci. 2017, 32, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Keshk, W.A.; Zahran, S.M. Mechanistic role of cAMP and hepatocyte growth factor signaling in thioacetamide-induced nephrotoxicity: Unraveling the role of platelet rich plasma. Biomed. Pharmacother 2019, 109, 1078–1084. [Google Scholar] [CrossRef]
- Uccelli, A.; Moretta, L.; Pistoia, V. Mesenchymal stem cells in health and disease. Nat. Rev. Immunol. 2008, 8, 726–736. [Google Scholar] [CrossRef]
- Liu, Z.-J.; Zhuge, Y.; Velazquez, O.C. Trafficking and differentiation of mesenchymal stem cells. J. Cell. Biochem. 2009, 106, 984–991. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Khalifa, A.M.; Mohamed, A.A.; Galhom, R.A.; Korayem, H.E.; El-Fadeal, N.M.A.; Tammam, A.A.-E.; Khalifa, M.M.; Elserafy, O.S.; Abdel-Karim, R.I. Bone-Marrow-Derived Mesenchymal Stem Cells, Their Conditioned Media, and Olive Leaf Extract Protect against Cisplatin-Induced Toxicity by Alleviating Oxidative Stress, Inflammation, and Apoptosis in Rats. Toxics 2022, 10, 526. [Google Scholar] [CrossRef] [PubMed]
- Perico, L.; Morigi, M.; Rota, C.; Breno, M.; Mele, C.; Noris, M.; Introna, M.; Capelli, C.; Longaretti, L.; Rottoli, D.; et al. Human mesenchymal stromal cells transplanted into mice stimulate renal tubular cells and enhance mitochondrial function. Nat. Commun. 2017, 8, 983. [Google Scholar] [CrossRef]
- Semedo, P.; Palasio, C.G.; Oliveira, C.D.; Feitoza, C.Q.; Gonçalves, G.M.; Cenedeze, M.A.; Wang, P.M.; Teixeira, V.P.; Reis, M.A.; Pacheco-Silva, A.; et al. Early modulation of inflammation by mesenchymal stem cell after acute kidney injury. Int. Immunopharmacol. 2009, 9, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Gad, A.M.; Hassan, W.A.; Fikry, E.M. Significant curative functions of the mesenchymal stem cells on methotrexate-induced kidney and liver injuries in rats. J. Biochem. Mol. Toxicol. 2017, 31, e21919. [Google Scholar] [CrossRef] [PubMed]
- E Weinblatt, M.; Maier, A.L.; A Fraser, P.; Coblyn, J.S. Longterm prospective study of methotrexate in rheumatoid arthritis: Conclusion after 132 months of therapy. J. Rheumatol. 1998, 25, 238–242. [Google Scholar]
- Yozai, K.; Shikata, K.; Sasaki, M.; Tone, A.; Ohga, S.; Usui, H.; Okada, S.; Wada, J.; Nagase, R.; Ogawa, D.; et al. Methotrexate Prevents Renal Injury in Experimental Diabetic Rats via Anti-Inflammatory Actions. J. Am. Soc. Nephrol. 2005, 16, 3326–3338. [Google Scholar] [CrossRef]
- Salem, N.; Helmi, N.; Assaf, N. Renoprotective Effect of Platelet-Rich Plasma on Cisplatin-Induced Nephrotoxicity in Rats. Oxidative Med. Cell. Longev. 2018, 2018, 1–10. [Google Scholar] [CrossRef]
- Hesami, Z.; Jamshidzadeh, A.; Ayatollahi, M.; Geramizadeh, B.; Farshad, O.; Vahdati, A. Effect of Platelet-Rich Plasma on CCl4-Induced Chronic Liver Injury in Male Rats. Int. J. Hepatol. 2014, 2014, 932930. [Google Scholar] [CrossRef] [PubMed]
