Study on Dihydromyricetin Improving Aflatoxin Induced Liver Injury Based on Network Pharmacology and Molecular Docking
Abstract
:1. Introduction
2. Materials and Methods
2.1. Network Pharmacological Analysis of DHM and Potential Targets for Liver Disease
2.1.1. Collection of Monomer Components and Targets Related to Aflatoxin-Induced Liver Damage
2.1.2. “Component-Target” Network Analysis
2.1.3. Construction and Analysis of Protein-Protein Interaction (PPI) Networks
2.1.4. GO and KEGG Enrichment Analysis
2.2. Molecular Docking
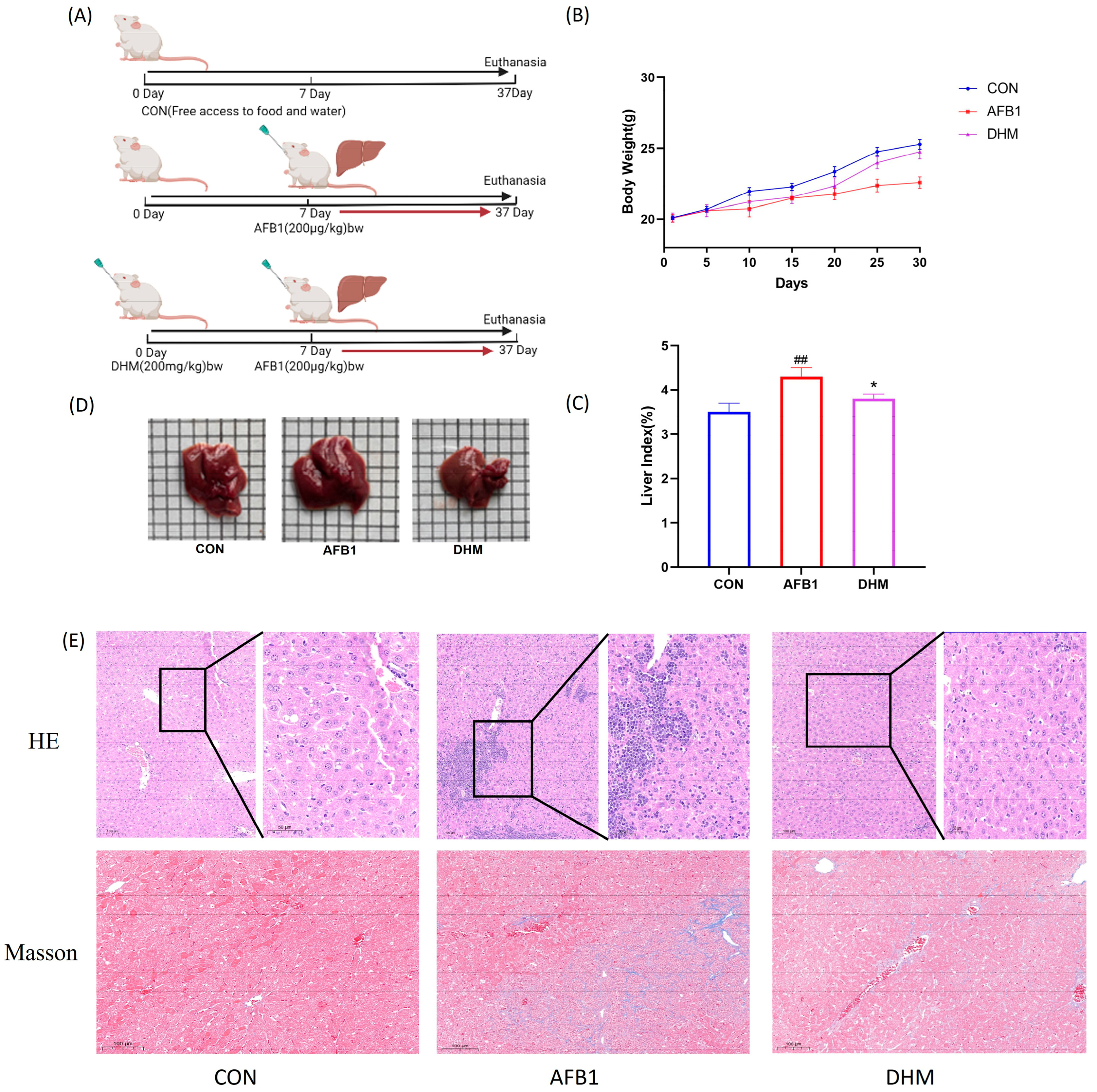
2.3. Animal Grouping and Drug Administration
2.4. Analysis of Biochemical Indices
2.5. Detection of Oxidative Stress Indicators
2.6. H&E and MASSON Colouring
2.7. TUNEL Fluorescent Staining
2.8. Western Blot Assay
2.9. Statistical Analysis
3. Results
3.1. Analysis of Potential Targets of DHM for the Treatment of Liver Injury
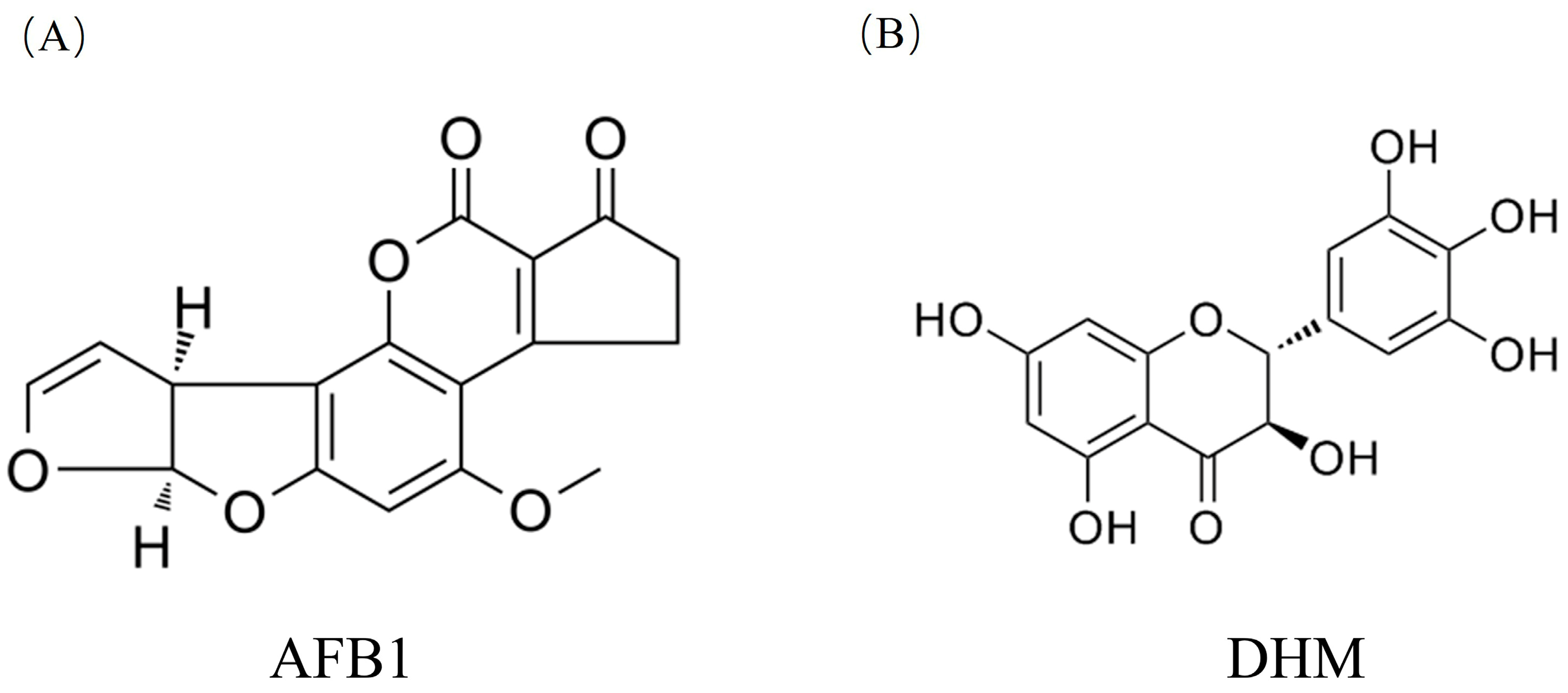
3.1.1. Collection of Monomer Components and Targets Related to Aflatoxin-Induced Liver Injury
3.1.2. Construction of Composite Target Networks
3.1.3. Construction and Analysis of Protein Interaction (PPI) Models
3.1.4. GO and KEGG Pathway Enrichment
3.2. Molecular Docking Analysis
3.3. Effect of DHM on AFB1-Induced Liver Injury in Mice
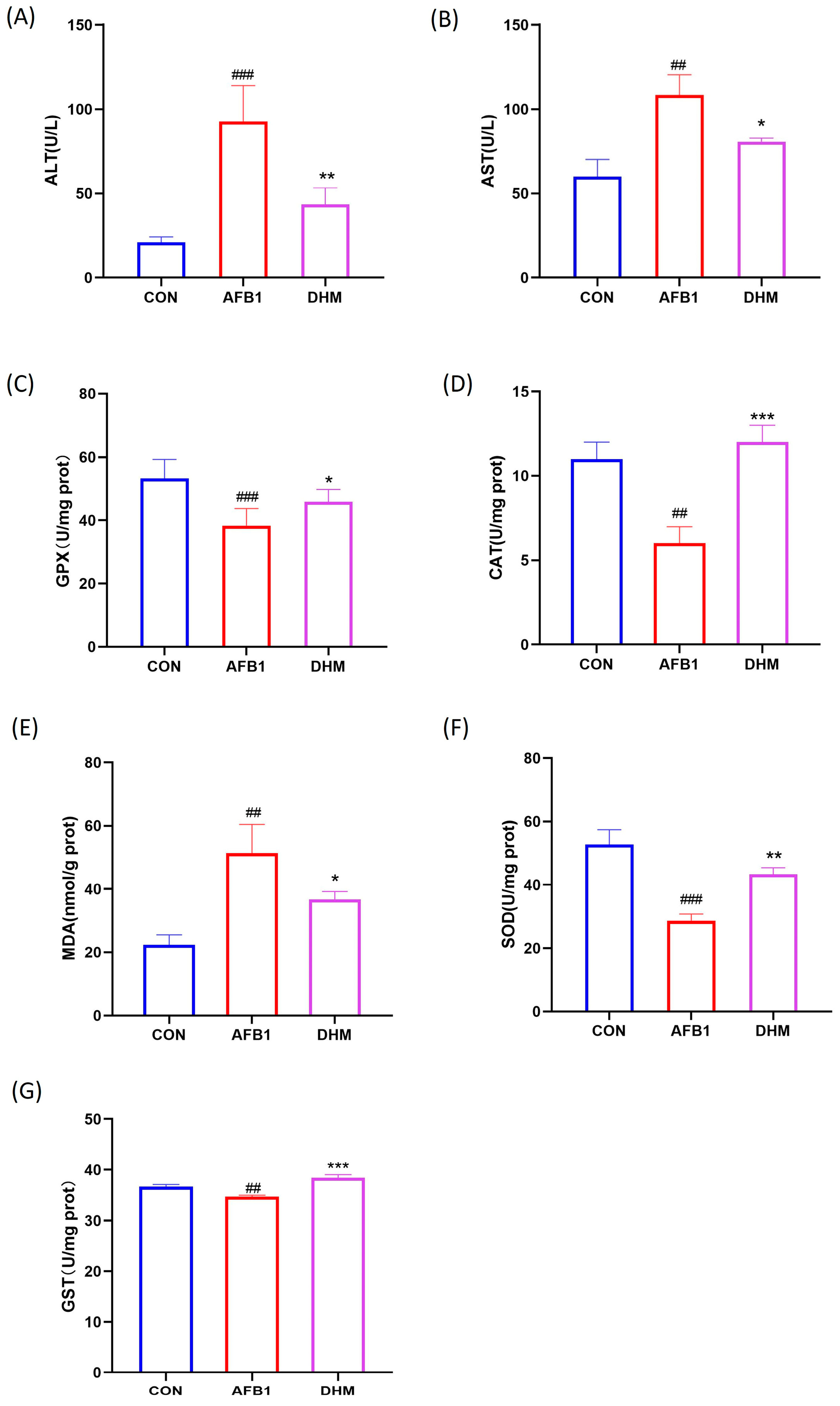
3.4. DHM Alleviates AFB1 Exposure-Induced Liver Dysfunction and Ameliorates AFB1-Induced Oxidative Stress
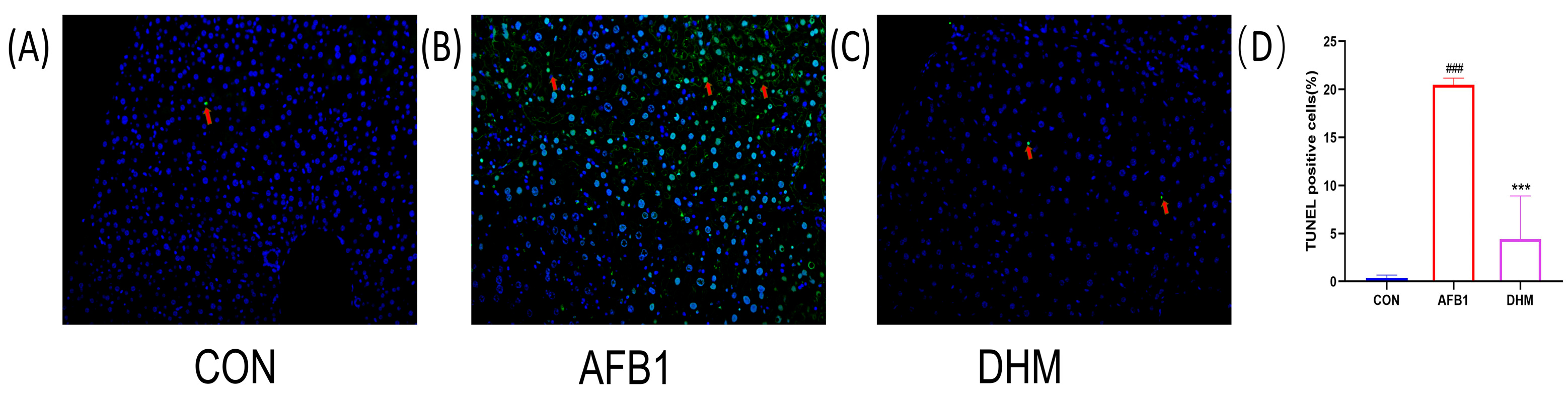
3.5. DHM Attenuates AFB1 Exposure-Induced Apoptosis in Liver Tissue
3.6. DHM Attenuates AFB1-Induced Liver Injury by Ameliorating Oxidative Stress and Apoptosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rahman, M.A.H.; Selamat, J.; Samsudin, N.I.P. Antagonism of nonaflatoxigenic Aspergillus flavus isolated from peanuts against aflatoxigenic A. flavus growth and aflatoxin B1 production in vitro. Food Sci. Nutr. 2022, 10, 3993–4002. