Potential Application Performance of Hydrochar from Kitchen Waste: Effects of Salt, Oil, Moisture, and pH
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Hydrothermalcarbonization of Synthetic KW
2.3. Adsorption Capacity of KWHC
2.3.1. Sorption Kinetics
2.3.2. Sorption Isotherms
2.4. Thermogravimetric Analysis of KWHC
2.5. Chemical Analysis of KWHC
2.6. Analytical Methods
2.7. Statistical Analysis
3. Results
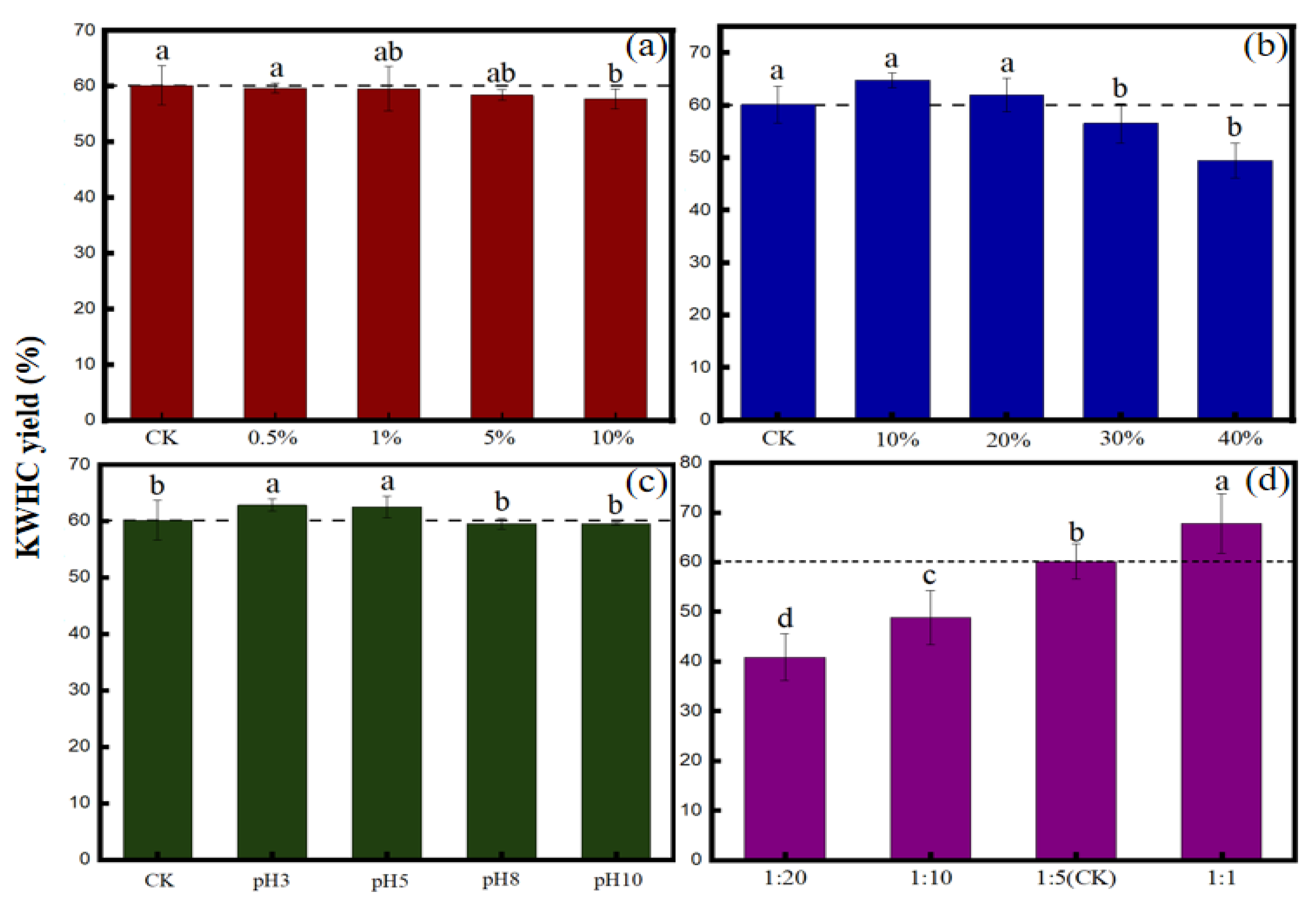
3.1. Effects of Salt, Oil, pH, and Solid-Liquid Ratio on KWHC Productivity
3.2. Elemental Analysis of the KWHC
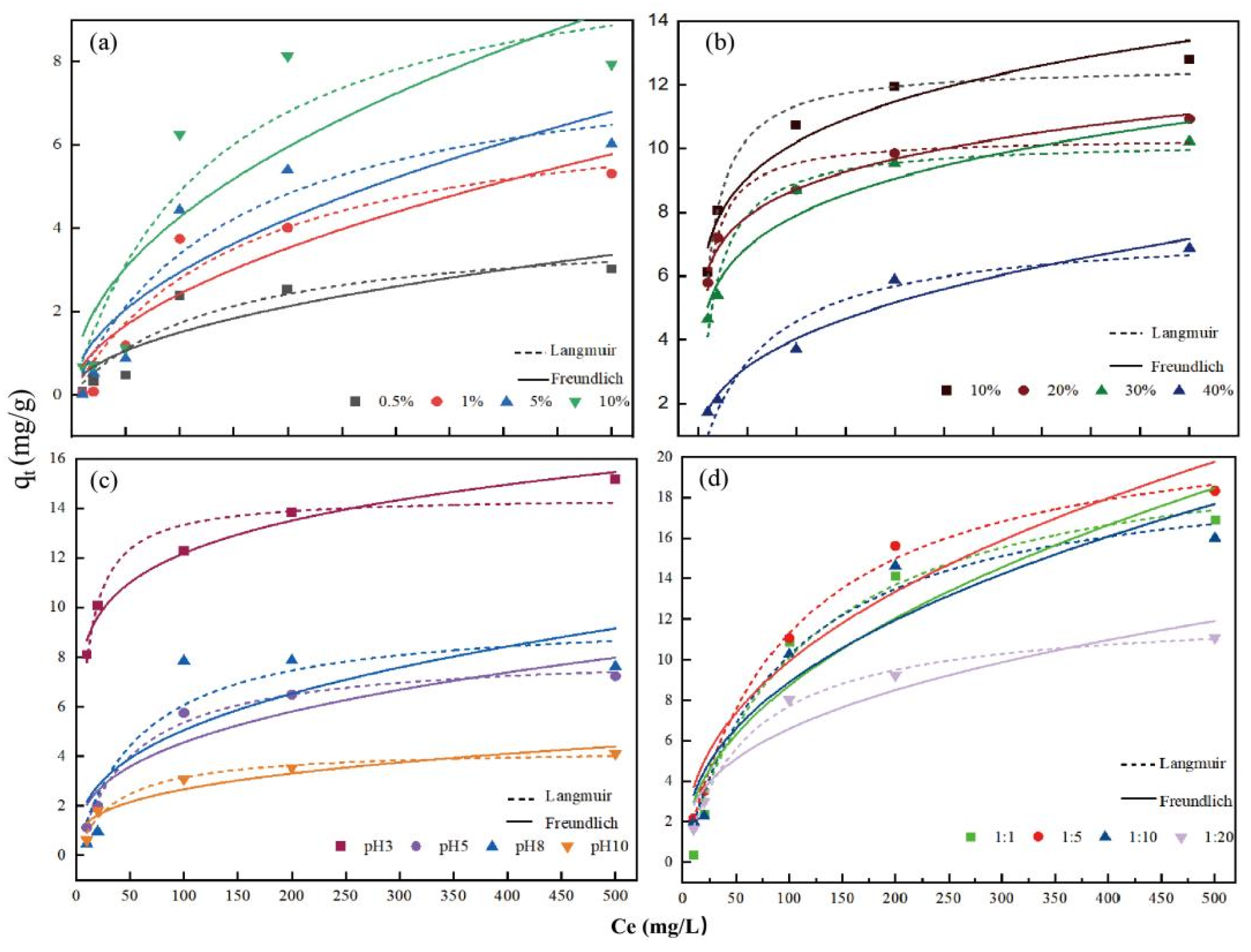
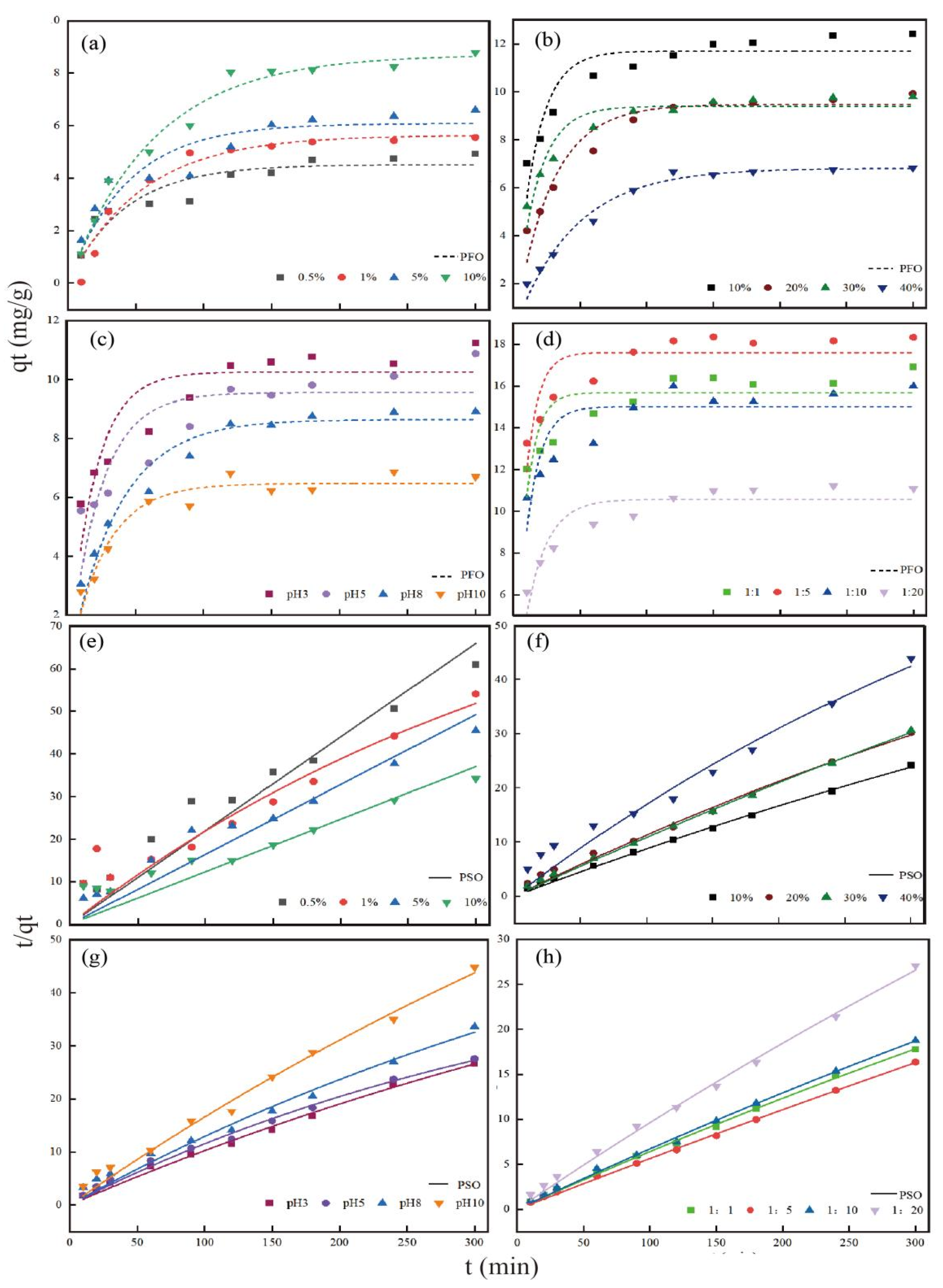
3.3. Adsorption of Cd (Ⅱ) on KWHC with Different Treatments
3.4. Combustion Characteristics Analysis
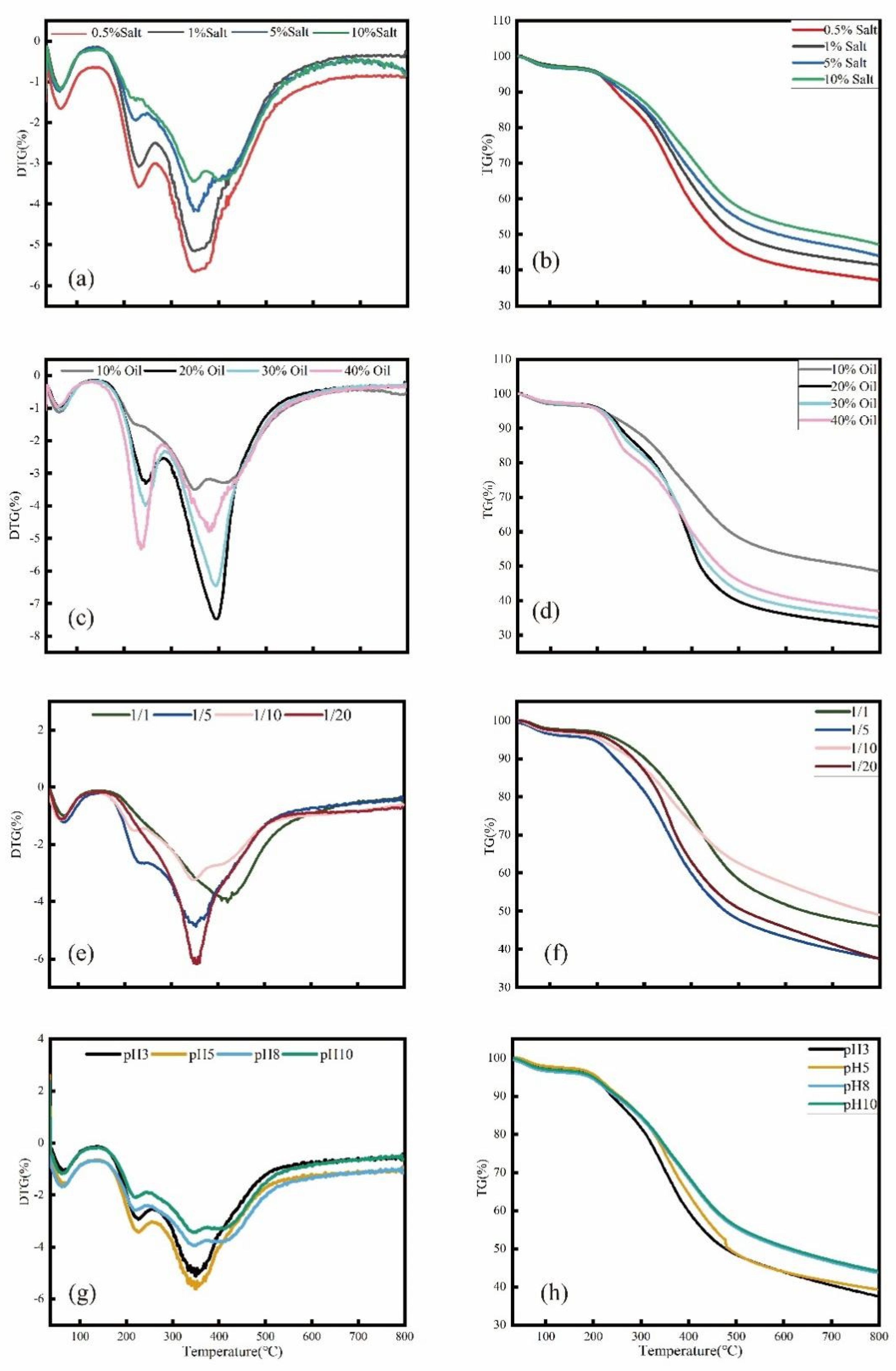
3.4.1. HHV Analysis of KWHC
3.4.2. Thermogravimetric Analysis of KWHC
3.4.3. FTIR Analysis of KWHC
3.4.4. XRD Analysis of KWHC
4. Discussion
4.1. Effects of Salt, Oil, pH, and Solid-Liquid Ratio on the Productivity of KWHC
4.1.1. Effect of Salt Content on the Productivity of KWHC
4.1.2. Effect of Oil Content on Productivity of KWHC
4.1.3. Effect of the pH of the Solution on the Productivity of KWHC
4.1.4. Effect of the Solid-Liquid Ratio on the Productivity of KWHC
4.2. Effects of the Salt, Oil, pH, and Solid-Liquid Ratio on Elemental Analysis of KWHC
4.2.1. Effects of Salt on the Elemental Analysis of KWHC
4.2.2. Effects of Oil on the Elemental Analysis of KWHC
4.2.3. Effects of pH on the Elemental Analysis of KWHC
4.2.4. Effects of the Solid-Liquid Ratio on the Elemental Analysis of KWHC
4.3. Adsorption of Cd (Ⅱ) on KWHC with Different Treatments
4.3.1. Effects of Salt on Adsorption of Cd (Ⅱ) on KWHC
4.3.2. Effects of Oil on Adsorption of Cd (Ⅱ) on KWHC
4.3.3. Effects of pH on Adsorption of Cd (Ⅱ) on KWHC
4.3.4. Effects of Solid-Liquid Ratio on Adsorption of Cd (Ⅱ) on KWHC
4.4. Adsorption Mechanism of KWHC
4.4.1. Sorption Isotherms
4.4.2. Sorption Kinetics
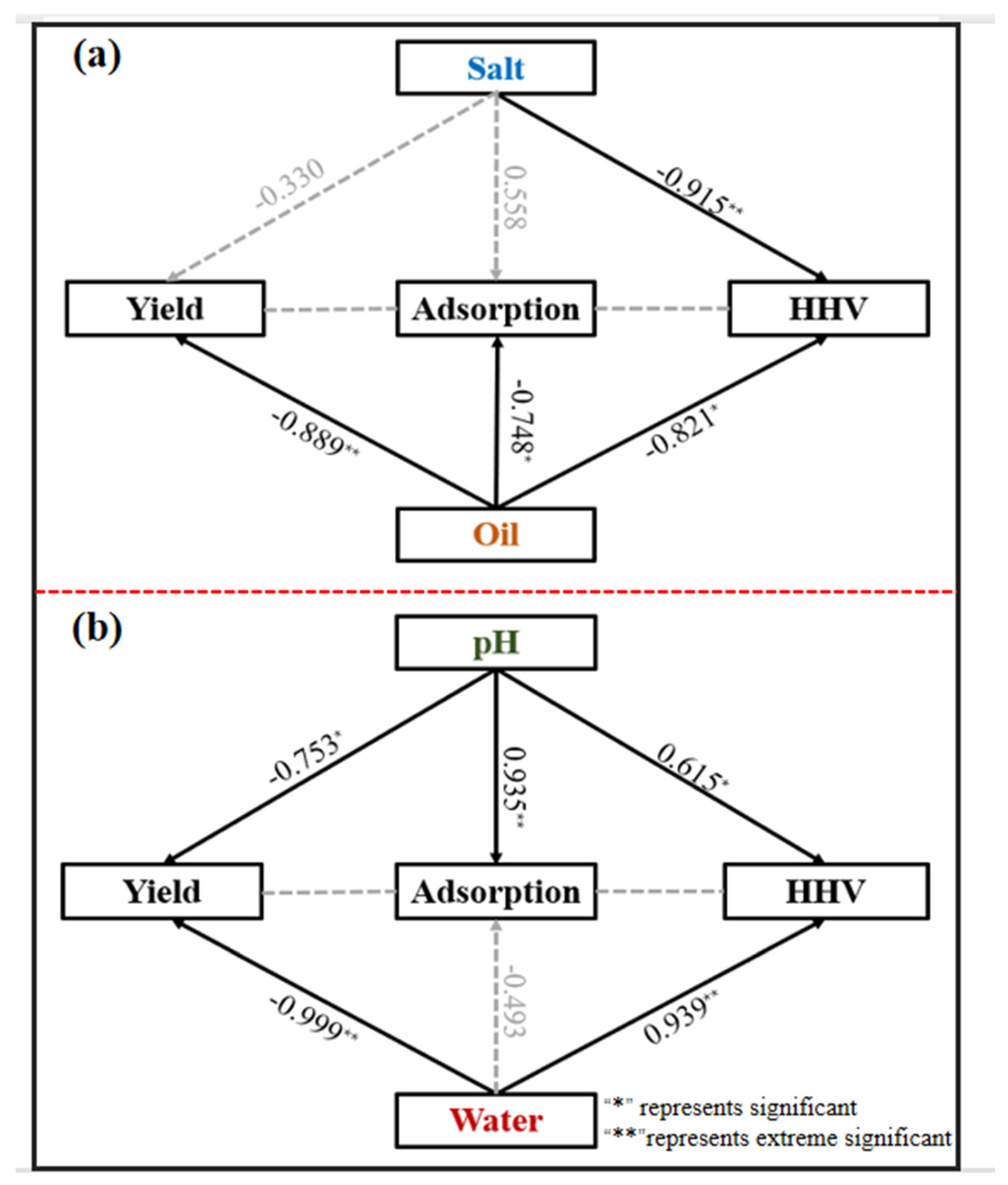
4.5. Correlation between Different Parameters and Application Performance
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cuervo, M.P.; Castillo, A.; Santiago-Connolly, L.M. Overview of Biological Hazards and Foodborne Diseases. Ref. Modul. Food Sci. 2023, 9, 00113-1. [Google Scholar] [CrossRef]
- Iranmanesh, M.; Ghobakhloo, M.; Nilashi, M.; Tseng, M.-L.; Senali, M.G.; Abbasi, G.A. Impacts of the COVID-19 pandemic on household food waste behaviour: A systematic review. Appetite 2022, 176, 106127. [Google Scholar] [CrossRef] [PubMed]
- Das, A.K.; Islam, N.; Billah, M.; Sarker, A. COVID-19 and municipal solid waste (MSW) management: A review. Environ. Sci. Pollut. Res. 2021, 28, 28993–29008. [Google Scholar] [CrossRef] [PubMed]
