Risk Threshold and Assessment of Chloramphenicol Antibiotics in Sediment in the Fenhe River Basin, China
Abstract
:1. Introduction
2. Sample Collection, Detection, and Analysis
2.1. Sample Collection and Detection
2.2. Partitioning Coefficients (Kp)
2.3. Predicted No-Effect Concentration
2.4. Ecological Risk Assessment
3. Results and Discussion
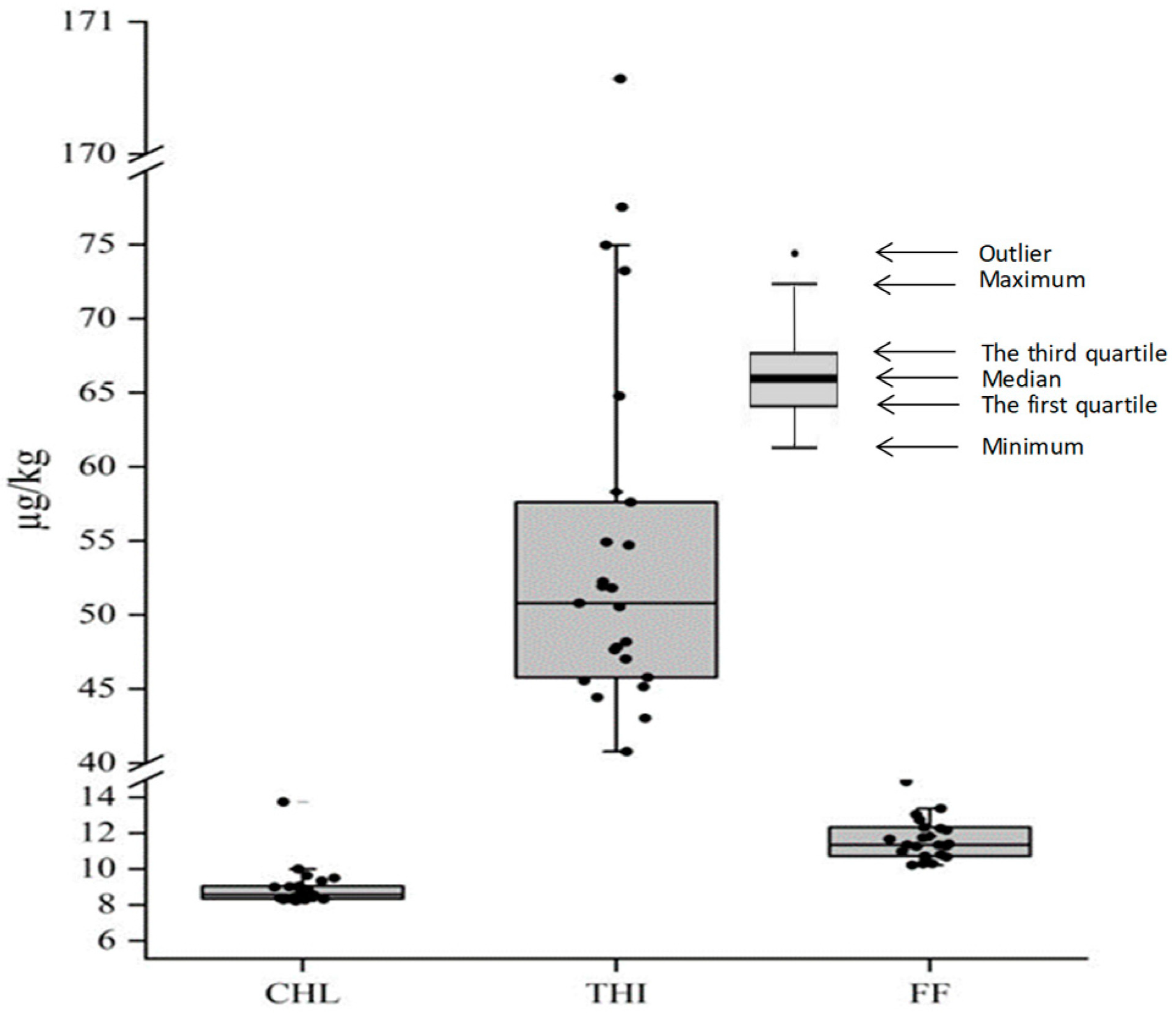
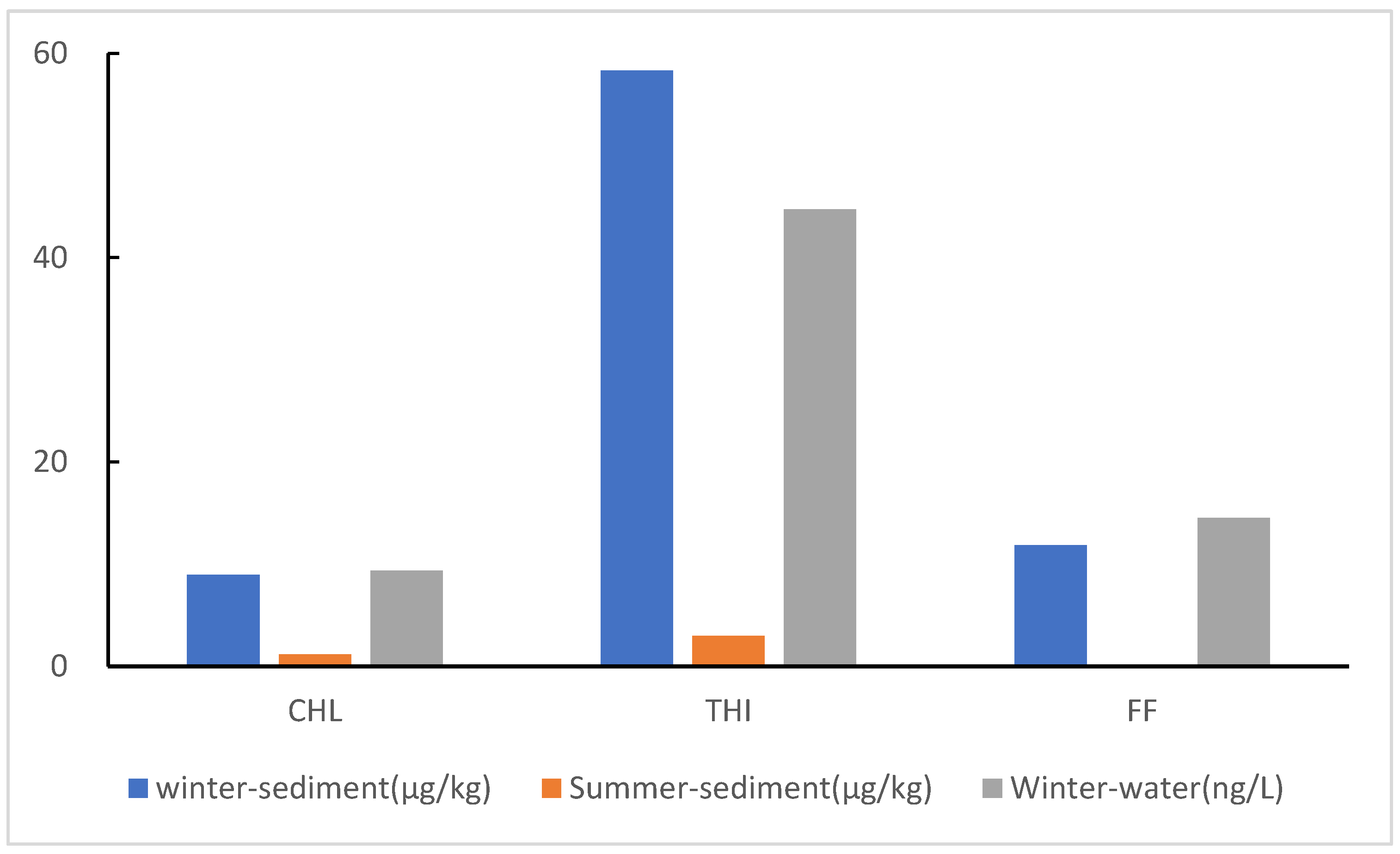
3.1. Concentration of CAs in Sediment and Temporal Variations
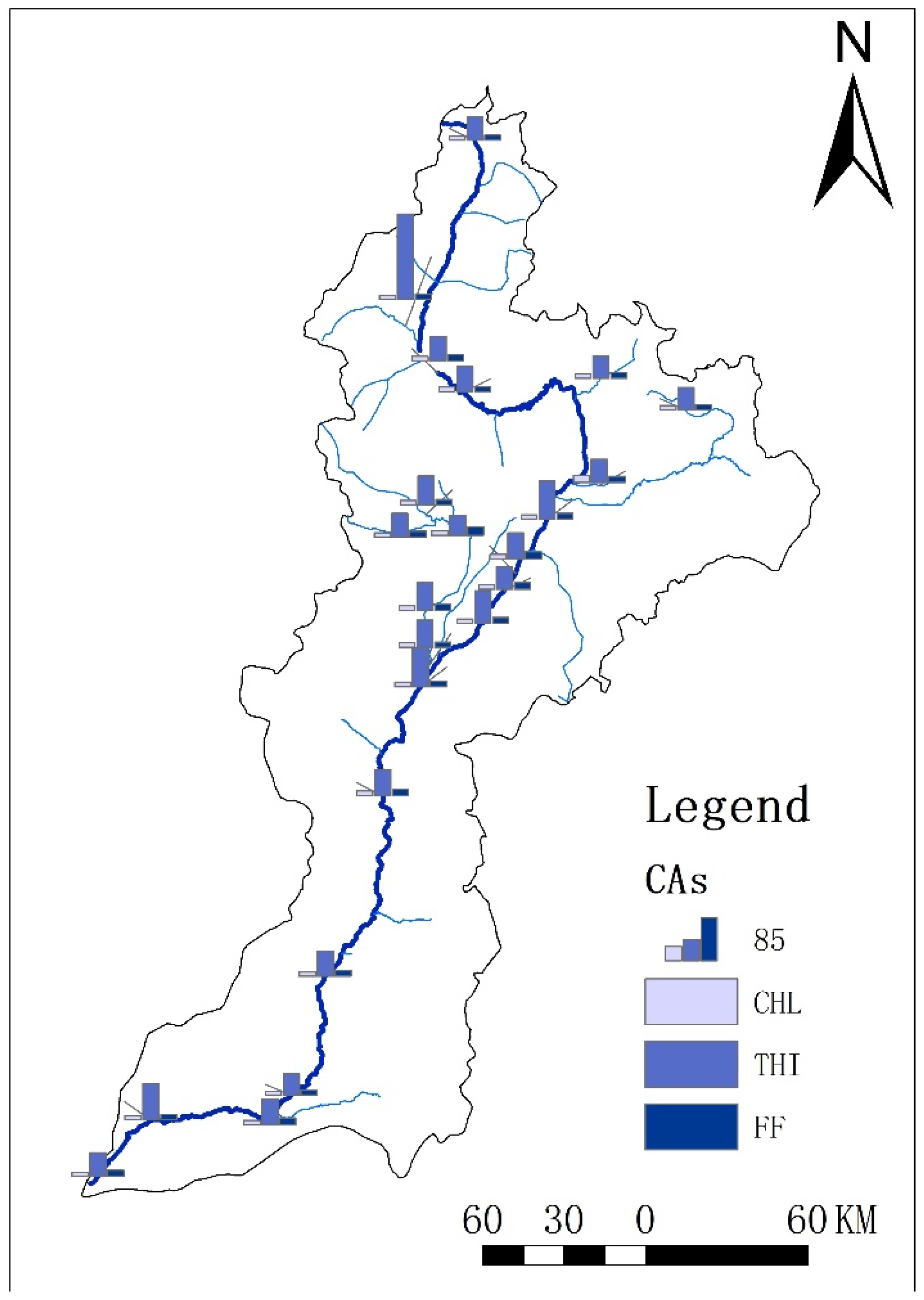
3.2. Spatial Distribution of CAs in Sediment
3.3. Analysis of Risk Threshold for CAs
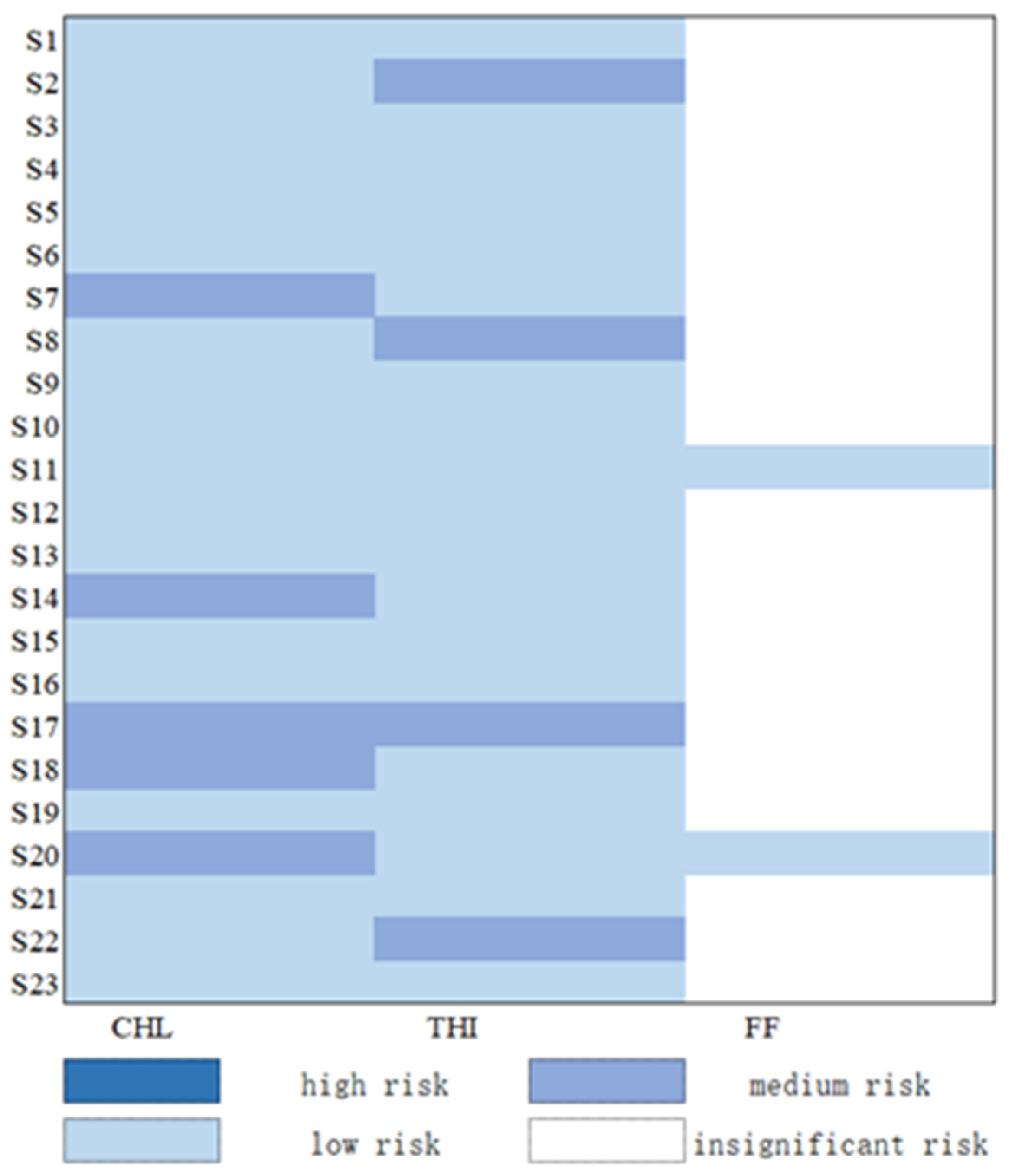
3.4. Risk Assessment of Chloramphenicol Antibiotics of Sediment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, L.; Li, H.; Liu, Y.; Cui, Y.; Li, Y.; Yang, Z. Distribution, residue level, sources, and phase partition of antibiotics in surface sediments from the inland river: A case study of the Xiangjiang River, south-central China. Environ. Sci. Pollut. Res. 2019, 27, 2273–2286. [Google Scholar] [CrossRef]
- Grossman, Z.; del Torso, S.; Hadjipanayis, A.; van Esso, D.; Drabik, A.; Sharland, M. Antibiotic prescribing for upper respiratory infections: European primary paediatricians’ knowledge, attitudes and practice. Acta Paediatr. 2012, 101, 935–940. [Google Scholar] [CrossRef]
- Liang, X.; Chen, B.; Nie, X.; Shi, Z.; Huang, X.; Li, X. The distribution and partitioning of common antibiotics in water and sediment of the Pearl River Estuary, South China. Chemosphere 2013, 92, 1410–1416. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Guo, S.; Dong, Q.; Song, Y. Development of an Immunochromatographic Test Strip for Rapid Simultaneous Detection of Enrofloxacin and Ofloxacin in Tissue of Chicken Muscle and Pork. Food Anal. Methods 2016, 9, 2807–2813. [Google Scholar] [CrossRef]
- Siedlewicz, G.; Białk-Bielińska, A.; Borecka, M.; Winogradow, A.; Stepnowski, P.; Pazdro, K. Presence, concentrations and riskassessment of selected antibiotic residues in sediments and near-bottom waters collected from the Polish coastal zone in the southern Baltic Sea—Summary of 3 years of studies. Mar. Pollut. Bull. 2018, 129, 787–801. [Google Scholar] [CrossRef]
