Early-Life Sublethal Thiacloprid Exposure to Honey Bee Larvae: Enduring Effects on Adult Bee Cognitive Abilities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Materials
2.2. Sample Preparation
2.3. Behavioral Experiments
2.4. Immunohistochemistry for Neural Apoptosis
2.5. RNA Extraction, Reverse Transcription, Quantitative qPCR, and Transcriptome Data Analysis
2.6. Statistical Analysis
3. Results
3.1. Thiacloprid Affects Honey Bee Survival and Body Weight
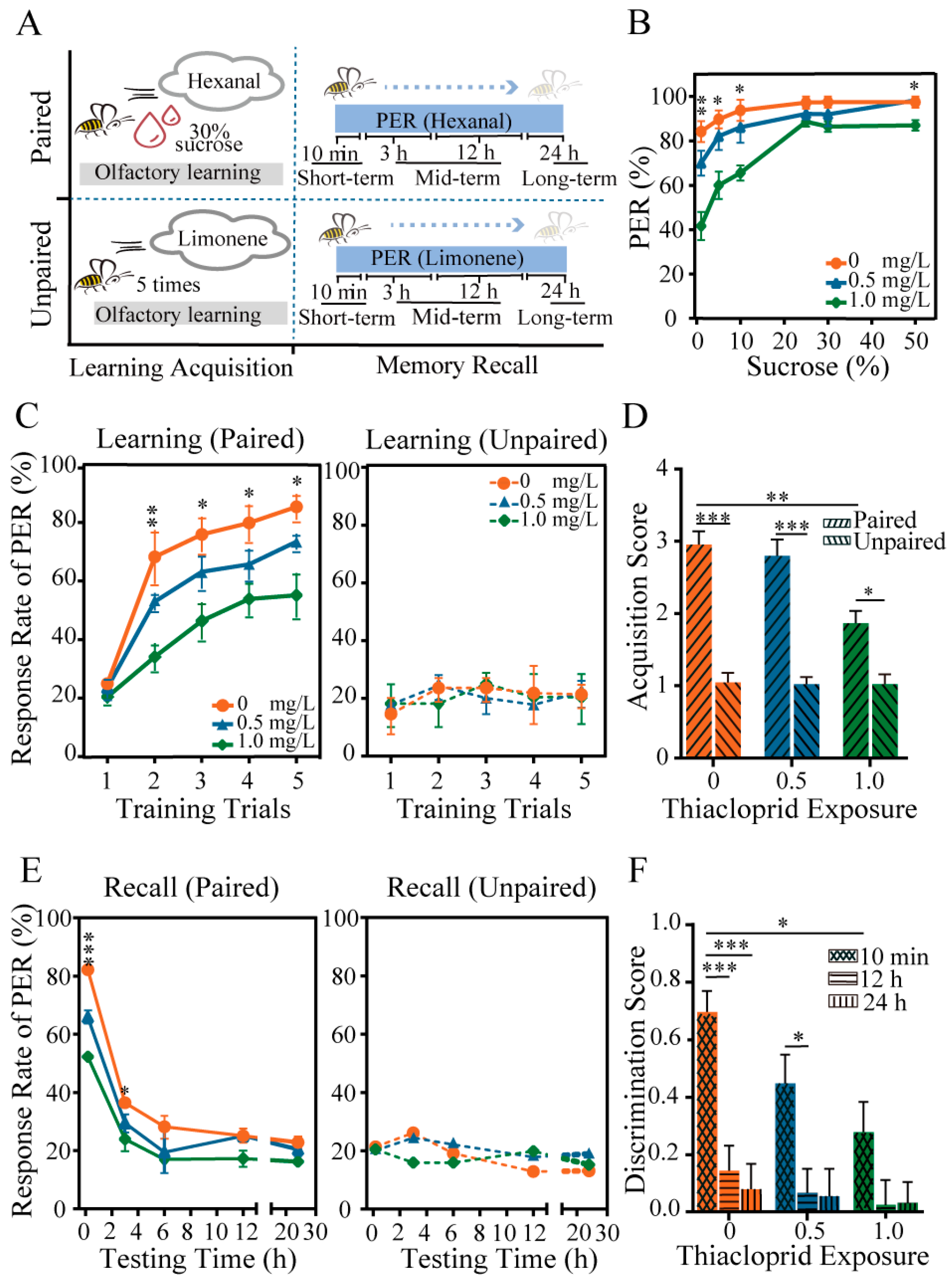
3.2. Thiacloprid Impaired Honey Bee Behaviors
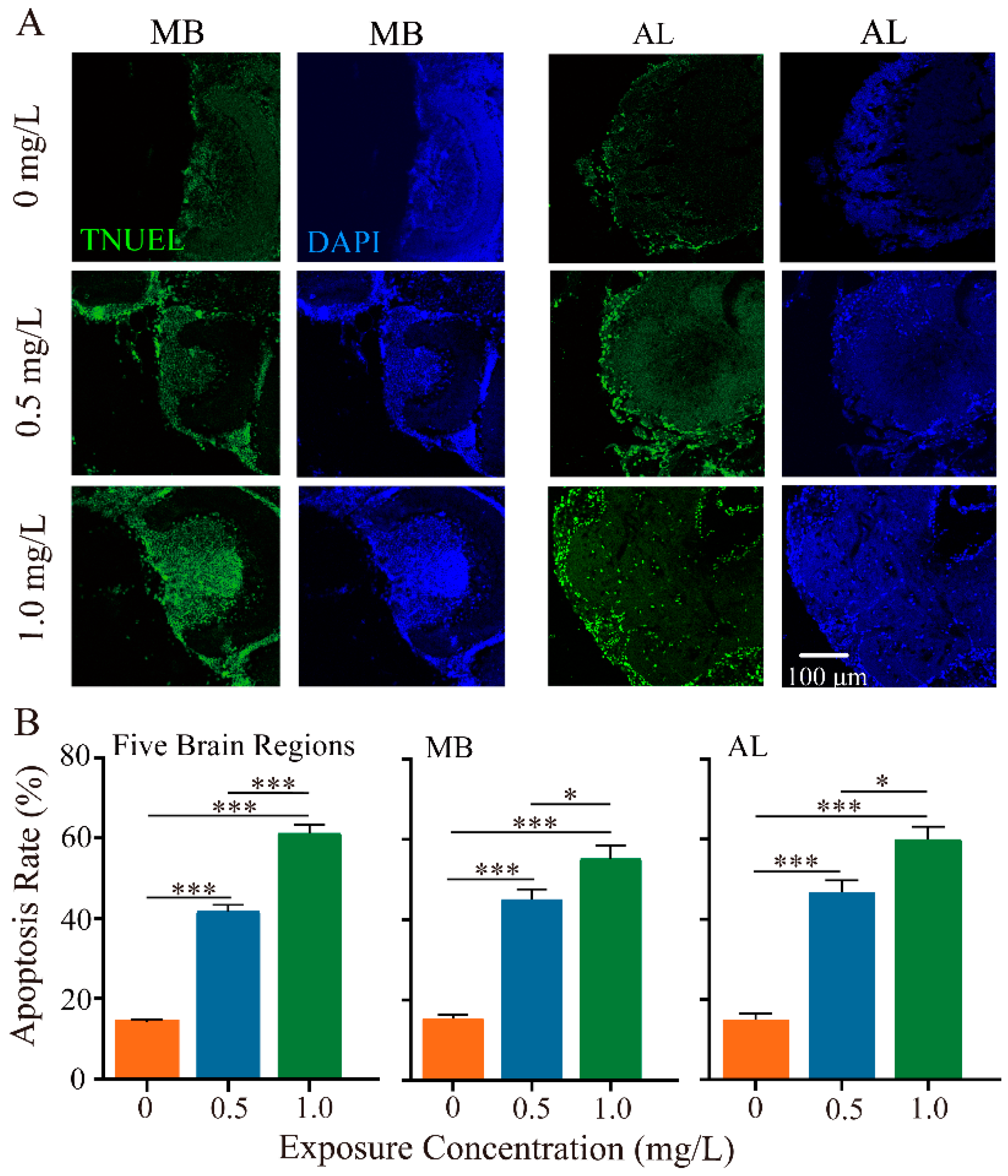
3.3. Thiacloprid Exposure Caused Honey Bee Neuronal Apoptosis
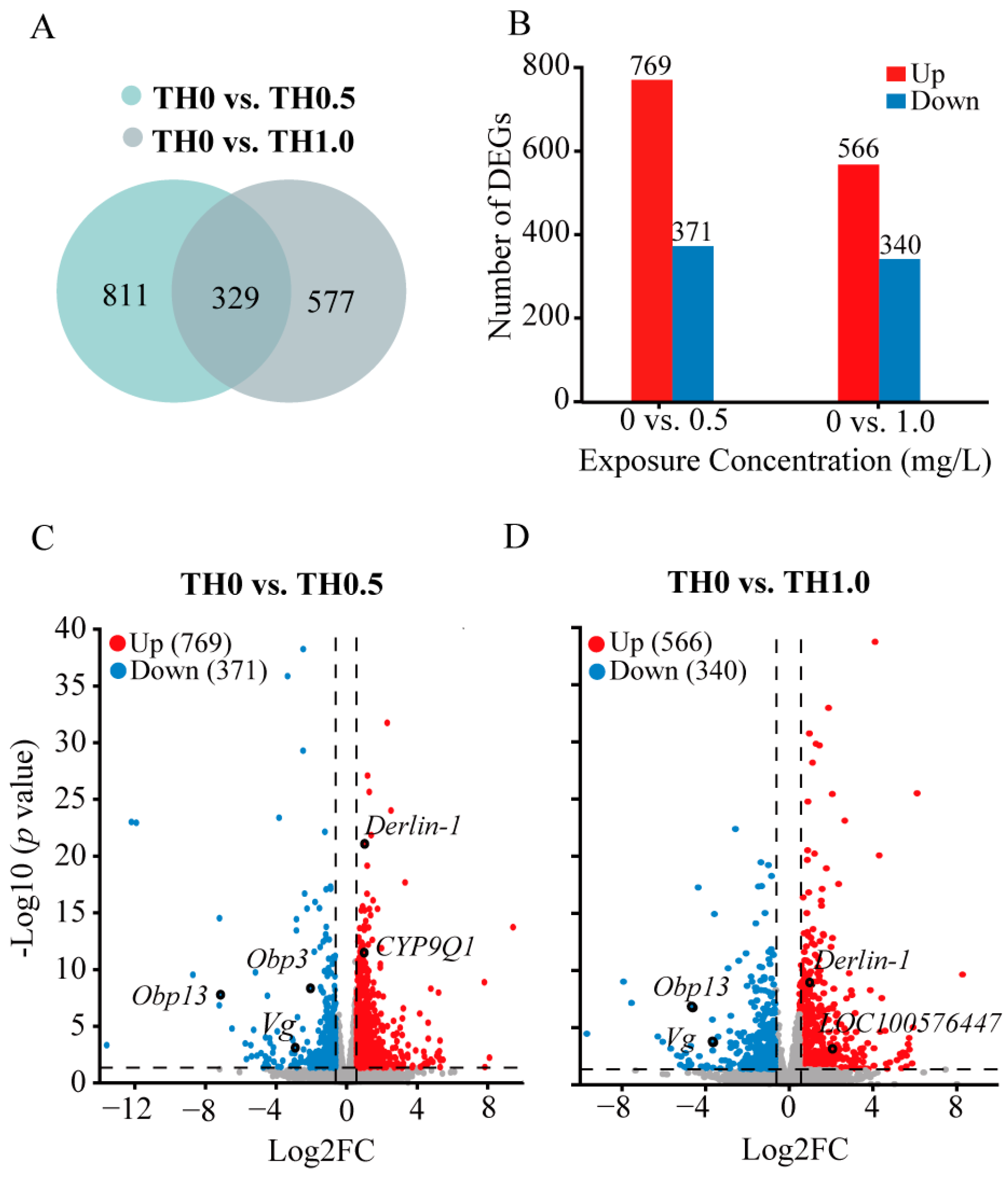
3.4. RNA-Seq Data Analysis of Honey Bees Exposed to Thiacloprid
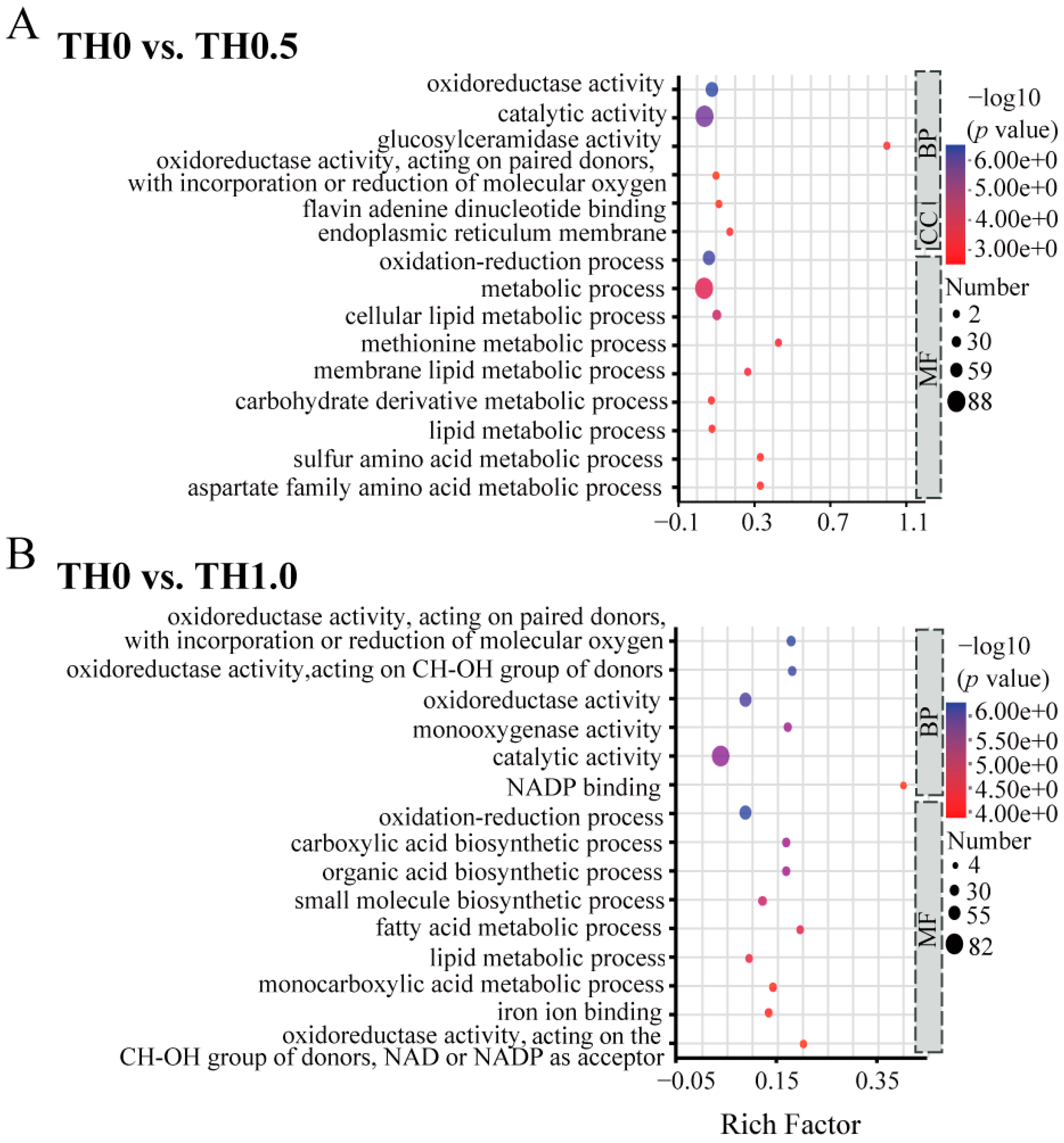
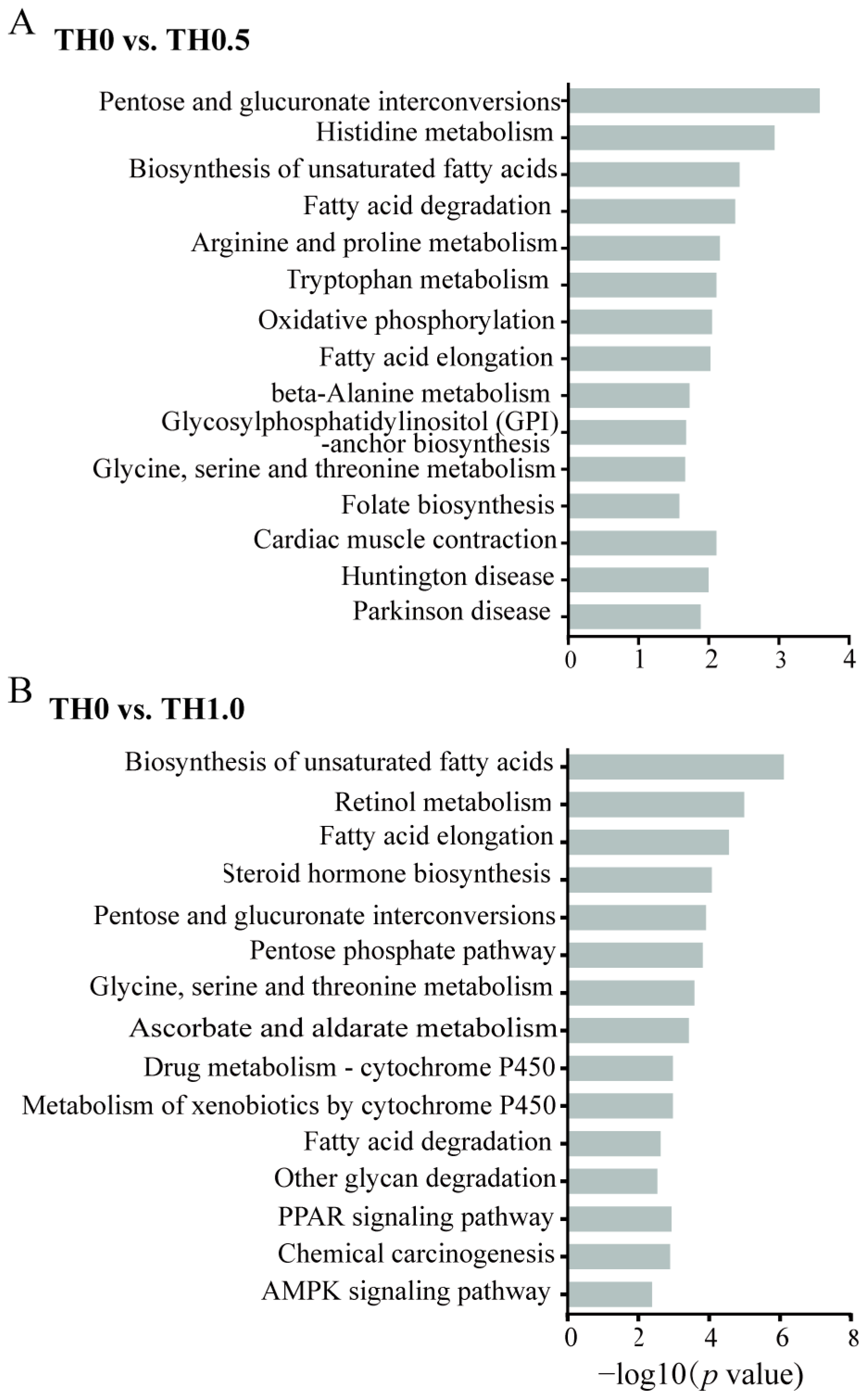
3.5. Functional Analysis of DEGs
3.6. qRT-PCR Validation Analysis
4. Discussion
4.1. Thiacloprid Disrupted Honey Bee Health
4.2. Thiacloprid Impaired Honey Bee Learning and Memory Ability
4.3. Larval Exposure to Thiacloprid Inducing Neuronal Apoptosis of Honey Bees
4.4. Thiacloprid Led to Transcriptome Changes in Honey Bees
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cilia, G.; Flaminio, S.; Zavatta, L.; Ranalli, R.; Quaranta, M.; Bortolotti, L.; Nanetti, A. Occurrence of honey bee (Apis mellifera L.) pathogens in wild pollinators in northern Italy. Front. Cell Infect. Microbiol. 2022, 12, 907489. [Google Scholar] [CrossRef] [PubMed]
