Effects of Fungicides and Nontarget Pesticides on Accumulation of the Mycotoxin Deoxynivlenol in Wheat
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals, Fungal Strains, and Culture Media
2.2. Pesticide Sensitivity Tests
2.3. In Vitro Induction Experiments
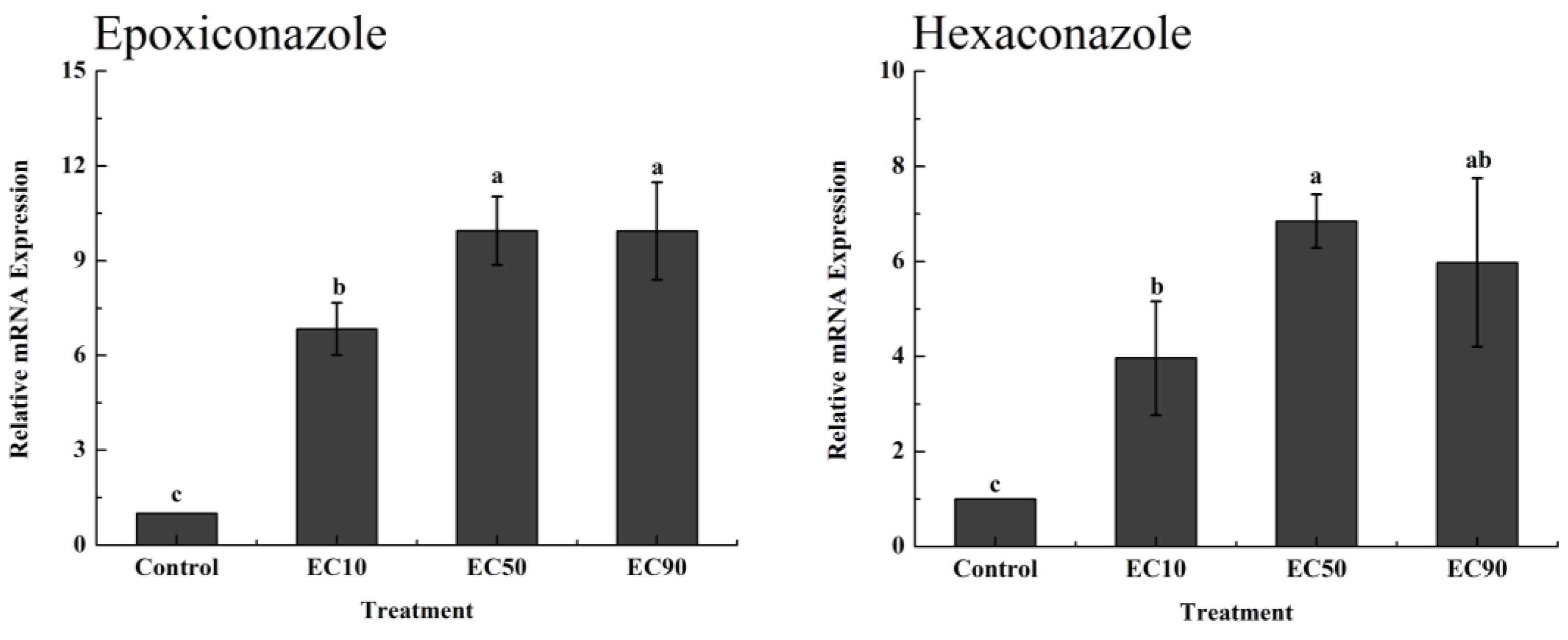
2.4. Relative Expression of Tri5
2.5. Validation of the Induction Effect In Vitro
2.6. Wheat Cultivation and Oxidative Stress Experiment
2.7. In Vivo Induction Experiments
2.8. DNA Extraction and Population Abundance Quantification of PH-1
2.9. Greenhouse Validation Experiment
2.10. Bioaccumulation of Pesticides by Wheat Plants
2.11. Extraction and Detection of H2O2, DON, and Pesticides from Different Matrices
2.12. Statistical Analysis
3. Results and Discussion
3.1. Pesticide Sensitivity Tests
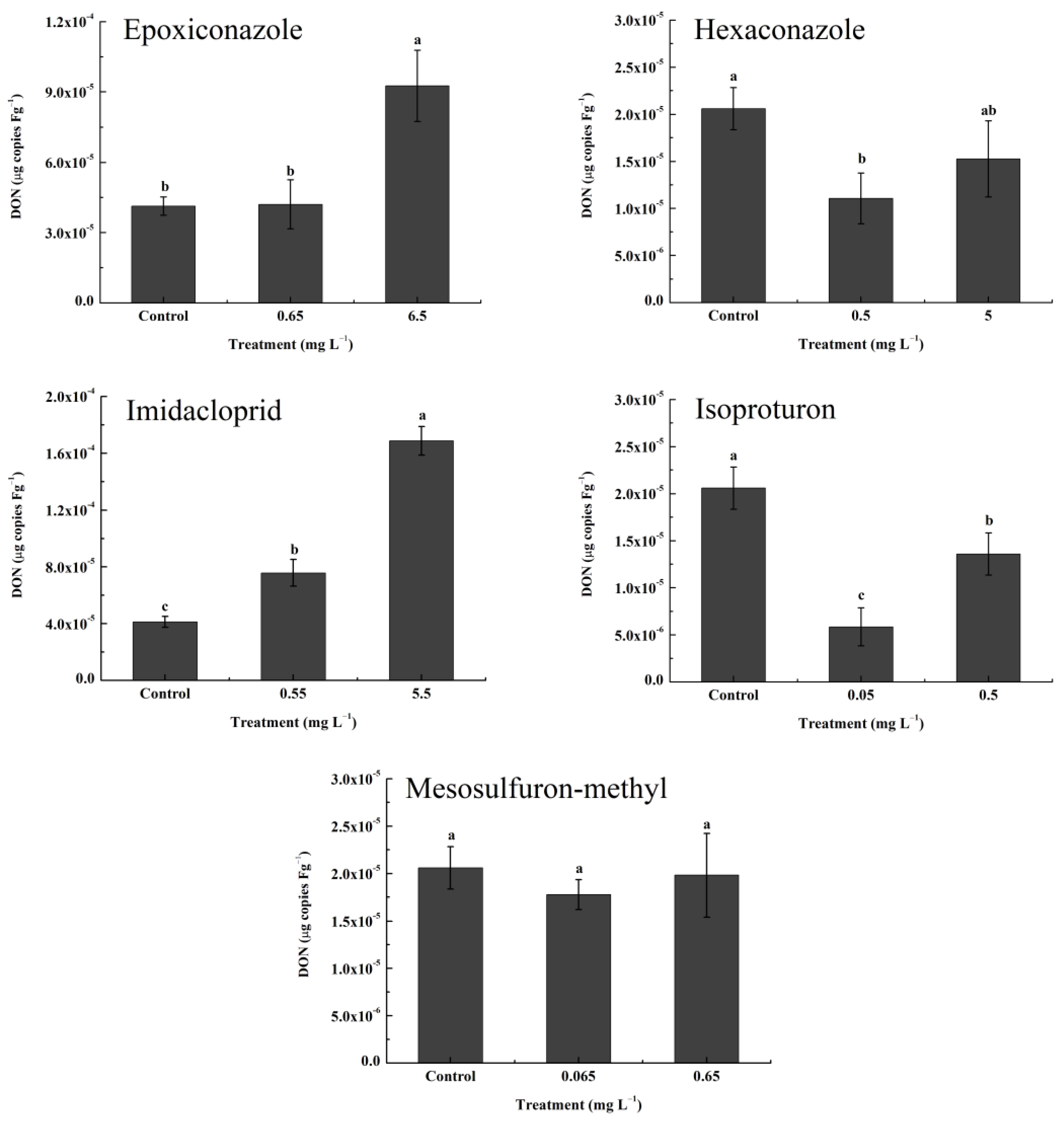
3.2. Accumulation of H2O2 and DON In Vitro and Expression of the Tri5 Gene after Pesticide Application
3.3. Validation of the Induction Effect of H2O2 Induced by Epoxiconazole and Hexaconazole In Vitro
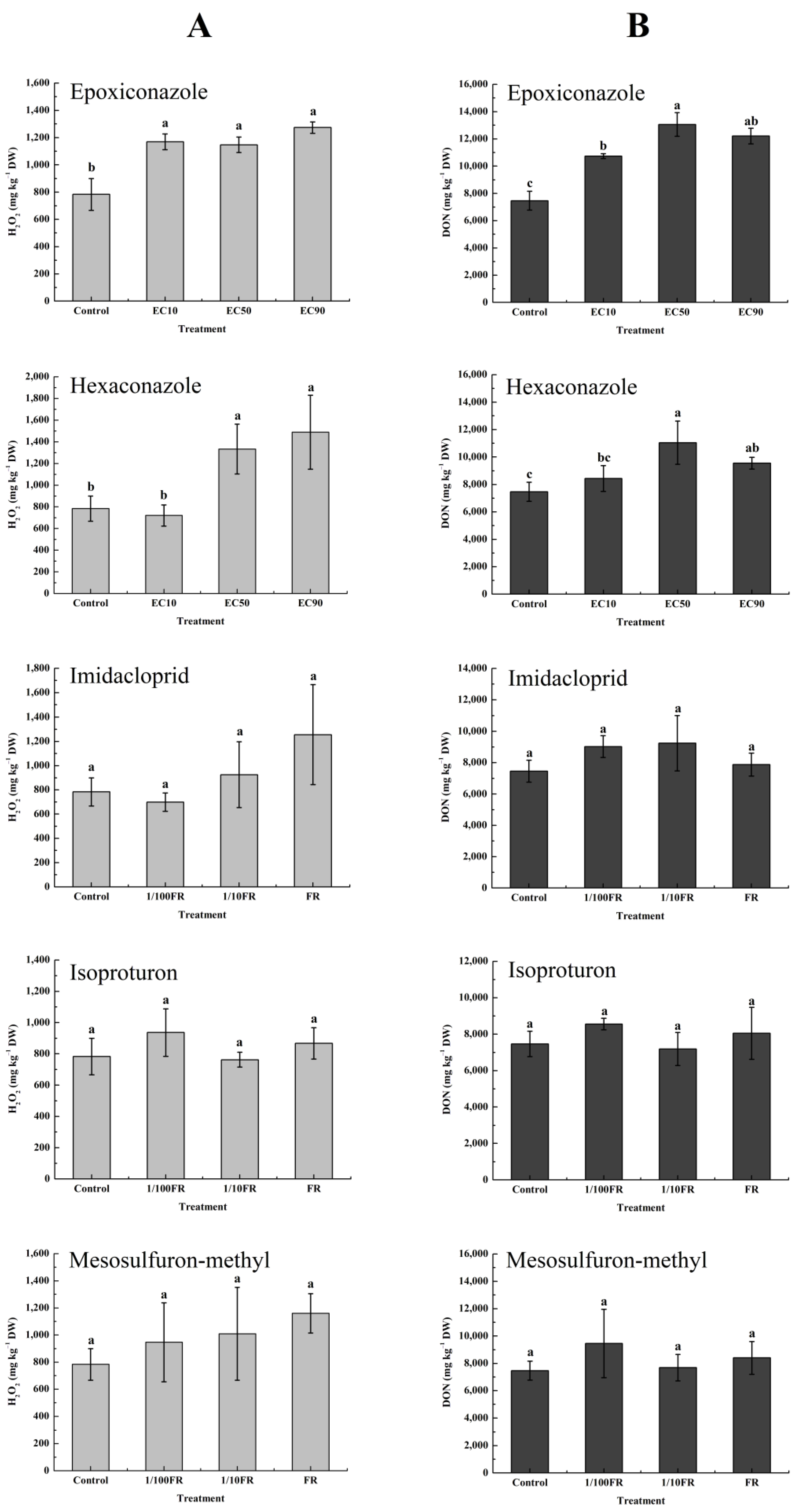
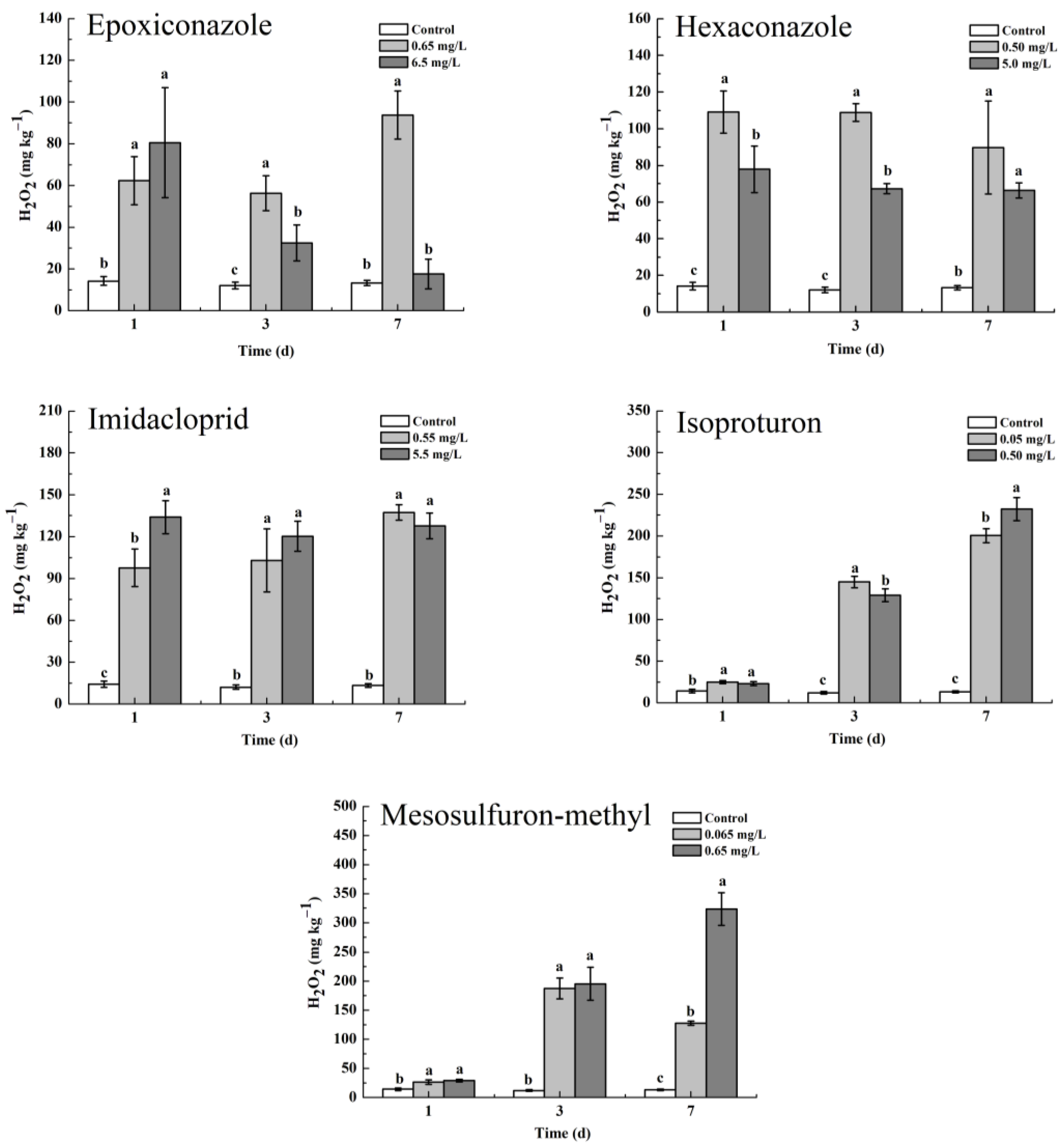
3.4. Oxidative Stress of Pesticides on Wheat Plants
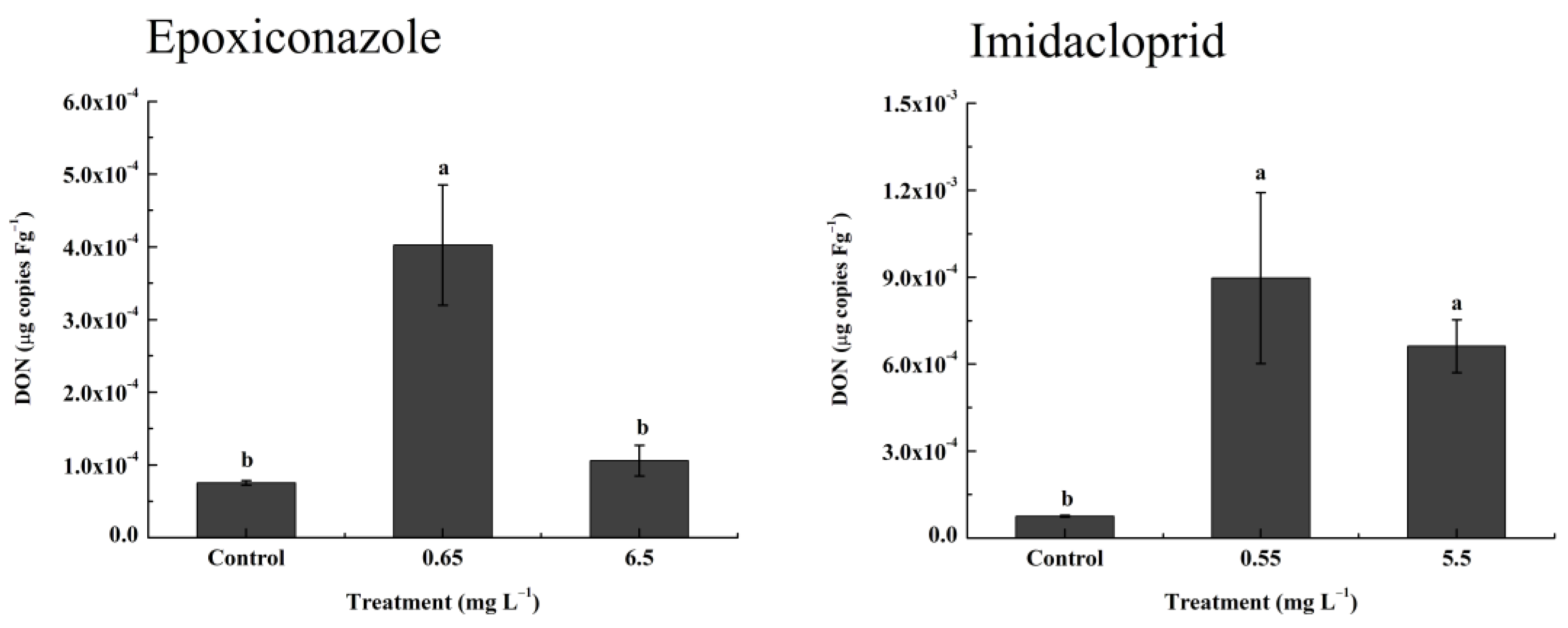
3.5. Effects of the Pesticide-Induced Oxidative Stress Response on DON Accumulation In Vivo
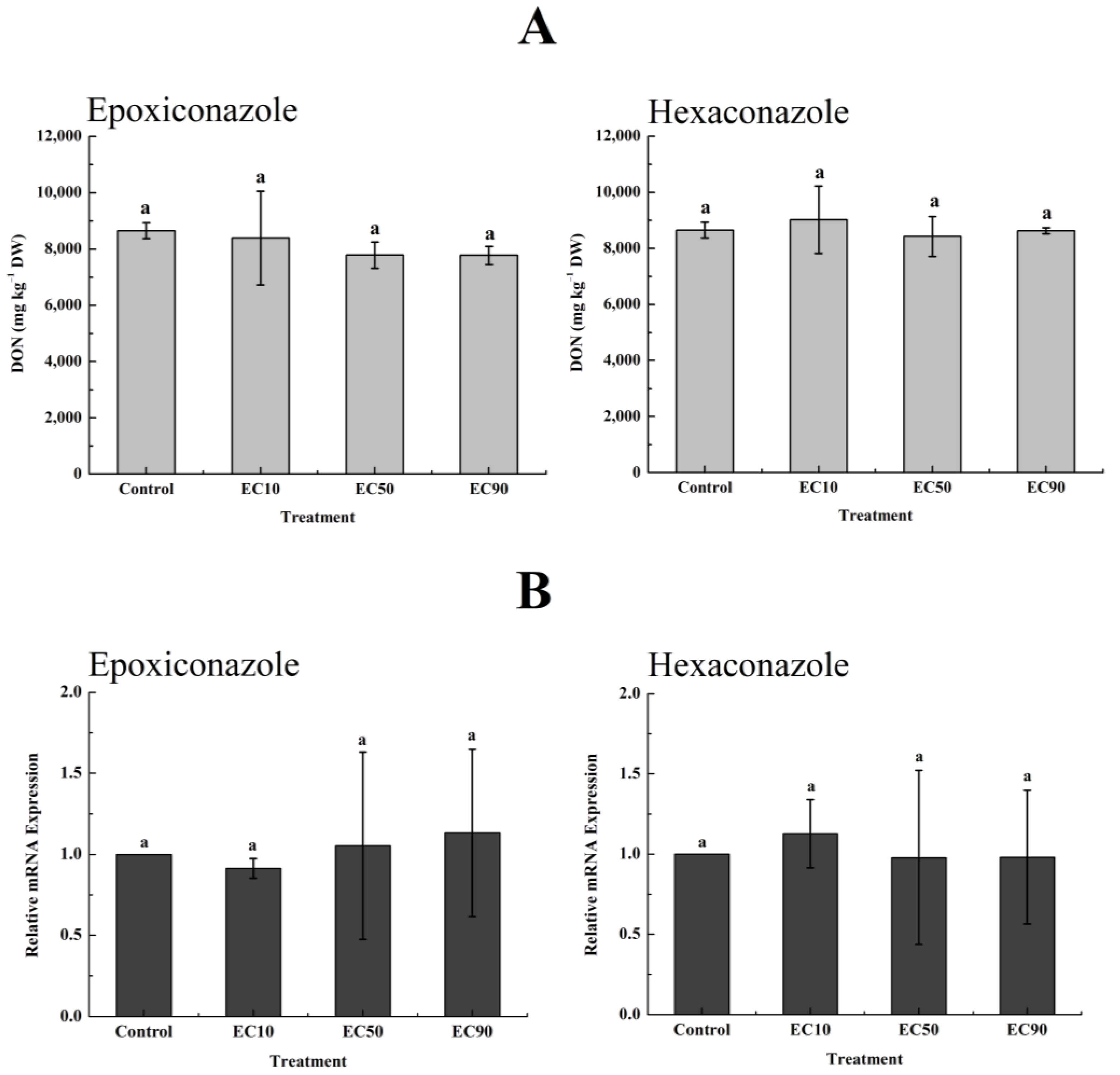
3.6. Greenhouse Validation Experiment
3.7. Risk Assessment of DON Based on Bioaccumulation of Pesticides by Wheat Plants
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, Z.; Zhang, J.; Shi, D.; Gao, B.; Wang, Z.; Zhang, Y.; Wang, M. Deoxynivalenol in Fusarium graminearum: Evaluation of cyproconazole stereoisomers in vitro and in Planta. J. Agric. Food Chem. 2021, 69, 9735–9742. [Google Scholar] [CrossRef]
- Chen, Y.; Kistler, H.C.; Ma, Z. Fusarium graminearum trichothecene mycotoxins: Biosynthesis, regulation, and management. Annu. Rev. Phytopathol. 2019, 57, 15–39. [Google Scholar] [CrossRef]
- Mudge, A.M.; Dill-Macky, R.; Dong, Y.; Gardiner, D.M.; White, R.G.; Manners, J.M. A role for the mycotoxin deoxynivalenol in stem colonisation during crown rot disease of wheat caused by Fusarium graminearum and Fusarium pseudograminearum. Physiol. Mol. Plant Pathol. 2006, 69, 73–85. [Google Scholar] [CrossRef]
