Comparative Assessment of the Toxicity of Brominated and Halogen-Free Flame Retardants to Zebrafish in Terms of Tail Coiling Activity, Biomarkers, and Locomotor Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Test Organism
2.3. Tail Coiling Assay
2.4. Locomotor Activity
2.5. Biochemical Analyses
2.5.1. Acetylcholinesterase (AChE)
2.5.2. Catalase (CAT)
2.5.3. Glutathione S-Transferase (GST)
2.5.4. Protein Quantification
2.6. Statistical Analysis
3. Results
3.1. Tail Coiling Assay
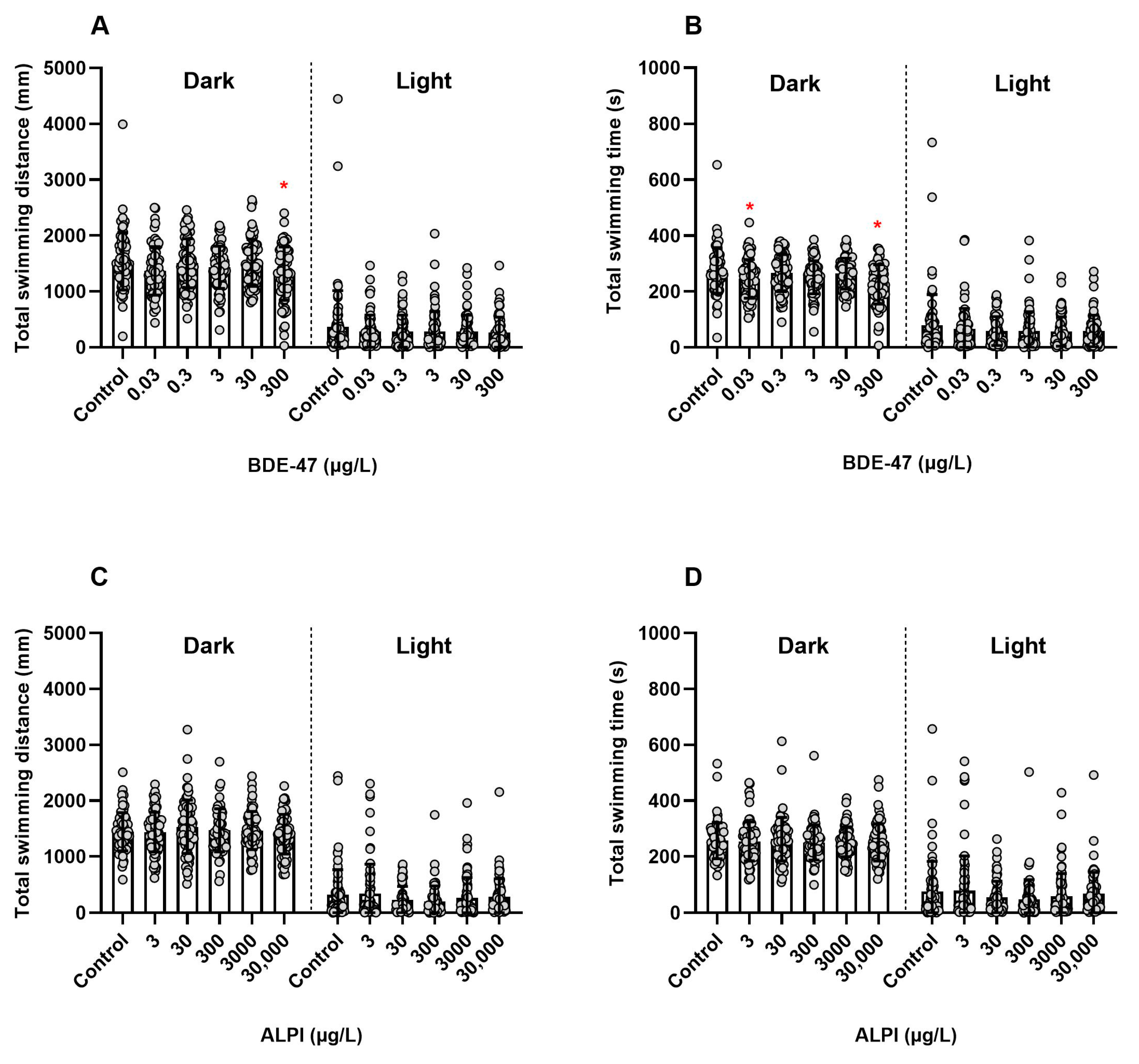
3.2. Locomotor Activity
