Vitamin E (α-Tocopherol) Does Not Ameliorate the Toxic Effect of Bisphenol S on the Metabolic Analytes and Pancreas Histoarchitecture of Diabetic Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
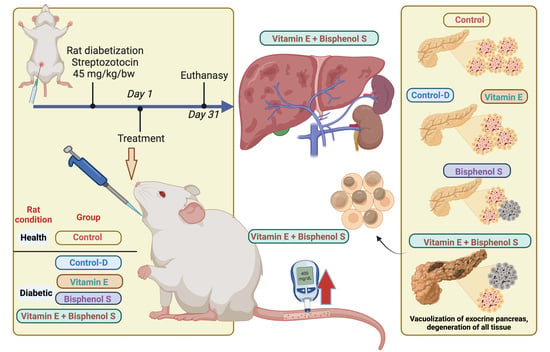
2.2. Experimental Design
2.3. Antioxidant Enzyme Activity
2.4. Glucose Tolerance Test
2.5. Biochemical Assays
2.6. Urinalysis
2.7. Nutrient Absorption and Digestibility
2.8. Pancreas Histology
2.9. Statistical Analysis
3. Results
3.1. Body Weight
3.2. Enzymatic Antioxidant Activities
3.3. Glucose Tolerance
3.4. Biochemical Assays
3.5. Pancreatic Histology
3.6. Urinalysis
3.7. Nutrient Absorption and Digestibility
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xing, J.; Zhang, S.; Zhang, M.; Hou, J. A critical review of presence, removal and potential impacts of endocrine disruptors bisphenol A. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2022, 254, 109275. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; Pang, W.K.; Ryu, D.Y.; Park, Y.J.; Pang, M.G. Multigenerational and transgenerational impact of paternal bisphenol A exposure on male fertility in a mouse model. Hum. Reprod. 2020, 35, 1740–1752. [Google Scholar] [CrossRef]
- Alboghobeish, S.; Mahdavinia, M.; Zeidooni, L.; Samimi, A.; Oroojan, A.A.; Alizadeh, S.; Dehghani, M.A.; Ahangarpour, A.; Khorsandi, L. Efficiency of naringin against reproductive toxicity and testicular damages induced by bisphenol A in rats. Iran. J. Basic. Med. Sci. 2019, 22, 315–523. [Google Scholar] [CrossRef] [PubMed]
- Othman, A.I.; Edrees, G.M.; El-Missiry, M.A.; Ali, D.A.; Aboel-Nour, M.; Dabdoub, B.R. Melatonin controlled apoptosis and protected the testes and sperm quality against bisphenol A-induced oxidative toxicity. Toxicol. Ind. Health 2016, 32, 1537–1549. [Google Scholar] [CrossRef]
- Karrer, C.; Roiss, T.; von Goetz, N.; Gramec Skledar, D.; Peterlin Mašič, L.; Hungerbühler, K. Physiologically Based Pharmacokinetic (PBPK) Modeling of the Bisphenols BPA, BPS, BPF, and BPAF with New Experimental Metabolic Parameters: Comparing the Pharmacokinetic Behavior of BPA with Its Substitutes. Environ. Health Perspect. 2018, 126, 077002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bousoumah, R.; Leso, V.; Iavicoli, I.; Huuskonen, P.; Viegas, S.; Porras, S.P.; Santonen, T.; Frery, N.; Robert, A.; Ndaw, S. Biomonitoring of occupational exposure to bisphenol A, bisphenol S and bisphenol F: A systematic review. Sci. Total Environ. 2021, 783, 146905. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.H.; Zhang, X.M.; Wang, F.; Gao, C.J.; Chen, D.; Palumbo, J.R.; Guo, Y.; Zeng, E.Y. Occurrence of bisphenol S in the environment and implications for human exposure: A short review. Sci. Total Environ. 2018, 615, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Kannan, K. Concentrations and profiles of bisphenol A and other bisphenol analogues in foodstuffs from the United States and their implications for human exposure. J. Agric. Food Chem. 2013, 61, 4655–4662. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Zhu, L. Occurrence and partitioning of bisphenol analogues in water and sediment from Liaohe River Basin and Taihu Lake, China. Water Res. 2016, 103, 343–351. [Google Scholar] [CrossRef]
- Yang, Y.; Lu, L.; Zhang, J.; Wu, Y.; Shao, B. Simultaneous determination of seven bisphenols in environmental water and solid samples by liquid chromatography-electrospray tandem mass spectrometry. J. Chromatogr. A 2014, 1328, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, E.; Yamashita, N.; Taniyasu, S.; Lam, J.; Lam, P.K.; Moon, H.B.; Jeong, Y.; Kannan, P.; Achyuthan, H.; Munuswamy, N.; et al. Bisphenol A and other bisphenol analogues including BPS and BPF in surface water samples from Japan, China, Korea and India. Ecotoxicol. Environ. Saf. 2015, 122, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Ivry Del Moral, L.; Le Corre, L.; Poirier, H.; Niot, I.; Truntzer, T.; Merlin, J.F.; Rouimi, P.; Besnard, P.; Rahmani, R.; Chagnon, M.C. Obesogen effects after perinatal exposure of 4,4′-sulfonyldiphenol (Bisphenol S) in C57BL/6 mice. Toxicology 2016, 357–358, 11–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rochester, J.R.; Bolden, A.L. Bisphenol S and F: A Systematic Review and Comparison of the Hormonal Activity of Bisphenol A Substitutes. Environ. Health Perspect. 2015, 123, 643–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mączka, W.; Grabarczyk, M.; Wińska, K. Can Antioxidants Reduce the Toxicity of Bisphenol? Antioxidants 2022, 11, 413. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Huang, G.; Guo, T.L. Bisphenol S Modulates Type 1 Diabetes Development in Non-Obese Diabetic (NOD) Mice with Diet- and Sex-Related Effects. Toxics 2019, 7, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogurtsova, K.; da Rocha Fernandes, J.D.; Huang, Y.; Linnenkamp, U.; Guariguata, L.; Cho, N.H.; Cavan, D.; Shaw, J.E.; Makaroff, L.E. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 2017, 128, 40–50. [Google Scholar] [CrossRef] [Green Version]
- Chiang, J.L.; Kirkman, M.S.; Laffel, L.M.; Peters, A.L.; on behalf of the Type 1 Diabetes Sourcebook Authors. Type 1 diabetes through the life span: A position statement of the American Diabetes Association. Diabetes Care 2014, 37, 2034–2054. [Google Scholar] [CrossRef] [Green Version]
- Alonso-Magdalena, P.; Morimoto, S.; Ripoll, C.; Fuentes, E.; Nadal, A. The estrogenic effect of bisphenol A disrupts pancreatic β-cell function in vivo and induces insulin resistance. Environ. Health Perspect. 2006, 114, 106–112. [Google Scholar] [CrossRef] [Green Version]