- Proctor, R.H.; Hohn, T.M.; McCormick, S.P. Reduced virulence of Gibberella zeae caused by disruption of a trichothecene toxin biosynthetic gene. Mol. Plant Microbe Interact. 1995, 8, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Lim, W.; Park, S.; Kim, J.; You, S.; Song, G. Deoxynivalenol induces apoptosis and disrupts cellular homeostasis through MAPK signaling pathways in bovine mammary epithelial cells. Environ. Pollut. 2019, 252, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Holanda, D.M.; Kim, S.W. Mycotoxin occurrence, toxicity, and detoxifying agents in pig production with an emphasis on deoxynivalenol. Toxins 2021, 13, 171. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Huang, W.; Sun, Y.; Li, Y. Deoxynivalenol induced spermatogenesis disorder by blood-testis barrier disruption associated with testosterone deficiency and inflammation in mice. Environ. Pollut. 2020, 264, 114748. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Chen, H.; Wu, Q.; Wu, Y.; Daly, P.; Chen, J.; Yang, H.; Wei, L.; Zhuang, Y. Trehalose-6-phosphate phosphatase inhibitor: N-(phenylthio) phthalimide, which can inhibit the DON biosynthesis of Fusarium graminearum. Pestic. Biochem. Physiol. 2021, 178, 104917. [Google Scholar] [CrossRef] [PubMed]
- Diao, X.; Han, Y.; Liu, C. The fungicidal activity of tebuconazole enantiomers against Fusarium graminearum and its selective effect on DON production under different conditions. J. Agric. Food Chem. 2018, 66, 3637–3643. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, Z.; Wang, J.; Yang, J.; E, H.; Chen, B.; He, P.; Tan, Y.; Zhou, C. Occurrence and risk assessment of dietary exposure to deoxynivalenol in wheat-based products based different wheat-producing area for the inhabitants in Shanghai, China. J. Fungi 2021, 7, 1015. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Jiang, D.; Zhou, J.; Chen, J.; Li, W.; Zheng, F. Mycotoxins in wheat flour and intake assessment in Shandong province of China. Food Addit. Contam. B. 2016, 9, 170–175. [Google Scholar] [CrossRef]