- Li, L.-H.; Li, Y.-L.; Hong, Y. New insights into the microalgal culture using kitchen waste: Enzyme pretreatment and mixed microalgae self-flocculation. Biochem. Eng. J. 2023, 195, 108904. [Google Scholar] [CrossRef]
- Zhou, L.; Huang, Y.; Qiu, W.; Sun, Z.; Liu, Z.; Song, Z. Adsorption Properties of Nano-MnO2–Biochar Composites for Copper in Aqueous Solution. Molecules 2017, 22, 173. [Google Scholar] [CrossRef]
- Sharma, P.; Gaur, V.K.; Kim, S.-H.; Pandey, A. Microbial strategies for bio-transforming food waste into resources. Bioresour. Technol. 2020, 299, 122580. [Google Scholar] [CrossRef]
- Tan, B.; He, L.; Dai, Z.; Sun, R.; Jiang, S.; Lu, Z.; Liang, Y.; Ren, L.; Sun, S.; Zhang, Y.; et al. Review on recent progress of bioremediation strategies in Landfill leachate—A green approach. J. Water Process. Eng. 2022, 50, 103229. [Google Scholar] [CrossRef]
- Zhu, K.; Zhang, L.; Mu, L.; Ma, J.; Li, C.; Li, A. Comprehensive investigation of soybean oil-derived LCFAs on anaerobic digestion of organic waste: Inhibitory effect and transformation. Biochem. Eng. J. 2019, 151, 107314. [Google Scholar] [CrossRef]
- Yang, G.; Liu, H.; Li, Y.; Zhou, Q.; Jin, M.; Xiao, H.; Yao, H. Kinetics of hydrothermal carbonization of kitchen waste based on multi-component reaction mechanism. Fuel 2022, 324, 124693. [Google Scholar] [CrossRef]
- Yan, H.; Hu, B.; Wang, R. Air-source heat pump heating based water vapor compression for localized steam sterilization applications during the COVID-19 pandemic. Renew. Sustain. Energy Rev. 2021, 145, 111026. [Google Scholar] [CrossRef]
- Ding, L.; Cheng, J.; Qiao, D.; Yue, L.; Li, Y.-Y.; Zhou, J.; Cen, K. Investigating hydrothermal pretreatment of food waste for two-stage fermentative hydrogen and methane co-production. Bioresour. Technol. 2017, 241, 491–499. [Google Scholar] [CrossRef]
- Liu, W.; Dong, Z.; Sun, D.; Chen, Y.; Wang, S.; Zhu, J.; Liu, C. Bioconversion of kitchen wastes into bioflocculant and its pilot-scale application in treating iron mineral processing wastewater. Bioresour. Technol. 2019, 288, 121505. [Google Scholar] [CrossRef]
- Gajera, Z.R.; Mungray, A.A.; Rene, E.R.; Mungray, A.K. Hydrothermal carbonization of cow dung with human urine as a solvent for hydrochar: An experimental and kinetic study. J. Environ. Manag. 2023, 327, 116854. [Google Scholar] [CrossRef]
- Lang, Q.; Liu, Z.; Li, Y.; Xu, J.; Li, J.; Liu, B.; Sun, Q. Combustion characteristics, kinetic and thermodynamic analyses of hydrochars derived from hydrothermal carbonization of cattle manure. J. Environ. Chem. Eng. 2022, 10, 106938. [Google Scholar] [CrossRef]
- Liu, X.; Shen, J.; Guo, Y.; Wang, S.; Chen, B.; Luo, L.; Zhang, H. Technical progress and perspective on the thermochemical conversion of kitchen waste and relevant applications: A comprehensive review. Fuel 2023, 331, 125803. [Google Scholar] [CrossRef]