- Eshak, M.G.; Osman, H.F. Effects of Chitosan against Bisphenol- A Induced EndocrineToxicity and Androgen Receptor Gene Expression Changes in Male Rats. Int. J. Pharm. Clin. Res. 2014, 6, 300–311. [Google Scholar]
- Sahu, C.; Charaya, A.; Singla, S.; Dwivedi, D.K.; Jena, G. Zinc deficient diet increases the toxicity of bisphenol A in rat testis. J. Biochem. Mol. Toxicol. 2020, 34, e22549. [Google Scholar] [CrossRef]
- Khalaf, A.A.; Ahmed, W.; Moselhy, W.A.; Abdel-Halim, B.R.; Ibrahim, M.A. Protective effects of selenium and nano-selenium on bisphenol-induced reproductive toxicity in male rats. Hum. Exp. Toxicol. 2019, 38, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Behmanesh, M.A.; Najafzadehvarzi, H.; Poormoosavi, S.M. Protective Effect of Aloe vera Extract against Bisphenol A Induced Testicular Toxicity in Wistar Rats. Cell J. 2018, 20, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Yousaf, B.; Amina; Liu, G.; Wang, R.; Qadir, A.; Ali, M.U.; Kanwal, Q.; Munir, B.; Asmatullah; Abbas, Z. Bisphenol A exposure and healing effects of Adiantum capillus-veneris L. plant extract (APE) in bisphenol A-induced reproductive toxicity in albino rats. Environ. Sci. Pollut. Res. Int. 2016, 23, 11645–11657. [Google Scholar] [CrossRef]
- Zahra, Z.; Khan, M.R.; Majid, M.; Maryam, S.; Sajid, M. Gonadoprotective ability of Vincetoxicum arnottianum extract against bisphenol A-induced testicular toxicity and hormonal imbalance in male Sprague Dawley rats. Andrologia 2020, 52, e13590. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Gupta, P. Alteration in apoptotic rate of testicular cells and sperms following administration of Bisphenol A (BPA) in Wistar albino rats. Environ. Sci. Pollut. Res. Int. 2018, 25, 21635–21643. [Google Scholar] [CrossRef]
- Gules, O.; Yildiz, M.; Naseer, Z.; Tatar, M. Effects of folic acid on testicular toxicity induced by bisphenol-A in male Wistar rats. Biotech. Histochem. 2019, 94, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Aydoğan, M.; Korkmaz, A.; Barlas, N.; Kolankaya, D. Pro-oxidant effect of vitamin C coadministration with bisphenol A, nonylphenol, and octylphenol on the reproductive tract of male rats. Drug Chem. Toxicol. 2010, 33, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K.; Khanna, S.; Roy, S. Tocotrienols: Vitamin E beyond tocopherols. Life Sci. 2006, 78, 2088–2098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Traber, M.G.; Atkinson, J. Vitamin E, antioxidant and nothing more. Free Radic. Biol. Med. 2007, 43, 4–15. [Google Scholar] [CrossRef] [Green Version]
- Al Shamsi, M.S.; Amin, A.; Adeghate, E. Beneficial effect of vitamin E on the metabolic parameters of diabetic rats. Mol. Cell Biochem. 2004, 261, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Mercuri, F.; Quagliaro, L.; Ceriello, A. Oxidative stress evaluation in diabetes. Diabetes Technol. Ther. 2000, 2, 589–600. [Google Scholar] [CrossRef]
- Nimisha, B.; Sendhilvadivu, M. Vitamin E Modulates the Oxidant-Antioxidant Imbalance of BPA induced Oxidative Stress in Albino Rats. Int. J. Sci. Res. 2018, 7, 900–906. [Google Scholar]
- Abdelrazik, E.; Hassan, H.M.; Abdallah, Z.; Magdy, A.; Farrag, E.A. Renoprotective effect of N-acetylcystein and vitamin E in bisphenol A-induced rat nephrotoxicity; Modulators of Nrf2/ NF-κB and ROS signaling pathway. Acta Biomed. 2022, 93, e2022301. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Sarkar, K.; Nath, P.P.; Mondal, M.; Khatun, A.; Paul, G. Bisphenol S impairs blood functions and induces cardiovascular risks in rats. Toxicol. Rep. 2017, 4, 560–565. [Google Scholar] [CrossRef] [PubMed]
- Rashad, S.; Ahmed, S.; El-Sayed, M.; Ahmed, D. The Toxic Effect of Bisphenol A on Albino Rat Testicles and the Possible Protective Value of Vitamin E and Melatonin. Egypt. Soc. Clin. Toxicol. J. 2021, 9, 1–12. [Google Scholar] [CrossRef]
- Amraoui, W.; Adjabi, N.; Bououza, F.; Boumendjel, M.; Taibi, F.; Boumendjel, A.; Abdennour, C.; Messarah, M. Modulatory Role of Selenium and Vitamin E, Natural Antioxidants, against Bisphenol A-Induced Oxidative Stress in Wistar Albinos Rats. Toxicol. Res. 2018, 34, 231–239. [Google Scholar] [CrossRef] [Green Version]
- Eid, R.A.; Zaki, M.S.A.; Alghamd, M.A.; Wares, A.; Eldeen, M.A.; Massoud, E.E.S.; Haidara, M.A. Ameliorative Effect of Vitamin E on Biochemical and Ultrastructural Changes in Artemether-induced Renal Toxicity in Rats. Int. J. Morphol. 2020, 38, 461–471. [Google Scholar] [CrossRef] [Green Version]
- Al-Mousawi, A.M.; Kulp, G.A.; Branski, L.K.; Kraft, R.; Mecott, G.A.; Williams, F.N.; Herndon, D.N.; Jeschke, M.G. Impact of anesthesia, analgesia, and euthanasia technique on the inflammatory cytokine profile in a rodent model of severe burn injury. Shock 2010, 34, 261–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Rojas, J.M.; Guerrero-Beltrán, C.E.; Cruz, C.; Sánchez-González, D.J.; Martínez-Martínez, C.M.; Pedraza-Chaverri, J. Preventive effect of tert-butylhydroquinone on cisplatin-induced nephrotoxicity in rats. Food Chem. Toxicol. 2011, 49, 2631–2637. [Google Scholar] [CrossRef] [PubMed]
- Bagi, C.M.; Edwards, K.; Berryman, E. Metabolic Syndrome and Bone: Pharmacologically Induced Diabetes has Deleterious Effect on Bone in Growing Obese Rats. Calcif. Tissue Int. 2018, 102, 683–694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giknis, M.; Clifford, C. Clinical Laboratory Parameters for Crl:Wi(Han). Available online: https://www.criver.com/sites/default/files/resources/rm_rm_r_Wistar_Han_clin_lab_parameters_08.pdf (accessed on 1 March 2023).