- Zhao, Y.; Guan, X.; Zong, Y.; Hua, X.; Xing, F.; Wang, Y.; Wang, F.; Liu, Y. Deoxynivalenol in wheat from the Northwestern region in China. Food Addit. Contam. B 2018, 11, 281–285. [Google Scholar] [CrossRef]
- Cui, L.; Selvaraj, J.N.; Xing, F.; Zhao, Y.; Zhou, L.; Liu, Y. A minor survey of deoxynivalenol in Fusarium infected wheat from Yangtze–Huaihe river basin region in China. Food Control. 2013, 30, 469–473. [Google Scholar] [CrossRef]
- Yao, Y.; Long, M. The biological detoxification of deoxynivalenol: A review. Food Chem. Toxicol. 2020, 145, 111649. [Google Scholar] [CrossRef] [PubMed]
- Edwards, S.; Pirgozliev, S.; Hare, M.; Jenkinson, P. Quantification of trichothecene-producing Fusarium species in harvested grain by competitive PCR to determine efficacies of fungicides against Fusarium head blight of winter wheat. Appl. Environ. Microb. 2001, 67, 1575–1580. [Google Scholar] [CrossRef]
- Tang, G.; Chen, Y.; Xu, J.R.; Kistler, H.C.; Ma, Z. The fungal myosin I is essential for Fusarium toxisome formation. PLoS Pathog. 2018, 14, e1006827. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Xiao, X.; Li, T.; Chen, W.; Wang, J.; Fraaije, B.A.; Zhou, M. Impact of epoxiconazole on Fusarium head blight control, grain yield and deoxynivalenol accumulation in wheat. Pestic. Biochem. Physiol. 2018, 152, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Lu, F.; Zhou, Z.; Zhao, H.; Zhang, J.; Mao, Y.; Li, M.; Wang, J.; Zhou, M. Quinone outside inhibitors affect DON biosynthesis, mitochondrial structure and toxisome formation in Fusarium graminearum. J. Hazard. Mater. 2020, 398, 122908. [Google Scholar] [CrossRef]
- Audenaert, K.; Callewaert, E.; Höfte, M.; De Saeger, S.; Haesaert, G. Hydrogen peroxide induced by the fungicide prothioconazole triggers deoxynivalenol (DON) production by Fusarium graminearum. BMC Microbiol. 2010, 10, 112. [Google Scholar] [CrossRef]