- Williams-Nguyen, J.; Sallach, J.B.; Bartelt-Hunt, S.; Boxall, A.B.; Durso, L.M.; McLain, J.E.; Singer, R.S.; Snow, D.D.; Zilles, J.L. Antibiotics and Antibiotic Resistance in Agroecosystems: State of the Science. J. Environ. Qual. 2016, 45, 394–406. [Google Scholar] [CrossRef] [Green Version]
- Bialk-Bielinska, A.; Stolte, S.; Arning, J.; Uebers, U.; Boeschen, A.; Stepnowski, P.; Matzke, M. Ecotoxicity evaluation of selected sulfonamides. Chemosphere 2011, 85, 928–933. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Kang, Y.; Zhang, R.; Han, M.; Zeng, W.; Wang, Y.; Yu, K.; Yang, Y. Occurrence, source, and the fate of antibiotics in mariculture ponds near the Maowei Sea, South China: Storm caused the increase of antibiotics usage. Sci. Total Environ. 2020, 752, 141882. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, H.; Dang, J.; Guo, H.; Zhu, Y.; Han, W. Occurrence, distribution, and partitioning of antibiotics in surface water and sediment in a typical tributary of Yellow River, China. Environ. Sci. Pollut. Res. 2021, 28, 28207–28221. [Google Scholar] [CrossRef]
- Chen, K.; Zhou, J. Occurrence and behavior of antibiotics in water and sediments from the Huangpu River, Shanghai, China. Chemosphere 2014, 95, 604–612. [Google Scholar] [CrossRef]
- Kim, S.-C.; Carlson, K. Temporal and spatial trends in the occurrence of human and veterinary antibiotics in aqueous and river sediment matrices. Environ. Sci. Technol. 2006, 41, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, G.; Liu, C.-Q.; Li, L.; Xiang, M. The occurrence of chloramphenicol and tetracyclines in municipal sewage and the Nanming River, Guiyang City, China. J. Environ. Monit. 2009, 11, 1199–1205. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Galan, M.J.; Diaz-Cruz, M.S.; Barcelo, D. Occurrence of sulfonamide residues along the Ebro river basin: Removal in wastewater treatment plants and environmental impact assessment. Environ. Int. 2011, 37, 462–473. [Google Scholar] [CrossRef] [PubMed]