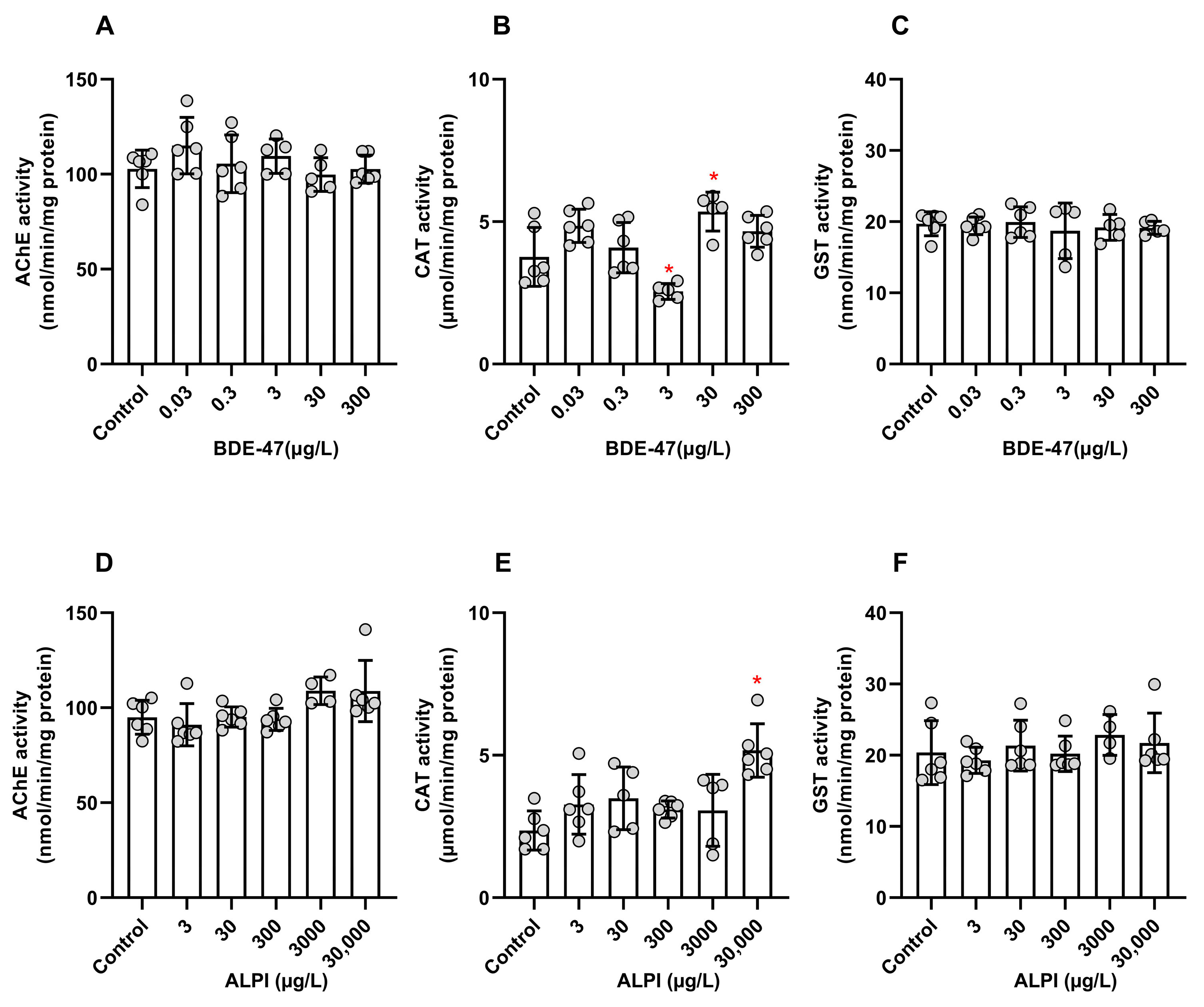
3.3. Biochemical Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United States Environmental Protection Agency. Polybrominated Diphenylethers (PBDEs) Significant New Use Rules (SNUR). 2023. Available online: https://www.epa.gov/assessing-and-managing-chemicals-under-tsca/polybrominated-diphenylethers-pbdes-significant-new-use#:~:text=The%20PBDEs%20are%20major%20components%20of%20commercial%20formulations,and%20plastics%20for%20personal%20computers%20and%20small%20appliances (accessed on 26 June 2023).