- Andallu, B.; Vinay Kumar, A.V.; Varadacharyulu, N.C. Lipid abnormalities in streptozotocin-diabetes: Amelioration by Morus indica L. cv Suguna leaves. Int. J. Diabetes Dev. Ctries. 2009, 29, 123–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barriga, S.A.; Peña-Corona, S.I.; Caballero, S.C.; Gracia, M.I.; Luna del Villar, J.; Escobar-Chavez, J.J.; Rivera, M.; Macias, L.; Garcia, H.; Vargas-Estrada, D. Effect of Tributyrin Supplementation on Glucose Levels, Liver and Kidney Integrity in an Experimental Model of Diabetes Mellitus. Int. J. Pharmacol. 2022, 18, 1387–1399. [Google Scholar] [CrossRef]
- GIMA. GIMAURS-10T Urinalysis Reagent Strips Instructions. Available online: https://manuals.plus/gima/gimaurs-10t-urinalysis-reagent-strips-manual.pdf (accessed on 1 March 2023).
- Queremel, D.A.; Ishiwarlal, M. Urianalisis; StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- AOAC Association Official Analytical Chemists. Official Methods of Analysis, 14th ed.; The Association: Arlington, VA, USA, 2011. [Google Scholar]
- Van Keulen, J.; Young, B.A. Evaluation of Acid-Insoluble Ash as a Natural Marker in Ruminant Digestibility Studies. J. Anim. Sci. 1977, 44, 282–287. [Google Scholar] [CrossRef]
- Zachary, J.F. Pathologic Basis of Veterinary Disease, 6th ed.; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Shubin, A.V.; Demidyuk, I.V.; Komissarov, A.A.; Rafieva, L.M.; Kostrov, S.V. Cytoplasmic vacuolization in cell death and survival. Oncotarget 2016, 7, 55863. [Google Scholar] [CrossRef] [Green Version]
- Reboul, E. Vitamin E Bioavailability: Mechanisms of Intestinal Absorption in the Spotlight. Antioxidants 2017, 6, 95. [Google Scholar] [CrossRef] [Green Version]
- Inoue, H.; Yuki, G.; Yokota, H.; Kato, S. Bisphenol A glucuronidation and absorption in rat intestine. Drug Metab. Dispos. 2003, 31, 140–144. [Google Scholar] [CrossRef] [Green Version]
- Brealey, D.; Singer, M. Hyperglycemia in critical illness: A review. J. Diabetes Sci. Technol. 2009, 3, 1250–1260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmeira, C.M.; Rolo, A.P.; Berthiaume, J.; Bjork, J.A.; Wallace, K.B. Hyperglycemia decreases mitochondrial function: The regulatory role of mitochondrial biogenesis. Toxicol. Appl. Pharmacol. 2007, 225, 214–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skledar, D.G.; Schmidt, J.; Fic, A.; Klopčič, I.; Trontelj, J.; Dolenc, M.S.; Finel, M.; Mašič, L.P. Influence of metabolism on endocrine activities of bisphenol S. Chemosphere 2016, 157, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wang, S.; Yang, B.; Zheng, J.; Cai, Y.; Yang, Z. Weight loss before a diagnosis of type 2 diabetes mellitus is a risk factor for diabetes complications. Medicine 2016, 95, e5618. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, A.M.; Arencibia, A.L.R.; García, M.L.G.; Cardoso, J.J.P.; Pérez, Q.Q. Estrés oxidativo en la diabetes mellitus papel de la vitamina E y antioxidantes endógenos. Rev. Cienc. Méd. 2015, 19, 973–985. [Google Scholar]
- Kaplanoglu, G.T.; Bahcelioglu, M.; Gozil, R.; Helvacioglu, F.; Buru, E.; Tekindal, M.A.; Erdogan, D.; Calguner, E. Effects of green tea and vitamin E in the testicular tissue of streptozotocin-induced diabetic rats. Saudi Med. J. 2013, 34, 734–743. [Google Scholar]
- Sharma, A.K.; Ponery, A.S.; Lawrence, P.A.; Ahmed, I.; Bastaki, S.M.; Dhanasekaran, S.; Sheen, R.S.; Adeghate, E. Effect of alpha-tocopherol supplementation on the ultrastructural abnormalities of peripheral nerves in experimental diabetes. J. Peripher. Nerv. Syst. 2001, 6, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Mandrah, K.; Jain, V.; Ansari, J.A.; Roy, S.K. Metabolomic perturbation precedes glycolytic dysfunction and procreates hyperglycemia in a rat model due to bisphenol S exposure. Environ. Toxicol. Pharmacol. 2020, 77, 103372. [Google Scholar] [CrossRef] [PubMed]
- Sahu, C.; Singla, S.; Jena, G. Studies on male gonadal toxicity of bisphenol A in diabetic rats: An example of exacerbation effect. J. Biochem. Mol. Toxicol. 2022, 36, e22996. [Google Scholar] [CrossRef]
- Peña-Corona, S.I.; Vásquez Aguire, W.S.; Vargas, D.; Juárez, I.; Mendoza-Rodríguez, C.A. Effects of bisphenols on Blood-Testis Barrier protein expression in vitro: A systematic review and meta-analysis. Reprod. Toxicol. 2021, 103, 139–148. [Google Scholar] [CrossRef]