- Megaloikonomos, P.D.; Panagopoulos, G.; Bami, M.; Igoumenou, V.G.; Dimopoulos, L.; Milonaki, A.; Kyriakidou, M.; Mitsiokapa, E.; Anastassopoulou, J.; Mavrogenis, A.F. Harvesting, Isolation and Differentiation of Rat Adipose-Derived Stem Cells. Curr. Pharm. Biotechnol. 2018, 19, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Bunnell, B.A.; Flaat, M.; Gagliardi, C.; Patel, B.; Ripoll, C. Adipose-derived stem cells: Isolation; expansion; differentiation. Methods 2008, 45, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Pazzini, J.M.; Nardi, A.B.D.; Huppes, R.R.; Gering, A.P.; Ferreira, M.G.; Silveira, C.P.; Luzzi, M.C.; Santos, R. Method to obtain platelet-rich plasma from rabbits (Oryctolagus cuniculus). Pesqui. Veterinária Bras. 2016, 36, 39–44. [Google Scholar] [CrossRef]
- Chung, B.H.; Lim, S.W.; Doh, K.C.; Piao, S.G.; Heo, S.B.; Yang, C.W. Human Adipose Tissue Derived Mesenchymal Stem Cells Aggravate Chronic Cyclosporin Nephrotoxicity by the Induction of Oxidative Stress. PLoS ONE 2013, 8, e59693. [Google Scholar] [CrossRef] [PubMed]
- Grobe, N.; Leiva, O.; Morris, M.; Elased, K.M. Loss of Prolyl Carboxypeptidase in Two-Kidney, One-Clip Goldblatt Hypertensive Mice. PLoS ONE 2015, 10, e0117899. [Google Scholar] [CrossRef]
- Sahin, H.; Yener, A.U.; Karaboga, I.; Sehitoglu, M.H.; Dogu, T.; Altinisik, H.B.; Altinisik, U.; Simsek, T. Protective effect of gel form of gastric gavage applicated aloe vera on ischemia reperfusion injury in renal and lung tissue. Cell. Mol. Biol. 2017, 63, 34–39. [Google Scholar] [CrossRef]
- Ohashi, R.; Shimizu, A.; Masuda, Y.; Kitamura, H.; Ishizaki, M.; Sugisaki, Y.; Yamanaka, N. Peritubular Capillary Regression during the Progression of Experimental Obstructive Nephropathy. J. Am. Soc. Nephrol. 2002, 13, 1795–1805. [Google Scholar] [CrossRef]
- Derakhshanfar, A.; Sadeghian, M.H.; Abbasabadi, N.; Imanian, M.H. Histopathologic and biochemical study of the effect of saffron extract on gentamicin-induced nephrotoxicity in rats. Comp. Clin. Pathol. 2015, 24, 1347–1351. [Google Scholar] [CrossRef]
- Beutler, E.; Duron, O.; Kelly, B.M. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 1963, 61, 882–888. [Google Scholar]
- Masayasu, M.; Hiroshi, Y. A simplified assay method of superoxide dismutase activity for clinical use. Clin. Chim. Acta 1979, 92, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in vitro. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1984; pp. 121–126. [Google Scholar]
- Kei, S. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin. Chim. Acta 1978, 90, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Andankar, P.; Shah, K.; Patki, V. A review of drug-induced renal injury. J. Pediatr. Crit. Care 2018, 5, 36. [Google Scholar] [CrossRef]
- Asci, H.; Ozmen, O.; Ellidag, H.Y.; Aydin, B.; Bas, E.; Yilmaz, N. The impact of gallic acid on the methotrexate-induced kidney damage in rats. J. Food Drug Anal. 2017, 25, 890–897. [Google Scholar] [CrossRef]