- Zhou, Y.; Engler, N.; Nelles, M. Symbiotic relationship between hydrothermal carbonization technology and anaerobic digestion for food waste in China. Bioresour. Technol. 2018, 260, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Guan, H.; Zhang, Y.; Liu, S.; Zhao, B.; Zhong, C.; Zhang, H.; Ding, W.; Song, A.; Zhu, D.; et al. Performance and mechanism of Pb2+ and Cd2+ ions’ adsorption via modified antibiotic residue-based hydrochar. Heliyon 2023, 9, e14930. [Google Scholar] [CrossRef]
- Sun, X.-N.; Yu, K.; He, J.-H.; Chen, Y.; Guo, J.-Z.; Li, B. Multiple roles of ferric chloride in preparing efficient magnetic hydrochar for sorption of methylene blue from water solutions. Bioresour. Technol. 2023, 373, 128715. [Google Scholar] [CrossRef]
- Yan, Y.; Qi, F.; Zhang, L.; Zhang, P.; Li, Q. Enhanced Cd adsorption by red mud modified bean-worm skin biochars in weakly alkali environment. Sep. Purif. Technol. 2022, 297, 121533. [Google Scholar] [CrossRef]
- Olszewski, M.P.; Arauzo, P.J.; Maziarka, P.A.; Ronsse, F.; Kruse, A. Pyrolysis Kinetics of Hydrochars Produced from Brewer’s Spent Grains. Catalysts 2019, 9, 625. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Wang, X.; Wu, W.; Cui, X.; Cheng, Z.; Yan, B.; Yang, X.; He, Z.; Chen, G.; et al. Hydrothermal conversion of Cd/Zn hyperaccumulator (Sedum alfredii) for heavy metal separation and hydrochar production. J. Hazard. Mater. 2022, 423 Pt B, 127122. [Google Scholar] [CrossRef]
- Liu, X.; Zhai, Y.; Li, S.; Wang, B.; Wang, T.; Liu, Y.; Qiu, Z.; Li, C. Hydrothermal carbonization of sewage sludge: Effect of feed-water pH on hydrochar’s physicochemical properties, organic component and thermal behavior. J. Hazard. Mater. 2020, 388, 122084. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Fan, Y.; Zhai, Y.; Liu, X.; Wang, Z.; Zhu, Y.; Shi, H.; Li, C.; Zhu, Y. Co-hydrothermal carbonization of rape straw and microalgae: pH-enhanced carbonization process to obtain clean hydrochar. Energy 2022, 257, 124733. [Google Scholar] [CrossRef]
- Periyavaram, S.R.; Uppala, L.; Sivaprakash, S.; Reddy, P.H.P. Thermal behaviour of hydrochar derived from hydrothermal carbonization of food waste using leachate as moisture source: Kinetic and thermodynamic analysis. Bioresour. Technol. 2023, 373, 128734. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.-Q.; Liao, J.-J.; Chen, D.-B.; Xu, Z.-X. Hydrothermal carbonization of sewage sludge: Catalytic effect of Cl− on hydrochars physicochemical properties. Mol. Catal. 2021, 513, 111789. [Google Scholar] [CrossRef]
- Cao, C.-Y.; Liang, C.-H.; Yin, Y.; Du, L.-Y. Thermal activation of serpentine for adsorption of cadmium. J. Hazard. Mater. 2017, 329, 222–229. [Google Scholar] [CrossRef]