- Wu, H.; Wei, Y.; Zhou, Y.; Long, C.; Hong, Y.; Fu, Y.; Zhao, T.; Wang, J.; Wu, Y.; Wu, S.; et al. Bisphenol S perturbs Sertoli cell junctions in male rats via alterations in cytoskeletal organization mediated by an imbalance between mTORC1 and mTORC2. Sci. Total Environ. 2021, 762, 144059. [Google Scholar] [CrossRef] [PubMed]
- Rosenmai, A.K.; Dybdahl, M.; Pedersen, M.; Alice van Vugt-Lussenburg, B.M.; Wedebye, E.B.; Taxvig, C.; Vinggaard, A.M. Are structural analogues to bisphenol a safe alternatives? Toxicol. Sci. 2014, 139, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, S.; Solís-Lemus, E.; Jiménez-Vivanco, J.; Castellanos-Ruiz, D.; Díaz-Díaz, E.; Mendoza-Rodríguez, C.A. Maternal perinatal exposure to bisphenol S induces an estrogenic like effect in glucose homeostasis in male offspring. Environ. Toxicol. 2022, 37, 2189–2200. [Google Scholar] [CrossRef] [PubMed]
- Benedé-Ubieto, R.; Estévez-Vázquez, O.; Ramadori, P.; Cubero, F.J.; Nevzorova, Y.A. Guidelines and Considerations for Metabolic Tolerance Tests in Mice. Diabetes Metab. Syndr. Obes. 2020, 13, 439–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phillips, P.J. Oral glucose tolerance testing. Aust. Fam. Physician 2012, 41, 391–393. [Google Scholar] [PubMed]
- Institute of Medicine (US) Panel on Dietary Antioxidants and Related Compounds. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. J. Acad. Nutr. Diet. 2000, 100, 637. [Google Scholar]
- Reaven, P.D.; Khouw, A.; Beltz, W.F.; Parthasarathy, S.; Witztum, J.L. Effect of dietary antioxidant combinations in humans. Protection of LDL by vitamin E but not by beta-carotene. Arterioscler. Thromb. 1993, 13, 590–600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takemoto, K.; Doi, W.; Masuoka, N. Protective effect of vitamin E against alloxan-induced mouse hyperglycemia. Biochim. Biophys. Acta 2016, 1862, 647–650. [Google Scholar] [CrossRef] [PubMed]
- Ceriello, A.; Giugliano, D.; Quatraro, A.; Donzella, C.; Dipalo, G.; Lefebvre, P.J. Vitamin E reduction of protein glycosylation in diabetes. New prospect for prevention of diabetic complications? Diabetes Care 1991, 14, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Tuñon, M.J.; Gonzalez, P.; Garcia-Pardo, L.A.; Gonzalez, J. Hepatic transport of bilirubin in rats with streptozotocin-induced diabetes. J. Hepatol. 1991, 13, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Gulle, K.; Ceri, N.G.; Akpolat, M.; Arasli, M.; Demirci, B. The effects of dexpanthenol in streptozotocin-induced diabetic rats: Histological, histochemical and immunological evidences. Histol. Histopathol. 2014, 29, 1305–1313. [Google Scholar] [CrossRef]
- Medina, M.; Meyer, W.; Echeverri, C.; Builes, N. Langerhans cell histiocytosis: Case report and literature review. Biomedica 2021, 41, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Collin, M.; Bigley, V.; McClain, K.L.; Allen, C.E. Cell(s) of Origin of Langerhans Cell Histiocytosis. Hematol. Oncol. Clin. N. Am. 2015, 29, 825–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamimura, W.; Doi, W.; Takemoto, K.; Ishihara, K.; Wang, D.H.; Sugiyama, H.; Oda, S.; Masuoka, N. Effect of vitamin E on alloxan-induced mouse diabetes. Clin. Biochem. 2013, 46, 795–798. [Google Scholar] [CrossRef]
- Gómez, J.A.; Molero, X.; Vaquero, E.; Alonso, A.; Salas, A.; Malagelada, J.R. Vitamin E attenuates biochemical and morphological features associated with development of chronic pancreatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 287, G162–G169. [Google Scholar] [CrossRef] [PubMed]
- Villar-Pazos, S.; Martinez-Pinna, J.; Castellano-Muñoz, M.; Alonso-Magdalena, P.; Marroqui, L.; Quesada, I.; Gustafsson, J.A.; Nadal, A. Molecular mechanisms involved in the non-monotonic effect of bisphenol-a on Ca2+ entry in mouse pancreatic β-cells. Sci. Rep. 2017, 7, 11770. [Google Scholar] [CrossRef] [Green Version]
- Ozaydın, T.; Oznurlu, Y.; Sur, E.; Celik, I.; Uluısık, D.; Dayan, M.O. Effects of bisphenol A on antioxidant system and lipid profile in rats. Biotech. Histochem. 2018, 93, 231–238. [Google Scholar] [CrossRef]
- Monteiro, T.H.; Silva, C.S.; Cordeiro Simões Ambrosio, L.M.; Zucoloto, S.; Vannucchi, H. Vitamin E alters inflammatory gene expression in alcoholic chronic pancreatitis. J. Nutr. Nutr. 2012, 5, 94–105. [Google Scholar] [CrossRef]