- Matthijs, S.; De Waele, V.; Vandenberge, V.; Verhoeven, B.; Evers, J.; Brunain, M.; Saegerman, C.; De Winter, P.J.J.; Roels, S.; de Graaf, D.C.; et al. Nationwide screening for bee viruses and parasites in belgian honey bees. Viruses 2020, 12, 890. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Chen, H.; Fan, R.L.; Lin, Z.G.; Niu, Q.S.; Wang, Z.; Ji, T. Effect of carbendazim on honey bee health: Assessment of survival, pollen consumption, and gut microbiome composition. Ecotoxicol. Environ. Saf. 2022, 239, 113648. [Google Scholar] [CrossRef] [PubMed]
- Branchiccela, B.; Castelli, L.; Corona, M.; Diaz-Cetti, S.; Invernizzi, C.; de la Escalera, G.M.; Mendoza, Y.; Santos, E.; Silva, C.; Zunino, P.; et al. Impact of nutritional stress on the honeybee colony health. Sci. Rep. 2019, 9, 10156. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Li, G.L.; Cui, X.P.; Wang, H.F.; Liu, Z.G.; Yang, Y.W.; Xu, B.H. Review on effects of some insecticides on honey bee health. Pestic. Biochem. Phys. 2022, 188, 105219. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, K.; Ihara, M.; Sattelle, D.B. Neonicotinoid insecticides: Molecular targets, resistance, and toxicity. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Woodcock, B.A.; Bullock, J.M.; Shore, R.F.; Heard, M.S.; Pereira, M.G.; Redhead, J.; Ridding, L.; Dean, H.; Sleep, D.; Henrys, P.; et al. Country-specific effects of neonicotinoid pesticides on honey bees and wild bees. Science 2017, 356, 1393–1395. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Goulson, D.; Chen, L.; Zhang, J.; Zhao, W.; Jin, Y.; Yang, S.; Li, Y.; Zhou, J. Occurrence of neonicotinoids in chinese apiculture and a corresponding risk exposure assessment. Environ. Sci. Technol. 2020, 54, 5021–5030. [Google Scholar] [CrossRef]