- Rechberger, M.V.; Kloss, S.; Wang, S.-L.; Lehmann, J.; Rennhofer, H.; Ottner, F.; Wriessnig, K.; Daudin, G.; Lichtenegger, H.; Soja, G.; et al. Enhanced Cu and Cd sorption after soil aging of woodchip-derived biochar: What were the driving factors? Chemosphere 2019, 216, 463–471. [Google Scholar] [CrossRef]
- Benedetti, V.; Pecchi, M.; Baratieri, M. Combustion kinetics of hydrochar from cow-manure digestate via thermogravimetric analysis and peak deconvolution. Bioresour. Technol. 2022, 353, 127142. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wei, Z.; Jia, X.; Li, M.; Zhu, C.; Xi, B.; Xia, T. Impacts of liquid substances transformation of kitchen waste with hydrothermal pretreatment. Res. Environ. 2015, 28, 440–446. [Google Scholar] [CrossRef]
- Jin, Y.; Li, Y.; Li, J. Influence of thermal pretreatment on physical and chemical properties of kitchen waste and the efficiency of anaerobic digestion. J. Environ. Manag. 2016, 180, 291–300. [Google Scholar] [CrossRef]
- Zhang, C.; Han, L.; Yan, M.; Xia, J.; Rong, N.; Baloch, H.A.; Guo, H.; Wu, P.; Xu, G.; Ma, K. Hydrothermal co-liquefaction of rice straw and waste cooking-oil model compound for bio-crude production. J. Anal. Appl. Pyrolysis 2021, 160, 105360. [Google Scholar] [CrossRef]
- Goswami, R.; Shim, J.; Deka, S.; Kumari, D.; Kataki, R.; Kumar, M. Characterization of cadmium removal from aqueous solution by biochar produced from Ipomoea fistulosa at different pyrolytic temperatures. Ecol. Eng. 2016, 97, 444–451. [Google Scholar] [CrossRef]
- Zhang, C.; Shao, M.; Wu, H.; Wang, N.; Chen, Q.; Xu, Q. Management and valorization of digestate from food waste via hydrothermal. Resour. Conserv. Recycl. 2021, 171, 105639. [Google Scholar] [CrossRef]
- Yang, W.; Shimanouchi, T.; Kimura, Y. Characterization of the residue and liquid products produced from husks of nuts from Carya cathayensis Sarg by hydrothermal carbonization. ACS Sustain. Chem. Eng. 2015, 3, 591–598. [Google Scholar] [CrossRef]
- Gong, M.; Zhu, W.; Xu, Z.R.; Zhang, H.W.; Yang, H.P. Influence of sludge properties on the direct gasification of dewatered sewage sludge in supercritical water. Renew. Energy. 2014, 66, 605–611. [Google Scholar] [CrossRef]
- Wang, L.; Chang, Y.; Li, A. Hydrothermal carbonization for energy-efficient processing of sewage sludge: A review. Renew. Sustain. Energy Rev. 2019, 108, 423–440. [Google Scholar] [CrossRef]
- Jiang, Z.; Fan, J.; Budarin, V.L.; Macquarrie, D.J.; Gao, Y.; Li, T.; Hu, C.; Clark, J.H. Mechanistic understanding of salt-assisted autocatalytic hydrolysis of cellulose. Sustain. Energy Fuels 2018, 2, 936–940. [Google Scholar] [CrossRef]
- Fan, Y.; Hornung, U.; Raffelt, K.; Dahmen, N. The influence of lipids on the fate of nitrogen during hydrothermal liquefaction of protein-containing biomass. J. Anal. Appl. Pyrolysis 2020, 147, 104798. [Google Scholar] [CrossRef]