- Managaki, S.; Murata, A.; Takada, H.; Tuyen, B.C.; Chiem, N.H. Distribution of macrolides, sulfonamides, and trimethoprim in tropical waters: Ubiquitous occurrence of veterinary antibiotics in the Mekong Delta. Environ. Sci. Technol. 2007, 41, 8004–8010. [Google Scholar] [CrossRef] [PubMed]
- Kafaei, R.; Papari, F.; Seyedabadi, M.; Sahebi, S.; Tahmasebi, R.; Ahmadi, M.; Sorial, G.A.; Asgari, G.; Ramavandi, B. Occurrence, distribution, and potential sources of antibiotics pollution in the water-sediment of the northern coastline of the Persian Gulf, Iran. Sci. Total Environ. 2018, 627, 703–712. [Google Scholar] [CrossRef]
- Ergie, A.A.; Leng, Y.; Wang, J. Antibiotics and Resistance Genes in Awash River Basin, Ethiopia. EcoHealth 2019, 16, 441–453. [Google Scholar] [CrossRef]
- Staples, C.A.; Woodburn, K.B.; Klecka, G.M.; Mihaich, E.M.; Hall, A.T.; Ortego, L.; Caspers, N.; Hentges, S.G. Comparison of four species sensitivity distribution methods to calculate predicted no effect concentrations for bisphenol A. Hum. Ecol. Risk Assess. Int. J. 2008, 14, 455–478. [Google Scholar] [CrossRef]
- Hickey, G.L.; Craig, P.S. Competing Statistical Methods for the Fitting of Normal Species Sensitivity Distributions: Recommendations for Practitioners. Risk Anal. 2012, 32, 1232–1243. [Google Scholar] [CrossRef]
- Cheng, J.X.; Jiang, L.; Sun, T.Q.; Tang, Y.; Du, Z.X.; Lee, L.J.; Zhao, Q.Y. Occurrence, Seasonal Variation and Risk Assessment of Antibiotics in the Surface Water of North China. Arch. Environ. Contam. Toxicol. 2019, 77, 88–97. [Google Scholar]
- Guérit, I.; Bocquené, G.; James, A.; Thybaud, E.; Minier, C. Environmental risk assessment: A critical approach of the European TGD in an in situ application. Ecotoxicol. Environ. Saf. 2008, 71, 291–300. [Google Scholar] [CrossRef] [Green Version]
- Dyer, S.D.; Versteeg, D.J.; Belanger, S.E.; Chaney, J.G.; Raimondo, S.; Barron, M.G. Comparison of species sensitivity distribtions derived from interspecies correlation models to distributions used to derive water quality criteria. Environ. Sci. Technol. 2008, 42, 3076–3083. [Google Scholar] [CrossRef]
- Chen, H.; Liu, S.; Xu, X.-R.; Zhou, G.-J.; Liu, S.-S.; Yue, W.-Z.; Sun, K.-F.; Ying, G.-G. Antibiotics in the coastal environment of the Hailing Bay region, South China Sea: Spatial distribution, source analysis and ecological risks. Mar. Pollut. Bull. 2015, 95, 365–373. [Google Scholar] [CrossRef]
