Serum Concentrations of Benzaldehyde, Isopentanaldehyde and Sex Hormones: Evidence from the National Health and Nutrition Examination Survey
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Measurement of Serum Benzaldehyde and Isopentanaldehyde
2.3. Measurement of Serum Sex Hormones
2.4. Covariates
2.5. Statistical Analysis
3. Results
3.1. Characteristics of Participants
3.2. Distributions of Characteristics according to the Tertile Groups of Benzaldehyde and Isopentanaldehyde
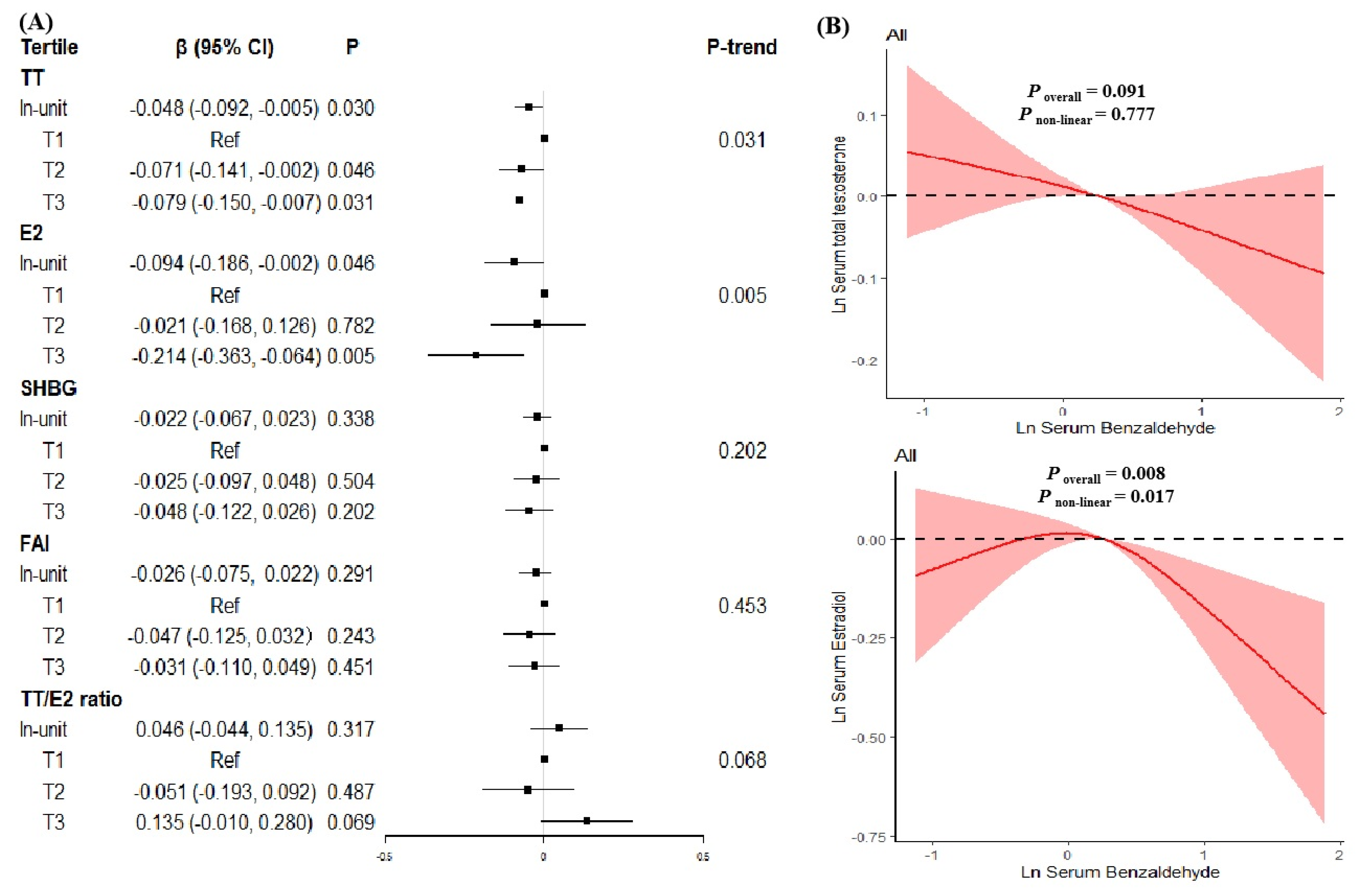
3.3. Associations of Benzaldehyde Exposure with Sex Hormones Levels
3.4. Associations of Isopentanaldehyde Exposure with Sex Hormones
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walker, W.H. Non-classical actions of testosterone and spermatogenesis. Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 2010, 365, 1557–1569. [Google Scholar] [CrossRef] [Green Version]
- Bond, A.; Davis, C. Sex hormone binding globulin in clinical perspective. Acta Obstet. Gynecol. Scand. 1987, 66, 255–262. [Google Scholar] [CrossRef]
- Dewailly, D.; Robin, G.; Peigne, M.; Decanter, C.; Pigny, P.; Catteau-Jonard, S. Interactions between androgens, FSH, anti-Müllerian hormone and estradiol during folliculogenesis in the human normal and polycystic ovary. Hum. Reprod. Update 2016, 22, 709–724. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.Y. Exogenous and endogenous hormones and breast cancer. Best Pract. Res. Clin. Endocrinol. Metab. 2008, 22, 573–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, E.L.; Song, Y.; Manson, J.E.; Hunter, D.J.; Lee, C.C.; Rifai, N.; Buring, J.E.; Gaziano, J.M.; Liu, S. Sex hormone-binding globulin and risk of type 2 diabetes in women and men. N. Engl. J. Med. 2009, 361, 1152–1163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, D.; Guallar, E.; Ouyang, P.; Subramanya, V.; Vaidya, D.; Ndumele, C.E.; Lima, J.A.; Allison, M.A.; Shah, S.J.; Bertoni, A.G.; et al. Endogenous Sex Hormones and Incident Cardiovascular Disease in Post-Menopausal Women. J. Am. Coll. Cardiol. 2018, 71, 2555–2566. [Google Scholar] [CrossRef] [PubMed]
- Moulton, V.R. Sex Hormones in Acquired Immunity and Autoimmune Disease. Front. Immunol. 2018, 9, 2279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saad, F.; Gooren, L. The role of testosterone in the metabolic syndrome: A review. J. Steroid Biochem. Mol. Biol. 2009, 114, 40–43. [Google Scholar] [CrossRef]
- Cherrier, M. Testosterone effects on cognition in health and disease. Front. Horm. Res. 2009, 37, 150–162. [Google Scholar]