- Ponts, N.; Pinson-Gadais, L.; Barreau, C.; Richard-Forget, F.; Ouellet, T. Exogenous H2O2 and catalase treatments interfere with Tri genes expression in liquid cultures of Fusarium graminearum. FEBS Lett. 2007, 581, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Ponts, N.; Pinson-Gadais, L.; Verdal-Bonnin, M.N.; Barreau, C.; Richard-Forget, F. Accumulation of deoxynivalenol and its 15-acetylated form is significantly modulated by oxidative stress in liquid cultures of Fusarium graminearum. FEMS Microbiol. Lett. 2006, 258, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Yang, X.; Huang, W.; Zhang, M.; Lu, W.; Zhao, H.; Wang, J.; Liu, H.; Dong, A.; Zhang, H. Effect of pesticide 1-[6-chloro-3-methyl-pyridyl-8-nitro-7-methyl-1 2 3 5 6 7-hexahydro imidazo (1,2a)]-pyridine when responding to a wheat plant’s antioxidant defense system. Food Chem. 2014, 146, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Yang, Y.; Jia, L.X.; Lin, J.L.; Liu, Y.; Pan, B.; Lin, Y. Biological responses of wheat (Triticum aestivum) plants to the herbicide simetryne in soils. Ecotoxicol. Environ. Saf. 2016, 127, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Fan, S.; Wen, Y.; Tan, Z.; Liu, C. Enantioselective effect of flutriafol on growth, deoxynivalenol production, and TRI gene transcript levels in Fusarium graminearum. J. Agric. Food Chem. 2021, 69, 1684–1692. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Gao, B.; He, Z.; Li, L.; Zhang, Q.; Kaziem, A.E.; Wang, M. Stereoselective bioactivity of the chiral triazole fungicide prothioconazole and its metabolite. Pestic. Biochem. Physiol. 2019, 160, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Feksa, H.; Do Couto, H.; Garozi, R.; De Almeida, J.; Gardiano, C.; Tessmann, D. Pre-and postinfection application of strobilurin-triazole premixes and single fungicides for control of fusarium head blight and deoxynivalenol mycotoxin in wheat. Crop Prot. 2019, 117, 128–134. [Google Scholar] [CrossRef]
- Robinson, M.A.; Cowbrough, M.J.; Sikkema, P.H.; Tardif, F.J. Winter wheat (Triticum aestivum L.) tolerance to mixtures of herbicides and fungicides applied at different timings. Can. J. Plant Sci. 2013, 93, 491–501. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, H.; Li, J.; Ju, C.; Huang, J. The transcription factor FgAtrR regulates asexual and sexual development, virulence, and DON production and contributes to intrinsic resistance to azole fungicides in Fusarium graminearum. Biology 2022, 11, 326. [Google Scholar] [CrossRef]