- Monti, G.S.; Filzmoser, P.; Deutsch, R.C. A Robust Approach to Risk Assessment Based on Species Sensitivity Distributions. Risk Anal. 2018, 38, 2073–2086. [Google Scholar] [CrossRef]
- Rico, A.; Oliveira, R.; McDonough, S.; Matser, A.; Khatikarn, J.; Satapornvanit, K.; Nogueira, A.J.; Soares, A.M.; Domingues, I.; Brink, P.J.V.D. Use, fate and ecological risks of antibiotics applied in tilapia cage farming in Thailand. Environ. Pollut. 2014, 191, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Yan, X.; Shen, Y.; Di, M.; Wang, J. Antibiotics in surface water and sediments from Hanjiang River, Central China: Occurrence, behavior and risk assessment. Ecotoxicol. Environ. Saf. 2018, 157, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, H.; Jing, L.; Teng, Y. Ecotoxicological risk assessment and source apportionment of antibiotics in the waters and sediments of a peri-urban river. Sci. Total Environ. 2020, 731, 139128. [Google Scholar] [CrossRef] [PubMed]
- Hiki, K.; Iwasaki, Y.; Watanabe, H.; Yamamoto, H. Comparison of Species Sensitivity Distributions for Sediment-Associated Nonionic Organic Chemicals through Equilibrium Partitioning Theory and Spiked-Sediment Toxicity Tests with Invertebrates. Environ. Toxicol. Chem. 2022, 41, 462–473. [Google Scholar] [CrossRef]
- van Beelen, P.; Verbruggen, E.M.; Peijnenburg, W.J. The evaluation of the equilibrium partitioning method using sensitivity distributions of species in water and soil. Chemosphere 2003, 52, 1153–1162. [Google Scholar] [CrossRef]
- Meng, Z.; Yang, Y.; Qin, Z.; Huang, L. Evaluating Temporal and Spatial Variation in Nitrogen Sources along the Lower Reach of The Fenhe River (Shanxi Province, China) Using Stable Isotope and Hydrochemical Tracers. Water 2018, 10, 231. [Google Scholar] [CrossRef] [Green Version]