- Wang, J.; Fan, X.; Yang, M.; Song, M.; Wang, K.; Giovannucci, E.; Ma, H.; Jin, G.; Hu, Z.; Shen, H.; et al. Sex-specific associations of circulating testosterone levels with all-cause and cause-specific mortality. Eur. J. Endocrinol. 2021, 184, 723–732. [Google Scholar] [CrossRef]
- Zhao, X.; Liang, S.; Wang, N.; Hong, T.; Sambou, M.L.; Fan, J.; Zhu, M.; Wang, C.; Hang, D.; Jiang, Y.; et al. Sex-Specific Associations of Testosterone and Genetic Factors with Health Span. Front. Endocrinol. 2021, 12, 773464. [Google Scholar] [CrossRef]
- Xita, N.; Tsatsoulis, A. Genetic variants of sex hormone-binding globulin and their biological consequences. Mol. Cell. Endocrinol. 2010, 316, 60–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marom-Haham, L.; Shulman, A. Cigarette smoking and hormones. Curr. Opin. Obstet. Gynecol. 2016, 28, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Hankinson, S.E.; Smith-Warner, S.A.; Wang, M.; Eliassen, A.H. Flavonoid Intake and Plasma Sex Steroid Hormones, Prolactin, and Sex Hormone-Binding Globulin in Premenopausal Women. Nutrients 2019, 11, 2669. [Google Scholar] [CrossRef] [Green Version]
- O’Brien, P.J.; Siraki, A.G.; Shangari, N. Aldehyde sources, metabolism, molecular toxicity mechanisms, and possible effects on human health. Crit. Rev. Toxicol. 2005, 35, 609–662. [Google Scholar] [CrossRef]
- Gordon, T.; Karey, E.; Rebuli, M.E.; Escobar, Y.H.; Jaspers, I.; Chen, L.C. E-Cigarette Toxicology. Annu. Rev. Pharmacol. Toxicol. 2022, 62, 301–322. [Google Scholar] [CrossRef]
- Pontel, L.B.; Rosado, I.V.; Burgos-Barragan, G.; Garaycoechea, J.I.; Yu, R.; Arends, M.J.; Chandrasekaran, G.; Broecker, V.; Wei, W.; Liu, L.; et al. Endogenous Formaldehyde Is a Hematopoietic Stem Cell Genotoxin and Metabolic Carcinogen. Mol. Cell 2015, 60, 177–188. [Google Scholar] [CrossRef] [Green Version]
- Weng, X.Q.; Chen, J.M.; Fei, Q.Y.; Guo, X.R.; Liu, S.; Wen, L.; Liang, H.Z.; Guo, C.C.; Nie, L.H.; Jing, C.X. The association of aldehydes exposure with diabetes mellitus in US population: NHANES 2013-2014. Chemosphere 2022, 291, 133019. [Google Scholar] [CrossRef]
- Liao, S.; Wu, N.; Gong, D.; Tang, X.; Yin, T.; Zhang, H.; Li, X. Association of aldehydes exposure with obesity in adults. Ecotoxicol. Environ. Saf. 2020, 201, 110785. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Xiong, X.; Chen, C.; Ran, J. Association of aldehyde exposure with bone mineral density in the national health and nutrition examination survey (NHANES 2013–2014). J. Endocrinol. Investig. 2022, 45, 2085–2096. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.K.; Espenship, M.F.; Newman, C.A.; Zhang, L.; Zhu, W.; Blount, B.C.; De Jesús, V.R. Assessment of Serum Concentrations of 12 Aldehydes in the U.S. Population from the 2013–2014 National Health and Nutrition Examination Survey. Environ. Sci. Technol. 2021, 55, 5076–5083. [Google Scholar] [CrossRef] [PubMed]