- Akoijam, R.; Singh, B. Metabolic degradation of imidacloprid in paddy field soil. Environ. Monit. Assess. 2014, 186, 5977. [Google Scholar] [CrossRef]
- Mohapatra, S.; Kumar, S.; Prakash, G.S. Residue evaluation of imidacloprid, spirotetramat, and spirotetramat-enol in/on grapes (Vitis vinifera L.) and soil. Environ. Monit. Assess. 2015, 187, 632. [Google Scholar] [CrossRef] [PubMed]
- Paul, R.; Sharma, R.; Kulshrestha, G.; Singh, S.B. Analysis of metsulfuron-methyl residues in wheat field soil: A comparison of HPLC and bioassay techniques. Pest. Manag. Sci. 2009, 65, 963–968. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, C.; Gao, J.; Wang, C.; Cui, L.; Li, A. Dissipation behavior of hexaconazole and kresoxim-methyl residues in ginseng and soil under field conditions. Environ. Monit. Assess. 2014, 187, 4126. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.J.; Ma, J.W.; Wang, H.; Zhou, L.; Tong, L.; Li, A.; Wu, J.K.; Zhang, J.F. Residue and dissipation of epoxiconazole in Triticum aestivum L. and soil under field conditions. Chin. J. Pestic. Sci. 2017, 19, 474–481. [Google Scholar]
- Yan, B.; Ye, F.; Gao, D. Residues of the fungicide epoxiconazole in rice and paddy in the Chinese field ecosystem. Pest. Manag. Sci. 2015, 71, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Song, N.H.; Le Yin, X.; Chen, G.F.; Yang, H. Biological responses of wheat (Triticum aestivum) plants to the herbicide chlorotoluron in soils. Chemosphere 2007, 68, 1779–1787. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, F.; Xue, C.; Wang, C.; Chi, S.; Zhang, J. Comparative toxicity of nonylphenol, nonylphenol-4-ethoxylate and nonylphenol-10-ethoxylate to wheat seedlings (Triticum aestivum L.). Ecotoxicol. Environ. Saf. 2016, 131, 7–13. [Google Scholar] [CrossRef]
- Wang, Y.S.; Yang, Z.M. Nitric oxide reduces aluminum toxicity by preventing oxidative stress in the roots of Cassia tora L. Plant Cell Physiol. 2005, 46, 1915–1923. [Google Scholar] [CrossRef]