- Menz, J.; Mueller, J.; Olsson, O.; Kuemmerer, K. Bioavailability of Antibiotics at Soil-Water Interfaces: A Comparison of Measured Activities and Equilibrium Partitioning Estimates. Environ. Sci. Technol. 2018, 52, 6555–6564. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Liu, R.; Li, L.; Cao, L.; Jiao, L.; Xia, X. Source-specific risk apportionment and critical risk source identification of antibiotic resistance in The Fenhe River basin, China. Chemosphere 2021, 287, 131997. [Google Scholar] [CrossRef] [PubMed]
- Jesus Garcia-Galan, M.; Silvia Diaz-Cruz, M.; Barcelo, D. Combining chemical analysis and ecotoxicity to determine environmental exposure and to assess risk from sulfonamides. TrAC-Trends Anal. Chem. 2009, 28, 804–819. [Google Scholar] [CrossRef]
- Eisentraeger, A.; Dott, W.; Klein, J.; Hahn, S. Comparative studies on algal toxicity testing using fluorometric microplate and Erlenmeyer flask growth-inhibition assays. Ecotoxicol. Environ. Saf. 2003, 54, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Ando, T.; Kusuhara, H.; Merino, G.; Alvarez, A.I.; Schinkel, A.H.; Sugiyama, Y. Involvement of breast cancer resistance protein (ABCG2) in the biliary excretion mechanism of fluoroquinolones. Drug Metab. Dispos. 2007, 35, 1873–1879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christensen, A.M.; Ingerslev, F.; Baun, A. Ecotoxicity of mixtures of antibiotics used in aquacultures. Environ. Toxicol. Chem. 2006, 25, 2208–2215. [Google Scholar] [CrossRef]
- Ando, T.; Nagase, H.; Eguchi, K.; Hirooka, T.; Nakamura, T.; Miyamoto, K.; Hirata, K. A novel method using cyanobacteria for ecotoxicity test of veterinary antimicrobial agents. Environ. Toxicol. Chem. 2007, 26, 601–606. [Google Scholar] [CrossRef]
- Luo, Y.; Xu, L.; Rysz, M.; Wang, Y.; Zhang, H.; Alvarez, P.J.J. Occurrence and Transport of Tetracycline, Sulfonamide, Quinolone, and Macrolide Antibiotics in the Haihe River Basin, China. Environ. Sci. Technol. 2011, 45, 1827–1833. [Google Scholar] [CrossRef]
- Ekpeghere, K.I.; Lee, J.-W.; Kim, H.-Y.; Shin, S.-K.; Oh, J.-E. Determination and characterization of pharmaceuticals in sludge from municipal and livestock wastewater treatment plants. Chemosphere 2017, 168, 1211–1221. [Google Scholar] [CrossRef]