- Kavanagh, S.; Henry, M.; Stout, J.C.; White, B. Neonicotinoid residues in honey from urban and rural environments. Environ. Sci. Pollut. Res. Int. 2021, 28, 28179–28190. [Google Scholar] [CrossRef]
- Purdy, J. Distribution of residues of neonicotinoids in the hive and in bees in relation to bee health. Jul.-Kuhn-Arch. 2018, 462, 32–37. [Google Scholar] [CrossRef]
- Jacob, C.R.O.; Malaquias, J.B.; Zanardi, O.Z.; Silva, C.A.S.; Jacob, J.F.O.; Yamamoto, P.T. Oral acute toxicity and impact of neonicotinoids on Apis mellifera L. and Scaptotrigona postica latreille (Hymenoptera: Apidae). Ecotoxicology 2019, 28, 744–753. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; Li, W.; Li, X.; Wang, D.; Weng, H.; Zhu, Y.-C.; Wang, Y. Mixture toxic effects of thiacloprid and cyproconazole on honey bees (Apis mellifera L.). Sci. Total Environ. 2023, 870, 161700. [Google Scholar] [CrossRef] [PubMed]
- Brandt, A.; Gorenflo, A.; Siede, R.; Meixner, M.; Buchler, R. The neonicotinoids thiacloprid, imidacloprid, and clothianidin affect the immunocompetence of honey bees (Apis mellifera L.). J. Insect Physiol. 2016, 86, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Qiao, N.H.; Diao, Q.Y.; Jing, Z.W.; Vukanti, R.; Dai, P.L.; Ge, Y. Thiacloprid exposure perturbs the gut microbiota and reduces the survival status in honeybees. J. Hazard. Mater. 2020, 389, 121818. [Google Scholar] [CrossRef] [PubMed]
- Fent, K.; Schmid, M.; Hettich, T.; Schmid, S. The neonicotinoid thiacloprid causes transcriptional alteration of genes associated with mitochondria at environmental concentrations in honey bees. Environ. Pollut. 2020, 266 Pt 1, 115297. [Google Scholar] [CrossRef] [PubMed]