- Brandfass, C.; Karlovsky, P. Upscaled CTAB-based DNA extraction and real-time PCR assays for Fusarium culmorum and F. graminearum DNA in plant material with reduced sampling error. Int. J. Mol. Sci. 2008, 9, 2306–2321. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Duan, Y.; Bian, C.; Pan, X.; Yao, C.; Wang, J.; Zhou, M. Effects of validamycin in controlling Fusarium head blight caused by Fusarium graminearum: Inhibition of DON biosynthesis and induction of host resistance. Pest. Manag. Sci. 2019, 153, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Kulik, T.; Łojko, M.; Jestoi, M.; Perkowski, J. Sublethal concentrations of azoles induce tri transcript levels and trichothecene production in Fusarium graminearum. FEMS Microbiol. Lett. 2012, 335, 58–67. [Google Scholar] [CrossRef]
- Liu, Z.; Jian, Y.; Chen, Y.; Kistler, H.C.; He, P.; Ma, Z.; Yin, Y. A phosphorylated transcription factor regulates sterol biosynthesis in Fusarium graminearum. Nat. Commun. 2019, 10, 1228. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Lu, Y.L.; Yang, H. Toxicology of isoproturon to the food crop wheat as affected by salicylic acid. Environ. Sci. Pollut. Res. 2012, 19, 2044–2054. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.X.; Tiedemann, A.V. Physiological effects of azoxystrobin and epoxiconazole on senescence and the oxidative status of wheat. Pestic. Biochem. Physiol. 2001, 71, 1–10. [Google Scholar] [CrossRef]
- Honorato Júnior, J.; Zambolim, L.; Aucique-Pérez, C.; Resende, R.; Rodrigues, F. Photosynthetic and antioxidative alterations in coffee leaves caused by epoxiconazole and pyraclostrobin sprays and Hemileia vastatrix infection. Pestic. Biochem. Physiol. 2015, 123, 31–39. [Google Scholar] [CrossRef]
- Dubey, P.; Mishra, A.K.; Singh, A.K. Comparative analyses of genotoxicity, oxidative stress and antioxidative defence system under exposure of methyl parathion and hexaconazole in barley (Hordeum vulgare L.). Environ. Sci. Pollut. Res. 2015, 22, 19848–19859. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kumar, V.; Kanwar, M.; Thukral, A.; Bhardwaj, R. Ameliorating imidacloprid induced oxidative stress by 24-epibrassinolide in Brassica juncea L. Russ. J. Plant Physiol. 2017, 64, 509–517. [Google Scholar] [CrossRef]