- Zhang, D.; Feng, Y.; Huang, H.; Khunjar, W.; Wang, Z.-W. Recalcitrant dissolved organic nitrogen formation in thermal hydrolysis pretreatment of municipal sludge. Environ. Int. 2020, 138, 105629. [Google Scholar] [CrossRef]
- Xu, S.; Chen, J.; Peng, H.; Leng, S.; Li, H.; Qu, W.; Hu, Y.; Li, H.; Jiang, S.; Zhou, W.; et al. Effect of biomass type and pyrolysis temperature on nitrogen in biochar, and the comparison with hydrochar. Fuel 2021, 291, 120128. [Google Scholar] [CrossRef]
- Khan, Z.H.; Gao, M.; Qiu, W.; Islam, S.; Song, Z. Mechanisms for cadmium adsorption by magnetic biochar composites in an aqueous solution. Chemosphere 2020, 246, 125701. [Google Scholar] [CrossRef] [PubMed]
- Usman, A.; Sallam, A.; Zhang, M.; Vithanage, M.; Ahmad, M.; Al-Farraj, A.; Ok, Y.S.; Abduljabbar, A.; Al-Wabel, M. Sorption Process of Date Palm Biochar for Aqueous Cd (II) Removal: Efficiency and Mechanisms. Water Air Soil Pollut. 2016, 227, 449. [Google Scholar] [CrossRef]
- Li, D.; Cui, H.; Cheng, Y.; Xue, L.; Wang, B.; He, H.; Hua, Y.; Chu, Q.; Feng, Y.; Yang, L. Chemical aging of hydrochar improves the Cd2+ adsorption capacity from aqueous solution. Environ. Pollut. 2021, 287, 117562. [Google Scholar] [CrossRef]
- Usman, A.R.; Almaroai, Y.A.; Ahmad, M.; Vithanage, M.; Ok, Y.S. Toxicity of synthetic chelators and metal availability in poultry manure amended Cd, Pb and As contaminated agricultural soil. J. Hazard. Mater. 2013, 262, 1022–1030. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Angeles, A.T.; Son, M.; Shim, J.; Chon, K.; Cho, K.H. Predicting the salt adsorption capacity of different capacitive deionization electrodes using random forest. Desalination 2022, 537, 115826. [Google Scholar] [CrossRef]
- Aich, S.; Behera, D.; Nandi, B.K.; Bhattacharya, S. Relationship between proximate analysis parameters and combustion behaviour of high ash Indian coal. Int. J. Coal Sci. Technol. 2020, 7, 766–777. [Google Scholar] [CrossRef]
- Phan, K.A.; Phihusut, D.; Tuntiwiwattanapun, N. Preparation of rice husk hydrochar as an atrazine adsorbent: Optimization, characterization, and adsorption mechanisms. J. Environ. Chem. Eng. 2022, 10, 107575. [Google Scholar] [CrossRef]
- Tong, X.-J.; Li, J.-Y.; Yuan, J.-H.; Xu, R.-K. Adsorption of Cu(II) by biochars generated from three crop straws. Chem. Eng. J. 2011, 172, 828–834. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, X.; Luo, W.; Sun, J.; Xu, Q.; Chen, F.; Zhao, J.; Wang, S.; Yao, F.; Wang, D.; et al. Effectiveness and mechanisms of phosphate adsorption on iron-modified biochars derived from waste activated sludge. Bioresour. Technol. 2018, 247, 537–544. [Google Scholar] [CrossRef]
- Wang, Z.; Lu, N.; Cao, X.; Li, Q.; Gong, S.; Lu, P.; Zhu, K.; Guan, J.; Feike, T. Interactions between Cr(VI) and the hydrochar: The electron transfer routes, adsorption mechanisms, and the accelerating effects of wood vinegar. Sci. Total Environ. 2023, 863, 160957. [Google Scholar] [CrossRef]