- Tison, L.; Hahn, M.L.; Holtz, S.; Rossner, A.; Greggers, U.; Bischoff, G.; Menzel, R. Honey bees’ behavior is impaired by chronic exposure to the neonicotinoid thiacloprid in the field. Environ. Sci. Technol. 2016, 50, 7218–7227. [Google Scholar] [CrossRef] [PubMed]
- Ke, L.; Chen, X.; Dai, P.; Liu, Y.-J. Chronic larval exposure to thiacloprid impairs honeybee antennal selectivity, learning and memory performances. Front. Physiol. 2023, 14, 1114488. [Google Scholar] [CrossRef]
- Sanchez-Bayo, F.; Goka, K. Pesticide residues and bees—A risk assessment. PLoS ONE 2014, 9, e94482. [Google Scholar] [CrossRef]
- Stuligross, C.; Williams, N.M. Past insecticide exposure reduces bee reproduction and population growth rate. Proc. Natl. Acad. Sci. USA 2021, 118, e2109909118. [Google Scholar] [CrossRef]
- Shi, J.; Zhang, R.; Pei, Y.; Liao, C.; Wu, X. Exposure to acetamiprid influences the development and survival ability of worker bees (Apis mellifera L.) from larvae to adults. Environ. Pollut. 2020, 266 Pt 2, 115345. [Google Scholar] [CrossRef]
- Claus, G.; Pisman, M.; Spanoghe, P.; Smagghe, G.; Eeraerts, M. Larval oral exposure to thiacloprid: Dose-response toxicity testing in solitary bees, Osmia spp. (Hymenoptera: Megachilidae). Ecotoxicol. Environ. Saf. 2021, 215, 112143. [Google Scholar] [CrossRef] [PubMed]
- Friol, P.S.; Catae, A.F.; Tavares, D.A.; Malaspina, O.; Roat, T.C. Can the exposure of Apis mellifera (Hymenoptera, Apiadae) larvae to a field concentration of thiamethoxam affect newly emerged bees? Chemosphere 2017, 185, 56–66. [Google Scholar] [CrossRef]