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Aziz, A.R.M.; Shehata, S.M.; Hisham, S.M. Molecular profile of aflatoxigenic and non-aflatoxigenic isolates of Aspergillus flavus isolated from stored maize. Saudi J. Biol. Sci. 2021, 28, 1383–1391. [Google Scholar] [CrossRef] [PubMed]
- Ghanem, K.M.; Lotfy, W.A.; EL-Shaer, M.M. The Inhibitory Effect of Wheat Husks Addition on Aflatoxins Production by Aspergillus flavus in Liquid Culture with Various Wheat Compositions as Carbon Sources. Front. Microbiol. 2020, 11, 1448. [Google Scholar] [CrossRef] [PubMed]
- Matumba, L.; Singano, L.; Pungulani, L. Aflatoxins, discolouration and insect damage in dried cowpea and pigeon pea in Malawi and the effectiveness of flotation/washing operation in eliminating the aflatoxins. Mycotoxin Res. 2017, 33, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Mahato, D.K.; Lee, K.E.; Kamle, M. Aflatoxins in Food and Feed: An Overview on Prevalence, Detection and Control Strategies. Front. Microbiol. 2019, 10, 2266. [Google Scholar] [CrossRef] [PubMed]
- Naveed, M.; Haleem, K.S.; Ghazanfar, S. Quantitative Estimation of Aflatoxin Level in Poultry Feed in Selected Poultry Farms. BioMed Res. Int. 2022, 2022, 5397561. [Google Scholar] [CrossRef]
- Yan, C.; Wang, Q.; Yang, Q. Recent Advances in Aflatoxins Detection Based on Nanomaterials. Nanomaterials. 2020, 10, 1626. [Google Scholar] [CrossRef]
- Kong, D.; Wang, G.; Tang, Y. Potential health risk of areca nut consumption: Hazardous effect of toxic alkaloids and aflatoxins on human digestive system. Food Res. Int. 2022, 162 Pt A, 112012. [Google Scholar] [CrossRef]
- Kiran Bhardwaj, A.; Julie, P.; Meneely, A.; Simon, A.; Haughey, A. Risk assessments for the dietary intake aflatoxins in food: A systematic review (2016–2022). Food Control 2023, 149, 109687. [Google Scholar] [CrossRef]
