A Review on the Use of Metal Oxide-Based Nanocomposites for the Remediation of Organics-Contaminated Water via Photocatalysis: Fundamentals, Bibliometric Study and Recent Advances
Abstract
1. Introduction
2. Metal Oxides and Photocatalysis
2.1. Fundamentals of Metal Oxides
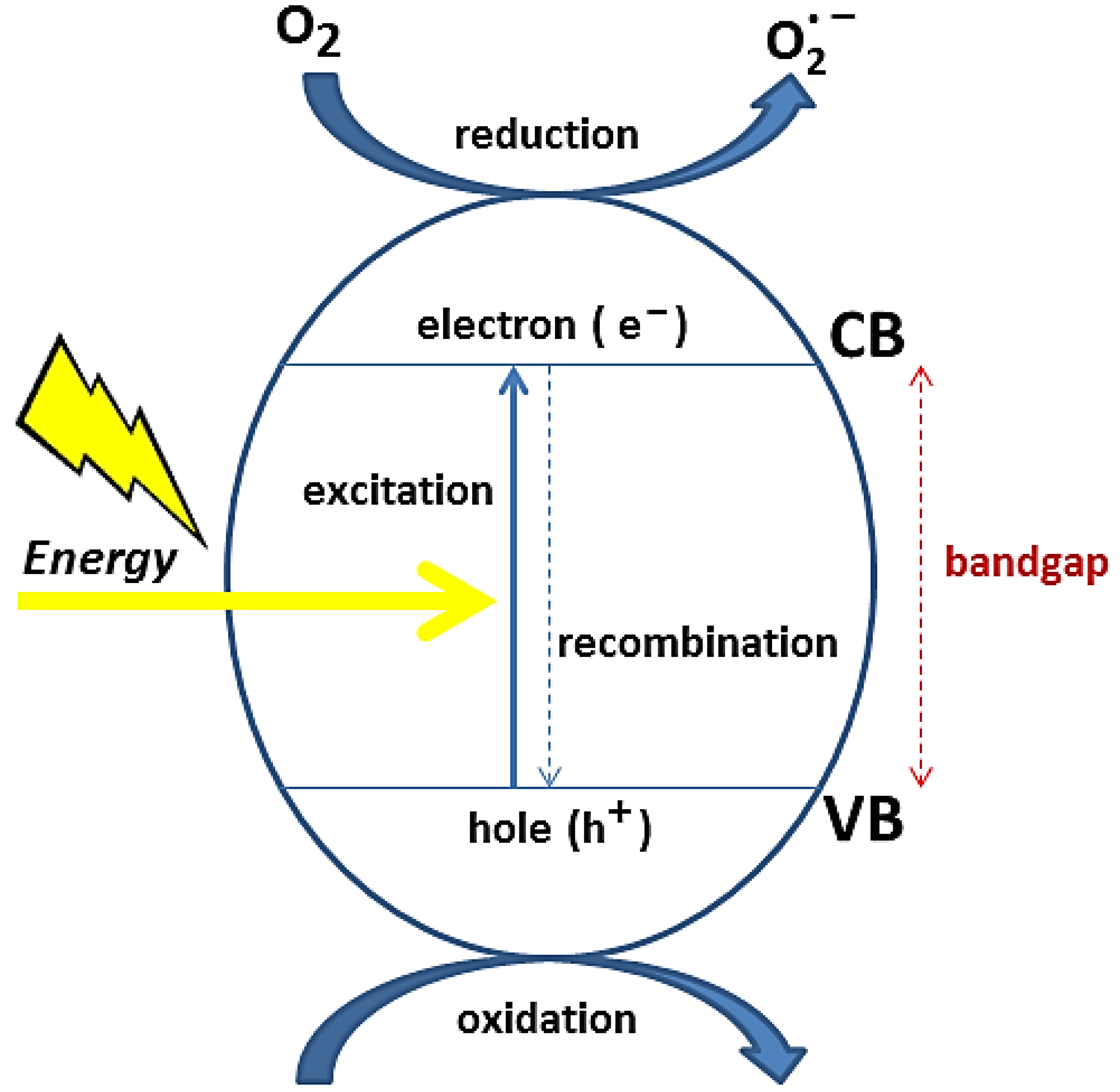
2.2. Electronic Structure
2.3. Metal Oxide-Based Photocatalysis
Charge Transfer in MON-Based Photocatalysts by Surface Tailoring
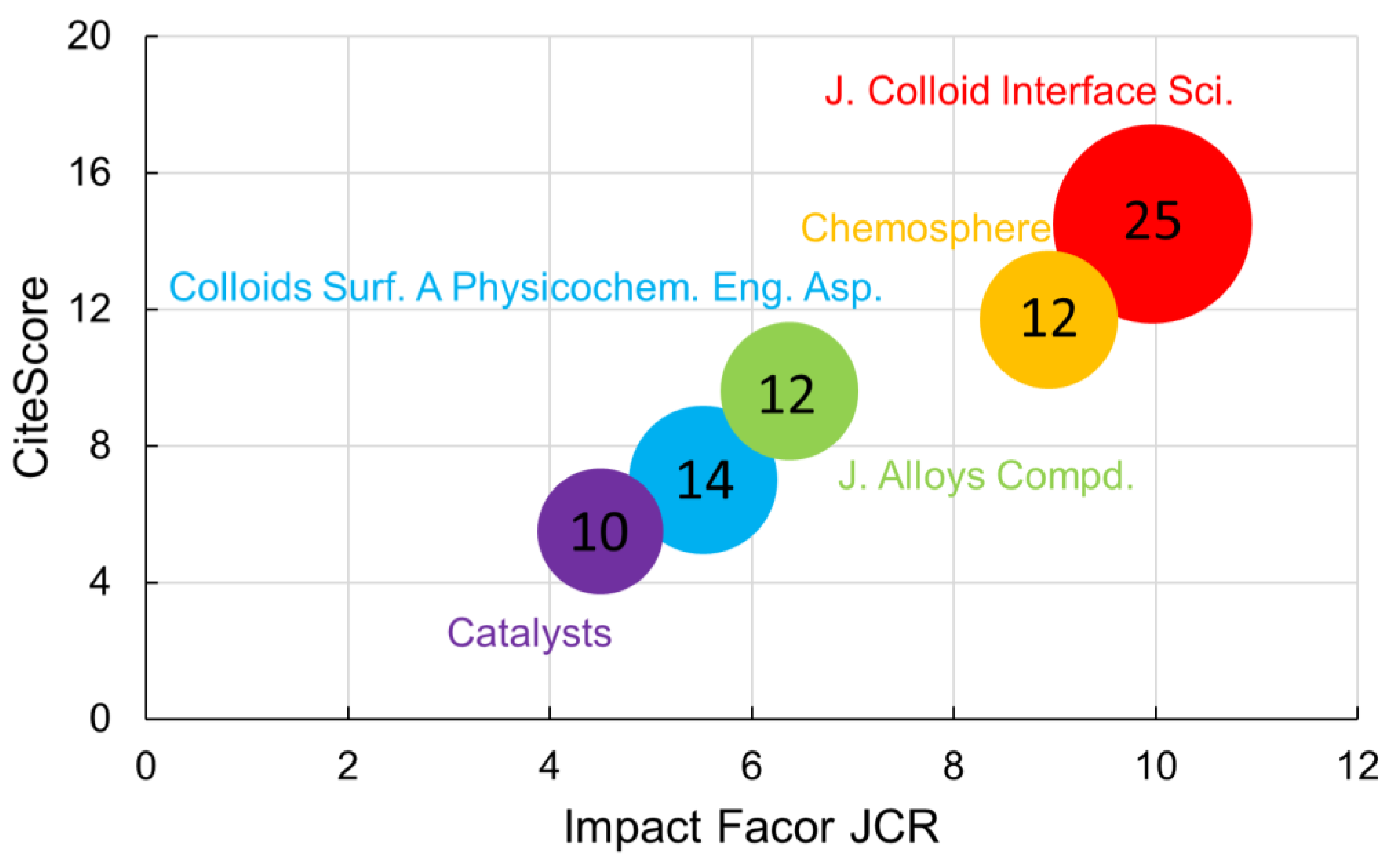
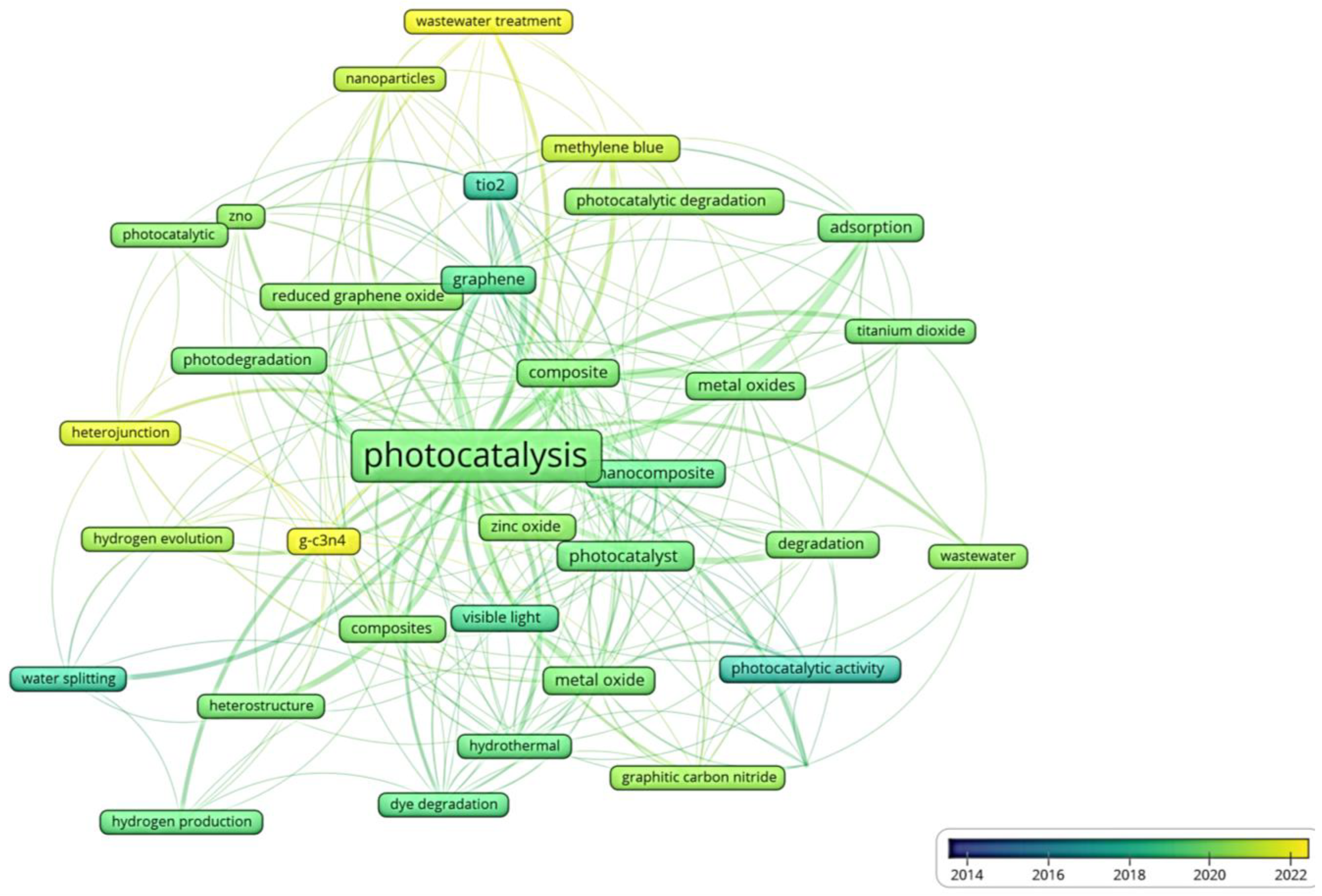
3. Bibliometric Study
4. Recent Advances on the Use of Metal Oxide-Based Nanocomposites (MON) in Photocatalysis of Organics
4.1. Mixed Metal Oxide-Based MON
4.2. (MO/Conducting Polymer)-Based MON
4.3. (MO/Carbon Materials)-Based MON
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Akhtar, N.; Ishak, M.I.S.; Bhawani, S.A.; Umar, K. Various Natural and Anthropogenic Factors Responsible for Water Quality Degradation: A Review. Water 2021, 13, 2660. [Google Scholar] [CrossRef]
- Breida, M.; Younssi, S.A.; Ouammou, M.; Bouhria, M.; Hafsi, M. Pollution of Water Sources from Agricultural and Industrial Effluents: Special Attention to NO3−, Cr(VI), and Cu(II). In Water Chemistry; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Chubarenko, I.; Bagaev, A.; Zobkov, M.; Esiukova, E. On some physical and dynamical properties of microplastic particles in marine environment. Mar. Pollut. Bull. 2016, 108, 105–112. [Google Scholar] [CrossRef]
- Lin, L.; Yang, H.; Xu, X. Effects of Water Pollution on Human Health and Disease Heterogeneity: A Review. Front. Environ. Sci. 2022, 10, 880246. [Google Scholar] [CrossRef]
- Rezai, B.; Allahkarami, E. Wastewater Treatment Processes-Techniques, Technologies, Challenges Faced, and Alternative Solutions. In Soft Computing Techniques in Solid Waste and Wastewater Management; Karri, R.R., Ravindran, G., Dehghani, M.H., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 35–53. [Google Scholar] [CrossRef]
- Zhao, W.; Chen, I.-W.; Huang, F. Toward large-scale water treatment using nanomaterials. Nano Today 2019, 27, 11–27. [Google Scholar] [CrossRef]
- Kotia, A. Cost-Effective Water Purification-Method. Encyclopedia. Available online: https://encyclopedia.pub/entry/6246 (accessed on 29 May 2023).