- Tison, L.; Holtz, S.; Adeoye, A.; Kalkan, O.; Irmisch, N.S.; Lehmann, N.; Menzel, R. Effects of sublethal doses of thiacloprid and its formulation Calypso (R) on the learning and memory performance of honey bees. J. Exp. Biol. 2017, 220, 3695–3705. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Yin, L.; Ke, L.; Diao, Q.-Y.; Wu, Y.; Dai, P.; Liu, Y.-J. Thiacloprid impairs honeybee worker learning and memory with inducing neuronal apoptosis and downregulating memory-related genes. Sci. Total Environ. 2023, 885, 163820. [Google Scholar] [CrossRef] [PubMed]
- Schmehl, D.R.; Tome, H.V.V.; Mortensen, A.N.; Martins, G.F.; Ellis, J.D. Protocol for the in vitro rearing of honey bee (Apis mellifera L.) workers. J. Apic. Res. 2016, 55, 113–129. [Google Scholar] [CrossRef]
- Li, B.; Ke, L.; Li, A.-R.; Diao, Q.Y.; Wang, Q.; Liu, Y.J. Exposure of larvae to sublethal thiacloprid delays bee development and affects transcriptional responses of newly emerged honey bees. Front. Insect Sci. 2022, 2, 844957. [Google Scholar] [CrossRef]
- Pohorecka, K.; Skubida, P.; Miszczak, A.; Semkiw, P.; Sikorski, P.; Zagibajlo, K.; Teper, D.; Koltowski, Z.; Skubida, M.; Zdanska, D.; et al. Residues of neonicotinoid insecticides in bee collected Plant materials from oilseed rape crops and their effect on bee colonies. J. Apic. Sci. 2012, 56, 115–134. [Google Scholar] [CrossRef]
- Ebeling, J.; Pieper, F.; Gobel, J.; Knispel, H.; McCarthy, M.; Goncalves, M.; Turner, M.; Rod Merrill, A.; Genersch, E. Anti-Virulence strategy against the honey bee pathogenic bacterium Paenibacillus larvae via small molecule inhibitors of the bacterial toxin Plx2A. Toxins 2021, 13, 607. [Google Scholar] [CrossRef]
- Denton, J.A.; Koludarov, I.; Thompson, M.; Bryk, J.; Velasque, M. Honey bee cognition as a tool for scientific engagement. Insects 2021, 12, 842. [Google Scholar] [CrossRef]