- Xing, W.L.; Gu, W.; Liang, M.Y.; Wang, Z.; Fan, D.L.; Zhang, B.; Wang, L. Association between aldehyde exposure and sex steroid hormones among adults. Environ. Sci. Pollut. Res. Int. 2023, 30, 30444–30461. [Google Scholar] [CrossRef]
- Georgiopoulos, G.A.; Lambrinoudaki, I.; Athanasouli, F.; Armeni, E.; Rizos, D.; Kazani, M.; Karamanou, M.; Manios, E.; Augoulea, A.; Stellos, K.; et al. Free androgen index as a predictor of blood pressure progression and accelerated vascular aging in menopause. Atherosclerosis 2016, 247, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Imani, B.; Eijkemans, M.J.; Jong, F.H.D.; Payne, N.N.; Bouchard, P.; Giudice, L.C.; Fauser, B.C. Free androgen index and leptin are the most prominent endocrine predictors of ovarian response during clomiphene citrate induction of ovulation in normogonadotropic oligoamenorrheic infertility. J. Clin. Endocrinol. Metab. 2000, 85, 676–682. [Google Scholar] [PubMed] [Green Version]
- Li, X.; Yu, X.; Luo, K.; Liu, H.; Fan, X.; Yin, X.; Zhao, Q.; Liu, X.; Yang, Y. Exposure to metals and the disruption of sex hormones in 6-19 years old children: An exploration of mixture effects. Ecotoxicol. Environ. Saf. 2023, 250, 114477. [Google Scholar] [CrossRef]
- Zhang, Q.F.; Bai, Q.; Yuan, Y.; Liu, P.; Qiao, J. Assessment of seminal estradiol and testosterone levels as predictors of human spermatogenesis. J. Androl. 2010, 31, 215–220. [Google Scholar] [CrossRef] [Green Version]
- Silva, L.K.; Hile, G.A.; Capella, K.M.; Espenship, M.F.; Smith, M.M.; De Jesus, V.R.; Blount, B.C. Quantification of 19 aldehydes in human serum by headspace SPME/GC/high-resolution mass spectrometry. Environ. Sci. Technol. 2018, 52, 10571–10579. [Google Scholar] [CrossRef]
- NCHS. Laboratory Procedure Manual for Serum Total Estradiol and Total Testosterone. National Center for Environmental Health 2013–2014a. Available online: https://wwwn.cdc.gov/nchs/data/nhanes/2013-2014/labmethods/TST_H_MET_Total_Estradiol_and_Total_Testosterone.pdf (accessed on 1 November 2018).
- NCHS. Laboratory Procedure Manual for Serum Sex Hormone Binding Globulin. National Center for Environmental Health 2013–2014b. Available online: https://wwwn.cdc.gov/nchs/data/nhanes/2013-2014/labmethods/TST_H_MET_Sex_Hormone_Binding_Globulin.pdf (accessed on 1 November 2018).