- Heidari, R.; Ahmadi, A.; Mohammadi, H.; Ommati, M.M.; Azarpira, N.; Niknahad, H. Mitochondrial dysfunction and oxida-tive stress are involved in the mechanism of methotrexate-induced renal injury and electrolytes imbalance. Biomed. Pharmacother. 2018, 107, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef]
- Ogrodnik, M.; Salmonowicz, H.; Gladyshev, V.N. Integrating cellular senescence with the concept of damage accumulation in aging: Relevance for clearance of senescent cells. Aging Cell 2019, 18, e12841. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Lin, W.; Liao, C.; Zhou, T. Nephroprotective Effect of Mesenchymal Stem Cell-Based Therapy of Kidney Disease In-duced by Toxicants. Stem Cells Int. 2020, 2020, 8819757. [Google Scholar] [CrossRef]
- Chen, Q.M.; Maltagliati, A.J. Nrf2 at the heart of oxidative stress and cardiac protection. Physiol. Genom. 2018, 50, 77–97. [Google Scholar] [CrossRef]
- Shen, Y.; Liu, X.; Shi, J.; Wu, X. Involvement of Nrf2 in myocardial ischemia and reperfusion injury. Int. J. Biol. Macromol. 2019, 125, 496–502. [Google Scholar] [CrossRef]
- Bellezza, I.; Giambanco, I.; Minelli, A.; Donato, R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2018, 1865, 721–733. [Google Scholar] [CrossRef]
- Yamamoto, M.; Kensler, T.W.; Motohashi, H. The KEAP1-NRF2 System: A Thiol-Based Sensor-Effector Apparatus for Main-taining Redox Homeostasis. Physiol. Rev. 2018, 98, 1169–1203. [Google Scholar] [CrossRef]
- Lian, F.Z.; Cheng, P.; Ruan, C.S.; Ling, X.X.; Wang, X.Y.; Pan, M.; Chen, M.L.; Shen, A.Z.; Gao, S. Xin-Ji-Er-Kang ameliorates kidney injury following myocardial infarction by inhibiting oxidative stress via Nrf2/HO-1 pathway in rats. Biomed. Pharmacother. 2019, 117, 109124. [Google Scholar] [CrossRef]
- Ding, M.; Li, M.; Zhang, E.M.; Yang, H.L. FULLEROL alleviates myocardial ischemia-reperfusion injury by reducing in-flammation and oxidative stress in cardiomyocytes via activating the Nrf2/HO-1 signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 9665–9674. [Google Scholar] [PubMed]
- Cheng, J.; Zhang, M.; Cheng, S.; Li, F.; Zhang, B.; Sun, X.; Hu, H.; Chen, L.; Zhao, Z.; Hu, H.; et al. Low-dose alcohol ameliorated high fat diet-induced anxiety-related behavior via enhancing adiponectin expression and activating the Nrf2 pathway. Food Funct. 2021, 12, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Qiao, H.; Sai, X.; Gai, L.; Huang, G.; Chen, X.; Tu, X.; Ding, Z. Association between heme oxygenase 1 gene promoter poly-morphisms and susceptibility to coronary artery disease: A HuGE review and meta-analysis. Am. J. Epidemiology 2014, 179, 1039–1048. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.-C.; Ji, J.-A.; Jiang, Y.-L.; Chen, Z.-Y.; Yuan, Z.-W.; You, Q.-D.; Jiang, Z.-Y. An inhibitor of the Keap1-Nrf2 protein-protein interaction protects NCM460 colonic cells and alleviates experimental colitis. Sci. Rep. 2016, 6, 26585. [Google Scholar] [CrossRef]