- Shakir, S.K.; Irfan, S.; Akhtar, B.; Daud, M.K.; Taimur, N.; Azizullah, A. Pesticide-induced oxidative stress and antioxidant responses in tomato (Solanum lycopersicum) seedlings. Ecotoxicology 2018, 27, 919–935. [Google Scholar] [CrossRef]
- Yin, X.L.; Jiang, L.; Song, N.H.; Yang, H. Toxic reactivity of wheat (Triticum aestivum) plants to herbicide isoproturon. J. Agric. Food Chem. 2008, 56, 4825–4831. [Google Scholar] [CrossRef]
- Anjum, N.A.; Gill, S.S.; Corpas, F.J.; Ortega-Villasante, C.; Hernandez, L.E.; Tuteja, N.; Sofo, A.; Hasanuzzaman, M.; Fujita, M. Editorial: Recent insights into the double role of hydrogen peroxide in plants. Front. Plant Sci. 2022, 13, 843274. [Google Scholar] [CrossRef]
- Bönnighausen, J.; Schauer, N.; Schäfer, W.; Bormann, J. Metabolic profiling of wheat rachis node infection by Fusarium graminearum–decoding deoxynivalenol-dependent susceptibility. New Phytol. 2019, 221, 459–469. [Google Scholar] [CrossRef]
- Etzerodt, T.; Gislum, R.; Laursen, B.B.; Heinrichson, K.; Gregersen, P.L.; Jørgensen, L.N.; Fomsgaard, I.S. Correlation of deoxynivalenol accumulation in Fusarium-infected winter and spring wheat cultivars with secondary metabolites at different growth stages. J. Agric. Food Chem. 2016, 64, 4545–4555. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Gu, S.; Han, C.; Lu, Y.; Ma, C.; Tian, J.; Bi, J.; Deng, Z.; Wang, Q.; Xu, Q. Targeted and untargeted metabolomics profiling of wheat reveals amino acids increase resistance to Fusarium head blight. Front. Plant Sci. 2021, 12, 762605. [Google Scholar] [CrossRef] [PubMed]
- Scarpino, V.; Reyneri, A.; Sulyok, M.; Krska, R.; Blandino, M. Impact of the insecticide application to maize cultivated in different environmental conditions on emerging mycotoxins. Field Crops Res. 2018, 217, 188–198. [Google Scholar] [CrossRef]
- Felix D’Mello, J.; Macdonald, A.; Postel, D.; Dijksma, W.T.; Dujardin, A.; Placinta, C.M. Pesticide use and mycotoxin production in Fusarium and Aspergillus phytopathogens. Eur. J. Plant Pathol. 1998, 104, 741–751. [Google Scholar] [CrossRef]