- Yan, C.; Yang, Y.; Zhou, J.; Liu, M.; Nie, M.; Shi, H.; Gu, L. Antibiotics in the surface water of the Yangtze Estuary: Occurrence, distribution and risk assessment. Environ. Pollut. 2013, 175, 22–29. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, G.; Zheng, Q.; Tang, J.; Chen, Y.; Xu, W.; Zou, Y.; Chen, X. Occurrence and risks of antibiotics in the Laizhou Bay, China: Impacts of river discharge. Ecotoxicol. Environ. Saf. 2012, 80, 208–215. [Google Scholar] [CrossRef]
- Adamek, E.; Baran, W.; Sobczak, A. Assessment of the biodegradability of selected sulfa drugs in two polluted rivers in Poland: Effects of seasonal variations, accidental contamination, turbidity and salinity. J. Hazard. Mater. 2016, 313, 147–158. [Google Scholar] [CrossRef]
- Rabølle, M.; Spliid, N.H. Sorption and mobility of metronidazole, olaquindox, oxytetracycline and tylosin in soil. Chemosphere 2000, 40, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Boxall, A.B.A.; Fogg, L.A.; Blackwell, P.A.; Kay, P.; Pemberton, E.J.; Croxford, A. Veterinary medicines in the environment. Rev. Environ. Contam. Toxicol. 2004, 180, 1–91. [Google Scholar] [PubMed]
- Kummerer, K. Drugs in the environment: Emission of drugs, diagnostic aids and disinfectants into wastewater by hospitals in relation to other sources—A review. Chemosphere 2001, 45, 957–969. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Liu, X.; Cheng, D.; Liu, G.; Liang, B.; Cui, B.; Bai, J. Temporal–spatial variation and partitioning prediction of antibiotics in surface water and sediments from the intertidal zones of the Yellow River Delta, China. Sci. Total Environ. 2016, 569, 1350–1358. [Google Scholar] [CrossRef]
- Bai, Y.; Meng, W.; Xu, J.; Zhang, Y.; Guo, C. Occurrence, distribution and bioaccumulation of antibiotics in the Liao River Basin in China. Environ. Sci. Processes Impacts 2013, 16, 586–593. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Zhang, Y.; Zhou, C.; Guo, C.; Wang, D.; Du, P.; Luo, Y.; Wan, J.; Meng, W. Distribution, sources and composition of antibiotics in sediment, overlying water and pore water from Taihu Lake, China. Sci. Total Environ. 2014, 497–498, 267–273. [Google Scholar] [CrossRef]