- Li, A.; Zhang, X.; Luo, Q. Neohesperidin alleviated pathological damage and immunological imbalance in rat myocardial ischemia-reperfusion injury via inactivation of JNK and NF-κB p65. Biosci. Biotechnol. Biochem. 2021, 85, 251–261. [Google Scholar] [CrossRef]
- Andia, I.; Abate, M. Platelet-rich plasma: Combinational treatment modalities for musculoskeletal conditions. Front. Med. 2018, 12, 139–152. [Google Scholar] [CrossRef]
- Nguyen, R.T.; Borg-Stein, J.; McInnis, K. Applications of platelet-rich plasma in musculoskeletal and sports medicine: An evi-dence-based approach. PM R J. Inj. Funct. Rehabil. 2011, 3, 226–250. [Google Scholar] [CrossRef]
- Qureshi, A.H.; Chaoji, V.; Maiguel, D.; Faridi, M.H.; Barth, C.J.; Salem, S.M.; Singhal, M.; Stoub, D.; Krastins, B.; Ogihara, M.; et al. Proteomic and Phospho-Proteomic Profile of Human Platelets in Basal, Resting State: Insights into Integrin Signaling. PLoS ONE 2009, 4, e7627. [Google Scholar] [CrossRef]
- Alsousou, J.; Ali, A.; Willett, K.; Harrison, P. The role of platelet-rich plasma in tissue regeneration. Platelets 2012, 24, 173–182. [Google Scholar] [CrossRef]
- Foster, T.E.; Puskas, B.L.; Mandelbaum, B.R.; Gerhardt, M.B.; Rodeo, S.A. Platelet-rich plasma: From basic science to clinical applications. Am. J. Sport. Med. 2009, 37, 2259–2272. [Google Scholar] [CrossRef] [PubMed]
- Madrazo, J.A.; Kelly, D.P. The PPAR trio: Regulators of myocardial energy metabolism in health and disease. J. Mol. Cell. Cardiol. 2008, 44, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Shi, M.; Wang, Y.; Liu, J. PPARγ and Its Agonists in Chronic Kidney Disease. Int. J. Nephrol. 2020, 2020, 2917474. [Google Scholar] [CrossRef]
- Borrione, P.; Gianfrancesco, A.D.; Pereira, M.T.; Pigozzi, F. Platelet-rich plasma in muscle healing. Am. J. Phys. Med. Rehabil. 2010, 89, 854–861. [Google Scholar] [CrossRef]
- Lubkowska, A.; Dolegowska, B.; Banfi, G. Growth factor content in PRP and their applicability in medicine. J. Biol. Regul. Homeost. Agents 2013, 26, 3S–22S. [Google Scholar]
- El-Sharkawy, H.; Kantarci, A.; Deady, J.; Hasturk, H.; Liu, H.; Alshahat, M.; Van Dyke, T.E. Platelet-Rich Plasma: Growth Factors and Pro- and Anti-Inflammatory Properties. J. Periodontol. 2007, 78, 661–669. [Google Scholar] [CrossRef]
- Moussa, M.; Lajeunesse, D.; Hilal, G.; El Atat, O.; Haykal, G.; Serhal, R.; Chalhoub, A.; Khalil, C.; Alaaeddine, N. Platelet rich plasma (PRP) induces chondroprotection via increasing autophagy, anti-inflammatory markers, and decreasing apoptosis in human osteoarthritic cartilage. Exp. Cell Res. 2017, 352, 146–156. [Google Scholar] [CrossRef]