- Khan, S.T.; Malik, A. Engineered nanomaterials for water decontamination and purification: From lab to products. J. Hazard. Mater. 2018, 363, 295–308. [Google Scholar] [CrossRef]
- de Jesus, R.A.; de Assis, G.C.; de Oliveira, R.J.; Bilal, M.; Bharagava, R.N.; Iqbal, H.M.; Ferreira, L.F.R.; Figueiredo, R.T. Metal oxide nanoparticles for environmental remediation. In Micro and Nano Technologies, Biodegradation and Biodeterioration at the Nanoscale; Hafiz, M.N.I., Bilal, M., Nguyen, T.A., Yasin, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 529–560. [Google Scholar] [CrossRef]
- Pattnaik, A.; Sahu, J.; Poonia, A.K.; Ghosh, P. Current perspective of nano-engineered metal oxide based photocatalysts in advanced oxidation processes for degradation of organic pollutants in wastewater. Chem. Eng. Res. Des. 2023, 190, 667–686. [Google Scholar] [CrossRef]
- Lu, F.; Didier, A. Nanocatalysts and other nanomaterials for water remediation from organic pollutants. Coordination Chemistry Reviews 2020, 408, 213180. [Google Scholar] [CrossRef]
- Shanaah, H.H.; Alzaimoor, E.F.H.; Rashdan, S.; Abdalhafith, A.A.; Kamel, A.H. Photocatalytic Degradation and Adsorptive Removal of Emerging Organic Pesticides Using Metal Oxide and Their Composites: Recent Trends and Future Perspectives. Sustainability 2023, 15, 7336. [Google Scholar] [CrossRef]
- Pereira, M.F.G.; Nascimento, M.M.; Cardoso, P.H.N.; Oliveira, C.Y.B.; Tavares, G.F.; Araújo, E.S. Preparation, Microstructural Characterization and Photocatalysis Tests of V5+-Doped TiO2/WO3 Nanocomposites Supported on Electrospun Membranes. Inorganics 2022, 10, 143. [Google Scholar] [CrossRef]
- Jamjoum, H.A.A.; Umar, K.; Adnan, R.; Razali, M.R.; Ibrahim, M.N.M. Synthesis, Characterization, and Photocatalytic Activities of Graphene Oxide/metal Oxides Nanocomposites: A Review. Front. Chem. 2021, 9, 752276. [Google Scholar] [CrossRef]
- Patil, S.R. Metal oxide-based composites as photocatalysts. In Metal Oxides, Advances in Metal Oxides and Their Composites for Emerging Applications; Delekar, S.D., Ed.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 633–672. [Google Scholar] [CrossRef]
- Böer, K.W.; Pohl, U.W. Bands and Bandgaps in Solids. In Semiconductor Physics; Springer: Singapore, 2023; pp. 257–317. [Google Scholar]
- He, H. 2-Metal oxide semiconductors and conductors. In Metal Oxides, Solution Processed Metal Oxide Thin Films for Electronic Applications; Zheng, C., Ghenadii, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 7–30. [Google Scholar] [CrossRef]
- Védrine, J.C. Metal Oxides in Heterogeneous Oxidation Catalysis: State of the Art and Challenges for a More Sustainable World. Chemsuschem 2019, 12, 577–588. [Google Scholar] [CrossRef]
- Melak, F.; Bogale, B.; Asere, T.G.; Yai, T. Photocatalytic degradation of methylene blue dye using cuprous oxide/graphene nanocomposite. Curr. Nanomater. 2022, 8, 182–193. [Google Scholar] [CrossRef]
- Dey, A. Semiconductor metal oxide gas sensors: A review. Mater. Sci. Eng. B 2018, 229, 206–217. [Google Scholar] [CrossRef]
- Sagar, D.; Delekar, S.D. (Eds.) Advances in Metal Oxides and Their Composites for Emerging Applications; Elsevier: Amsterdam, The Netherlands, 2022; 746p, ISBN 9780323857062. [Google Scholar]
- Samriti; Rajput, V.; Gupta, R.K.; Prakash, J. Engineering metal oxide semiconductor nanostructures for enhanced charge transfer: Fundamentals and emerging SERS applications. J. Mater. Chem. C 2021, 10, 73–95. [Google Scholar] [CrossRef]
- Nikolic, M.V.; Milovanovic, V.; Vasiljevic, Z.Z.; Stamenkovic, Z. Semiconductor Gas Sensors: Materials, Technology, Design, and Application. Sensors 2020, 20, 6694. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Rhimi, B.; Wang, H.; Wang, C. Efficient photocatalytic degradation of gaseous toluene over F-doped TiO2/exfoliated bentonite. Appl. Surf. Sci. 2020, 530, 147286. [Google Scholar] [CrossRef]
- Pham, T.-H.; Jung, S.H.; Kim, T. Enhanced photodegradation of toxic volatile organic pollutants using Ni-doped graphitic carbon nitride under natural solar light. Sol. Energy 2021, 224, 18–26. [Google Scholar] [CrossRef]
- Abo-Dief, H.M.; Hussein, O.K.; Ihsan, A.; El-Bahy, S.M.; Raslan, A.M.; Shahid, M.; Warsi, M.F. Ternary metal oxide WO3.NiO.ZnO nanoparticles and their composite with CNTs for organic dye photocatalytic degradation. Ceram. Int. 2022, 48, 22228–22236. [Google Scholar] [CrossRef]
- Perera, M.; Wijenayaka, L.A.; Siriwardana, K.; Dahanayake, D.; de Silva, K.M.N. Gold nanoparticle decorated titania for sustainable environmental remediation: Green synthesis, enhanced surface adsorption and synergistic photocatalysis. RSC Adv. 2020, 10, 29594–29602. [Google Scholar] [CrossRef]
- Li, S.S. Energy Band Theory. In Semiconductor Physical Electronics; Li, S.S., Ed.; Springer: New York, NY, USA, 2006. [Google Scholar] [CrossRef]
- Callaway, J. Quantum Theory of the Solid State: Pt. A; Academic Press: Cambridge, UK, 2013. [Google Scholar]
- Ge, L.; Han, C.; Liu, J. Novel visible light-induced g-C3N4/Bi2WO6 composite photocatalysts for efficient degradation of methyl orange. Appl. Catal. B Environ. 2011, 108–109, 100–107. [Google Scholar] [CrossRef]
- Li, D.; Li, R.; Zeng, F.; Yan, W.; Deng, M.; Cai, S. The photoexcited electron transfer and photocatalytic mechanism of g-C3N4/TiO2 heterojunctions: Time-domain ab initio analysis. Appl. Surf. Sci. 2023, 614, 156104. [Google Scholar] [CrossRef]
- He, X.; Xiao, Z.; Katase, T.; Ide, K.; Hosono, H.; Kamiya, T. Intrinsic and Extrinsic Defects in Layered Nitride Semiconductor SrTiN2. J. Phys. Chem. C 2019, 123, 19307–19314. [Google Scholar] [CrossRef]
- Karthikeyan, C.; Arunachalam, P.; Ramachandran, K.; Al-Mayouf, A.M.; Karuppuchamy, S. Recent advances in semiconductor metal oxides with enhanced methods for solar photocatalytic applications. J. Alloys Compd. 2020, 828, 154281. [Google Scholar] [CrossRef]
- Yang, H. A short review on heterojunction photocatalysts: Carrier transfer behavior and photocatalytic mechanisms. Mater. Res. Bull. 2021, 142, 111406. [Google Scholar] [CrossRef]
- Singh, A.K.; Singh, A.K.; Sinha, S.R.P. Fermi-Level Modulation of Chemical Vapor Deposition-Grown Monolayer Graphene via Nanoparticles to Macromolecular Dopants. ACS Omega 2021, 7, 744–751. [Google Scholar] [CrossRef]
- Siu, C. Semiconductor Physics. Electronic Devices, Circuits, and Applications; Springer International Publishing: Cham, Switzerland, 2022; pp. 35–39. [Google Scholar] [CrossRef]
- Sagadevan, S.; Marlinda, A.; Johan, M.R.; Umar, A.; Fouad, H.; Alothman, O.Y.; Khaled, U.; Akhtar, M.; Shahid, M. Reduced graphene/nanostructured cobalt oxide nanocomposite for enhanced electrochemical performance of supercapacitor applications. J. Colloid Interface Sci. 2020, 558, 68–77. [Google Scholar] [CrossRef]
- Sharma, S.; Sudhakara, P.; Omran, A.A.B.; Singh, J.; Ilyas, R.A. Recent Trends and Developments in Conducting Polymer Nanocomposites for Multifunctional Applications. Polymers 2021, 13, 2898. [Google Scholar] [CrossRef]
- Kotowska, U.; Karpińska, J.; Kiejza, D.; Ratkiewicz, A.; Piekutin, J.; Makarova, K.; Olchowik-Grabarek, E. Oxidation of contaminants of emerging concern by combination of peracetic acid with iron ions and various types of light radiation–Optimization, kinetics, removal efficiency and mechanism investigation. J. Mol. Liq. 2023, 369, 120859. [Google Scholar] [CrossRef]
- Coronado, J.M. A Historical Introduction to Photocatalysis. In Design of Advanced Photocatalytic Materials for Energy and Environmental Applications. Green Energy and Technology; Coronado, J., Fresno, F., Hernández-Alonso, M., Portela, R., Eds.; Springer: London, UK, 2013. [Google Scholar] [CrossRef]
- Zhu, D.; Zhou, Q. Action and mechanism of semiconductor photocatalysis on degradation of organic pollutants in water treatment: A review. Environ. Nanotechnology, Monit. Manag. 2019, 12, 100255. [Google Scholar] [CrossRef]
- Friehs, E.; AlSalka, Y.; Jonczyk, R.; Lavrentieva, A.; Jochums, A.; Walter, J.-G.; Stahl, F.; Scheper, T.; Bahnemann, D. Toxicity, phototoxicity and biocidal activity of nanoparticles employed in photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 2016, 29, 1–28. [Google Scholar] [CrossRef]