- Zheng, S.; Zhang, Q.; Wu, R.; Shi, X.; Peng, J.; Tan, W.; Huang, W.; Wu, K.; Liu, C. Behavioral changes and transcriptomic effects at embryonic and post-embryonic stages reveal the toxic effects of 2,2′,4,4′-tetrabromodiphenyl ether on neurodevelopment in zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 2022, 248, 114310. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Han, W.; Yang, X.; Li, Y.; Wang, Y. The occurrence of polybrominated diphenyl ether (PBDE) contamination in soil, water/sediment, and air. Environ. Sci. Pollut. Res. Int. 2019, 26, 23219–23241. [Google Scholar] [CrossRef]
- Abe, F.R.; Oliveira, A.Á.S.d.; Marino, R.V.; Rialto, T.C.R.; Oliveira, D.P.; Dorta, D.J. A comparison of developmental toxicity of brominated and halogen-free flame retardant on zebrafish. Ecotoxicol. Environ. Saf. 2021, 208, 111745. [Google Scholar] [CrossRef] [PubMed]
- Hites, R.A. Polybrominated Diphenyl Ethers in the Environment and in People: A Meta-Analysis of Concentrations. Environ. Sci. Technol. 2004, 38, 945–956. [Google Scholar] [CrossRef]
- Sacks, V.P.; Lohmann, R. Freely dissolved PBDEs in water and porewater of an urban estuary. Environmental pollution 2012, 162, 287–293. [Google Scholar] [CrossRef]
- Moon, H.-B.; Choi, M.; Yu, J.; Jung, R.-H.; Choi, H.-G. Contamination and potential sources of polybrominated diphenyl ethers (PBDEs) in water and sediment from the artificial Lake Shihwa, Korea. Chemosphere 2012, 88, 837–843. [Google Scholar] [CrossRef]
- Wang, J.; Lin, Z.; Lin, K.; Wang, C.; Zhang, W.; Cui, C.; Lin, J.; Dong, Q.; Huang, C. Polybrominated diphenyl ethers in water, sediment, soil, and biological samples from different industrial areas in Zhejiang, China. J. Hazard. Mater. 2011, 197, 211–219. [Google Scholar] [CrossRef]
- Pei, J.; Yao, H.; Wang, H.; Li, H.; Lu, S.; Zhang, X.; Xiang, X. Polybrominated diphenyl ethers (PBDEs) in water, surface sediment, and suspended particulate matter from the Yellow River, China: Levels, spatial and seasonal distribution, and source contribution. Mar. Pollut. Bull. 2018, 129, 106–113. [Google Scholar] [CrossRef]
- Liu, J.; Lu, G.; Zhang, F.; Nkoom, M.; Yan, Z.; Wu, D. Polybrominated Diphenyl Ethers (PBDEs) in a Large, Highly Polluted Freshwater Lake, China: Occurrence, Fate, and Risk Assessment. Int. J. Environ. Res. Public Health 2018, 15, 1529. [Google Scholar] [CrossRef]
- Xu, L.; Gao, S.; Zhao, H.; Wang, L.; Cao, Y.; Xi, J.; Zhang, X.; Dong, X.; Luan, Y. Integrated Proteomic and Metabolomic Analysis of the Testes Characterizes BDE-47-Induced Reproductive Toxicity in Mice. Biomolecules 2021, 11, 821. [Google Scholar] [CrossRef]
- Ji, F.; Sreenivasmurthy, S.G.; Wei, J.; Shao, X.; Luan, H.; Zhu, L.; Song, J.; Liu, L.; Li, M.; Cai, Z. Study of BDE-47 induced Parkinson’s disease-like metabolic changes in C57BL/6 mice by integrated metabolomic, lipidomic and proteomic analysis. J. Hazard. Mater. 2019, 378, 120738. [Google Scholar] [CrossRef]
- Yang, C.; Zhu, L.; Kang, Q.; Lee, H.K.; Li, D.; Chung, A.C.K.; Cai, Z. Chronic exposure to tetrabromodiphenyl ether (BDE-47) aggravates hepatic steatosis and liver fibrosis in diet-induced obese mice. J. Hazard. Mater. 2019, 378, 120766. [Google Scholar] [CrossRef]