- Li, S.; Zhou, J.; Xu, S.; Li, J.; Liu, J.; Lu, Y.; Shi, J.; Zhou, S.; Wu, Q. Induction of Nrf2 pathway by Dendrobium nobile Lindl. alkaloids protects against carbon tetrachloride induced acute liver injury. Biomed. Pharmacother. 2019, 117, 109073. [Google Scholar] [CrossRef]
- Ghafoor, S.; Abbasi, M.H.; Khawar, M.B.; Tayyeb, A.; Saleem, T.; Ashfaq, I.; Sheikh, N. Bisphenol S induced dysregulations in liver; iron regulatory genes and inflammatory mediators in male Wistar rats. Environ. Sci. Pollut. Res. Int. 2022, 29, 83711–83722. [Google Scholar] [CrossRef]
- Nagarajan, S.R.; Paul-Heng, M.; Krycer, J.R.; Fazakerley, D.J.; Sharland, A.F.; Hoy, A.J. Lipid and glucose metabolism in hepatocyte cell lines and primary mouse hepatocytes: A comprehensive resource for in vitro studies of hepatic metabolism. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E578–E589. [Google Scholar] [CrossRef]
- Al-Shamsi, M.; Amin, A.; Adeghate, E. Vitamin E ameliorates some biochemical parameters in normal and diabetic rats. Ann. N. Y. Acad. Sci. 2006, 1084, 411–431. [Google Scholar] [CrossRef]
- Hassan, Z.K.; Elobeid, M.A.; Virk, P.; Omer, S.A.; ElAmin, M.; Daghestani, M.H.; AlOlayan, E.M. Bisphenol A induces hepatotoxicity through oxidative stress in rat model. Oxid. Med. Cell Longev. 2012, 2012, 194829. [Google Scholar] [CrossRef] [Green Version]
- Mahdavinia, M.; Alizadeh, S.; Raesi Vanani, A.; Dehghani, M.A.; Shirani, M.; Alipour, M.; Shahmohammadi, H.A.; Rafiei Asl, S. Effects of quercetin on bisphenol A-induced mitochondrial toxicity in rat liver. Iran. J. Basic. Med. Sci. 2019, 22, 499–505. [Google Scholar] [CrossRef]
- Figueira, M.F.; Castiglione, R.C.; de Lemos Barbosa, C.M.; Ornellas, F.M.; da Silva Feltran, G.; Morales, M.M.; da Fonseca, R.N.; de Souza-Menezes, J. Diabetic rats present higher urinary loss of proteins and lower renal expression of megalin, cubilin, ClC-5, and CFTR. Physiol. Rep. 2017, 5, e13335. [Google Scholar] [CrossRef]
- Tang, S.C.W.; Leung, J.C.K.; Lai, K.N. Diabetic tubulopathy: An emerging entity. Contrib. Nephrol. 2011, 170, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Warren, B.L.; Pak, R.; Finlayson, M.; Gontovnick, L.; Sunahara, G.; Bellward, G.D. Differential effects of diabetes on microsomal metabolism of various substrates. Comparison of streptozotocin and spontaneously diabetic Wistar rats. Biochem. Pharmacol. 1983, 32, 327–335. [Google Scholar] [CrossRef]
- Dilworth, L.; Facey, A.; Omoruyi, F. Diabetes Mellitus and Its Metabolic Complications: The Role of Adipose Tissues. Int. J. Mol. Sci. 2021, 22, 7644. [Google Scholar] [CrossRef] [PubMed]
- Barzegar-Amini, M.; Ghazizadeh, H.; Seyedi, S.M.R.; Sadeghnia, H.R.; Mohammadi, A.; Hassanzade-Daloee, M.; Barati, E.; Kharazmi-Khorassani, S.; Kharazmi-Khorassani, J.; Mohammadi-Bajgiran, M.; et al. Serum vitamin E as a significant prognostic factor in patients with dyslipidemia disorders. Diabetes Metab. Syndr. 2019, 13, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Fuller, C.J.; Chandalia, M.; Garg, A.; Grundy, S.M.; Jialal, I. RRR-alpha-tocopheryl acetate supplementation at pharmacologic doses decreases low-density-lipoprotein oxidative susceptibility but not protein glycation in patients with diabetes mellitus. Am. J. Clin. Nutr. 1996, 63, 753–759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baydas, G.; Canatan, H.; Turkoglu, A. Comparative analysis of the protective effects of melatonin and vitamin E on streptozocin-induced diabetes mellitus. J. Pineal Res. 2002, 32, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Fedorak, R.N.; Chang, E.B.; Madara, J.L.; Field, M. Intestinal adaptation to diabetes. Altered Na-dependent nutrient absorption in streptozocin-treated chronically diabetic rats. J. Clin. Investig. 1987, 79, 1571–1578. [Google Scholar] [CrossRef]
- Rayner, C.K.; Schwartz, M.P.; van Dam, P.S.; Renooij, W.; de Smet, M.; Horowitz, M.; Smout, A.J.; Samsom, M. Small intestinal glucose absorption and duodenal motility in type 1 diabetes mellitus. Am. J. Gastroenterol. 2002, 97, 3123–3130. [Google Scholar] [CrossRef] [PubMed]
- Vergès, B. Intestinal lipid absorption and transport in type 2 diabetes. Diabetologia 2022, 65, 1587–1600. [Google Scholar] [CrossRef]