- Sadeghinia, A.; Davaran, S.; Salehi, R.; Jamalpoor, Z. Nano-hydroxy apatite/chitosan/gelatin scaffolds enriched by a combi-nation of platelet-rich plasma and fibrin glue enhance proliferation and differentiation of seeded human dental pulp stem cells. Biomed. Pharmacother. 2019, 109, 1924–1931. [Google Scholar] [CrossRef] [PubMed]
- Sundman, E.A.; Cole, B.J.; Karas, V.; Della Valle, C.; Tetreault, M.W.; Mohammed, H.O.; Fortier, L.A. The Anti-inflammatory and Matrix Restorative Mechanisms of Platelet-Rich Plasma in Osteoarthritis. Am. J. Sports Med. 2014, 42, 35–41. [Google Scholar] [CrossRef] [PubMed]
- El-Twab, S.M.A.; Hussein, O.E.; Hozayen, W.G.; Bin-Jumah, M.; Mahmoud, A. MChicoric acid prevents methotrexate-induced kidney injury by suppressing NF-κB/NLRP3 inflammasome activation and up-regulating Nrf2/ARE/HO-1 signaling. Inflamm. Res. Off. J. Eur. Histamine Res. Soc. 2019, 68, 511–523. [Google Scholar]
- Mahmoud, A.M.; Hussein, O.E.; Hozayen, W.G.; Bin-Jumah, M.; El-Twab, S.M.A. Ferulic acid prevents oxidative stress, in-flammation, and liver injury via upregulation of Nrf2/HO-1 signaling in methotrexate-induced rats. Environ. Sci. Pollut. Res. Int. 2020, 27, 7910–7921. [Google Scholar] [CrossRef] [PubMed]
- Rahnavard, M.; Hassanpour, M.; Ahmadi, M.; Heidarzadeh, M.; Amini, H.; Javanmard, M.Z.; Nouri, M.; Rahbarghazi, R.; Safaie, N. Curcumin ameliorated myocardial infarction by inhibition of cardiotoxicity in the rat model. J. Cell. Biochem. 2019, 120, 11965–11972. [Google Scholar] [CrossRef]
- Zhang, W.; Li, Y.; Ge, Z. Cardiaprotective effect of crocetin by attenuating apoptosis in isoproterenol induced myocardial infarction rat model. Biomed. Pharmacother. 2017, 93, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Liu, X.; Li, Z.; Huang, Q.; Li, F.; Li, C.-Y. Caspase-3 regulates the migration, invasion and metastasis of colon cancer cells. Int. J. Cancer 2018, 143, 921–930. [Google Scholar] [CrossRef]
- Akhigbe, R.; Ajayi, A. Testicular toxicity following chronic codeine administration is via oxidative DNA damage and up-regulation of NO/TNF-α and caspase 3 activities. PLoS ONE 2020, 15, e0224052. [Google Scholar] [CrossRef]
- Yang, C.-C.; Chen, Y.-T.; Wallace, C.G.; Chen, K.-H.; Cheng, B.-C.; Sung, P.-H.; Li, Y.-C.; Ko, S.-F.; Chang, H.-W.; Yip, H.-K. Early administration of empagliflozin preserved heart function in cardiorenal syndrome in rat. Biomed. Pharmacother. 2019, 109, 658–670. [Google Scholar] [CrossRef]
- Zhang, F.-X.; Yuan, Y.-L.; Cui, S.-S.; Li, M.; Tan, X.; Qiu, Z.-C.; Li, R.-M. Dissection of the potential pharmacological function of neohesperidin dihydrochalcone–a food additive–by in vivo substances profiling and network pharmacology. Food Funct. 2021, 12, 4325–4336. [Google Scholar] [CrossRef]
- Seo, H.Y.; Lee, S.H.; Lee, J.H.; Hwang, J.S.; Kim, M.K.; Jang, B.K. Kahweol activates the Nrf2/HO-1 pathway by decreasing Keap1 expression independently of p62 and autophagy pathways. PLoS ONE 2020, 15, e0240478. [Google Scholar] [CrossRef] [PubMed]