- Bora, L.V.; Mewada, R.K. Visible/solar light active photocatalysts for organic effluent treatment: Fundamentals, mechanisms and parametric review. Renew. Sustain. Energy Rev. 2017, 76, 1393–1421. [Google Scholar] [CrossRef]
- Karim, A.V.; Shriwastav, A. Degradation of amoxicillin with sono, photo, and sonophotocatalytic oxidation under low-frequency ultrasound and visible light. Environ. Res. 2021, 200, 111515. [Google Scholar] [CrossRef]
- Bharathi, P.; Harish, S.; Archana, J.; Navaneethan, M.; Ponnusamy, S.; Muthamizhchelvan, C.; Shimomura, M.; Hayakawa, Y. Enhanced charge transfer and separation of hierarchical CuO/ZnO composites: The synergistic effect of photocatalysis for the mineralization of organic pollutant in water. Appl. Surf. Sci. 2019, 484, 884–891. [Google Scholar] [CrossRef]
- Menon, N.G.; Tatiparti, S.S.V.; Mukherji, S. Synthesis, characterization and photocatalytic activity evaluation of TiO2–ZnO nanocomposites: Elucidating effect of varying Ti:Zn molar ratio. Colloids Surf. A Physicochem. Eng. Asp. 2018, 565, 47–58. [Google Scholar] [CrossRef]
- Alam, U.; Shah, T.A.; Khan, A.; Muneer, M. One-pot ultrasonic assisted sol-gel synthesis of spindle-like Nd and V codoped ZnO for efficient photocatalytic degradation of organic pollutants. Sep. Purif. Technol. 2018, 212, 427–437. [Google Scholar] [CrossRef]
- Natarajan, T.S.; Mozhiarasi, V.; Tayade, R.J. Nitrogen Doped Titanium Dioxide (N-TiO2): Synopsis of Synthesis Methodologies, Doping Mechanisms, Property Evaluation and Visible Light Photocatalytic Applications. Photochemistry 2021, 1, 371–410. [Google Scholar] [CrossRef]
- Huang, J.; Dou, L.; Li, J.; Zhong, J.; Li, M.; Wang, T. Excellent visible light responsive photocatalytic behavior of N-doped TiO2 toward decontamination of organic pollutants. J. Hazard. Mater. 2020, 403, 123857. [Google Scholar] [CrossRef]
- Olana, M.H.; Sabir, F.K.; Bekele, E.T.; Gonfa, B.A. Citrus sinensis and Musa acuminata Peel Waste Extract Mediated Synthesis of TiO2/rGO Nanocomposites for Photocatalytic Degradation of Methylene Blue under Visible Light Irradiation. Bioinorg. Chem. Appl. 2022, 2022, 5978707. [Google Scholar] [CrossRef]
- Sivakami, A.; Sarankumar, R.; Sudhagar, P. Graphene–Metal Oxides Nanocomposite Heterojunction as an Efficient Photocatalyst for Energy and Environmental Applications. In Heterojunction Photocatalytic Materials; Jenny Stanford Publishing: New York, NY, USA, 2022; pp. 227–255. [Google Scholar]
- Kusiak-Nejman, E.; Wanag, A.; Kapica-Kozar, J.; Kowalczyk, Ł.; Zgrzebnicki, M.; Tryba, B.; Przepiórski, J.; Morawski, A.W. Methylene blue decomposition on TiO2/reduced graphene oxide hybrid photocatalysts obtained by a two-step hydrothermal and calcination synthesis. Catal. Today 2019, 357, 630–637. [Google Scholar] [CrossRef]
- Usman, A.K.; Cursaru, D.-L.; Brănoiu, G.; Şomoghi, R.; Manta, A.-M.; Matei, D.; Mihai, S. A Modified Sol–Gel Synthesis of Anatase {001}-TiO2/Au Hybrid Nanocomposites for Enhanced Photodegradation of Organic Contaminants. Gels 2022, 8, 728. [Google Scholar] [CrossRef]
- Tahir, M.B.; Farman, S.; Rafique, M.; Shakil, M.; Khan, M.I.; Ijaz, M.; Mubeen, I.; Ashraf, M.; Riaz, K.N. Photocatalytic performance of hybrid WO3/TiO2 nanomaterials for the degradation of methylene blue under visible light irradiation. Int. J. Environ. Anal. Chem. 2021, 101, 1448–1460. [Google Scholar] [CrossRef]
- Younas, U.; Ahmad, A.; Islam, A.; Ali, F.; Pervaiz, M.; Saleem, A.; Waseem, M.; Aljuwayid, A.M.; Habila, M.A.; Naqvi, S.R. Fabrication of a novel nanocomposite (TiO2/WO3/V2O5) by hydrothermal method as catalyst for hazardous waste treatment. Fuel 2023, 349, 128668. [Google Scholar] [CrossRef]
- Han, X.; Kuang, Q.; Jin, M.; Xie, Z.; Zheng, L. Synthesis of Titania Nanosheets with a High Percentage of Exposed (001) Facets and Related Photocatalytic Properties. J. Am. Chem. Soc. 2009, 131, 3152–3153. [Google Scholar] [CrossRef]
- Dai, Y.; Cobley, C.M.; Zeng, J.; Sun, Y.; Xia, Y. Synthesis of Anatase TiO2 Nanocrystals with Exposed {001} Facets. Nano Lett. 2009, 9, 2455–2459. [Google Scholar] [CrossRef]
- Liu, M.; Piao, L.; Zhao, L.; Ju, S.; Yan, Z.; He, T.; Zhou, C.; Wang, W. Anatase TiO2 single crystals with exposed {001} and {110} facets: Facile synthesis and enhanced photocatalysis. Chem. Commun. 2010, 46, 1664–1666. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Qi, L.; Jaroniec, M. Hydrogen Production by Photocatalytic Water Splitting over Pt/TiO2 Nanosheets with Exposed (001) Facets. J. Phys. Chem. C 2010, 114, 13118–13125. [Google Scholar] [CrossRef]
- Sosnowchik, B.D.; Chiamori, H.C.; Ding, Y.; Ha, J.-Y.; Wang, Z.L.; Lin, L. Titanium dioxide nanoswords with highly reactive, photocatalytic facets. Nanotechnology 2010, 21, 485601. [Google Scholar] [CrossRef]
- Batzill, M. Fundamental aspects of surface engineering of transition metal oxide photocatalysts. Energy Environ. Sci. 2011, 4, 3275–3286. [Google Scholar] [CrossRef]
- Sydorchuk, V.; Levytska, S.; Shcherban, N.; Khalameida, S. Transition metal oxides supported onto silica gel as visible light-driven photocatalysts. Res. Chem. Intermed. 2020, 46, 3997–4015. [Google Scholar] [CrossRef]
- Bandaranayake, S.; Hruska, E.; Londo, S.; Biswas, S.; Baker, L.R. Small Polarons and Surface Defects in Metal Oxide Photocatalysts Studied Using XUV Reflection–Absorption Spectroscopy. J. Phys. Chem. C 2020, 124, 22853–22870. [Google Scholar] [CrossRef]
- Koutavarapu, R.; Tamtam, M.R.; Rao, M.; Peera, S.G.; Shim, J. Recent progress in transition metal oxide/sulfide quantum dots-based nanocomposites for the removal of toxic organic pollutants. Chemosphere 2021, 272, 129849. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X.; Tryk, D.A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Thompson, T.L.; Yates, J.T. TiO2-based Photocatalysis: Surface Defects, Oxygen and Charge Transfer. Top. Catal. 2005, 35, 197–210. [Google Scholar] [CrossRef]
- Hotsenpiller, P.A.M.; Bolt, J.D.; Farneth, W.E.; Lowekamp, J.B.; Rohrer, G.S. Orientation Dependence of Photochemical Reactions on TiO2 Surfaces. J. Phys. Chem. B 1998, 102, 3216–3226. [Google Scholar] [CrossRef]
- Giocondi, J.L.; Salvador, P.A.; Rohrer, G.S. The origin of photochemical anisotropy in SrTiO3. Top. Catal. 2007, 44, 529–533. [Google Scholar] [CrossRef][Green Version]
- Giocondi, J.L.; Rohrer, G.S. Spatial Separation of Photochemical Oxidation and Reduction Reactions on the Surface of Ferroelectric BaTiO3. J. Phys. Chem. B 2001, 105, 8275–8277. [Google Scholar] [CrossRef]
- Giocondi, J.L.; Rohrer, G.S. The Influence of the Dipolar Field Effect on the Photochemical Reactivity of Sr2Nb2O7 and BaTiO3 Microcrystals. Top. Catal. 2008, 49, 18–23. [Google Scholar] [CrossRef]
- Kalinin, S.V.; Bonnell, D.A.; Alvarez, T.; Lei, X.; Hu, Z.; Ferris, J.H.; Zhang, Q.; Dunn, S. Atomic Polarization and Local Reactivity on Ferroelectric Surfaces: A New Route toward Complex Nanostructures. Nano Lett. 2002, 2, 589–593. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Burbure, N.V.; Rohrer, G.S. Enhanced Photochemical Reactivity at the Ferroelectric Phase Transition in Ba1−xSrxTiO3. J. Am. Ceram. Soc. 2010, 93, 4129–4134. [Google Scholar] [CrossRef]
- Bullard, J.W.; Cima, M.J. Orientation Dependence of the Isoelectric Point of TiO2 (Rutile) Surfaces. Langmuir 2006, 22, 10264–10271. [Google Scholar] [CrossRef]
- Beck, T.; Klust, A.; Batzill, M.; Diebold, U.; Di Valentin, C.; Selloni, A. Surface Structure of TiO2(011)-(2×1). Phys. Rev. Lett. 2004, 93, 036104. [Google Scholar] [CrossRef]
- Tao, J.; Luttrell, T.; Batzill, M. A two-dimensional phase of TiO2 with a reduced bandgap. Nat. Chem. 2011, 3, 296–300. [Google Scholar] [CrossRef]
- Lahiri, J.; Batzill, M. Surface Functionalization of ZnO Photocatalysts with Monolayer ZnS. J. Phys. Chem. C 2008, 112, 4304–4307. [Google Scholar] [CrossRef]
- Lahiri, J.; Senanayake, S.; Batzill, M. Soft X-ray photoemission of clean and sulfur-covered polar ZnO surfaces: A view of the stabilization of polar oxide surfaces. Phys. Rev. B 2008, 78, 155414. [Google Scholar] [CrossRef]