- da Rosa Lima, T.; Ávila, E.T.P.; Fraga, G.A.; de Souza Sena, M.; de Souza Dias, A.B.; de Almeida, P.C.; Dos Santos Trombeta, J.C.; Junior, R.C.V.; Damazo, A.S.; Navalta, J.W.; et al. Effect of administration of high-protein diet in rats submitted to resistance training. Eur. J. Nutr. 2018, 57, 1083–1096. [Google Scholar] [CrossRef]
- Torres-García, J.L.; Ahuactzin-Pérez, M.; Fernández, F.J.; Cortés-Espinosa, D.V. Bisphenol A in the environment and recent advances in biodegradation by fungi. Chemosphere 2022, 303, 134940. [Google Scholar] [CrossRef] [PubMed]
- Ogata, Y.; Goda, S.; Toyama, T.; Sei, K.; Ike, M. The 4-tert-butylphenol-utilizing bacterium Sphingobium fuliginis OMI can degrade bisphenols via phenolic ring hydroxylation and meta-cleavage pathway. Environ. Sci. Technol. 2013, 47, 1017–1023. [Google Scholar] [CrossRef]
- Ike, M.; Chen, M.Y.; Danzl, E.; Sei, K.; Fujita, M. Biodegradation of a variety of bisphenols under aerobic and anaerobic conditions. Water Sci. Technol. 2006, 53, 153–159. [Google Scholar] [CrossRef] [Green Version]
- Cao, G.; He, R.; Cai, Z.; Liu, J. Photolysis of bisphenol S in aqueous solutions and the effects of different surfactants. React. Kinet. Mech. Catal. 2013, 109, 259–271. [Google Scholar] [CrossRef]
- Korkmaz, A.; Aydoğan, M.; Kolankaya, D.; Barlas, N. Vitamin C coadministration augments bisphenol A, nonylphenol, and octylphenol induced oxidative damage on kidney of rats. Environ. Toxicol. 2011, 26, 325–337. [Google Scholar] [CrossRef]
- Korkmaz, A.; Ahbab, M.A.; Kolankaya, D.; Barlas, N. Influence of vitamin C on bisphenol A, nonylphenol and octylphenol induced oxidative damages in liver of male rats. Food Chem. Toxicol. 2010, 48, 2865–2871. [Google Scholar] [CrossRef]
- Young, A.J.; Lowe, G.M. Antioxidant and prooxidant properties of carotenoids. Arch. Biochem. Biophys. 2001, 385, 20–27. [Google Scholar] [CrossRef]
- Baltusnikiene, A.; Staneviciene, I.; Jansen, E. Beneficial and adverse effects of vitamin E on the kidney. Front. Physiol. 2023, 14, 1145216. [Google Scholar] [CrossRef] [PubMed]
- Abudu, N.; Miller, J.J.; Attaelmannan, M.; Levinson, S.S. Vitamins in human arteriosclerosis with emphasis on vitamin C and vitamin E. Clin. Chim. Acta 2004, 339, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Rahal, A.; Kumar, A.; Singh, V.; Yadav, B.; Tiwari, R.; Chakraborty, S.; Dhama, K. Oxidative stress, prooxidants, and antioxidants: The interplay. Biomed. Res. Int. 2014, 2014, 761264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, L.; Kang, Y.J. Oxidative stress and diabetic cardiomyopathy: A brief review. Cardiovasc. Toxicol. 2001, 1, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Pricci, F.; Leto, G.; Amadio, L.; Iacobini, C.; Cordone, S.; Catalano, S.; Zicari, A.; Sorcini, M.; Di Mario, U.; Pugliese, G. Oxidative stress in diabetes-induced endothelial dysfunction involvement of nitric oxide and protein kinase C. Free Radic. Biol. Med. 2003, 35, 683–694. [Google Scholar] [CrossRef]
- Baynes, J.W. Role of oxidative stress in development of complications in diabetes. Diabetes 1991, 40, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Mullarkey, C.J.; Edelstein, D.; Brownlee, M. Free radical generation by early glycation products: A mechanism for accelerated atherogenesis in diabetes. Biochem. Biophys. Res. Commun. 1990, 173, 932–939. [Google Scholar] [CrossRef] [PubMed]
- Je, H.D.; Shin, C.Y.; Park, H.S.; Huh, I.H.; Sohn, U.D. The comparison of vitamin C and vitamin E on the protein oxidation of diabetic rats. J. Auton. Pharmacol. 2001, 21, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Uzunhisarcikli, M.; Aslanturk, A. Hepatoprotective effects of curcumin and taurine against bisphenol A-induced liver injury in rats. Environ. Sci. Pollut. Res. Int. 2019, 26, 37242–37253. [Google Scholar] [CrossRef]
- Shirani, M.; Alizadeh, S.; Mahdavinia, M.; Dehghani, M.A. The ameliorative effect of quercetin on bisphenol A-induced toxicity in mitochondria isolated from rats. Environ. Sci. Pollut. Res. Int. 2019, 26, 7688–7696. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi-Motamayel, F.; Goodarzi, M.T.; Mahdavinezhad, A.; Jamshidi, Z.; Darvishi, M. Salivary and Serum Antioxidant and Oxidative Stress Markers in Dental Caries. Caries Res. 2018, 52, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Morrison, M.H.; Hawksworth, G.M. Glucuronic acid conjugation by hepatic microsomal fractions isolated from streptozotocin-induced diabetic rats. Biochem. Pharmacol. 1984, 33, 3833–3838. [Google Scholar] [CrossRef] [PubMed]