- Hafez, E.; Abd El-Aziz, N.M.; Darwish, A.M.G. Validation of New ELISA Technique for Detection of Aflatoxin B1 Contamination in Food Products versus HPLC and VICAM. Toxins 2021, 13, 747. [Google Scholar] [CrossRef]
- Nazhand, A.; Durazzo, A.; Lucarini, M. Characteristics, Occurrence, Detection and Detoxification of Aflatoxins in Foods and Feeds. Foods 2020, 9, 644. [Google Scholar] [CrossRef]
- Qin, X.; Xin, Y.; Zou, J. Efficient Degradation of Aflatoxin B1 and Zearalenone by Laccase-like Multicopper Oxidase from Streptomyces thermocarboxydus in the Presence of Mediators. Toxins 2021, 13, 754. [Google Scholar] [CrossRef]
- Mottaghianpour, E.; Nazari, F.; Mehrasbi, M.R. Occurrence of aflatoxin B1 in baby foods marketed in Iran. J. Sci. Food Agric. 2017, 97, 2690–2694. [Google Scholar] [CrossRef]
- Allameh, A.; Khanian, M.; Karimi-Torshizi, M.A. Hepatoprotective effects of Lactobacillus plantarum 299v supplemented via drinking water against aflatoxin-induced liver damage. Avian Pathol. J. WVPA 2021, 50, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Shi, W.; Lv, P. Critical role of caveolin-1 in aflatoxin B1-induced hepatotoxicity via the regulation of oxidation and autophagy. Cell Death Dis. 2020, 11, 6. [Google Scholar] [CrossRef]
- Liu, F.; Wang, Y.; Zhou, X. Resveratrol Relieved Acute Liver Damage in Ducks (Anas platyrhynchos) Induced by AFB1 via Modulation of Apoptosis and Nrf2 Signaling Pathways. Animals 2021, 11, 3516. [Google Scholar] [CrossRef]
- Carry, E.; Kshatriya, D.; Silva, J. Identification of Dihydromyricetin and Metabolites in Serum and Brain Associated with Acute Anti-Ethanol Intoxicating Effects in Mice. Int. J. Mol. Sci. 2021, 22, 7460. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, Y.; Luo, H. Recent Update on the Pharmacological Effects and Mechanisms of Dihydromyricetin. Front. Pharmacol. 2018, 9, 1204. [Google Scholar] [CrossRef]