- Zioga, E.; Kelly, R.; White, B.; Stout, J.C. Plant protection product residues in plant pollen and nectar: A review of current knowledge. Environ. Res. 2020, 189, 109873. [Google Scholar] [CrossRef]
- Christen, V.; Grossar, D.; Charrière, J.-D.; Eyer, M.; Jeker, L. Correlation between increased homing flight duration and altered gene expression in the brain of honey bee foragers after acute oral exposure to thiacloprid and thiamethoxam. Front. Insect Sci. 2021, 1, 765570. [Google Scholar] [CrossRef]
- Forfert, N.; Moritz, R.F.A. Thiacloprid alters social interactions among honey bee workers (Apis mellifera). J. Apic. Res. 2017, 56, 467–474. [Google Scholar] [CrossRef]
- Tison, L.; Rossner, A.; Gerschewski, S.; Menzel, R. The neonicotinoid clothianidin impairs memory processing in honey bees. Ecotoxicol. Environ. Saf. 2019, 180, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Ohlinger, B.D.; Schurch, R.; Durzi, S.; Kietzman, P.M.; Silliman, M.R.; Couvillon, M.J. Honey bees (Hymenoptera: Apidae) decrease foraging but not recruitment after neonicotinoid exposure. J. Insect Sci. 2022, 22, 16. [Google Scholar] [CrossRef]
- Demares, F.J.; Pirk, C.W.W.; Nicolson, S.W.; Human, H. Neonicotinoids decrease sucrose responsiveness of honey bees at first contact. J. Insect Physiol. 2018, 108, 25–30. [Google Scholar] [CrossRef]
- Andrione, M.; Vallortigara, G.; Antolini, R.; Haase, A. Neonicotinoid-induced impairment of odour coding in the honeybee. Sci. Rep. 2016, 6, 38110. [Google Scholar] [CrossRef]