- Grant, M.H.; Duthie, S.J. Conjugation reactions in hepatocytes isolated from streptozotocin-induced diabetic rats. Biochem. Pharmacol. 1987, 36, 3647–3655. [Google Scholar] [CrossRef] [PubMed]
- Brigelius-Flohé, R. Adverse effects of vitamin E by induction of drug metabolism. Genes. Nutr. 2007, 2, 249–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, S.; Tezuka, Y.; Ushiyama, A.; Kawashima, C.; Kitagawara, Y.; Takahashi, K.; Ohta, S.; Mashino, T. Ipso substitution of bisphenol A catalyzed by microsomal cytochrome P450 and enhancement of estrogenic activity. Toxicol. Lett. 2011, 203, 92–95. [Google Scholar] [CrossRef]
- Ungurianu, A.; Zanfirescu, A.; Nițulescu, G.; Margină, D. Vitamin E beyond Its Antioxidant Label. Antioxidants 2021, 10, 634. [Google Scholar] [CrossRef] [PubMed]
- Hermano Sampaio Dias, A.; Yadav, R.; Mokkawes, T.; Kumar, A.; Skaf, M.S.; Sastri, C.V.; Kumar, D.; de Visser, S.P. Biotransformation of Bisphenol by Human Cytochrome P450 2C9 Enzymes: A Density Functional Theory Study. Inorg. Chem. 2023, 62, 2244–2256. [Google Scholar] [CrossRef] [PubMed]
- Santos Musachio, E.A.; Poetini, M.R.; Janner, D.E.; Fernandes, E.J.; Meichtry, L.B.; Mustafa Dahleh, M.M.; Guerra, G.P.; Prigol, M. Safer alternatives? Bisphenol F and Bisphenol S induce oxidative stress in Drosophila melanogaster larvae and trigger developmental damage. Food Chem. Toxicol. 2023, 175, 113701. [Google Scholar] [CrossRef]
- Abe, C.; Uchida, T.; Ohta, M.; Ichikawa, T.; Yamashita, K.; Ikeda, S. Cytochrome P450-dependent metabolism of vitamin E isoforms is a critical determinant of their tissue concentrations in rats. Lipids 2007, 42, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Herrera, E.; Barbas, C. Vitamin E: Action, metabolism and perspectives. J. Physiol. Biochem. 2001, 57, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Uchida, T.; Nomura, S.; Ichikawa, T.; Abe, C.; Ikeda, S. Tissue distribution of vitamin E metabolites in rats after oral administration of tocopherol or tocotrienol. J. Nutr. Sci. Vitaminol. 2011, 57, 326–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Q.; Shao, W.; Weng, Z.; Zhang, X.; Ding, G.; Xu, C.; Xu, J.; Jiang, Z.; Gu, A. In vitro evaluation of the hepatic lipid accumulation of bisphenol analogs: A high-content screening assay. Toxicol. In Vitro 2020, 68, 104959. [Google Scholar] [CrossRef]
- Omran, B.; Abdallah, E.; Abdelwahab, M. Study of Probable Toxic Effects of Bisphenol A & the Protective Role of Vitamin E on Testes and Prostate of Adult Male Albino Rats. Ain Shams J. Forensic Med. Clin. Toxicol. 2017, 29, 7–18. [Google Scholar] [CrossRef] [Green Version]
- Radwa, M.; Al-Sayed, M.D.; Heba, O.; Mohammed, M.D.; Azza, I.; Farag, M.D.; Khaled, A.; Abulfadle, M.D. Potential Protective Role of Vitamin E in Lung of Adult Male Albino Rat Exposed to Bisphenol A. Med. J. Cairo Univ. 2019, 87, 4901–4915. [Google Scholar] [CrossRef]
- El-Hak, H.N.G.; ELaraby, E.E.; Hassan, A.K.; Abbas, O.A. Study of the toxic effect and safety of vitamin E supplement in male albino rats after 30 days of repeated treatment. Heliyon 2019, 5, e02645. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Brand | Name | Catalog Number | Lot | Expiration |
|---|---|---|---|---|
| SEKISUI Diagnostics® | Albumin | 200-05 | 59489 | 29 February 2024 |
| ALT | 318-30 | 61318 | 11 February 2023 | |
| AST | 319-10 | 60989 | 8 April 2023 | |
| BUN | 283-30 | 61232 | 31 July 2023 | |
| Creatinine | 221-30 | 58181 | 31 August 2023 | |
| HDL-c | 1001-80 | 59195 | 27 January 2023 | |
| LDL-c | 1014-80 | 58142 | 6 October 2022 | |
| Total protein | 200-55 | 61229 | 30 November 2024 | |
| SYNER-MED® | Total bilirubin | IR701 | 242105 | 24 May 2023 |
| Analyte | RV | Ctrl | Ctrl-D | VitE-D | BPS-D | VitE + BPS-D |
|---|---|---|---|---|---|---|
| Creatinine (μmol/L) | 09.00–70.00 | 58.50 ± 3.32 | 55.40 ± 3.01 | 52.20 ± 1.59 | 56.40 ± 3.44 | 58.60 ± 7.90 |
| AST (U/L) | 46.00–245.0 | 213.17 ± 17.99 | 379.20 ± 92.38 | 283.00 ± 53.85 | 349.40 ± 93.67 | 423.50 ± 82.12 |
| HDL-c (mmol/L) | 0.6–0.75 | 0.69 ± 0.05 | 1.16 ± 0.10 * | 1.05 ± 0.08 * | 0.95 ± 0.05 | 1.05 ± 0.15 * |
| LDL-c (mmol/L) | 0.49–0.05 | 0.36 ± 0.03 | 0.50 ± 0.11 | 0.47 ± 0.04 | 0.54 ± 0.02 | 0.80 ± 0.15 *,& |