- Hacioglu, C.; Kar, F.; Kacar, S.; Sahinturk, V.; Kanbak, G. Bexarotene inhibits cell proliferation by inducing oxidative stress, DNA damage and apoptosis via PPARγ/NF-κB signaling pathway in C6 glioma cells. Med. Oncol. 2021, 38, 31. [Google Scholar] [CrossRef]
- Gendy, A.M.; Amin, M.M.; Al-Mokaddem, A.K.; Ellah, M.F.A. Cilostazol mitigates mesenteric ischemia/reperfusion-induced lung lesion: Contribution of PPAR-γ, NF-κB, and STAT3 crosstalk. Life Sci. 2021, 266, 118882. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Kuse, Y.; Inoue, Y.; Nakamura, S.; Hara, H.; Shimazawa, M. Transient acceleration of autophagic degradation by pharmacological Nrf2 activation is important for retinal pigment epithelium cell survival. Redox Biol. 2018, 19, 354–363. [Google Scholar] [CrossRef]
- Hussein, O.E.; Hozayen, W.G.; Bin-Jumah, M.N.; Germoush, M.O.; El-Twab, S.M.A.; Mahmoud, A.M. Chicoric acid prevents methotrexate hepatotoxicity via attenuation of oxidative stress and inflammation and up-regulation of PPARγ and Nrf2/HO-1 signaling. Environ. Sci. Pollut. Res. Int. 2020, 27, 20725–20735. [Google Scholar] [CrossRef]
- Li, C.; Zhang, J.; Xue, M.; Li, X.; Han, F.; Liu, X.; Xu, L.; Lu, Y.; Cheng, Y.; Li, T.; et al. SGLT2 inhibition with empagliflozin attenuates myocardial oxidative stress and fibrosis in diabetic mice heart. Cardiovasc. Diabetol. 2019, 18, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Czapla, J.; Matuszczak, S.; Kulik, K.; Wiśniewska, E.; Pilny, E.; Jarosz-Biej, M.; Smolarczyk, R.; Sirek, T.; Zembala, M.O.; Zembala, M.; et al. The effect of culture media on large-scale expansion and characteristic of adipose tis-sue-derived mesenchymal stromal cells. Stem Cell Res. Ther. 2019, 10, 235. [Google Scholar] [CrossRef]
- El-Agawy, M.S.E.-D.; Badawy, A.M.M.; Rabei, M.R.; Elshaer, M.M.A.; El Nashar, E.M.; Alghamdi, M.A.; Alshehri, M.A.; Elsayed, H.R.H. Methotrexate-Induced Alteration of Renal Aquaporins 1 and 2, Oxidative Stress and Tubular Apoptosis Can Be Attenuated by Omega-3 Fatty Acids Supplementation. Int. J. Mol. Sci. 2022, 23, 12794. [Google Scholar] [CrossRef]
- Dabak, D.O.; Kocaman, N. Effects of silymarin on methotrexate-induced nephrotoxicity in rats. Ren. Fail. 2015, 37, 734–739. [Google Scholar] [CrossRef]
- Elsawy, H.; Alzahrani, A.M.; Alfwuaires, M.; Abdel-Moneim, A.M.; Khalil, M. Nephroprotective effect of naringin in metho-trexate induced renal toxicity in male rats. Biomed. Pharmacother. 2021, 143, 112180. [Google Scholar] [CrossRef]
- Wei, X.; Wu, Y.; Tang, H.; Wang, B.; Wang, Y.; Sun, W.; Asenso, J.; Xiao, F.; Wang, C. CP-25 ameliorates methotrexate induced nephrotoxicity via improving renal apoptosis and methotrexate excretion. J. Pharmacol. Sci. 2021, 146, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Babiak, R.M.V.; Campello, A.P.; Carnieri, E.G.; Oliveira, M.B.M. Methotrexate: Pentose cycle and oxidative stress. Cell Biochem. Funct. Cell. Biochem. Its Modul. Act. Agents Dis. 1998, 16, 283–293. [Google Scholar] [CrossRef]