- Gyesi, J.N.; Nyaaba, B.A.; Darko, G.; Mills-Robertson, F.C.; Miezah, K.; Acheampong, N.A.; Frimpong, F.; Gyimah, G.; Quansah, B.; Borquaye, L.S. Occurrence of Pharmaceutical Residues and Antibiotic-Resistant Bacteria in Water and Sediments from Major Reservoirs (Owabi and Barekese Dams) in Ghana. J. Chem. 2022, 2022, 1802204. [Google Scholar] [CrossRef]
- Kummerer, K. Antibiotics in the aquatic environment—A review—Part I. Chemosphere 2009, 75, 417–434. [Google Scholar] [CrossRef]
- Li, N.; Zhang, X.; Wu, W.; Zhao, X. Occurrence, seasonal variation and risk assessment of antibiotics in the reservoirs in North China. Chemosphere 2014, 111, 327–335. [Google Scholar] [CrossRef]
- Tang, X.; Lou, C.; Wang, S.; Lu, Y.; Liu, M.; Hashmi, M.Z.; Liang, X.; Li, Z.; Liao, Y.; Qin, W.; et al. Effects of long-term manure applications on the occurrence of antibiotics and antibiotic resistance genes (ARGs) in paddy soils: Evidence from four field experiments in south of China. Soil Biol. Biochem. 2015, 90, 179–187. [Google Scholar] [CrossRef]
- Leibold, A.M.; Suie, T. Invitro effects of sulfacetamide on ocular strains of bacteria. Am. J. Ophthalmol. 1958, 45, 383–386. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, D.; Zhang, Q.; Song, K.; Zhou, X.; Tang, Z.; Zhou, X. Occurrence and ecological risk assessment of selected antibiotics in the freshwater lakes along the middle and lower reaches of Yangtze River Basin. J. Environ. Manag. 2019, 249, 109396. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.P.; Ci, M.W.; Yan, X.S.; Wang, Y.Q.; Zhang, G.D.; Xu, W.F.; Gao, X.G.; Xie, K.; Wang, W.L. Antibiotics in the surface water and sediment from the tributaries of the Xiaoqing River, China: Occurrence, distribution and risk assessment. Desalination Water Treat. 2022, 247, 229–243. [Google Scholar] [CrossRef]
- Kaeseberg, T.; Zhang, J.; Schubert, S.; Oertel, R.; Siedel, H.; Krebs, P. Sewer sediment-bound antibiotics as a potential environmental risk: Adsorption and desorption affinity of 14 antibiotics and one metabolite. Environ. Pollut. 2018, 239, 638–647. [Google Scholar] [CrossRef]
- González-Gaya, B.; García-Bueno, N.; Buelow, E.; Marin, A.; Rico, A. Effects of aquaculture waste feeds and antibiotics on marine benthic ecosystems in the Mediterranean Sea. Sci. Total Environ. 2021, 806, 151190. [Google Scholar] [CrossRef]