- Xing, X.; Kang, J.; Qiu, J.; Zhong, X.; Shi, X.; Zhou, B.; Wei, Y. Waterborne exposure to low concentrations of BDE-47 impedes early vascular development in zebrafish embryos/larvae. Aquat. Toxicol. 2018, 203, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Fujiwara, M.; Shindo, A.; Yin, G.; Kitazawa, T.; Teraoka, H. Aroclor 1254 and BDE-47 inhibit dopaminergic function manifesting as changes in locomotion behaviors in zebrafish embryos. Chemosphere 2018, 193, 1207–1215. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Wu, R.; Wang, X.; Huang, W.; Zheng, S.; Zhang, Q.; Peng, J.; Tan, W.; Wu, K. Effects of 2,2′,4,4′-tetrabromodiphenyl ether (BDE-47) on reproductive and endocrine function in female zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 2022, 248, 114326. [Google Scholar] [CrossRef]
- Clariant. EXOLIT® OVERVIEW. An extensive range of non-halogenated flame retardants [Brochure]. Clariant Plastics & Coatings (Deutschland) Gmbh: 2022. Available online: https://www.clariant.com/en/Solutions/Products/2014/03/18/16/31/Exolit-OP-1230 (accessed on 26 June 2023).
- Bao, J.; Liu, Y.; Li, L.; Chen, M.; Liu, J.; Niu, Y.; Liu, J.; Liang, Y. Biological effects of new-generation dialkyl phosphinate flame retardants and their hydrolysates in BALB/C mice. Environ. Toxicol. 2017, 32, 1578–1586. [Google Scholar] [CrossRef]
- Niu, Y.; Liu, J.; Liang, Y.; Hao, Z.; Liu, J.; Liu, Y.; Sun, X. Aluminum Dialkyl Phosphinate Flame Retardants and Their Hydrolysates: Analytical Method and Occurrence in Soil and Sediment Samples from a Manufacturing Site. Environ. Sci. Technol. 2014, 48, 3336–3343. [Google Scholar] [CrossRef]
- Shen, C.; Zuo, Z. Zebrafish (Danio rerio) as an excellent vertebrate model for the development, reproductive, cardiovascular, and neural and ocular development toxicity study of hazardous chemicals. Environ. Sci. Pollut. Res. Int. 2020, 27, 43599–43614. [Google Scholar] [CrossRef] [PubMed]
- Hahn, M.E.; Timme-Laragy, A.R.; Karchner, S.I.; Stegeman, J.J. Nrf2 and Nrf2-related proteins in development and developmental toxicity: Insights from studies in zebrafish (Danio rerio). Free. Radic. Biol. Med. 2015, 88 Pt B, 275–289. [Google Scholar] [CrossRef]
- de Oliveira, A.A.S.; Brigante, T.A.V.; Oliveira, D.P. Tail Coiling Assay in Zebrafish (Danio rerio) Embryos: Stage of Development, Promising Positive Control Candidates, and Selection of an Appropriate Organic Solvent for Screening of Developmental Neurotoxicity (DNT). Water 2021, 13, 119. [Google Scholar] [CrossRef]
- MacPhail, R.C.; Brooks, J.; Hunter, D.L.; Padnos, B.; Irons, T.D.; Padilla, S. Locomotion in larval zebrafish: Influence of time of day, lighting and ethanol. Neurotoxicology 2009, 30, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, J.A.; Könemann, S.; Krümpelmann, L.; Županič, A.; Berg, C.V. Approaches to Test the Neurotoxicity of Environmental Contaminants in the Zebrafish Model: From Behavior to Molecular Mechanisms. Environ. Toxicol. Chem. 2021, 40, 989–1006. [Google Scholar] [CrossRef] [PubMed]
- Durieux, E.D.H.; Farver, T.B.; Fitzgerald, P.S.; Eder, K.J.; Ostrach, D.J. Natural factors to consider when using acetylcholinesterase activity as neurotoxicity biomarker in Young-Of-Year striped bass (Morone saxatilis). Fish Physiol. Biochem. 2011, 37, 21–29. [Google Scholar] [CrossRef][Green Version]
- Kumari, K.; Singh, A.; Swamy, S.; Singhar, R.S.; Thakur, S. Use of enzymatic biomarkers of Labeo rohita to study the effect of polybrominated diphenyl ether (BDE- 209) via dietary exposure in laboratory conditions. Environ. Monit. Assess. 2022, 194, 499. [Google Scholar] [CrossRef] [PubMed]