- Alaghmandfard, A.; Ghandi, K. A Comprehensive Review of Graphitic Carbon Nitride (g-C3N4)–Metal Oxide-Based Nanocomposites: Potential for Photocatalysis and Sensing. Nanomaterials 2022, 12, 294. [Google Scholar] [CrossRef]
- Wudil, Y.; Ahmad, U.; Gondal, M.; Al-Osta, M.A.; Almohammedi, A.; Sa’Id, R.; Hrahsheh, F.; Haruna, K.; Mohamed, M. Tuning of graphitic carbon nitride (g-C3N4) for photocatalysis: A critical review. Arab. J. Chem. 2023, 16, 104542. [Google Scholar] [CrossRef]
- Aboualigaledari, N.; Rahmani, M. A review on the synthesis of the TiO2-based photocatalyst for the environmental purification. J. Compos. Compd. 2020, 2, 25–42. [Google Scholar] [CrossRef]
- Huang, L.; Chen, X.; Wu, Y. The interfacial ionic transport of two-dimensional ZnAl-mixed metal oxides nanocomposite. J. Alloys Compd. 2022, 921, 166118. [Google Scholar] [CrossRef]
- Lu, H.; Wright, D.S.; Pike, S.D. The use of mixed-metal single source precursors for the synthesis of complex metal oxides. Chem. Commun. 2019, 56, 854–871. [Google Scholar] [CrossRef]
- Yadav, G.D.; Mewada, R.K.; Wagh, D.P.; Manyar, H.G. Advances and future trends in selective oxidation catalysis: A critical review. Catal. Sci. Technol. 2022, 12, 7245–7269. [Google Scholar] [CrossRef]
- Arjun, A.; Dharr, A.; Raguram, T.; Rajni, K.S. Study of Copper Doped Zirconium Dioxide Nanoparticles Synthesized via Sol–Gel Technique for Photocatalytic Applications. J. Inorg. Organomet. Polym. Mater. 2020, 30, 4989–4998. [Google Scholar] [CrossRef]
- Joshi, N.; da Silva, L.F.; Shimizu, F.M.; Mastelaro, V.R.; M’peko, J.-C.; Lin, L.; Oliveira, O.N. UV-assisted chemiresistors made with gold-modified ZnO nanorods to detect ozone gas at room temperature. Microchim. Acta 2019, 186, 418. [Google Scholar] [CrossRef]
- Song, G.; Zhang, Q.; Liu, F.; Wang, C.; Yang, R.; Chen, Z.; Ma, D. Mixed-Phase ZnIn2S4 Nanosheets Grown on TiO2 Nanotrees for the Visible-Light Photocatalytic Degradation of Organic Dyes. ACS Appl. Nano Mater. 2022, 5, 380–390. [Google Scholar] [CrossRef]
- Ma, R.; Xiang, L.; Zhao, X.; Yin, J. Progress in Preparation of Sea Urchin-like Micro-/Nanoparticles. Materials 2022, 15, 2846. [Google Scholar] [CrossRef]
- Bhat, A.H.; Chisti, H.-T. Facile fabrication of ternary metal oxide ZnO/CuO/SnO2 nanocomposite for excellent photocatalytic degradation of fast green dye. Int. J. Environ. Anal. Chem. 2021, 1–22. [Google Scholar] [CrossRef]
- Hariharalakshmanan, R.K.; Watanabe, F.; Karabacak, T. In Situ Growth and UV Photocatalytic Effect of ZnO Nanostructures on a Zn Plate Immersed in Methylene Blue. Catalysts 2022, 12, 1657. [Google Scholar] [CrossRef]
- Zhou, L.; Bainglass, E.; Masroor, M.; Giri, B.; Li, G.; Carl, A.D.; Grimm, R.L.; Huda, M.N.; Titova, L.V.; Rao, P.M. Synthesis and optoelectronic properties of a promising quaternary metal oxide light absorber CuBiW2O8. J. Mater. Chem. A 2020, 9, 1643–1654. [Google Scholar] [CrossRef]
- Muneer, I.; Farrukh, M.A.; Ali, D.; Bashir, F. Heterogeneous photocatalytic degradation of organic dyes by highly efficient GdCoSnO3. Mater. Sci. Eng. B 2021, 265, 115028. [Google Scholar] [CrossRef]
- Lohar, S.; Vijay, A.; Kataria, B.; Kumawat, A.S.; Bhardwaj, S. Visible light driven photocatalytic degradation of organic pollutant: Cleaning the environment by novel ZrCdPbO4. Indian J. Chem. Technol. 2021, 28, 460–466. [Google Scholar]
- Okab, A.A.; Alwared, A.I. Photodegradation of tetracycline antibiotic by ternary recyclable Z-scheme g-C3N4/Fe3O4/Bi2WO6/Bi2S3 photocatalyst with improved charge separation efficiency: Characterization and mechanism studies. Environ. Nanotechnol. Monit. Manag. 2023, 19, 100767. [Google Scholar] [CrossRef]
- Ramos, P.G.; Sánchez, L.A.; Rodriguez, J.M. A review on improving the efficiency of photocatalytic water decontamination using ZnO nanorods. J. Sol-Gel Sci. Technol. 2022, 102, 105–124. [Google Scholar] [CrossRef]
- Araújo, E.S.; da Costa, B.P.; Oliveira, R.A.; Libardi, J.; Faia, P.M.; de Oliveira, H.P. TiO2/ZnO hierarchical heteronanostructures: Synthesis, characterization and application as photocatalysts. J. Environ. Chem. Eng. 2016, 4, 2820–2829. [Google Scholar] [CrossRef]
- Bao, Y.; Guo, R.; Gao, M.; Kang, Q.; Ma, J. Morphology control of 3D hierarchical urchin-like hollow SiO2@TiO2 spheres for photocatalytic degradation: Influence of calcination temperature. J. Alloys Compd. 2020, 853, 157202. [Google Scholar] [CrossRef]
- Luo, J.; Guo, W.H.; Zhang, Q.; Wang, X.H.; Shen, L.; Fu, H.C.; Wu, L.L.; Chen, X.H.; Luo, H.Q.; Li, N.B. One-pot synthesis of Mn–Fe bimetallic oxide heterostructures as bifunctional electrodes for efficient overall water splitting. Nanoscale 2020, 12, 19992–20001. [Google Scholar] [CrossRef]
- Xie, J.; Yamaguchi, T.; Oh, J.-M. Synthesis of a mesoporous Mg–Al–mixed metal oxide with P123 template for effective removal of Congo red via aggregation-driven adsorption. J. Solid State Chem. 2020, 293, 121758. [Google Scholar] [CrossRef]
- Ahmadi, M.; Dorraji, M.S.; Hajimiri, I.; Rasoulifard, M. The main role of CuO loading against electron-hole recombination of SrTiO3: Improvement and investigation of photocatalytic activity, modeling and optimization by response surface methodology. J. Photochem. Photobiol. A Chem. 2020, 404, 112886. [Google Scholar] [CrossRef]
- Wolff, N.; Braniste, T.; Krüger, H.; Mangelsen, S.; Islam, R.; Schürmann, U.; Saure, L.M.; Schütt, F.; Hansen, S.; Terraschke, H.; et al. Synthesis and Nanostructure Investigation of Hybrid β-Ga2O3/ZnGa2O4 Nanocomposite Networks with Narrow-Band Green Luminescence and High Initial Electrochemical Capacity. Small 2023, 19, e2207492. [Google Scholar] [CrossRef]
- Danish, M.S.S.; Estrella, L.L.; Alemaida, I.M.A.; Lisin, A.; Moiseev, N.; Ahmadi, M.; Nazari, M.; Wali, M.; Zaheb, H.; Senjyu, T. Photocatalytic Applications of Metal Oxides for Sustainable Environmental Remediation. Metals 2021, 11, 80. [Google Scholar] [CrossRef]
- Handojo, L.; Ikhsan, N.A.; Mukti, R.R.; Indarto, A. Nanomaterials for remediations of agrochemicals. In Agrochemicals Detection, Treatment and Remediation; Elsevier: Amsterdam, The Netherlands, 2020; pp. 535–567. [Google Scholar] [CrossRef]
- Suganthi, N.; Thangavel, S.; Kannan, K. Hibiscus subdariffa leaf extract mediated 2-D fern-like ZnO/TiO2 hierarchical nanoleaf for photocatalytic degradation. Flatchem 2020, 24, 100197. [Google Scholar] [CrossRef]
- Song, J.; Sun, G.; Yu, J.; Si, Y.; Ding, B. Construction of ternary Ag@ZnO/TiO2 fibrous membranes with hierarchical nanostructures and mechanical flexibility for water purification. Ceram. Int. 2019, 46, 468–475. [Google Scholar] [CrossRef]
- Adesoye, S.; Dellinger, K. ZnO and TiO2 nanostructures for surface-enhanced Raman scattering-based bio-sensing: A review. Sens. Bio-Sens. Res. 2022, 37, 100499. [Google Scholar] [CrossRef]
- Siwińska-Stefańska, K.; Kubiak, A.; Piasecki, A.; Goscianska, J.; Nowaczyk, G.; Jurga, S.; Jesionowski, T. TiO2-ZnO Binary Oxide Systems: Comprehensive Characterization and Tests of Photocatalytic Activity. Materials 2018, 11, 841. [Google Scholar] [CrossRef]
- Araújo, E.S.; Libardi, J.; Faia, P.M.; de Oliveira, H.P. Hybrid ZnO/TiO2 Loaded in Electrospun Polymeric Fibers as Photocatalyst. J. Chem. 2015, 2015, 1–10. [Google Scholar] [CrossRef]
- Upadhyay, G.K.; Rajput, J.K.; Pathak, T.K.; Kumar, V.; Purohit, L. Synthesis of ZnO:TiO2 nanocomposites for photocatalyst application in visible light. Vacuum 2018, 160, 154–163. [Google Scholar] [CrossRef]
- Yan, J.; Wu, G.; Guan, N.; Li, L. Nb2O5/TiO2 heterojunctions: Synthesis strategy and photocatalytic activity. Appl. Catal. B Environ. 2014, 152-153, 280–288. [Google Scholar] [CrossRef]
- Mandal, R.K.; Kundu, S.; Sain, S.; Pradhan, S.K. Enhanced photocatalytic performance of V2O5–TiO2 nanocomposites synthesized by mechanical alloying with morphological hierarchy. N. J. Chem. 2019, 43, 2804–2816. [Google Scholar] [CrossRef]
- Marcolongo, D.M.S.; Nocito, F.; Ditaranto, N.; Comparelli, R.; Aresta, M.; Dibenedetto, A. Opto-Electronic Characterization of Photocatalysts Based on p,n-Junction Ternary and Quaternary Mixed Oxides Semiconductors (Cu2O-In2O3 and Cu2O-In2O3-TiO2). Catalysts 2022, 12, 153. [Google Scholar] [CrossRef]