- Ogden, C.L.; Carroll, M.D.; Fakhouri, T.H.; Hales, C.M.; Fryar, C.D.; Li, X.F.; Freedman, D.S. Prevalence of Obesity among Youths by Household Income and Education Level of Head of Household—United States 2011–2014. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 186–189. [Google Scholar] [CrossRef]
- Ahmed Laskar, A.; Younus, H. Aldehyde toxicity and metabolism: The role of aldehyde dehydrogenases in detoxification, drug resistance and carcinogenesis. Drug Metab. Rev. 2019, 51, 42–64. [Google Scholar] [CrossRef]
- Ge, P.; Zhang, X.; Yang, Y.Q.; Lv, M.Q.; Zhou, D.X. Long-term exposure to formaldehyde induced down-regulation of SPO11 in rats. Inhal. Toxicol. 2021, 33, 8–17. [Google Scholar] [CrossRef]
- Morohashi, K.; Baba, T.; Tanaka, M. Steroid hormones and the development of reproductive organs. Sex. Dev. 2013, 7, 61–79. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, A.R.; Gautam, A.K.; Patel, K.G.; Trivedi, H.S. Steroidogenic inhibition in testicular tissue of formaldehyde exposed rats. Indian J. Physiol. Pharmacol. 1992, 36, 162–168. [Google Scholar] [PubMed]
- Duong, A.; Steinmaus, C.; McHale, C.M.; Vaughan, C.P.; Zhang, L. Reproductive and developmental toxicity of formaldehyde: A systematic review. Mutat. Res. 2011, 728, 118–138. [Google Scholar] [CrossRef] [Green Version]
- Darbandi, M.; Darbandi, S.; Agarwal, A.; Sengupta, P.; Durairajanayagam, D.; Henkel, R.; Sadeghi, M.R. Reactive oxygen species and male reproductive hormones. Reprod. Biol. Endocrinol. 2018, 16, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozen, O.A.; Akpolat, N.; Songur, A.; Kuş, I.; Zararsiz, I.; Ozaçmak, V.H.; Sarsilmaz, M. Effect of formaldehyde inhalation on Hsp70 in seminiferous tubules of rat testes: An immunohistochemical study. Toxicol. Ind. Health 2005, 21, 249–254. [Google Scholar] [CrossRef]
- Razandi, M.; Pedram, A.; Levin, E.R. Heat shock protein 27 is required for sex steroid receptor trafficking to and functioning at the plasma membrane. Mol. Cell. Biol. 2010, 30, 3249–3261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tunc, O.; Tremellen, K. Oxidative DNA damage impairs global sperm DNA methylation in infertile men. J. Assist. Reprod. Genet. 2009, 26, 537–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyne, D.J.; Friedenreich, C.M.; McIntyre, J.B.; Stanczyk, F.Z.; Courneya, K.S.; King, W.D. Endogenous sex hormone exposure and repetitive element DNA methylation in healthy postmenopausal women. Cancer Causes Control. 2017, 28, 1369–1379. [Google Scholar] [CrossRef]
- Dai, W.S.; Gutai, J.P.; Kuller, L.H.; Cauley, J.A. Cigarette smoking and serum sex hormones in men. Am. J. Epidemiol. 1988, 128, 796–805. [Google Scholar] [CrossRef]
- Brand, J.S.; Chan, M.F.; Dowsett, M.; Folkerd, E.; Wareham, N.J.; Luben, R.N.; van der Schouw, Y.T.; Khaw, K.T. Cigarette smoking and endogenous sex hormones in postmenopausal women. J. Clin. Endocrinol. Metab. 2011, 96, 3184–3192. [Google Scholar] [CrossRef] [Green Version]




| Characteristics | Benzaldehyde, ng/mL | |||
|---|---|---|---|---|