- Henriques, J.F.; Almeida, A.R.; Andrade, T.; Koba, O.; Golovko, O.; Soares, A.M.; Oliveira, M.; Domingues, I. Effects of the lipid regulator drug gemfibrozil: A toxicological and behavioral perspective. Aquat. Toxicol. 2016, 170, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Abe, F.R.; Mendonça, J.N.; Moraes, L.A.B.; Oliveira, G.A.R.d.; Gravato, C.; Soares, A.M.V.M.; Oliveira, D.P. Toxicological and behavioral responses as a tool to assess the effects of natural and synthetic dyes on zebrafish early life. Chemosphere 2017, 178, 282–290. [Google Scholar] [CrossRef]
- Kristofco, L.A.; Cruz, L.C.; Haddad, S.P.; Behra, M.L.; Chambliss, C.K.; Brooks, B.W. Age matters: Developmental stage of Danio rerio larvae influences photomotor response thresholds to diazinion or diphenhydramine. Aquat. Toxicol. 2016, 170, 344–354. [Google Scholar] [CrossRef]
- Strähle, U.; Scholz, S.; Geisler, R.; Greiner, P.; Hollert, H.; Rastegar, S.; Schumacher, A.; Selderslaghs, I.; Weiss, C.; Witters, H.; et al. Zebrafish embryos as an alternative to animal experiments--a commentary on the definition of the onset of protected life stages in animal welfare regulations. Reprod. Toxicol. 2012, 33, 128–132. [Google Scholar] [CrossRef]
- Abe, F.R.; Gravato, C.; Soares, A.M.V.M.; Oliveira, D.P. Biochemical approaches to assess oxidative stress induced by exposure to natural and synthetic dyes in early life stages in zebrafish. J. Toxicol. Environ. Health Part A 2017, 80, 1259–1268. [Google Scholar] [CrossRef]
- Abe, F.R.; Soares, A.M.V.M.; Oliveira, D.P.d.; Gravato, C. Toxicity of dyes to zebrafish at the biochemical level: Cellular energy allocation and neurotoxicity. Environ. Pollut. 2018, 235, 255–262. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Feather-Stone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Gravato, C.; Abe, F.R.; Oliveira, D.a.P.d.; Soares, A.M.V.M.; Domingues, I. Acetylcholinesterase (AChE) Activity in Embryos of Zebrafish. Methods Mol. Biol. 2021, 2240, 119–124. [Google Scholar] [CrossRef]
- Guilhermino, L.; Lopes, M.C.; Carvalho, A.P.; Soares, A.M. Acetylcholinesterase activity in juveniles of Daphnia magna Straus. Bull. Environ. Contam. Toxicol. 1996, 57, 979–985. [Google Scholar] [CrossRef] [PubMed]
- Claiborne, A. Catalase Activity. In CRC Handbook of Methods for Oxygen Radical Research; CRC Press: Boca Raton, FL, USA, 1985; pp. 283–284. [Google Scholar]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef] [PubMed]
- Frasco, M.F.; Guilhermino, L. Effects of dimethoate and beta-naphthoflavone on selected biomarkers of Poecilia reticulata. Fish Physiol. Biochem. 2002, 26, 149–156. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Usenko, C.Y.; Robinson, E.M.; Usenko, S.; Brooks, B.W.; Bruce, E.D. PBDE developmental effects on embryonic zebrafish. Environ. Toxicol. Chem. 2011, 30, 1865–1872. [Google Scholar] [CrossRef]
- Zhuang, J.; Pan, Z.-J.; Qin, Y.; Liang, H.; Zhang, W.-F.; Sun, Z.-Y.; Shi, H.-B. Evaluation of BDE-47-induced neurodevelopmental toxicity in zebrafish embryos. Environ. Sci. Pollut. Res. Int. 2023, 30, 54022–54034. [Google Scholar] [CrossRef]
- Chen, X.; Huang, C.; Wang, X.; Chen, J.; Bai, C.; Chen, Y.; Chen, X.; Dong, Q.; Yang, D. BDE-47 disrupts axonal growth and motor behavior in developing zebrafish. Aquat. Toxicol. 2012, 120–121, 135–144. [Google Scholar] [CrossRef]
- Wang, F.; Fang, M.; Hinton, D.E.; Chernick, M.; Jia, S.; Zhang, Y.; Xie, L.; Dong, W.; Dong, W. Increased coiling frequency linked to apoptosis in the brain and altered thyroid signaling in zebrafish embryos (Danio rerio) exposed to the PBDE metabolite 6-OH-BDE-47. Chemosphere 2018, 198, 342–350. [Google Scholar] [CrossRef]