- Ifthikar, J.; Wang, J.; Wang, Q.; Wang, T.; Wang, H.; Khan, A.; Jawad, A.; Sun, T.; Jiao, X.; Chen, Z. Highly Efficient Lead Distribution by Magnetic Sewage Sludge Biochar: Sorption Mechanisms and Bench Applications. Bioresour. Technol. 2017, 238, 399–406. [Google Scholar] [CrossRef]
- Poomsawat, S.; Wijittra, P. Analysis of hydrochar fuel characterization and combustion behavior derived from aquatic biomass via hydrothermal carbonization process. Case Stud. Therm. Eng. 2021, 27, 101255. [Google Scholar] [CrossRef]
- Su, H.; Zhou, X.; Zheng, R.; Zhou, Z.; Zhang, Y.; Zhu, G.; Yan, M. Hydrothermal carbonization of food waste after oil extraction pre-treatment: Study on hydrochar fuel characteristics, combustion behavior, and removal behavior of sodium and potassium. Sci. Total Environ. 2021, 754, 142192. [Google Scholar] [CrossRef]
- Wilk, M.; Śliz, M.; Lubieniecki, B. Hydrothermal co-carbonization of sewage sludge and fuel additives: Combustion performance of hydrochar. Renew. Energy 2021, 178, 1046–1056. [Google Scholar] [CrossRef]
- Arauzo, P.J.; Atiena-Martinez, M.; Ábrego, J.; Olszewski, M.P.; Cao, Z.; Kruse, A. Combustion Characteristics of Hydrochar and Pyrochar Derived from Digested Sewage Sludge. Energies 2020, 13, 4164. [Google Scholar] [CrossRef]
- Ponnusamy, V.K.; Nagappan, S.; Bhosale, R.R.; Lay, C.-H.; Nguyen, D.D.; Pugazhendhi, A.; Chang, S.W.; Kumar, G. Review on sustainable production of biochar through hydrothermal liquefaction: Physico-chemical properties and applications. Bioresour. Technol. 2020, 310, 123414. [Google Scholar] [CrossRef]
- Xu, J.; Tang, H.; Su, S.; Liu, J.; Xu, K.; Qian, K.; Wang, Y.; Zhou, Y.; Hu, S.; Zhang, A.; et al. A study of the relationships between coal structures and combustion characteristics: The insights from micro-Raman spectroscopy based on 32 kinds of Chinese coals. Appl. Energy 2018, 212, 46–56. [Google Scholar] [CrossRef]


| Samples | Rice (g) | Egg (g) | Vegetables (g) | Salt (g) | Oil (g) | Water (g) | pH |
|---|---|---|---|---|---|---|---|
| S1 | 9.99 ± 0.02 | 2.67 ± 0.02 | 0.67 ± 0.00 | 0.067 ± 0.00 | 0.00 | 66.67 ± 0.01 | 7 |
| S2 | 9.99 ± 0.03 | 2.67 ± 0.00 | 0.67 ± 0.00 | 0.13 ± 0.00 | 0.00 | 66.67 ± 0.16 | 7 |
| S3 | 9.99 ± 0.01 | 2.67 ± 0.00 | 0.67 ± 0.00 | 0.67 ± 0.00 | 0.00 | 66.67 ± 0.03 | 7 |
| S4 | 9.99 ± 0.01 | 2.67 ± 0.00 | 0.67 ± 0.01 | 1.33 ± 0.00 | 0.00 | 66.67 ± 0.03 | 7 |
| O1 | 9.99 ± 0.00 | 2.67 ± 0.02 | 0.67 ± 0.03 | 0.00 | 1.33 ± 0.02 | 66.67 ± 0.02 | 7 |
| O2 | 9.99 ± 0.01 | 2.67 ± 0.00 | 0.67 ± 0.00 | 0.00 | 2.67 ± 0.02 | 66.67 ± 0.03 | 7 |
| O3 | 9.99 ± 0.00 | 2.67 ± 0.00 | 0.67 ± 0.00 | 0.00 | 3.99 ± 0.01 | 66.67 ± 0.01 | 7 |
| O4 | 9.99 ± 0.01 | 2.67 ± 0.00 | 0.67 ± 0.00 | 0.00 | 5.33 ± 0.01 | 66.67 ± 0.01 | 7 |