| Tertile1 (n = 354) | Tertile2 (n = 360) | Tertile3 (n = 350) | P | |
| <0.961 | 0.961–1.680 | >1.680 | ||
| Age, years, median (IQR) | 45 (35–60) | 45 (32–59) | 47 (34.25–59.75) | 0.605 |
| Gender, n (%) | 0.394 | |||
| Male | 209 (59.04%) | 201 (55.83%) | 189 (54.00%) | |
| Female | 145 (40.96%) | 159 (44.17%) | 161 (46.00%) | |
| Ethnicity, n (%) | 0.005 | |||
| Mexican Americans | 38 (10.73%) a | 40 (11.11%) a | 63 (18.00%) b | |
| Other Hispanic | 28 (7.91%) a | 30 (8.33%) a | 19 (5.43%) a | |
| Non-Hispanic White | 176 (49.72%) a | 169 (46.94%) a | 163 (46.57%) a | |
| Non-Hispanic Black | 76 (21.47%) a | 70 (19.44%) a,b | 49 (14.00%) b | |
| Others | 36 (10.17%) a | 51 (14.17%) a,b | 56 (16.00%) b | |
| BMI, kg/m2, median (IQR) | 27.80 (24.60–32.58) | 27.50 (23.60–32.50) | 27.60 (23.75–32.15) | 0.575 |
| <25, n (%) | 99 (27.97%) | 117 (32.50%) | 115 (32.86%) | 0.554 |
| 25.0–30.0, n (%) | 125 (35.31%) | 111 (30.83%) | 111 (31.71%) | |
| ≥30.0, n (%) | 130 (36.72%) | 132 (36.67%) | 124 (35.43%) | |
| Serum Cotinine | <0.001 | |||
| ≥LOD | 292 (82.49%) a | 272 (75.56%) a,b | 238 (68.00%) b | |
| <LOD | 62 (17.51%) | 88 (24.44%) | 112 (32.00%) | |
| Education, n (%) | 0.003 | |||
| Less than high school | 105 (29.66%) a | 72 (20.00%) b | 73 (20.86%) b | |
| High school or equivalent | 88 (24.86%) a | 82 (22.78%) a | 74 (21.14%) a | |
| College or above | 161 (45.48%) a | 206 (57.22%) b | 203 (58.00%) b | |
| Family income-to-poverty ratio, n (%) | 0.003 | |||
| Low | 169 (47.74%) a | 132 (36.67%) b | 123 (35.14%) b | |
| Medium | 110 (31.07%) a | 121 (33.61%) a | 119 (34.00%) a | |
| High | 75 (21.19%) a | 107 (29.72%) b | 108 (30.86%) b | |
| Six-month time period | 0.068 | |||
| November 1 through April 30 | 187 (52.82%) | 159 (44.17%) | 171 (48.86%) | |
| May 1 through October 31 | 167 (47.18%) | 201 (55.83%) | 179 (51.14%) | |
| Time of blood draw | 0.129 | |||
| Morning | 166 (46.89%) | 180 (50.00%) | 156 (44.57%) | |
| Afternoon | 125 (35.31%) | 133 (36.94%) | 148 (42.29%) | |
| Evening | 63 (17.8%) | 47 (13.06%) | 46 (13.14%) | |
| Sex hormones, median (IQR) | ||||
| TT,ng/dL | 274.90 (26.18–446.75) a | 223.58 (23.21–407.89) a,b | 207.50 (20.11–396.50) b | 0.031 |
| E2, pg/mL | 25.60 (16.50–39.50) a | 25.15 (16.43- 40.83) a | 22.50 (13.93–32.78) b | 0.010 |
| SHBG, nmol/L | 49.68 (32.24–71.99) | 47.25 (32.08–73.36) | 45.49 (31.43–72.31) | 0.727 |
| FAI | 23.91 (1.49–36.22) | 19.15 (1.44–34.88) | 18.07 (1.19–36.48) | 0.298 |
| TT/E2 ratio | 114.60 (8.85–196.19) | 99.31 (7.12–176.30) | 98.41 (13.72–180.50) | 0.264 |
| Characteristics | Isopentanaldehyde, ng/mL | |||
|---|---|---|---|---|
| Tertile1 (n = 354) | Tertile2 (n = 355) | Tertile3 (n = 355) | P | |
| <0.391 | 0.391–0.861 | >0.861 | ||
| Age, years, median (IQR) | 45 (34–61) a,b | 47 (35–63) a | 45 (33–56) b | 0.048 |
| Gender, n (%) | ||||
| Male | 168 (47.46%) a | 219 (61.69%) b | 212 (59.72%) b | <0.001 |
| Female | 186 (52.54%) | 136 (38.31%) | 143 (40.28%) | |
| Ethnicity, n (%) | <0.001 | |||