- Wu, Y.Y.; Zhou, T.; Wang, Q.; Dai, P.L.; Xu, S.F.; Jia, H.R.; Wang, X. Programmed cell death in the honey bee (Apis mellifera) (Hymenoptera: Apidae) worker brain induced by imidacloprid. J. Econ. Entomol. 2015, 108, 1486–1494. [Google Scholar] [CrossRef]
- Tavares, D.A.; Roat, T.C.; Silva-Zacarin, E.C.M.; Nocelli, R.C.F.; Malaspina, O. Exposure to thiamethoxam during the larval phase affects synapsin levels in the brain of the honey bee. Ecotoxicol. Environ. Saf. 2019, 169, 523–528. [Google Scholar] [CrossRef]
- Li, Z.; Yu, T.; Chen, Y.; Heerman, M.; He, J.; Huang, J.; Nie, H.; Su, S. Brain transcriptome of honey bees (Apis mellifera) exhibiting impaired olfactory learning induced by a sublethal dose of imidacloprid. Pestic. Biochem. Physiol. 2019, 156, 36–43. [Google Scholar] [CrossRef]
- Shi, T.F.; Burton, S.; Wang, Y.F.; Xu, S.Y.; Zhang, W.X.; Yu, L.S. Metabolomic analysis of honey bee, Apis mellifera L. response to thiacloprid. Pestic. Biochem. Physiol. 2018, 152, 17–23. [Google Scholar] [CrossRef]
- Foret, S.; Maleszka, R. Function and evolution of a gene family encoding odorant binding-like proteins in a social insect, the honey bee (Apis mellifera). Genome Res. 2006, 16, 1404–1413. [Google Scholar] [CrossRef] [PubMed]
- Manjon, C.; Troczka, B.J.; Zaworra, M.; Beadle, K.; Randall, E.; Hertlein, G.; Singh, K.S.; Zimmer, C.T.; Homem, R.A.; Lueke, B.; et al. Unravelling the molecular determinants of bee sensitivity to neonicotinoid insecticides. Curr. Biol. 2018, 28, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Tsvetkov, N.; Bahia, S.; Calla, B.; Berenbaum, M.R.; Zayed, A. Genetics of tolerance in honeybees to the neonicotinoid clothianidin. iScience 2023, 26, 106084. [Google Scholar] [CrossRef] [PubMed]