| Albumin /globulin | 0.44–2.68 | 1.08 ± 0.04 | 0.82 ± 0.07 * | 0.92 ± 0.08 | 0.98 ± 0.02 | 0.80 ± 0.06 * |
| Group | ID | Langerhans Islets Atrophy | Endocrine Degeneration | Endocrine Lipid Infiltration | Exocrine Degeneration | Exocrine Vacuolization | Findings |
|---|---|---|---|---|---|---|---|
| Ctrl | 1 | − | − | − | − | − | NF |
| 2 | − | − | − | − | − | NF | |
| 3 | − | − | − | − | − | NF | |
| 4 | − | − | − | − | − | NF | |
| 5 | − | − | − | − | − | NF | |
| 6 | − | − | − | − | − | NF | |
| Ctrl−D | 1 | +++ | ++ | ++ | − | ++ | NF |
| 2 | +++ | ++ | − | − | ++ | NF | |
| 3 | ++ | ++ | ++ | − | ++ | NF | |
| 4 | ++ | − | − | − | − | NF | |
| 5 | +++ | − | − | − | − | NF | |
| VitE−D | 1 | + | − | − | − | − | NF |
| 2 | − | − | ++ | − | − | NF | |
| 3 | − | + | + | − | − | NF | |
| 4 | ++ | − | − | − | +++ | LE | |
| 5 | − | + | − | − | − | NF | |
| BPS−D | 1 | − | − | − | + | − | NF |
| 2 | ++ | − | − | + | − | NF | |
| 3 | − | ++ | − | + | − | LNH | |
| 4 | ++ | − | − | − | − | NF | |
| 5 | − | ++ | − | ++ | − | NF | |
| VitE + BPS−D | 1 | ++ | − | − | +++ | − | NF |
| 2 | − | − | − | ++ | − | NF | |
| 3 | ++ | − | − | − | ++ | NF | |
| 4 | − | − | − | + | − | LE | |
| 5 | − | − | − | − | − | LE |
| Analyte | Ctrl | Ctrl-D | VitE-D | BPS-D | VitE + BPS-D |
|---|---|---|---|---|---|
| Specific gravity | 1.01 ± 0.00 | 1.06 ± 0.00 * | 1.04 ± 0.01 *,#,$ | 1.07 ± 0.00 * | 1.05 ± 0.01 *,$,& |
| Leukocytes (cells/μL) | 6.00 ± 3.67 | 92.00 ± 13.47 | 178.00 ± 22.00 * | 103.00 ± 83.27 | 59.00 ± 13.47 |
| pH | 6.90 ± 0.25 | 5.50 ± 0.27 | 5.90 ± 0.40 | 5.80 ± 0.34 | 5.60 ± 0.40 |
| Protein (g/L) | 0.14 ± 0.04 | 0.10 ± 0.00 | 0.26 ± 0.04 | 4.38 ± 3.90 | 0.10 ± 0.00 |
| Glucose (mmol/L) | 6.00 ± 3.67 | 48.00 ± 7.35 * | 42.00 ± 7.35 * | 27.00 ± 3.00 | 64.00 ± 12.88 *,$ |
| Nutrient | FS | Food | Ctrl | Ctrl-D | VitE-D | BPS-D | VitE + BPS-D |
|---|---|---|---|---|---|---|---|
| Humidity (%) | 12.0 | 11.03 | 56.47 | 60.95 | 46.64 | 57.60 | 46.05 |
| Dry matter (%) | 88.0 | 88.97 | 43.53 | 39.05 | 53.36 | 42.40 | 53.95 |
| Raw protein 1 (%) | 23.5 | 21.10 | 12.45 | 8.77 | 10.06 | 8.51 | 10.47 |
| Raw fat 1 (%) | 6.0 | 5.47 | 2.59 | 1.29 | 1.92 | 1.47 | 1.89 |
| Ash 1 (%) | 8.0 | 9.67 | 13.01 | 9.04 | 11.91 | 9.47 | 11.77 |
| Raw fiber 1 (%) | 4.0 | 5.58 | 3.97 | 3.99 | 4.51 | 3.53 | 5.05 |
| NFE 1 (%) | 46.5 | 47.15 | 11.51 | 15.96 | 24.96 | 19.42 | 24.77 |
| AIA (%) | - | 0.84 | 2.31 | 1.10 | 2.07 | 1.62 | 1.70 |
| Digestibility (%) | - | - | 83.84 | 43.84 | 79.62 | 68.35 | 70.79 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peña-Corona, S.I.; Vargas-Estrada, D.; Chávez-Corona, J.I.; Mendoza-Rodríguez, C.A.; Caballero-Chacón, S.; Pedraza-Chaverri, J.; Gracia-Mora, M.I.; Galván-Vela, D.P.; García-Rodríguez, H.; Sánchez-Bartez, F.; et al. Vitamin E (α-Tocopherol) Does Not Ameliorate the Toxic Effect of Bisphenol S on the Metabolic Analytes and Pancreas Histoarchitecture of Diabetic Rats. Toxics 2023, 11, 626. https://doi.org/10.3390/toxics11070626
Peña-Corona SI, Vargas-Estrada D, Chávez-Corona JI, Mendoza-Rodríguez CA, Caballero-Chacón S, Pedraza-Chaverri J, Gracia-Mora MI, Galván-Vela DP, García-Rodríguez H, Sánchez-Bartez F, et al. Vitamin E (α-Tocopherol) Does Not Ameliorate the Toxic Effect of Bisphenol S on the Metabolic Analytes and Pancreas Histoarchitecture of Diabetic Rats. Toxics. 2023; 11(7):626. https://doi.org/10.3390/toxics11070626
Chicago/Turabian StylePeña-Corona, Sheila I., Dinorah Vargas-Estrada, Juan I. Chávez-Corona, C. Adriana Mendoza-Rodríguez, Sara Caballero-Chacón, José Pedraza-Chaverri, María Isabel Gracia-Mora, Diana Patricia Galván-Vela, Helena García-Rodríguez, Francisco Sánchez-Bartez, and et al. 2023. "Vitamin E (α-Tocopherol) Does Not Ameliorate the Toxic Effect of Bisphenol S on the Metabolic Analytes and Pancreas Histoarchitecture of Diabetic Rats" Toxics 11, no. 7: 626. https://doi.org/10.3390/toxics11070626
APA StylePeña-Corona, S. I., Vargas-Estrada, D., Chávez-Corona, J. I., Mendoza-Rodríguez, C. A., Caballero-Chacón, S., Pedraza-Chaverri, J., Gracia-Mora, M. I., Galván-Vela, D. P., García-Rodríguez, H., Sánchez-Bartez, F., Vergara-Onofre, M., & Leyva-Gómez, G. (2023). Vitamin E (α-Tocopherol) Does Not Ameliorate the Toxic Effect of Bisphenol S on the Metabolic Analytes and Pancreas Histoarchitecture of Diabetic Rats. Toxics, 11(7), 626. https://doi.org/10.3390/toxics11070626