- Cragg, G.M.; Newman, D.J.J.P.B. Natural Product Drug Discovery in the Next Millennium. Pharm. Biol. 2011, 39 (Suppl. S1), 8–17. [Google Scholar]
- Teng, J.; Liu, X.; Hu, X. Dihydromyricetin as a Functional Additive to Enhance Antioxidant Capacity and Inhibit the Formation of Thermally Induced Food Toxicants in a Cookie Model. Molecules 2018, 23, 2184. [Google Scholar] [CrossRef]
- Hou, L.; Jiang, F.; Huang, B. Dihydromyricetin Ameliorates Inflammation-Induced Insulin Resistance via Phospholipase C-CaMKK-AMPK Signal Pathway. Oxid. Med. Cell. Longev. 2021, 2021, 8542809. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yang, Z.S.; Zhou, Y.Z. Dihydromyricetin inhibits cell proliferation, migration, invasion and promotes apoptosis via regulating miR-21 in Human Cholangiocarcinoma Cells. J. Cancer 2020, 11, 5689–5699. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Li, X.; Yang, X. Dihydromyricetin promotes GLP-1 release and glucose uptake by STC-1 cells and enhances the effects of metformin upon STC-1 cells and diabetic mouse model. Tissue Cell 2023, 82, 102108. [Google Scholar] [CrossRef]
- Martínez-Coria, H.; Mendoza-Rojas, M.X.; Arrieta-Cruz, I. Preclinical Research of Dihydromyricetin for Brain Aging and Neurodegenerative Diseases. Front. Pharmacol. 2019, 10, 1334. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, X.; Xia, T. Molecular mechanisms and therapeutic implications of dihydromyricetin in liver disease. Biomed. Pharmacother. 2021, 142, 111927. [Google Scholar] [CrossRef]
- Qiu, P.; Dong, Y.; Li, B. Dihydromyricetin modulates p62 and autophagy crosstalk with the Keap-1/Nrf2 pathway to alleviate ethanol-induced hepatic injury. Toxicol. Lett. 2017, 274, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Mao, Y.; Ding, L. Dihydromyricetin: A review on identification and quantification methods, biological activities, chemical stability, metabolism and approaches to enhance its bioavailability. Trends Food Sci. Technol. 2019, 91, 586–597. [Google Scholar] [CrossRef]