- Vardi, N.; Parlakpinar, H.; Ates, B.; Cetin, A.; Otlu, A. The protective effects of Prunus armeniaca L (apricot) against metho-trexate-induced oxidative damage and apoptosis in rat kidney. J. Physiol. Biochem. 2013, 69, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Karbaat, L.; Wu, L.; Leijten, J.; Both, S.K.; Karperien, M. Trophic Effects of Mesenchymal Stem Cells in Tissue Regeneration. Tissue Eng. Part B Rev. 2017, 23, 515–528. [Google Scholar] [CrossRef]
- Ankrum, J.; Karp, J.M. Mesenchymal stem cell therapy: Two steps forward, one step back. Trends Mol. Med. 2010, 16, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.M.; Fouad, F.E. Possible protective effect of platelet-rich plasma on a model of cisplatin-induced nephrotoxicity in rats: A light and transmission electron microscopic study. J. Cell. Physiol. 2019, 234, 10470–10480. [Google Scholar] [CrossRef]
- Afifi, N.M.; Reyad, O.N. Role of mesenchymal stem cell therapy in restoring ovarian function in a rat model of chemothera-py-induced ovarian failure: A histological and immunohistochemical study. Egypt. J. Histol. 2013, 36, 114–126. [Google Scholar] [CrossRef]
- Choi, M.R.; Kim, H.Y.; Park, J.-Y.; Lee, T.Y.; Baik, C.S.; Chai, Y.G.; Jung, K.H.; Park, K.S.; Roh, W.; Kim, K.S.; et al. Selection of optimal passage of bone marrow-derived mesenchymal stem cells for stem cell therapy in patients with amyotrophic lateral sclerosis. Neurosci. Lett. 2010, 472, 94–98. [Google Scholar] [CrossRef]
- Ebrahimi, R.; Sepand, M.R.; Seyednejad, S.A.; Omidi, A.; Akbariani, M.; Gholami, M.; Sabzevari, O. Ellagic acid reduces methotrexate-induced apoptosis and mitochondrial dysfunction via up-regulating Nrf2 expression and inhibiting the IĸBα/NFĸB in rats. DARU J. Pharm. Sci. 2019, 27, 721–733. [Google Scholar] [CrossRef]
- Morsy, M.A.; Abdel-Aziz, A.M.; Abdel-Hafez, S.M.N.; Venugopala, K.N.; Nair, A.B.; Abdel-Gaber, S.A. The Possible Contri-bution of P-Glycoprotein in the Protective Effect of Paeonol against Methotrexate-Induced Testicular Injury in Rats. Pharmaceuticals 2020, 13, 223. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.; El-Sherbeney, S.; Ahmed, R. The Possible Ameliorative effect of Zafirlukast on Renal Toxicity Induced by Methotrexate in Experimental Animals. Ain Shams J. Forensic Med. Clin. Toxicol. 2016, 27, 92–104. [Google Scholar] [CrossRef]















| Gene | Primer Sequence |
|---|---|
| Caspase-3 | F5′-GGTATTGAGACAGACAGTGG-3′ R:5′-CATGGGATCTGTTTCTTTGC-3′ |
| Keap1 | F:5′-GGATGGTAACCGAACCTTCA-3′ R:5′-AAGCCCGTTGGTGAACATAG-3′ |
| NF-κB | F:5′-CTGGCAGCTCTTCTCAAAGC-3′ R:5′-CCAGGTCATAGAGAGGCTCAA -3′ |
| Nrf2 | F:5′-CCCATTGAGGGCTGTGAT-3′ R:5′-TTGGCTGTGCTTTAGGTC-3′ |