| P1 | 9.99 ± 0.02 | 2.67 ± 0.02 | 0.67 ± 0.01 | 0.00 | 0.00 | 66.67 ± 0.05 | 3 |
| P2 | 9.99 ± 0.00 | 2.67 ± 0.00 | 0.67 ± 0.01 | 0.00 | 0.00 | 66.67 ± 0.03 | 5 |
| P3 | 9.99 ± 0.00 | 2.67 ± 0.00 | 0.67 ± 0.01 | 0.00 | 0.00 | 66.67 ± 0.05 | 8 |
| P4 | 9.99 ± 0.00 | 2.67 ± 0.00 | 0.67 ± 0.01 | 0.00 | 0.00 | 66.67 ± 0.01 | 10 |
| R1 | 30.00 ± 0.02 | 8.00 ± 0.02 | 2.00 ± 0.02 | 0.00 | 0.00 | 40.00 ± 0.01 | 7 |
| R2(CK) | 9.99 ± 0.02 | 2.67 ± 0.02 | 0.67 ± 0.01 | 0.00 | 0.00 | 66.67 ± 0.01 | 7 |
| R3 | 5.45 ± 0.01 | 1.15 ± 0.02 | 0.36 ± 0.02 | 0.00 | 0.00 | 72.72 ± 0.02 | 7 |
| R4 | 2.86 ± 0.01 | 0.76 ± 0.02 | 0.19 ± 0.02 | 0.00 | 0.00 | 76.19 ± 0.03 | 7 |
| Ultimate Analysis (% d.b) | Atomic Ratio | ||||||
|---|---|---|---|---|---|---|---|
| Samples | C | N | H | O | H/C | O/C | C/N |
| S1 | 64.01 | 4.69 | 5.72 | 16.19 | 1.072 | 0.190 | 15.596 |
| S2 | 62.13 | 4.65 | 5.84 | 18.78 | 1.128 | 0.227 | 15.913 |
| S3 | 63.18 | 4.32 | 5.56 | 19.21 | 1.056 | 0.228 | 17.050 |
| S4 | 59.65 | 4.07 | 5.59 | 18.84 | 1.125 | 0.237 | 17.097 |
| O1 | 68.80 | 4.24 | 7.07 | 18.56 | 1.234 | 0.202 | 18.940 |
| O2 | 66.90 | 3.82 | 7.18 | 18.18 | 1.287 | 0.204 | 20.430 |
| O3 | 67.28 | 3.82 | 6.80 | 18.82 | 1.212 | 0.210 | 20.547 |
| O4 | 47.33 | 2.64 | 4.58 | 16.97 | 1.160 | 0.269 | 20.913 |
| P1 | 64.06 | 5.08 | 6.30 | 21.69 | 1.181 | 0.254 | 14.707 |
| P2 | 62.01 | 4.52 | 6.30 | 19.11 | 1.220 | 0.231 | 16.016 |
| P3 | 69.73 | 4.97 | 5.56 | 18.32 | 0.957 | 0.197 | 16.374 |
| P4 | 66.17 | 4.55 | 5.78 | 18.66 | 1.048 | 0.212 | 16.986 |
| R1 | 65.26 | 3.03 | 5.64 | 21.14 | 1.036 | 0.243 | 25.145 |
| R2 (CK) | 63.67 | 4.27 | 5.99 | 20.60 | 1.129 | 0.243 | 17.401 |
| R3 | 64.47 | 4.52 | 6.17 | 18.22 | 1.149 | 0.212 | 16.626 |
| R4 | 66.61 | 5.19 | 5.90 | 20.14 | 1.063 | 0.227 | 14.975 |
| Sample | Ti (°C) | Tp (°C) | Tb (°C) | S (%2/°C−3∙min2) | HHV(MJ/Kg) |
|---|---|---|---|---|---|
| S1 | 246.74 | 349.98 | 481.95 | 6.86 × 10−7 | 26.886 |
| S2 | 246.52 | 353.73 | 505.43 | 5.01 × 10−7 | 25.969 |
| S3 | 253.84 | 352.02 | 503.87 | 5.26 × 10−7 | 25.846 |
| S4 | 256.70 | 407.13 | 541.00 | 4.87 × 10−7 | 24.768 |
| O1 | 286.38 | 379.78 | 468.32 | 7.74 × 10−7 | 30.014 |
| O2 | 281.38 | 379.78 | 472.32 | 7.58 × 10−7 | 29.583 |
| O3 | 280.10 | 386.93 | 474.60 | 5.12 × 10−7 | 29.060 |
| O4 | 243.92 | 382.33 | 543.91 | 4.93 × 10−7 | 19.488 |
| P1 | 248.91 | 357.84 | 475.68 | 7.36 × 10−7 | 26.762 |
| P2 | 247.82 | 364.92 | 506.87 | 5.48 × 10−7 | 26.525 |
| P3 | 228.98 | 377.19 | 504.17 | 5.85 × 10−7 | 28.217 |
| P4 | 227.89 | 377.19 | 549.33 | 4.66 × 10−7 | 27.266 |
| R1 | 278.79 | 412.44 | 548.03 | 4.70 × 10−7 | 26.316 |
| R2(CK) | 252.80 | 349.84 | 536.76 | 4.59 × 10−7 | 26.376 |
| R3 | 273.81 | 353.44 | 478.28 | 6.45 × 10−7 | 27.331 |
| R4 | 235.26 | 351.14 | 500.81 | 7.10 × 10−7 | 27.330 |