- Abbas, A.; Yli-Mattila, T. Biocontrol of Fusarium graminearum, a Causal Agent of Fusarium Head Blight of Wheat, and Deoxynivalenol Accumulation: From In Vitro to In Planta. Toxins 2022, 14, 299. [Google Scholar] [CrossRef]

| Sublethal Concentrations | Value (mg L−1) | |
|---|---|---|
| EC10 | 0.274 ± 0.013 | |
| Epoxiconazole | EC50 | 0.723 ± 0.008 |
| EC90 | 3.610 ± 0.129 | |
| EC10 | 0.184 ± 0.013 | |
| Hexaconazole | EC50 | 1.170 ± 0.079 |
| EC90 | 7.490 ± 0.515 |
| Pesticides | Application Concentrations (mg L−1) | Accumulation Concentrations (mg kg−1) |
|---|---|---|
| Epoxiconazole | 0.650 | 0.080 ± 0.004 |
| 6.500 | 0.331 ± 0.015 | |
| Imidacloprid | 0.550 | 2.070 ± 0.374 |
| 5.500 | 14.300 ± 0.270 |
| Pesticides | Application Concentrations (mg kg−1) | Accumulation Concentrations (μg kg−1) |
|---|---|---|
| Epoxiconazole | 0.65 | 25.10 ± 3.60 |
| 6.50 | 36.80 ± 1.93 | |
| Imidacloprid | 0.55 | 9.45 ± 1.80 |
| 5.50 | 957.00 ± 67.90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ju, C.; Jiang, F.; Gao, Y.; Chen, T.; Cao, J.; Lv, J.; Zhao, Y.; Zheng, Y.; Guo, W.; Huang, J. Effects of Fungicides and Nontarget Pesticides on Accumulation of the Mycotoxin Deoxynivlenol in Wheat. Toxics 2023, 11, 768. https://doi.org/10.3390/toxics11090768
Ju C, Jiang F, Gao Y, Chen T, Cao J, Lv J, Zhao Y, Zheng Y, Guo W, Huang J. Effects of Fungicides and Nontarget Pesticides on Accumulation of the Mycotoxin Deoxynivlenol in Wheat. Toxics. 2023; 11(9):768. https://doi.org/10.3390/toxics11090768
Chicago/Turabian StyleJu, Chao, Fan Jiang, Yuan Gao, Tongwu Chen, Jiakuo Cao, Junbo Lv, Yanxiang Zhao, Yongquan Zheng, Wei Guo, and Jinguang Huang. 2023. "Effects of Fungicides and Nontarget Pesticides on Accumulation of the Mycotoxin Deoxynivlenol in Wheat" Toxics 11, no. 9: 768. https://doi.org/10.3390/toxics11090768
APA StyleJu, C., Jiang, F., Gao, Y., Chen, T., Cao, J., Lv, J., Zhao, Y., Zheng, Y., Guo, W., & Huang, J. (2023). Effects of Fungicides and Nontarget Pesticides on Accumulation of the Mycotoxin Deoxynivlenol in Wheat. Toxics, 11(9), 768. https://doi.org/10.3390/toxics11090768