- Pan, D.; Ge, S.; Zhao, J.; Shao, Q.; Guo, L.; Zhang, X.; Lin, J.; Xu, G.; Guo, Z. Synthesis, characterization and photocatalytic activity of mixed-metal oxides derived from NiCoFe ternary layered double hydroxides. Dalton Trans. 2018, 47, 9765–9778. [Google Scholar] [CrossRef] [PubMed]
- Bibi, S.; Ahmad, A.; Anjum, M.A.R.; Haleem, A.; Siddiq, M.; Shah, S.S.; Al Kahtani, A. Photocatalytic degradation of malachite green and methylene blue over reduced graphene oxide (rGO) based metal oxides (rGO-Fe3O4/TiO2) nanocomposite under UV-visible light irradiation. J. Environ. Chem. Eng. 2021, 9, 105580. [Google Scholar] [CrossRef]
- Zhang, L.; Dai, C.-H.; Zhang, X.-X.; Liu, Y.-N.; Yan, J.-H. Synthesis and highly efficient photocatalytic activity of mixed oxides derived from ZnNiAl layered double hydroxides. Trans. Nonferrous Met. Soc. China 2016, 26, 2380–2389. [Google Scholar] [CrossRef]
- Doan, V.-D.; Huynh, B.-A.; Le Pham, H.A.; Vasseghian, Y.; Le, V.T. Cu2O/Fe3O4/MIL-101(Fe) nanocomposite as a highly efficient and recyclable visible-light-driven catalyst for degradation of ciprofloxacin. Environ. Res. 2021, 201, 111593. [Google Scholar] [CrossRef]
- Dehghan, S.; Jafari, A.J.; FarzadKia, M.; Esrafili, A.; Kalantary, R.R. Visible-light-driven photocatalytic degradation of Metalaxyl by reduced graphene oxide/Fe3O4/ZnO ternary nanohybrid: Influential factors, mechanism and toxicity bioassay. J. Photochem. Photobiol. A Chem. 2019, 375, 280–292. [Google Scholar] [CrossRef]
- Farrukh, M.A.; Butt, K.M.; Altaf, A.; Khadim, S. Influence of pH and Temperature on Structural, Optical and Catalytical Investigations of CeO2-SiO2 Nanoparticles. Silicon 2019, 11, 2591–2598. [Google Scholar] [CrossRef]
- Saljooqi, A.; Shamspur, T.; Mostafavi, A. Synthesis and photocatalytic activity of porous ZnO stabilized by TiO2 and Fe3O4 nanoparticles: Investigation of pesticide degradation reaction in water treatment. Environ. Sci. Pollut. Res. 2020, 28, 9146–9156. [Google Scholar] [CrossRef]
- Issa, M.A.; Zentou, H.; Jabbar, Z.H.; Abidin, Z.Z.; Harun, H.; Halim, N.A.A.; Alkhabet, M.M.; Pudza, M.Y. Ecofriendly adsorption and sensitive detection of Hg (II) by biomass-derived nitrogen-doped carbon dots: Process modelling using central composite design. Environ. Sci. Pollut. Res. 2022, 29, 86859–86872. [Google Scholar] [CrossRef]
- Di, G.; Zhu, Z.; Huang, Q.; Zhang, H.; Zhu, J.; Qiu, Y.; Yin, D.; Zhao, J. Targeted modulation of g-C3N4 photocatalytic performance for pharmaceutical pollutants in water using ZnFe-LDH derived mixed metal oxides: Structure-activity and mechanism. Sci. Total Environ. 2018, 650, 1112–1121. [Google Scholar] [CrossRef]
- Di, G.; Zhu, Z.; Zhang, H.; Zhu, J.; Lu, H.; Zhang, W.; Qiu, Y.; Zhu, L.; Küppers, S. Simultaneous removal of several pharmaceuticals and arsenic on Zn-Fe mixed metal oxides: Combination of photocatalysis and adsorption. Chem. Eng. J. 2017, 328, 141–151. [Google Scholar] [CrossRef]
- Kumari, V.; Sharma, S.; Sharma, A.; Kumari, K.; Kumar, N. Hydrothermal synthesis conditions effect on hierarchical ZnO/CuO hybrid materials and their photocatalytic activity. J. Mater. Sci. Mater. Electron. 2021, 32, 9596–9610. [Google Scholar] [CrossRef]
- Manav, N.; Dwivedi, V.; Bhagi, A.K. Degradation of DDT, a Pesticide by Mixed Metal Oxides Nanoparticles. In Green Chemistry in Environmental Sustainability and Chemical Education; Springer: Singapore, 2018. [Google Scholar] [CrossRef]
- Fu, L.; Wu, D.; Wen, M.; Zhu, Y.; Wu, Q.; Zhou, T.; Fu, Y.Q. 2D hetero-nanostructured reduced-CuNiFe-oxides with self-produced H2O2 Fenton-like photocatalysis for tetracycline degradation. Inorg. Chem. Front. 2022, 10, 567–578. [Google Scholar] [CrossRef]
- Heris, S.Z.; Etemadi, M.; Mousavi, S.B.; Mohammadpourfard, M.; Ramavandi, B. Preparation and characterizations of TiO2/ZnO nanohybrid and its application in photocatalytic degradation of tetracycline in wastewater. J. Photochem. Photobiol. A Chem. 2023, 443, 114893. [Google Scholar] [CrossRef]
- Xia, D.; Lo, I.M. Synthesis of magnetically separable Bi2O4/Fe3O4 hybrid nanocomposites with enhanced photocatalytic removal of ibuprofen under visible light irradiation. Water Res. 2016, 100, 393–404. [Google Scholar] [CrossRef]
- Castro, L.V.; Manriquez, M.E.; Ortiz-Islas, E.; Bahena-Gutierrez, G.M. Kinetic study of the photodegradation of ibuprofen using tertiary oxide ZnO–Al2O3–TiO2. React. Kinet. Catal. Lett. 2023, 136, 1705–1721. [Google Scholar] [CrossRef]
- Sabit, D.A.; Ebrahim, S.E.; Jabbar, Z.H. Immobilization of 0D CuO/ZnFe2O4 nanoparticles onto 2D BiOBr nanoplates as dual S-scheme heterostructure for boosting photocatalytic oxidation of levofloxacin in wastewater: Magnetic reusability and mechanism insights. J. Photochem. Photobiol. A Chem. 2023, 443, 114849. [Google Scholar] [CrossRef]
- Jabbar, Z.H.; Graimed, B.H.; Okab, A.A.; Alsunbuli, M.M.; Al-Husseiny, R.A. Construction of 3D flower-like Bi5O7I/Bi/Bi2WO6 heterostructure decorated NiFe2O4 nanoparticles for photocatalytic destruction of Levofloxacin in aqueous solution: Synergistic effect between S-scheme and SPR action. J. Photochem. Photobiol. A Chem. 2023, 441, 114734. [Google Scholar] [CrossRef]
- Xue, X.; Liao, W.; Liu, D.; Zhang, X.; Huang, Y. MgO/Co3O4 composite activated peroxymonosulfate for levofloxacin degradation: Role of surface hydroxyl and oxygen vacancies. Sep. Purif. Technol. 2023, 306, 122560. [Google Scholar] [CrossRef]
- Mahjoore, M.; Honarmand, M.; Aryafar, A. Plant-based green fabrication of CuO-CdO-bentonite S-scheme heterojunction with enhanced photocatalytic performance for the degradation of levofloxacin. Environ. Sci. Pollut. Res. 2023, 30, 44439–44456. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, G.A.; Rasool, R.T.; Rasool, R.U.; Saleem, M.F.; Ali, J.; Ghernaout, D.; Hassan, M.; Aljuwayid, A.M.; Habila, M.A.; Guo, H. Photocatalytic stimulation of peroxymonosulfate by novel MoO3@ZrO2 with Z-scheme heterojunction for diclofenac sodium degradation. J. Water Process. Eng. 2023, 51, 103435. [Google Scholar] [CrossRef]
- Adam, M.S.S.; Sikander, S.; Qamar, M.T.; Iqbal, S.; Khalil, A.; Taha, A.M.; Abdel-Rahman, O.S.; Elkaeed, E.B. Photocatalytic removal of imidacloprid containing frequently applied insecticide in agriculture industry using Co3O4 modified MoO3 composites. Front. Chem. 2023, 11, 1125835. [Google Scholar] [CrossRef]
- Gervasi, S.; Blangetti, N.; Freyria, F.S.; Guastella, S.; Bonelli, B. Undoped and Fe-Doped Anatase/Brookite TiO2 Mixed Phases, Obtained by a Simple Template-Free Synthesis Method: Physico-Chemical Characterization and Photocatalytic Activity towards Simazine Degradation. Catalysts 2023, 13, 667. [Google Scholar] [CrossRef]
- Hosseinzadeh, G. Innovative fabrication of Ceo2 nanoparticles/WO3 nanoplates S-Scheme heterojunction for visible light photocatalytic degradation of nitenpyram insecticide. Int. J. Nano Dimens. 2023, 14, 50–59. [Google Scholar] [CrossRef]
- Shoneye, A.; Jiao, H.; Tang, J. Bimetallic FeOx–MOx Loaded TiO2 (M = Cu, Co) Nanocomposite Photocatalysts for Complete Mineralization of Herbicides. J. Phys. Chem. C 2023, 127, 1388–1396. [Google Scholar] [CrossRef]
- Abla, F.; Elsayed, Y.; Abu Farha, N.; Obaideen, K.; Mohamed, A.A.; Lee, H.; Han, C.; Egilmez, M.; Kanan, S. Fabrication of High Surface Area TiO2-MoO3 Nanocomposite as a Photocatalyst for Organic Pollutants Removal from Water Bodies. Catalysts 2023, 13, 362. [Google Scholar] [CrossRef]
- Basaleh, A.S.; El-Hout, S.I.; Mahmoud, M. Li2MnO3@ZrO2 heterojunctions for highly efficient catalytic photodegradation of Atrazine herbicide. Ceram. Int. 2022, 48, 30978–30987. [Google Scholar] [CrossRef]
- Yadav, J.; Rani, M.; Zhang, T.C.; Shanker, U. Efficient photo-adsorptive eradication of endocrine disrupting pesticides by chitosan co- decorated metal oxide bio-nanocomposite. Environ. Sci. Pollut. Res. 2023, 30, 72523–72538. [Google Scholar] [CrossRef] [PubMed]
- Ullah, H.; Tahir, A.A.; Mallick, T.K. Polypyrrole/TiO2 composites for the application of photocatalysis. Sens. Actuators B Chem. 2017, 241, 1161–1169. [Google Scholar] [CrossRef]