- Fiala, A.; Muller, U.; Menzel, R. Reversible downregulation of protein kinase a during olfactory learning using antisense technique impairs long-term memory formation in the honeybee, Apis mellifera. J. Neurosci. 1999, 19, 10125–10134. [Google Scholar] [CrossRef]
- Finley, J. Facilitation of hippocampal long-term potentiation and reactivation of latent HIV-1 via AMPK activation: Common mechanism of action linking learning, memory, and the potential eradication of HIV-1. Med. Hypotheses 2018, 116, 61–73. [Google Scholar] [CrossRef]
- Christen, V.; Mittner, F.; Fent, K. Molecular effects of neonicotinoids in honey bees (Apis mellifera). Environ. Sci. Technol. 2016, 50, 4071–4081. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Li, A.; Yin, L.; Ke, L.; Dai, P.; Liu, Y.-J. Early-Life Sublethal Thiacloprid Exposure to Honey Bee Larvae: Enduring Effects on Adult Bee Cognitive Abilities. Toxics 2024, 12, 18. https://doi.org/10.3390/toxics12010018
Chen X, Li A, Yin L, Ke L, Dai P, Liu Y-J. Early-Life Sublethal Thiacloprid Exposure to Honey Bee Larvae: Enduring Effects on Adult Bee Cognitive Abilities. Toxics. 2024; 12(1):18. https://doi.org/10.3390/toxics12010018
Chicago/Turabian StyleChen, Xiasang, Airui Li, Linghong Yin, Li Ke, Pingli Dai, and Yong-Jun Liu. 2024. "Early-Life Sublethal Thiacloprid Exposure to Honey Bee Larvae: Enduring Effects on Adult Bee Cognitive Abilities" Toxics 12, no. 1: 18. https://doi.org/10.3390/toxics12010018
APA StyleChen, X., Li, A., Yin, L., Ke, L., Dai, P., & Liu, Y.-J. (2024). Early-Life Sublethal Thiacloprid Exposure to Honey Bee Larvae: Enduring Effects on Adult Bee Cognitive Abilities. Toxics, 12(1), 18. https://doi.org/10.3390/toxics12010018