| Treatment | Qmax (mg/g) | Langmuir | Freundlich | ||
|---|---|---|---|---|---|
| b | R2 | n | R2 | ||
| S1 | 4.31 | 4.06 | 0.89 | 0.50 | 0.80 |
| S2 | 5.31 | 7.22 | 0.92 | 0.54 | 0.85 |
| S3 | 6.02 | 8.41 | 0.90 | 0.52 | 0.81 |
| S4 | 8.13 | 11.14 | 0.86 | 0.48 | 0.76 |
| O1 | 12.80 | 11.27 | 0.98 | 0.17 | 0.95 |
| O2 | 10.93 | 8.61 | 0.92 | 0.15 | 0.98 |
| O3 | 10.22 | 14.98 | 0.97 | 0.20 | 0.94 |
| O4 | 6.87 | 7.51 | 0.93 | 0.36 | 0.96 |
| P1 | 15.16 | 8.60 | 0.94 | 0.15 | 0.98 |
| P2 | 7.23 | 8.19 | 0.99 | 0.35 | 0.88 |
| P3 | 7.62 | 9.69 | 0.88 | 0.37 | 0.70 |
| P4 | 4.10 | 4.30 | 0.97 | 0.31 | 0.90 |
| R1 | 16.90 | 21.22 | 0.98 | 0.47 | 0.90 |
| R2 (CK) | 18.33 | 22.24 | 1.00 | 0.43 | 0.94 |
| R3 | 15.99 | 19.86 | 0.98 | 0.43 | 0.90 |
| R4 | 11.08 | 12.35 | 1.00 | 0.37 | 0.92 |
| Treatment | Qmax (mg/g) | Pseudo-First-Order | Pseudo-Second-Order | ||
|---|---|---|---|---|---|
| K1 (h−1) | R2 | K2 (h−1) | R2 | ||
| S1 | 4.92 | 0.02 | 0.84 | 2.28 | 0.91 |
| S2 | 5.55 | 0.02 | 0.95 | 0.00 | 0.87 |
| S3 | 6.59 | 0.02 | 0.85 | 3.35 | 0.90 |
| S4 | 8.78 | 0.02 | 0.98 | 2.90 | 0.78 |
| O1 | 12.41 | 0.06 | 0.84 | 0.00 | 1.00 |
| O2 | 9.93 | 0.04 | 0.93 | 0.00 | 0.99 |
| O3 | 9.80 | 0.06 | 0.90 | 7.96 | 1.00 |
| O4 | 6.83 | 0.02 | 0.98 | 0.00 | 0.95 |
| P1 | 11.24 | 0.05 | 0.77 | 0.00 | 0.99 |
| P2 | 10.88 | 0.04 | 0.07 | 0.00 | 0.99 |
| P3 | 8.92 | 0.03 | 0.95 | 0.00 | 0.98 |
| P4 | 6.86 | 0.04 | 0.93 | 0.00 | 0.99 |
| R1 | 16.90 | 0.11 | 0.59 | 7.85 | 1.00 |
| R2 (CK) | 18.35 | 0.11 | 0.68 | 3.83 | 1.00 |
| R3 | 15.99 | 0.09 | 0.67 | 7.65 | 1.00 |
| R4 | 11.22 | 0.06 | 0.84 | 8.66 | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, X.; He, J.; Khan, M.A.; Chang, K.; Liu, Y.; Guo, G.; Li, X.; Jin, F.; Kuang, M.; Gouda, S.; et al. Potential Application Performance of Hydrochar from Kitchen Waste: Effects of Salt, Oil, Moisture, and pH. Toxics 2023, 11, 679. https://doi.org/10.3390/toxics11080679
Su X, He J, Khan MA, Chang K, Liu Y, Guo G, Li X, Jin F, Kuang M, Gouda S, et al. Potential Application Performance of Hydrochar from Kitchen Waste: Effects of Salt, Oil, Moisture, and pH. Toxics. 2023; 11(8):679. https://doi.org/10.3390/toxics11080679
Chicago/Turabian StyleSu, Xuesong, Jizu He, Muhammad Amjad Khan, Kenlin Chang, Yin Liu, Genmao Guo, Xiaohui Li, Fangming Jin, Meijuan Kuang, Shaban Gouda, and et al. 2023. "Potential Application Performance of Hydrochar from Kitchen Waste: Effects of Salt, Oil, Moisture, and pH" Toxics 11, no. 8: 679. https://doi.org/10.3390/toxics11080679
APA StyleSu, X., He, J., Khan, M. A., Chang, K., Liu, Y., Guo, G., Li, X., Jin, F., Kuang, M., Gouda, S., & Huang, Q. (2023). Potential Application Performance of Hydrochar from Kitchen Waste: Effects of Salt, Oil, Moisture, and pH. Toxics, 11(8), 679. https://doi.org/10.3390/toxics11080679