- Hong, W.; Liu, Y.; Liang, J. Molecular Mechanisms of Selenium Mitigating Lead Toxicity in Chickens via Mitochondrial Pathway: Selenoproteins, Oxidative Stress, HSPs, and Apoptosis. Toxics 2023, 11, 734. [Google Scholar] [CrossRef]
- Shi, X.; Xu, W.; Che, X. Effect of arsenic stress on the intestinal structural integrity and intestinal flora abundance of Cyprinus carpio. Front. Microbiol. 2023, 14, 1179397. [Google Scholar] [CrossRef]
- Dong, S.; Ji, J.; Hu, L. Dihydromyricetin alleviates acetaminophen-induced liver injury via the regulation of transformation, lipid homeostasis, cell death and regeneration. Life Sci. 2019, 227, 20–29. [Google Scholar] [CrossRef]
- Mao, X.Q.; Feng, Y.; Wang, N. Hypoglycemic effect of polysaccharide enriched extract of Astragalus membranaceus in diet induced insulin resistant C57BL/6J mice and its potential mechanism. Phytomedicine 2009, 16, 416–425. [Google Scholar] [CrossRef]
- Win, S.; Than, T.A.; Min, R. c-Jun N-terminal kinase mediates mouse liver injury through a novel Sab (SH3BP5)-dependent pathway leading to inactivation of intramitochondrial Src. Hepatology 2016, 63, 1987–2003. [Google Scholar] [CrossRef] [PubMed]
- Rao, X.; Zhong, J.; Sun, Q.J.L.S. The heterogenic properties of monocytes/macrophages and neutrophils in inflammatory response in diabetes. Life Sci. 2014, 116, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Bi, D.; Xie, R. Correction To: Fusobacterium nucleatum enhances the efficacy of PD-L1 blockade in colorectal cancer. Signal Transduct. Target. Ther. 2022, 1, 7. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.; Xu, W.; Zhang, Y. Transcriptome analysis revealed the mechanism of Luciobarbus capito (L. capito) adapting high salinity: Antioxidant capacity, heat shock proteins, immunity. Mar. Pollut. Bull. 2023, 192, 115017. [Google Scholar] [CrossRef]