| PPARγ | F:5′-GGACGCTGAAGAAGAGACCTG-3′ R:5′-CCGGGTCCTGTCTGAGTATG-3′ |
| HO-1 | F:5′-GCATGTCCCAGGATTTGTCC-3′ R:5′-GGTTCTGCTTGTTTCGCTCT-3′ |
| β-actin | F:5′-GGGAAATCGTGCGTGACATT-3′ R:5′-GCGGCAGTGGCCATCTC-3′ |
| Parameters | Control | MTX | MTX + PRP | MTX + MSC |
|---|---|---|---|---|
| MDA | 43.5 ± 4.04 | 90.33 ± 4.84 a | 55.50 ± 4.8 b | 49.00 ± 5.22 b |
| SOD | 279.2 ± 6.01 | 220.83 ± 12.88 a | 266.17 ± 5.95 b | 276.83 ± 5.23 b |
| GSH | 6.8 ± 0.36 | 4.05 ± 0.15 a | 6.25 ± 0.29 b | 6.43 ± 0.34 b |
| CAT | 329. ± 10.98 | 214.67 ± 8.24 a | 308.83 ± 8.45 b | 326.83 ± 5.34 bc |
| TAC | 90.3 ± 5.89 | 46.7 ± 4.89 a | 74.2 ± 5.78 ab | 83.2 ± 3.49 b |
| Parameters | Control | MTX | MTX + PRP | MTX + MSC |
|---|---|---|---|---|
| IL-1β | 14.9 ± 0.83 | 32.3 ± 1.60 a | 17.8 ± 0.87 b | 16.0 ± 0.66 b |
| IL-6 | 99.2 ± 6.34 | 172.0 ± 5.55 a | 112.5 ± 10.11 b | 106.8 ± 10.55 b |
| TNF-α | 2364 ± 23.44 | 3280 ± 55.75 a | 2495 ± 57.11 b | 2424 ± 52.47 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wani, F.A.; Ibrahim, M.A.; Ameen, S.H.; Farage, A.E.; Ali, Z.A.-E.; Saleh, K.; Farag, M.M.; Sayeed, M.U.; Alruwaili, M.A.Y.; Alruwaili, A.H.F.; et al. Platelet Rich Plasma and Adipose-Derived Mesenchymal Stem Cells Mitigate Methotrexate-Induced Nephrotoxicity in Rat via Nrf2/Pparγ/HO-1 and NF-Κb/Keap1/Caspase-3 Signaling Pathways: Oxidative Stress and Apoptosis Interplay. Toxics 2023, 11, 398. https://doi.org/10.3390/toxics11050398
Wani FA, Ibrahim MA, Ameen SH, Farage AE, Ali ZA-E, Saleh K, Farag MM, Sayeed MU, Alruwaili MAY, Alruwaili AHF, et al. Platelet Rich Plasma and Adipose-Derived Mesenchymal Stem Cells Mitigate Methotrexate-Induced Nephrotoxicity in Rat via Nrf2/Pparγ/HO-1 and NF-Κb/Keap1/Caspase-3 Signaling Pathways: Oxidative Stress and Apoptosis Interplay. Toxics. 2023; 11(5):398. https://doi.org/10.3390/toxics11050398
Chicago/Turabian StyleWani, Farooq A., Mahrous A. Ibrahim, Shimaa H. Ameen, Amira E. Farage, Zinab Abd-Elhady Ali, Khaldoon Saleh, Medhat M. Farag, Mohammed U. Sayeed, Muhannad A. Y. Alruwaili, Abdulsalam H. F. Alruwaili, and et al. 2023. "Platelet Rich Plasma and Adipose-Derived Mesenchymal Stem Cells Mitigate Methotrexate-Induced Nephrotoxicity in Rat via Nrf2/Pparγ/HO-1 and NF-Κb/Keap1/Caspase-3 Signaling Pathways: Oxidative Stress and Apoptosis Interplay" Toxics 11, no. 5: 398. https://doi.org/10.3390/toxics11050398
APA StyleWani, F. A., Ibrahim, M. A., Ameen, S. H., Farage, A. E., Ali, Z. A.-E., Saleh, K., Farag, M. M., Sayeed, M. U., Alruwaili, M. A. Y., Alruwaili, A. H. F., Aljared, A. Z. A., & Galhom, R. A. (2023). Platelet Rich Plasma and Adipose-Derived Mesenchymal Stem Cells Mitigate Methotrexate-Induced Nephrotoxicity in Rat via Nrf2/Pparγ/HO-1 and NF-Κb/Keap1/Caspase-3 Signaling Pathways: Oxidative Stress and Apoptosis Interplay. Toxics, 11(5), 398. https://doi.org/10.3390/toxics11050398