| Mexican Americans | 63 (17.8%) a | 59 (16.62%) a | 19 (5.35%) b | |
| Other Hispanic | 32 (9.04%) a | 27 (7.61%) a,b | 18 (5.07%) b | |
| Non-Hispanic White | 140 (39.55%) a | 168 (47.32%) b | 200 (56.34%) c | |
| Non-Hispanic Black | 60 (16.95%) a | 51 (14.37%) a | 84 (23.66%) b | |
| Others | 59 (16.67%) a | 50 (14.08%) a,b | 34 (9.58%) b | |
| BMI, kg/m2, median (IQR) | 28.20 (24.73–34.18) a | 28.10 (24.90–32.80) a | 26.70 (22.70–30.55) b | <0.001 |
| <25, n (%) | 99 (27.97%) a | 91 (25.63%) a | 141 (39.72%) b | <0.001 |
| 25.0–30.0, n (%) | 101 (28.53%) a | 125 (35.21%) a | 121 (34.08%) a | |
| ≥30.0, n (%) | 154 (43.5%) a | 139 (39.15%) a | 93 (26.2%) b | |
| Serum Cotinine | <0.001 | |||
| ≥LOD | 226 (63.84%) a | 230 (64.79%) a | 346 (97.46%) b | |
| <LOD | 128 (36.16%) | 125 (35.21%) | 9 (2.54%) | |
| Education, n (%) | <0.001 | |||
| Less than high school | 77 (21.75%) a | 67 (18.87%) a | 106 (29.86%) b | |
| High school or equivalent | 69 (19.49%) a | 72 (20.28%) a | 103 (29.01%) b | |
| College or above | 208 (58.76%) a | 216 (60.85%) a | 146 (41.13%) b | |
| Family income-to-poverty ratio, n (%) | <0.001 | |||
| Low | 118 (33.33%) a | 110 (30.99%) a | 196 (55.21%) b | |
| Medium | 123 (34.75%) a | 116 (32.68%) a | 111 (31.27%) a | |
| High | 113 (31.92%) a | 129 (36.34%) a | 48 (13.52%) b | |
| Six-month time period | 0.443 | |||
| November 1 through April 30 | 172 (48.59%) | 181 (50.99%) | 164 (46.2%) | |
| May 1 through October 31 | 182 (51.41%) | 174 (49.01%) | 191 (53.8%) | |
| Time of blood draw | 0.020 | |||
| Morning | 189 (53.39%) a | 168 (47.32%) a,b | 145 (40.85%) b | |
| Afternoon | 116 (32.77%) a | 138 (38.87%) a,b | 152 (42.82%) b | |
| Evening | 49 (13.84%) a | 49 (13.8%) a | 58 (16.34%) a | |
| Sex hormones, median (IQR) | ||||
| TT,ng/dL | 55.99 (19.75–380.75) a | 256.79 (26.30–405.50) b | 284.00 (25.18–499.84) b | <0.001 |
| E2, pg/mL | 25.40 (15.35–45.93) | 24.20 (16.85–34.45) | 23.00 (14.60–34.85) | 0.142 |
| SHBG, nmol/L | 45.97 (29.01–69.61) a | 44.61 (29.96–66.22) a | 51.98 (36.30–79.33) b | <0.001 |
| FAI | 5.72 (1.18–35.33) a | 23.72 (1.59–36.41) b | 21.92 (1.43–36.24) a,b | 0.022 |
| TT/E2 ratio | 61.55 (5.67–157.61) a | 116.79 (12.21–178.99) b | 130.67 (21.02–216.30) b | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, Z.; Yuan, R.; Wang, X.; Xie, K.; Xu, B. Serum Concentrations of Benzaldehyde, Isopentanaldehyde and Sex Hormones: Evidence from the National Health and Nutrition Examination Survey. Toxics 2023, 11, 573. https://doi.org/10.3390/toxics11070573
Mao Z, Yuan R, Wang X, Xie K, Xu B. Serum Concentrations of Benzaldehyde, Isopentanaldehyde and Sex Hormones: Evidence from the National Health and Nutrition Examination Survey. Toxics. 2023; 11(7):573. https://doi.org/10.3390/toxics11070573
Chicago/Turabian StyleMao, Zhilei, Rui Yuan, Xu Wang, Kaipeng Xie, and Bo Xu. 2023. "Serum Concentrations of Benzaldehyde, Isopentanaldehyde and Sex Hormones: Evidence from the National Health and Nutrition Examination Survey" Toxics 11, no. 7: 573. https://doi.org/10.3390/toxics11070573