- Seike, T.; Boontem, P.; Yanagi, M. Hydroxynonenal Causes Hepatocyte Death by Disrupting Lysosomal Integrity in Nonalcoholic Steatohepatitis. Cell. Mol. Gastroenterol. Hepatol. 2022, 14, 925–944. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Teng, X.; Yue, W. The effect of acute toxicity from tributyltin on Liza haematocheila liver: Energy metabolic disturbance, oxidative stress, and apoptosis. Aquat. Toxicol. 2023, 258, 106506. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Hao, Z.; Zhou, Q. Chlorpyrifos induced autophagy and mitophagy in common carp livers through AMPK pathway activated by energy metabolism disorder. Ecotoxicol. Environ. Saf. 2023, 258, 114983. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Kang, W. Aflatoxin B1 induces liver injury by disturbing gut microbiota-bile acid-FXR axis in mice. Food Chem. Toxicol. 2023, 176, 113751. [Google Scholar] [CrossRef]
- Cao, W.; Yu, P.; Yang, K. Aflatoxin B1: Metabolism, toxicology, and its involvement in oxidative stress and cancer development. Toxicol. Mech. Methods 2022, 32, 395–419. [Google Scholar] [CrossRef]
- Cui, J.; Qiu, M.; Liu, Y. Nano-selenium protects grass carp hepatocytes against 4-tert-butylphenol-induced mitochondrial apoptosis and necroptosis via suppressing ROS-PARP1 axis. Fish Shellfish Immunol. 2023, 135, 108682. [Google Scholar] [CrossRef] [PubMed]