- Nadeem, M.S.; Munawar, T.; Mukhtar, F.; Rabbani, A.W.; Ur Rehman, N.; Mahmood, K.; Iqbal, F. Facile synthesis of PANI and rGO supported Y/Pr co-doped ZnO: Boosted solar light-driven photocatalysis. Appl. Phys. A 2023, 129, 450. [Google Scholar] [CrossRef]
- Patil, P.H.; Kulkarni, V.V.; Jadhav, S.A. An Overview of Recent Advancements in Conducting Polymer–Metal Oxide Nanocomposites for Supercapacitor Application. J. Compos. Sci. 2022, 6, 363. [Google Scholar] [CrossRef]
- Li, C.; Zhou, T.; Zhu, T.; Li, X. Enhanced visible light photocatalytic activity of polyaniline–crystalline TiO2–halloysite composite nanotubes by tuning the acid dopant in the preparation. RSC Adv. 2015, 5, 98482–98491. [Google Scholar] [CrossRef]
- Van Tran, V.; Nu, T.T.V.; Jung, H.-R.; Chang, M. Advanced Photocatalysts Based on Conducting Polymer/Metal Oxide Composites for Environmental Applications. Polymers 2021, 13, 3031. [Google Scholar] [CrossRef]
- Liu, J.; Tang, Z.; Zeng, J.; Zhong, Y.; Long, Z.; Wang, Z.; Feng, L. Degradation of Acid Orange 8 through Photocatalysis in the Presence of ZnO/polyaniline Nanocomposite. Int. J. Electrochem. Sci. 2022, 17, 220857. [Google Scholar] [CrossRef]
- Mukhtar, F.; Munawar, T.; Nadeem, M.S.; Khan, S.A.; Koc, M.; Batool, S.; Hasan, M.; Iqbal, F. Enhanced sunlight-absorption of Fe2O3 covered by PANI for the photodegradation of organic pollutants and antimicrobial inactivation. Adv. Powder Technol. 2022, 33, 103708. [Google Scholar] [CrossRef]
- Meng, S.; Zhang, J.; Chen, S.; Zhang, S.; Huang, W. Perspective on construction of heterojunction photocatalysts and the complete utilization of photogenerated charge carriers. Appl. Surf. Sci. 2019, 476, 982–992. [Google Scholar] [CrossRef]
- Xiong, P.; Wang, L.; Sun, X.; Binhai, X.; Wang, X. Ternary Titania–Cobalt Ferrite–Polyaniline Nanocomposite: A Magnetically Recyclable Hybrid for Adsorption and Photodegradation of Dyes under Visible Light. Ind. Eng. Chem. Res. 2013, 52, 10105–10113. [Google Scholar] [CrossRef]
- Li, J.; Xiao, Q.; Li, L.; Shen, J.; Hu, D. Novel ternary composites: Preparation, performance and application of ZnFe2O4/TiO2/polyaniline. Appl. Surf. Sci. 2015, 331, 108–114. [Google Scholar] [CrossRef]
- Feng, J.; Hou, Y.; Wang, X.; Quan, W.; Zhang, J.; Wang, Y.; Li, L. In-depth study on adsorption and photocatalytic performance of novel reduced graphene oxide-ZnFe2O4-polyaniline composites. J. Alloys Compd. 2016, 681, 157–166. [Google Scholar] [CrossRef]
- Park, Y.; Numan, A.; Ponomarev, N.; Iqbal, J.; Khalid, M. Enhanced photocatalytic performance of PANI-rGO-MnO2 ternary composite for degradation of organic contaminants under visible light. J. Environ. Chem. Eng. 2021, 9, 106006. [Google Scholar] [CrossRef]
- Sharma, S.; Sharma, A.; Chauhan, N.S.; Tahir, M.; Kumari, K.; Mittal, A.; Kumar, N. TiO2/Bi2O3/PANI nanocomposite materials for enhanced photocatalytic decontamination of organic pollutants. Inorg. Chem. Commun. 2022, 146, 110093. [Google Scholar] [CrossRef]
- Chen, J.; Feng, J.; Yan, W. Influence of metal oxides on the adsorption characteristics of PPy/metal oxides for Methylene Blue. J. Colloid Interface Sci. 2016, 475, 26–35. [Google Scholar] [CrossRef]
- Zhang, M.; Chang, L.; Zhao, Y.; Yu, Z. Fabrication of Zinc Oxide/Polypyrrole Nanocomposites for Brilliant Green Removal from Aqueous Phase. Arab. J. Sci. Eng. 2018, 44, 111–121. [Google Scholar] [CrossRef]
- Yadav, M.; Arora, R.; Dhanda, M.; Ahlawat, S.; Shoran, S.; Ahlawat, S.; Nehra, S.P.; Singh, G.; Lata, S. Ppy/TiO2-SiO2 nanohybrid series: Synthesis, characterization, photocatalytic activity, and antimicrobial potentiality. J. Environ. Health Sci. Eng. 2023, 21, 239–254. [Google Scholar] [CrossRef]
- Li, X.; Wang, J.; Hu, Z.; Li, M.; Ogino, K. In situ polypyrrole polymerization enhances the photocatalytic activity of nanofibrous TiO2/SiO2 membranes. Chin. Chem. Lett. 2018, 29, 166–170. [Google Scholar] [CrossRef]
- Sagadevan, S.; Lett, J.A.; Weldegebrieal, G.K.; Imteyaz, S.; Johan, M.R. Synthesis, characterization, and photocatalytic activity of PPy/SnO2 nanocomposite. Chem. Phys. Lett. 2021, 783, 139051. [Google Scholar] [CrossRef]
- Parveen, A.; Surumbarkuzhali, N.; Shkir, M.; Massoud, E.E.S.; Manjunath, V.; Ahn, C.-H.; Park, S.-H. Design of SnO2 nanorods/polypyrrole nanocomposite photocatalysts for photocatalytic activity towards various organic pollutants under the visible light irradiation. Inorg. Chem. Commun. 2022, 142, 109685. [Google Scholar] [CrossRef]
- Silvestri, S.; Ferreira, C.D.; Oliveira, V.; Varejão, J.M.; Labrincha, J.A.; Tobaldi, D.M. Synthesis of PPy-ZnO composite used as photocatalyst for the degradation of diclofenac under simulated solar irradiation. J. Photochem. Photobiol. A Chem. 2019, 375, 261–269. [Google Scholar] [CrossRef]
- Jadoun, S.; Yáñez, J.; Mansilla, H.D.; Riaz, U.; Chauhan, N.P.S. Conducting polymers/zinc oxide-based photocatalysts for environmental remediation: A review. Environ. Chem. Lett. 2022, 20, 2063–2083. [Google Scholar] [CrossRef] [PubMed]
- Ceretta, M.B.; Vieira, Y.; Wolski, E.A.; Foletto, E.L.; Silvestri, S. Biological degradation coupled to photocatalysis by ZnO/polypyrrole composite for the treatment of real textile wastewater. J. Water Process. Eng. 2020, 35, 101230. [Google Scholar] [CrossRef]
- Biju, R.; Ravikumar, R.; Thomas, C.; Indulal, C.R. Enhanced photocatalytic degradation of Metanil Yellow dye using polypyrrole-based copper oxide–zinc oxide nanocomposites under visible light. J. Nanoparticle Res. 2022, 24, 117. [Google Scholar] [CrossRef]
- Singh, P.; Shandilya, P.; Raizada, P.; Sudhaik, A.; Rahmani-Sani, A.; Hosseini-Bandegharaei, A. Review on various strategies for enhancing photocatalytic activity of graphene based nanocomposites for water purification. Arab. J. Chem. 2018, 13, 3498–3520. [Google Scholar] [CrossRef]
- Yao, S.; Yuan, X.; Jiang, L.; Xiong, T.; Zhang, J. Recent Progress on Fullerene-Based Materials: Synthesis, Properties, Modifications, and Photocatalytic Applications. Materials 2020, 13, 2924. [Google Scholar] [CrossRef]
- Alsafari, I.A.; Fatima, R.; Warsi, M.F.; Ayman, I.; Jamil, A.; Shahid, M.; Irshad, A. Photocatalytic and antibacterial activity study of ternary oxide of Ni-Al-Cd and their nanocomposite with carbon nanotubes. J. Taibah Univ. Sci. 2022, 16, 1016–1025. [Google Scholar] [CrossRef]
- Mallakpour, S.; Khadem, E. Carbon nanotube–metal oxide nanocomposites: Fabrication, properties and applications. Chem. Eng. J. 2016, 302, 344–367. [Google Scholar] [CrossRef]
- Nair, R.G.; Das, A.; Paul, S.; Rajbongshi, B.; Samdarshi, S. MWCNT decorated V-doped titania: An efficient visible active photocatalyst. J. Alloys Compd. 2017, 695, 3511–3516. [Google Scholar] [CrossRef]
- Hu, G.; Tang, B. Photocatalytic mechanism of graphene/titanate nanotubes photocatalyst under visible-light irradiation. Mater. Chem. Phys. 2013, 138, 608–614. [Google Scholar] [CrossRef]
- Giovannetti, R.; Rommozzi, E.; Zannotti, M.; D’Amato, C.A. Recent Advances in Graphene Based TiO2 Nanocomposites (GTiO2Ns) for Photocatalytic Degradation of Synthetic Dyes. Catalysts 2017, 7, 305. [Google Scholar] [CrossRef]
- Cruz, M.; Gomez, C.; Duran-Valle, C.J.; Pastrana-Martínez, L.M.; Faria, J.L.; Silva, A.M.; Faraldos, M.; Bahamonde, A. Bare TiO2 and graphene oxide TiO2 photocatalysts on the degradation of selected pesticides and influence of the water matrix. Appl. Surf. Sci. 2017, 416, 1013–1021. [Google Scholar] [CrossRef]
- Li, Y.-H.; Tang, Z.-R.; Xu, Y.-J. Multifunctional graphene-based composite photocatalysts oriented by multifaced roles of graphene in photocatalysis. Chin. J. Catal. 2022, 43, 708–730. [Google Scholar] [CrossRef]
- Hosseinzadeh, G.; Ghasemian, N.; Zinatloo-Ajabshir, S. TiO2/graphene nanocomposite supported on clinoptilolite nanoplate and its enhanced visible light photocatalytic activity. Inorg. Chem. Commun. 2022, 136, 109144. [Google Scholar] [CrossRef]
- Le, T.-L.T.; Van, K.N.; Van Bui, H.; Le, T.G.; Vo, V. Controlled growth of TiO2 nanoparticles on graphene by hydrothermal method for visible-light photocatalysis. J. Sci. Adv. Mater. Devices 2021, 6, 516–527. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, M.; Wang, L.; Fei, Y.; Wang, S.; Núñez-Delgado, A.; Bokhari, A.; Race, M.; Khataee, A.; Klemeš, J.J.; et al. Photocatalytic degradation of xanthate in flotation plant tailings by TiO2/graphene nanocomposites. Chem. Eng. J. 2021, 431, 134104. [Google Scholar] [CrossRef]