- Schuijt, L.M.; Peng, F.-J.; Berg, S.J.P.v.d.; Dingemans, M.M.L.; Brink, P.J.V.d. (Eco)toxicological tests for assessing impacts of chemical stress to aquatic ecosystems: Facts, challenges, and future. Sci. Total Environ. 2021, 795, 148776. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.M.; Fero, K.; Arrenberg, A.B.; Bergeron, S.A.; Driever, W.; Burgess, H.A. Deep brain photoreceptors control light-seeking behavior in zebrafish larvae. Curr. Biol. CB 2012, 22, 2042–2047. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Xu, T.; Yin, D.-Q. Locomotor activity changes on zebrafish larvae with different 2,2′,4,4′-tetrabromodiphenyl ether (PBDE-47) embryonic exposure modes. Chemosphere 2014, 94, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Glazer, L.; Wells, C.N.; Drastal, M.; Odamah, K.-A.; Galat, R.E.; Behl, M.; Levin, E.D. Developmental exposure to low concentrations of two brominated flame retardants, BDE-47 and BDE-99, causes life-long behavioral alterations in zebrafish. Neurotoxicology 2018, 66, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Brewer, S.K.; Little, E.E.; DeLonay, A.J.; Beauvais, S.L.; Jones, S.B.; Ellersieck, M.R. Behavioral dysfunctions correlate to altered physiology in rainbow trout (Oncorynchus mykiss) exposed to cholinesterase-inhibiting chemicals. Arch. Environ. Contam. Toxicol. 2001, 40, 70–76. [Google Scholar] [CrossRef]
- Faimali, M.; Gambardella, C.; Costa, E.; Piazza, V.; Morgana, S.; Estévez-Calvar, N.; Garaventa, F. Old model organisms and new behavioral end-points: Swimming alteration as an ecotoxicological response. Mar. Environ. Res. 2017, 128, 36–45. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, L.; Shi, Q.; Guo, Y.; Hua, J.; Han, J.; Yang, L. DE-71 affected the cholinergic system and locomotor activity via disrupting calcium homeostasis in zebrafish larvae. Aquat. Toxicol. 2022, 250, 106237. [Google Scholar] [CrossRef]
- Kalyn, M.; Lee, H.; Curry, J.; Tu, W.; Ekker, M.; Mennigen, J.A. Effects of PFOS, F-53B and OBS on locomotor behaviour, the dopaminergic system and mitochondrial function in developing zebrafish (Danio rerio). Environ. Pollut. 2023, 326, 121479. [Google Scholar] [CrossRef]
- Wang, X.H.; Souders II, C.L.; Zhao, Y.H.; Martyniuk, C.J. Paraquat affects mitochondrial bioenergetics, dopamine system expression, and locomotor activity in zebrafish (Danio rerio). Chemosphere 2018, 191, 106–117. [Google Scholar] [CrossRef]
- Chen, L.; Yu, K.; Huang, C.; Yu, L.; Zhu, B.; Lam, P.K.S.; Lam, J.C.W.; Zhou, B. Prenatal transfer of polybrominated diphenyl ethers (PBDEs) results in developmental neurotoxicity in zebrafish larvae. Environ. Sci. Technol. 2012, 46, 9727–9734. [Google Scholar] [CrossRef]
- Xie, Z.; Lu, G.; Qi, P. Effects of BDE-209 and its mixtures with BDE-47 and BDE-99 on multiple biomarkers in Carassius auratus. Environ. Toxicol. Pharmacol. 2014, 38, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Liñán, L.; Bellas, J.; Fumega, J.; Beiras, R. Bioaccumulation of BDE-47 and effects on molecular biomarkers acetylcholinesterase, glutathione-S-transferase and glutathione peroxidase in Mytilus galloprovincialis mussels. Ecotoxicology 2015, 24, 292–300. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency. Flame Retardants in Pressed Circuit Boards. Final Report. EPA Publication 744-R-15-001. 2015. Available online: https://www.epa.gov/sites/default/files/2015-08/documents/pcb_final_report.pdf (accessed on 26 June 2023).