- Qiao, B.; He, Y.; Gao, X. Curcumin attenuates AFB1-induced duck liver injury by inhibiting oxidative stress and lysosomal damage. Food Chem. Toxicol. 2023, 172, 113593. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lin, X.; Hao, Z. Cadmium exposure caused cardiotoxicity in common carps (Cyprinus carpio L.): miR-9-5p, oxidative stress, energetic impairment, mitochondrial division/fusion imbalance, inflammation, and autophagy. Fish Shellfish Immunol. 2023, 138, 108853. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; He, Y.; Tian, J. Ferulic acid prevents aflatoxin B1-induced liver injury in rats via inhibiting cytochrome P450 enzyme, activating Nrf2/GST pathway and regulating mitochondrial pathway. Ecotoxicol. Environ. Saf. 2021, 224, 112624. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhao, Y.; Xu, X. Lactiplantibacillus plantarum P101 alleviates alcoholic liver injury by modulating the Nrf2/HO-1 pathway in mice. J. Appl. Microbiol. 2023, 134, lxac032. [Google Scholar] [CrossRef] [PubMed]
- Mathew, T.; Sarada, S.K.S. Intonation of Nrf2 and Hif1-α pathway by curcumin prophylaxis: A potential strategy to augment survival signaling under hypoxia. Respir. Physiol. Neurobiol. 2018, 258, 12–24. [Google Scholar] [CrossRef]
- Miao, Z.; Miao, Z.; Teng, X. Melatonin alleviates lead-induced intestinal epithelial cell pyroptosis in the common carps (Cyprinus carpio) via miR-17-5p/TXNIP axis. Fish Shellfish. Immunol. 2022, 131, 127–136. [Google Scholar] [CrossRef]
- Polat, L.O.; Gungor, A.J.E.S.; Research, P. WEEE closed-loop supply chain network management considering the damage levels of returned products. Environ. Sci. Pollut. Res. Int. 2021, 28, 7786–7804. [Google Scholar] [CrossRef]
- Jiao, W.; Han, Q.; Xu, Y. Impaired immune function and structural integrity in the gills of common carp (Cyprinus carpio L.) caused by chlorpyrifos exposure: Through oxidative stress and apoptosis. Fish Shellfish Immunol. 2019, 86, 239–245. [Google Scholar] [CrossRef]
- Li, H.; Xing, L.; Zhang, M. The Toxic Effects of Aflatoxin B1 and Aflatoxin M1 on Kidney through Regulating L-Proline and Downstream Apoptosis. BioMed Res. Int. 2018, 2018, 9074861. [Google Scholar] [CrossRef]