- Chen, L.; Huang, C.-P.; Chuang, Y.; Nguyen, T.-B.; Chen, C.-W.; Dong, C.-D. Z-Scheme MoS2/TiO2/graphene nanohybrid photocatalysts for visible light-induced degradation for highly efficient water disinfection and antibacterial activity. New J. Chem. 2022, 46, 14159–14169. [Google Scholar] [CrossRef]
- Kiarii, E.M.; Govender, K.K.; Ndungu, P.G.; Govender, P.P. Recent advances in titanium dioxide/graphene photocatalyst materials as potentials of energy generation. Bull. Mater. Sci. 2018, 41, 75. [Google Scholar] [CrossRef]
- Wang, Z.; Du, Y.; Zhang, F.; Zheng, Z.; Zhang, X.; Feng, Q.; Wang, C. Photocatalytic degradation of pendimethalin over Cu2O/SnO2/graphene and SnO2/graphene nanocomposite photocatalysts under visible light irradiation. Mater. Chem. Phys. 2013, 140, 373–381. [Google Scholar] [CrossRef]
- Ou, R.; Zeng, Z.; Ning, X.; Zeng, B.; Wu, C. Improved photocatalytic performance of N-doped ZnO/graphene/ZnO sandwich composites. Appl. Surf. Sci. 2021, 577, 151856. [Google Scholar] [CrossRef]
- Shanmugam, M.; Alsalme, A.; Alghamdi, A.; Jayavel, R. Enhanced Photocatalytic Performance of the Graphene-V2O5 Nanocomposite in the Degradation of Methylene Blue Dye under Direct Sunlight. ACS Appl. Mater. Interfaces 2015, 7, 14905–14911. [Google Scholar] [CrossRef]
- Hu, X.; Xu, P.; Gong, H.; Yin, G. Synthesis and Characterization of WO3/Graphene Nanocomposites for Enhanced Photocatalytic Activities by One-Step In-Situ Hydrothermal Reaction. Materials 2018, 11, 147. [Google Scholar] [CrossRef]
- Qi, S.; Fei, L.; Zuo, R.; Wang, Y.; Wu, Y. Graphene nanocluster decorated niobium oxide nanofibers for visible light photocatalytic applications. J. Mater. Chem. A 2014, 2, 8190–8195. [Google Scholar] [CrossRef]
- Noor, S.; Sajjad, S.; Leghari, S.A.K.; Flox, C.; Kallio, T.; Kauppinen, E.I.; Ahmad, S. Electronic transitions of SWCNTs in comparison to GO on Mn3O4/TiO2nanocomposites for hydrogen energy generation and solar photocatalysis. New J. Chem. 2021, 45, 2431–2442. [Google Scholar] [CrossRef]
- Shaban, M.; Ashraf, A.M.; Abukhadra, M.R. TiO2 Nanoribbons/Carbon Nanotubes Composite with Enhanced Photocatalytic Activity; Fabrication, Characterization, and Application. Sci. Rep. 2018, 8, 781. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, R.; Chen, D.; Hu, X.; Chen, P.; Chen, Z.; Li, D. Synthesis and Characterization of CNT/TiO2/ZnO Composites with High Photocatalytic Performance. Catalysts 2018, 8, 151. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, D.; Hu, X.; Qian, Y.; Li, D. Preparation of TiO2/Carbon Nanotubes/Reduced Graphene Oxide Composites with Enhanced Photocatalytic Activity for the Degradation of Rhodamine B. Nanomaterials 2018, 8, 431. [Google Scholar] [CrossRef]
- Farhadian, M.; Sangpour, P.; Hosseinzadeh, G. Preparation and photocatalytic activity of WO3–MWCNT nanocomposite for degradation of naphthalene under visible light irradiation. RSC Adv. 2016, 6, 39063–39073. [Google Scholar] [CrossRef]
- Iqbal, S.; Bokhari, T.H.; Latif, S.; Imran, M.; Javaid, A.; Mitu, L. Structural and Morphological Studies of V2O5/MWCNTs and ZrO2/MWCNTs Composites as Photocatalysts. J. Chem. 2021, 2021, 9922726. [Google Scholar] [CrossRef]
- Munawar, T.; Bashir, A.; Nadeem, M.S.; Mukhtar, F.; Manzoor, S.; Ashiq, M.N.; Khan, S.A.; Koc, M.; Iqbal, F. Core-shell CeO2@C60 hybrid serves as a dual-functional catalyst: Photocatalyst for organic pollutant degradation and electrocatalyst for oxygen evolution reaction. Ceram. Int. 2023, 49, 8447–8462. [Google Scholar] [CrossRef]
- Sathish, M.; Miyazawa, K.; Ye, J. Fullerene nanowhiskers at liquid–liquid interface: A facile template for metal oxide (TiO2, CeO2) nanofibers and their photocatalytic activity. Mater. Chem. Phys. 2011, 130, 211–217. [Google Scholar] [CrossRef]
- Li, G.; Jiang, B.; Li, X.; Lian, Z.; Xiao, S.; Zhu, J.; Zhang, D.; Li, H. C60/Bi2TiO4F2 Heterojunction Photocatalysts with Enhanced Visible-Light Activity for Environmental Remediation. ACS Appl. Mater. Interfaces 2013, 5, 7190–7197. [Google Scholar] [CrossRef]
- Sapkota, K.P.; Islam, A.; Hanif, A.; Akter, J.; Lee, I.; Hahn, J.R. Hierarchical Nanocauliflower Chemical Assembly Composed of Copper Oxide and Single-Walled Carbon Nanotubes for Enhanced Photocatalytic Dye Degradation. Nanomaterials 2021, 11, 696. [Google Scholar] [CrossRef]
- Sapkota, K.P.; Lee, I.; Hanif, A.; Islam, A.; Akter, J.; Hahn, J.R. Enhanced Visible-Light Photocatalysis of Nanocomposites of Copper Oxide and Single-Walled Carbon Nanotubes for the Degradation of Methylene Blue. Catalysts 2020, 10, 297. [Google Scholar] [CrossRef]
- Sapkota, K.P.; Lee, I.; Hanif, A.; Islam, A.; Hahn, J.R. Solar-Light-Driven Efficient ZnO–Single-Walled Carbon Nanotube Photocatalyst for the Degradation of a Persistent Water Pollutant Organic Dye. Catalysts 2019, 9, 498. [Google Scholar] [CrossRef]
- Hanif, A.; Kim, Y.-S.; Akter, J.; Kim, H.G.; Kwac, L.K. Fabrication of Robust and Stable N-Doped ZnO/Single-Walled Carbon Nanotubes: Characterization, Photocatalytic Application, Kinetics, Degradation Products, and Toxicity Analysis. ACS Omega 2023, 8, 16174–16185. [Google Scholar] [CrossRef]
- de la Flor, M.P.; Camarillo, R.; Martínez, F.; Jiménez, C.; Quiles, R.; Rincón, J. Synthesis and characterization of TiO2/CNT/Pd: An effective sunlight photocatalyst for neonicotinoids degradation. J. Environ. Chem. Eng. 2021, 9, 106278. [Google Scholar] [CrossRef]
- Tanase, T.; Nakamae, K.; Kitagawa, Y.; Nakajima, T. Octapalladium Strings Trap C 60 and C 70 Fullerenes Affording Metal-Chain-Wired Bucky Balls. Chem.–A Eur. J. 2021, 27, 12953–12958. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Q.; Zou, L.-H.; You, J.-W. Facile fabrication of titanium dioxide/fullerene nanocomposite and its enhanced visible photocatalytic activity. J. Colloid Interface Sci. 2016, 466, 56–61. [Google Scholar] [CrossRef]
- Munawar, T.; Sardar, S.; Mukhtar, F.; Nadeem, M.S.; Manzoor, S.; Ashiq, M.N.; Khan, S.A.; Koc, M.; Iqbal, F. Fabrication of fullerene-supported La2O3–C60 nanocomposites: Dual-functional materials for photocatalysis and supercapacitor electrodes. Phys. Chem. Chem. Phys. 2023, 25, 7010–7027. [Google Scholar] [CrossRef] [PubMed]
- Maldaner, L.; Jardim, I.C. Determination of some organic contaminants in water samples by solid-phase extraction and liquid chromatography–tandem mass spectrometry. Talanta 2012, 100, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, I.; Writer, J.; Keen, O.; Lester, Y.; Padilla-Sánchez, J.; Fernández-Ramos, C.; Thurman, E. Chapter 8-LC-TOF-MS for the Identification of Environmental Metabolites and Degradation Products. In Comprehensive Analytical Chemistry; Pérez, S., Eichhorn, P., Barceló, D., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 71, pp. 231–261. [Google Scholar] [CrossRef]
- Kotthoff, L.; Keller, J.; Lörchner, D.; Mekonnen, T.F.; Koch, M. Transformation Products of Organic Contaminants and Residues—Overview of Current Simulation Methods. Molecules 2019, 24, 753. [Google Scholar] [CrossRef] [PubMed]
- Pica, M.; Calzuola, S.; Donnadio, A.; Gentili, P.L.; Nocchetti, M.; Casciola, M. De-Ethylation and Cleavage of Rhodamine B by a Zirconium Phosphate/Silver Bromide Composite Photocatalyst. Catalysts 2018, 9, 3. [Google Scholar] [CrossRef]
- Gaudino, E.C.; Canova, E.; Liu, P.; Wu, Z.; Cravotto, G. Degradation of Antibiotics in Wastewater: New Advances in Cavitational Treatments. Molecules 2021, 26, 617. [Google Scholar] [CrossRef]
- Ahmad, F.; Zhu, D.; Sun, J. Environmental fate of tetracycline antibiotics: Degradation pathway mechanisms, challenges, and perspectives. Environ. Sci. Eur. 2021, 33, 1–17. [Google Scholar] [CrossRef]
- Matsunami, D.; Yamanaka, K.; Mizoguchi, T.; Kojima, K. Comparison of photodegradation of methylene blue using various TiO2 films and WO3 powders under ultraviolet and visible-light irradiation. J. Photochem. Photobiol. A Chem. 2018, 369, 106–114. [Google Scholar] [CrossRef]