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Dong, M.; Zhu, L.; Shao, B.; Zhu, S.; Wang, J.; Xie, H.; Wang, J.; Wang, F. The effects of endosulfan on cytochrome P450 enzymes and glutathione S-transferases in zebrafish (Danio rerio) livers. Ecotoxicol. Environ. Saf. 2013, 92, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tierbach, A.; Groh, K.J.; Schönenberger, R.; Schirmer, K.; Suter, M.J.-F. Glutathione S-Transferase Protein Expression in Different Life Stages of Zebrafish (Danio rerio). Toxicol. Sci. Off. J. Soc. Toxicol. 2018, 162, 702–712. [Google Scholar] [CrossRef]
- Usenko, C.Y.; Abel, E.L.; Kudela, M.; Janise, A.; Bruce, E.D. Comparison of PBDE congeners as inducers of oxidative stress in zebrafish. Environ. Toxicol. Chem. 2015, 34, 1154–1160. [Google Scholar] [CrossRef]
- Zhuang, J.; Pan, Z.-J.; Li, M.; Hong, F.-S.; Zhu, C.-K.; Wu, N.; Chang, G.; Wang, H.; Zhao, X.-X. BDE-47 induced apoptosis in zebrafish embryos through mitochondrial ROS-mediated JNK signaling. Chemosphere 2020, 258, 127385. [Google Scholar] [CrossRef]
- Meng, S.; Chen, X.; Gyimah, E.; Xu, H.; Chen, J. Hepatic oxidative stress, DNA damage and apoptosis in adult zebrafish following sub-chronic exposure to BDE-47 and BDE-153. Environ. Toxicol. 2020, 35, 1202–1211. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, X.; Ren, X.; Duan, X. Antagonistic effects of multi-walled carbon nanotubes and BDE-47 in zebrafish (Danio rerio): Oxidative stress, apoptosis and DNA damage. Aquat. Toxicol. 2020, 225, 105546. [Google Scholar] [CrossRef]
- Hendriks, H.S.; Meijer, M.; Muilwijk, M.; van den Berg, M.; Westerink, R.H.S. A comparison of the in vitro cyto- and neurotoxicity of brominated and halogen-free flame retardants: Prioritization in search for safe(r) alternatives. Arch. Toxicol. 2014, 88, 857–869. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rialto, T.C.R.; Marino, R.V.; Abe, F.R.; Dorta, D.J.; Oliveira, D.P. Comparative Assessment of the Toxicity of Brominated and Halogen-Free Flame Retardants to Zebrafish in Terms of Tail Coiling Activity, Biomarkers, and Locomotor Activity. Toxics 2023, 11, 732. https://doi.org/10.3390/toxics11090732
Rialto TCR, Marino RV, Abe FR, Dorta DJ, Oliveira DP. Comparative Assessment of the Toxicity of Brominated and Halogen-Free Flame Retardants to Zebrafish in Terms of Tail Coiling Activity, Biomarkers, and Locomotor Activity. Toxics. 2023; 11(9):732. https://doi.org/10.3390/toxics11090732
Chicago/Turabian StyleRialto, Taisa Carla Rizzi, Renan Vieira Marino, Flavia Renata Abe, Daniel Junqueira Dorta, and Danielle Palma Oliveira. 2023. "Comparative Assessment of the Toxicity of Brominated and Halogen-Free Flame Retardants to Zebrafish in Terms of Tail Coiling Activity, Biomarkers, and Locomotor Activity" Toxics 11, no. 9: 732. https://doi.org/10.3390/toxics11090732
APA StyleRialto, T. C. R., Marino, R. V., Abe, F. R., Dorta, D. J., & Oliveira, D. P. (2023). Comparative Assessment of the Toxicity of Brominated and Halogen-Free Flame Retardants to Zebrafish in Terms of Tail Coiling Activity, Biomarkers, and Locomotor Activity. Toxics, 11(9), 732. https://doi.org/10.3390/toxics11090732