- Cui, J.; Liu, Y.; Hao, Z. Cadmium induced time-dependent kidney injury in common carp via mitochondrial pathway: Impaired mitochondrial energy metabolism and mitochondrion-dependent apoptosis. Aquat. Toxicol. 2023, 261, 106570. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Duan, S.; Guan, T. Vitexin protects against ethanol-induced liver injury through Sirt1/p53 signaling pathway. Eur. J. Pharmacol. 2020, 873, 173007. [Google Scholar] [CrossRef] [PubMed]



| Target Protein | Combined Heat and Power (kcal/mol) |
|---|---|
| CASP3 | −5.03 |
| HIF1A | −7.8 |
| SRC | −6.4 |
| VEGFA | −5.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, X.; Liu, S.; Pei, H.; Chen, W.; Zong, Y.; Zhao, Y.; Li, J.; Du, R.; He, Z. Study on Dihydromyricetin Improving Aflatoxin Induced Liver Injury Based on Network Pharmacology and Molecular Docking. Toxics 2023, 11, 760. https://doi.org/10.3390/toxics11090760
Zhu X, Liu S, Pei H, Chen W, Zong Y, Zhao Y, Li J, Du R, He Z. Study on Dihydromyricetin Improving Aflatoxin Induced Liver Injury Based on Network Pharmacology and Molecular Docking. Toxics. 2023; 11(9):760. https://doi.org/10.3390/toxics11090760
Chicago/Turabian StyleZhu, Xiaoying, Silu Liu, Hongyan Pei, Weijia Chen, Ying Zong, Yan Zhao, Jianming Li, Rui Du, and Zhongmei He. 2023. "Study on Dihydromyricetin Improving Aflatoxin Induced Liver Injury Based on Network Pharmacology and Molecular Docking" Toxics 11, no. 9: 760. https://doi.org/10.3390/toxics11090760
APA StyleZhu, X., Liu, S., Pei, H., Chen, W., Zong, Y., Zhao, Y., Li, J., Du, R., & He, Z. (2023). Study on Dihydromyricetin Improving Aflatoxin Induced Liver Injury Based on Network Pharmacology and Molecular Docking. Toxics, 11(9), 760. https://doi.org/10.3390/toxics11090760