- Ditoro, D.M.; Zarba, C.S.; Hansen, D.J.; Berry, W.J.; Swartz, R.C.; Cowan, C.E.; Pavlou, S.P.; Allen, H.E.; Thomas, N.A.; Paquin, P.R. Technical basis for establishing sediment quality criteria for nonionic organic chemicals using equilibrium partitioning. Environ. Toxicol. Chem. 1991, 10, 1541–1583. [Google Scholar] [CrossRef]
- Xu, J. Grain-size characteristics of suspended sediment in the Yellow River, China. Catena 2000, 38, 243–263. [Google Scholar] [CrossRef]
- Brock, T.; Belgers, J.; Boerwinkel, M.-C.; Jollie, L.; Kraak, M.; Papo, M.; Vonk, J.; Roessink, I. Toxicity of sediment-bound lufenuron to benthic arthropods in laboratory bioassays. Aquat. Toxicol. 2018, 198, 118–128. [Google Scholar] [CrossRef] [Green Version]


| Antibiotics | Molecular Formula | CAS | Molecular Weight (g/mol) | |
|---|---|---|---|---|
| Chloramphenicol | CHL | C11H12Cl2FN2O5 | 154–75–2 | 323.1 |
| Thiamphenicol | THI | C12H15Cl2NO5S | 15,318–45–3 | 356.2 |
| Florfenicol | FF | C12H14Cl2FNO4S | 73,231–34–2 | 358.2 |
| Antibiotics | Summer (L/kg) | Winter (L/kg) |
|---|---|---|
| CHL | 294 | 963 |
| THI | 188 | 2972 |
| FF | - | 865 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Dang, D.; Cao, L.; Wang, H.; Liu, R. Risk Threshold and Assessment of Chloramphenicol Antibiotics in Sediment in the Fenhe River Basin, China. Toxics 2023, 11, 570. https://doi.org/10.3390/toxics11070570
Wang L, Dang D, Cao L, Wang H, Liu R. Risk Threshold and Assessment of Chloramphenicol Antibiotics in Sediment in the Fenhe River Basin, China. Toxics. 2023; 11(7):570. https://doi.org/10.3390/toxics11070570
Chicago/Turabian StyleWang, Linfang, Dexuan Dang, Leiping Cao, Huiyan Wang, and Ruimin Liu. 2023. "Risk Threshold and Assessment of Chloramphenicol Antibiotics in Sediment in the Fenhe River Basin, China" Toxics 11, no. 7: 570. https://doi.org/10.3390/toxics11070570
APA StyleWang, L., Dang, D., Cao, L., Wang, H., & Liu, R. (2023). Risk Threshold and Assessment of Chloramphenicol Antibiotics in Sediment in the Fenhe River Basin, China. Toxics, 11(7), 570. https://doi.org/10.3390/toxics11070570