- Tsui, T.-H.; van Loosdrecht, M.C.; Dai, Y.; Tong, Y.W. Machine learning and circular bioeconomy: Building new resource efficiency from diverse waste streams. Bioresour. Technol. 2023, 369, 128445. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, J.; Yang, X.; Zhang, Y.; Zhang, L.; Ren, H.; Wu, B.; Ye, L. A review of the application of machine learning in water quality evaluation. Eco-Environ. Health 2022, 1, 107–116. [Google Scholar] [CrossRef]
- Bottausci, S.; Midence, R.; Serrano-Bernardo, F.; Bonoli, A. Organic Waste Management and Circular Bioeconomy: A Literature Review Comparison between Latin America and the European Union. Sustainability 2022, 14, 1661. [Google Scholar] [CrossRef]
- Tsui, T.-H.; Zhang, L.; Zhang, J.; Dai, Y.; Tong, Y.W. Methodological framework for wastewater treatment plants delivering expanded service: Economic tradeoffs and technological decisions. Sci. Total Environ. 2022, 823, 153616. [Google Scholar] [CrossRef] [PubMed]







| Mixed MO-Based MON | Light Stimulus/ Amount of Catalyst | Pollutant/Initial Concentration/ % Degradation/Number of Cycles Reuse | [Ref.] |
|---|---|---|---|
| grain bi-phase Fe3O4/Bi2WO6 supported on g-C3N4 nanosheets | Visible light/ 100 mg | Tetracycline antibiotic/20 mg L−1/ 98% in 105 min/6 cycles reuse | [93] |
| grain bi-phase Cu2O/Fe3O4 supported on Fe MOFs | Visible light/ 50 mg | Ciprofloxacin antibiotic/20 mg L−1/ 99.2% in 105 min/5 cycles reuse | [115] |
| heterogeneous nanoleaves of Cu/Ni/Fe oxides | Visible light/ 25 mg | Tetracycline antibiotic/10 mg L¯1 100% in 4 min/12 cycles reuse | [124] |
| grain bi-phase TiO2/ZnO heteronanostructures | UV light/ 512 mg | Tetracycline antibiotic/20 mg L−1/ 82% after 165 min/6 cycles reuse | [125] |
| Fe3O4 nanoparticles on Bi2O4 nanorods | Visible light/ 10 mg | Ibuprofen antibiotic/500 μM/ 100%, after 240 h/4 cycles reuse | [126] |
| grain three-phase ZnO/Al2O3/TiO2 heteronanostructures | UV light/ 100 mg | Ibuprofen antibiotic/60 mg L−1/ 95%, after 210 min/no reuse | [127] |
| CuO/ZnFe2O4 nanoparticles on BiOBr nanoplates | Visible light/ 150 mg | Levofloxacin antibiotic/25 mg L−1 91% in 90 min/5 cycles reuse | [128] |
| flower-like Bi5O7I/Bi/Bi2WO6 decorated NiFe2O4 nanoparticles | Visible light/ 75 mg | Levofloxacin antibiotic/28 mg L−1/ 97.5% in 90 min/5 cycles reuse | [129] |
| coral-like MgO/Co3O4 spherical nanostructures | Visible light/ 50 mg L−1 | Levofloxacin antibiotic/10 mg L−1/ 96.9% in 20 min/6 cycles reuse | [130] |
| grain bi-phase CuO/CdO supported onto bentonite nanoleads | Sunlight/ 400 mg L−1 | Levofloxacin antibiotic/10 mg L−1/ 96.1% in 30 min/3 cycles reuse | [131] |
| ZrO2 nanoparticles coated on MoO3 nanoplates | Visible light/ 250 mg L−1 | Diclofenac sodium antibiotic/n.i./ 91% in 120 min/5 cycles reuse | [132] |
| Co3O4 nanoparticles dispersed on MoO3 surface | Sunlight/ 150 mg | Imidacloprid insecticide/15 mg L−1/ 98% in 150 min/no reuse | [133] |
| Fe-doped anatase/brookite TiO2 heteronanostructures | UV light/ 1 g L−1 | Simazine herbicide/1.73 × 10−5 M 65% in 180 min/no reuse | [134] |
| CeO2 nanoparticles/WO3 nanoplates heteronanostructures | Visible light/ 30 mg | Nitenpyram insecticide/n.i./ 100% in 180 min/no reuse | [135] |
| FeO/CoO nanoparticles loaded onto the TiO2 surface | UV light/ 100 mg | 2,4,6-Trichlorophenol herbicide/25 mg L−1/100% in 180 min/no reuse; 2,4-Dichlorophenoxyacetic acid herbicide/25 mg L−1/100% in 120 min/no reuse | [136] |
| grain bi-phase TiO2-MoO3 heteronanostructures | Visible light/ 50 mg | Carbaryl and Fenoxycarb insecticides/60 mg L−1/100% in 60 min/no reuse | [137] |
| Li2MnO3@ZrO2 core-shell Heterostructures | Visible light/ 1 g L−1 | Atrazine herbicide/50 mg L−1/ 100% in 60 min/5 cycles reuse | [138] |
| NiO/ZnO heterostructures embedded in the chitosan pores | Sunlight/ 30 mg | Malathion insecticide/20 mg L−1/ 94% in 5 h/5 cycles reuse | [139] |
| Metal Oxide/Carbon Material-Based MON | Light Light Stimulus/ Amount of Catalyst | Pollutant/Initial Concentration/ Pollutant/Initial Concentration/ % Degradation/Number of Cycles Reuse | [Ref.] |
|---|---|---|---|
| Cu2O/G Nanostructures | Visible light/ XXX mg | MB dye/900 mg L−1/94%, after 180 min/3 cycles reuse | [19] |
| TiO2/G (supported on clinoptilolite nanoplates) | Visible light (xenon lamp) | Nitenpyram insecticide/n.i./100%, after 80 min/no reuse | [169] |
| TiO2 on G surface | Visible light (tungsten lamp)/30 mg | RhB dye/30 mg L−1/84% in 6 h/ 2 cycles reuse | [173] |
| TiO2 on G surface | Visible light (xenon lamp)/10 mg | Xanthate (pollutant from the mineral industry)/20 mg L−1/97%, after 100 min/no reuse | [174] |
| TiO2/MoS2 on G surface | Visible light/n.i. | Tetracycline antibiotic/10 mg L−1/95%, after 60 min/no reuse | [175] |
| sandwich-structured N-doped (ZnO/G/ZnO) nanosheets | Visible light (xenon lamp)/300 mg | MOrange dye/10 mg L−1/80%, after 80 min/no reuse | [178] |
| V2O5 nanorods on G surface | Visible light (sunlight)/10 mg | MB dye/n.i/100% in 90 min/no reuse | [179] |
| WO3 nanorods on G nanosheets | visible light (xenon lamp)/20 mg | MB dye/10 mg L−1/83% in 70 min/no reuse | [180] |
| G nanocluster decorated Nb2O5 Nanofibers | Visible light (metal-halide lamp)/1 g | MOrange dye/20 mg L−1/95% in 5 h/ 3 cycles reuse | [181] |
| single-walled CNT on Mn3O4-TiO2 surface | Visible light (sunlight)/1 g | MOrange dye/20 mg L−1/98% in 150 min/no reuse | [182] |
| WO3.ZnO.NiO on CNT | Visible light (sunlight)/50 mg | MB dye/5 ppm/66.19% in 105 min/ 4 cycles reuse | [26] |
| multi-walled CNT decorated V-doped TiO2 | Visible light (sunlight)/500 mg | MB dye/12.8 mg L−1/65%, after 60 min/no reuse | [167] |
| TiO2 nanoribbons/multi-walled CNT nanostructures | Visible light (sunlight)/20 mg | MB dye/10 mg L−1/97.3%, after 180 min/3 cycles reuse | [183] |
| TiO2/ZnO covered multi-walled CNT | UV; visible light/n.i. | RhB dye/5 mg L−1/100% in 40 min/no reuse | [184] |
| TiO2/CNTs/reduced graphene oxide (rGO) nanostructures | Visible light (xenon lamp)/10 mg | RhB dye/10 mg L−1/100%, after 60 min/no reuse | [185] |
| WO3 on multi-walled CNT surface | Visible light (xenon lamp)/50 mg | Naphthalene insecticide/10 ppm/65% in 240 min/no reuse | [186] |
| V2O5 on multi-walled CNT surface | UV light/10 mg | MB dye/100 ppm/96%, after 60 min/no reuse | [187] |
| CeO2@C60 core-shell nanostructures | Visible light (sunlight)/1 g | P-nitroaniline/10 ppm/100% in 75 min/7 cycles reuse | [188] |
| TiO2 and CeO2 nanofibers embedded in C60 nanowhiskers matrix | UV light/75 mg | Isopropyl alcohol (IPA)/200 ppm/ 90%, after 120 min/no reuse | [189] |
| Bi2TiO4F2-C60 hierarchical Spheres | Visible light (xenon lamp)/10 mg | RhB and Eosin Y/20 ppm/80% and 90%, respectively, after 60 min/ 3 cycles reuse | [190] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araújo, E.S.; Pereira, M.F.G.; da Silva, G.M.G.; Tavares, G.F.; Oliveira, C.Y.B.; Faia, P.M. A Review on the Use of Metal Oxide-Based Nanocomposites for the Remediation of Organics-Contaminated Water via Photocatalysis: Fundamentals, Bibliometric Study and Recent Advances. Toxics 2023, 11, 658. https://doi.org/10.3390/toxics11080658
Araújo ES, Pereira MFG, da Silva GMG, Tavares GF, Oliveira CYB, Faia PM. A Review on the Use of Metal Oxide-Based Nanocomposites for the Remediation of Organics-Contaminated Water via Photocatalysis: Fundamentals, Bibliometric Study and Recent Advances. Toxics. 2023; 11(8):658. https://doi.org/10.3390/toxics11080658
Chicago/Turabian StyleAraújo, Evando S., Michel F. G. Pereira, Georgenes M. G. da Silva, Ginetton F. Tavares, Carlos Y. B. Oliveira, and Pedro M. Faia. 2023. "A Review on the Use of Metal Oxide-Based Nanocomposites for the Remediation of Organics-Contaminated Water via Photocatalysis: Fundamentals, Bibliometric Study and Recent Advances" Toxics 11, no. 8: 658. https://doi.org/10.3390/toxics11080658
APA StyleAraújo, E. S., Pereira, M. F. G., da Silva, G. M. G., Tavares, G. F., Oliveira, C. Y. B., & Faia, P. M. (2023). A Review on the Use of Metal Oxide-Based Nanocomposites for the Remediation of Organics-Contaminated Water via Photocatalysis: Fundamentals, Bibliometric Study and Recent Advances. Toxics, 11(8), 658. https://doi.org/10.3390/toxics11080658









