Lactic Acid Salts of Nicotine Potentiate the Transfer of Toxic Metals into Electronic Cigarette Aerosols
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Aerosol Collection and Preparation for Analysis
2.3. Sample Analysis by ICP-MS
2.4. Microscopy
3. Results
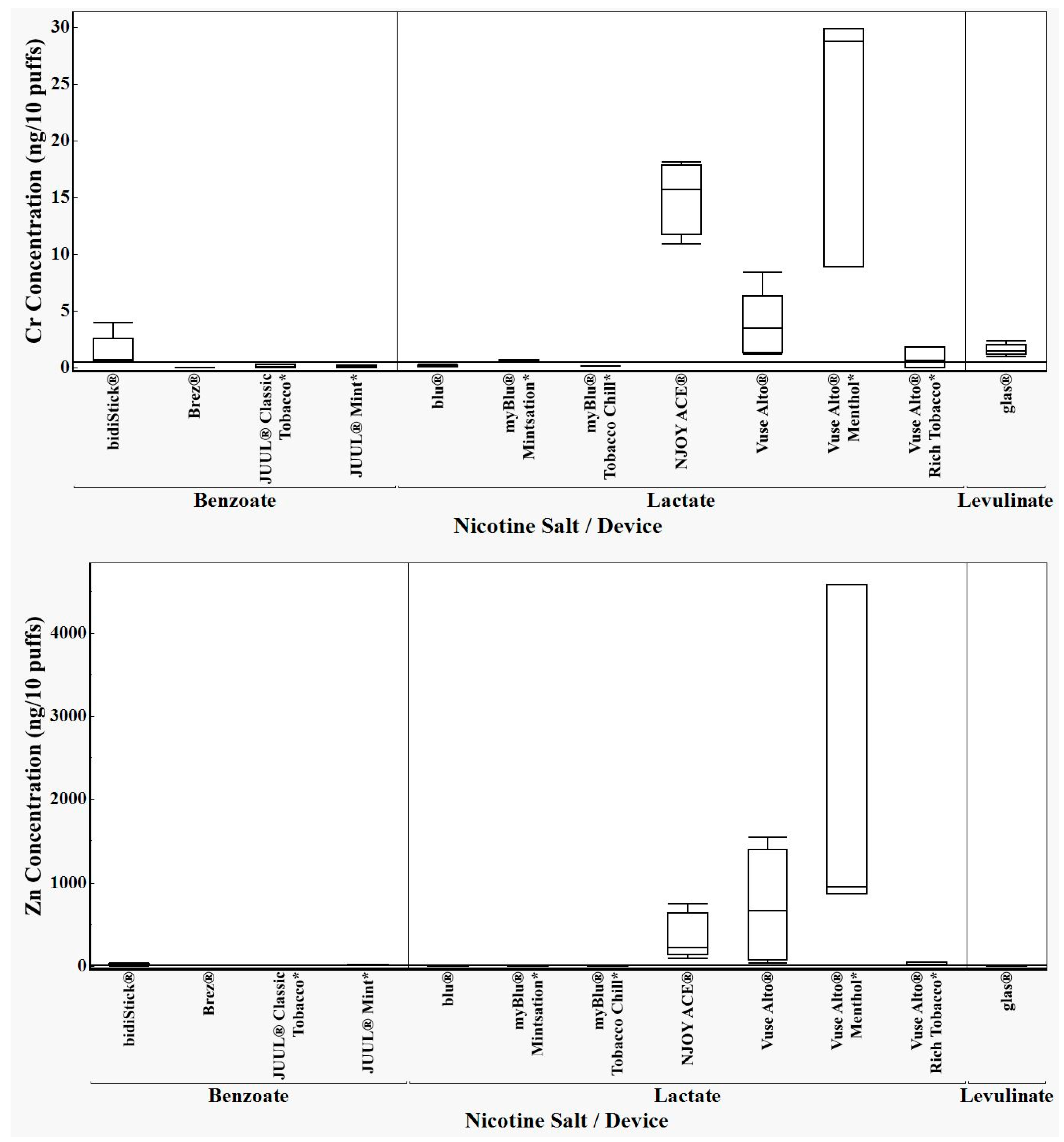
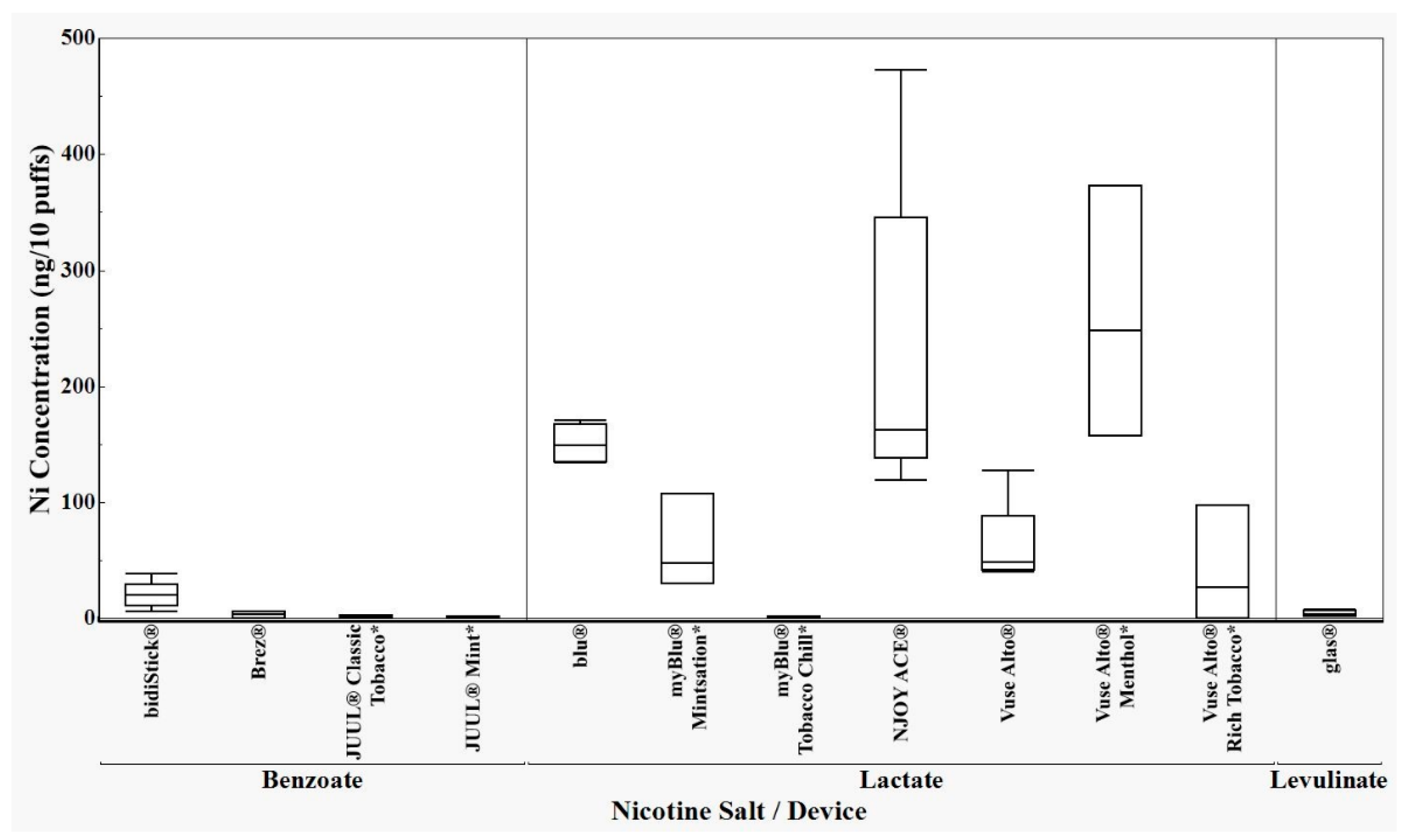
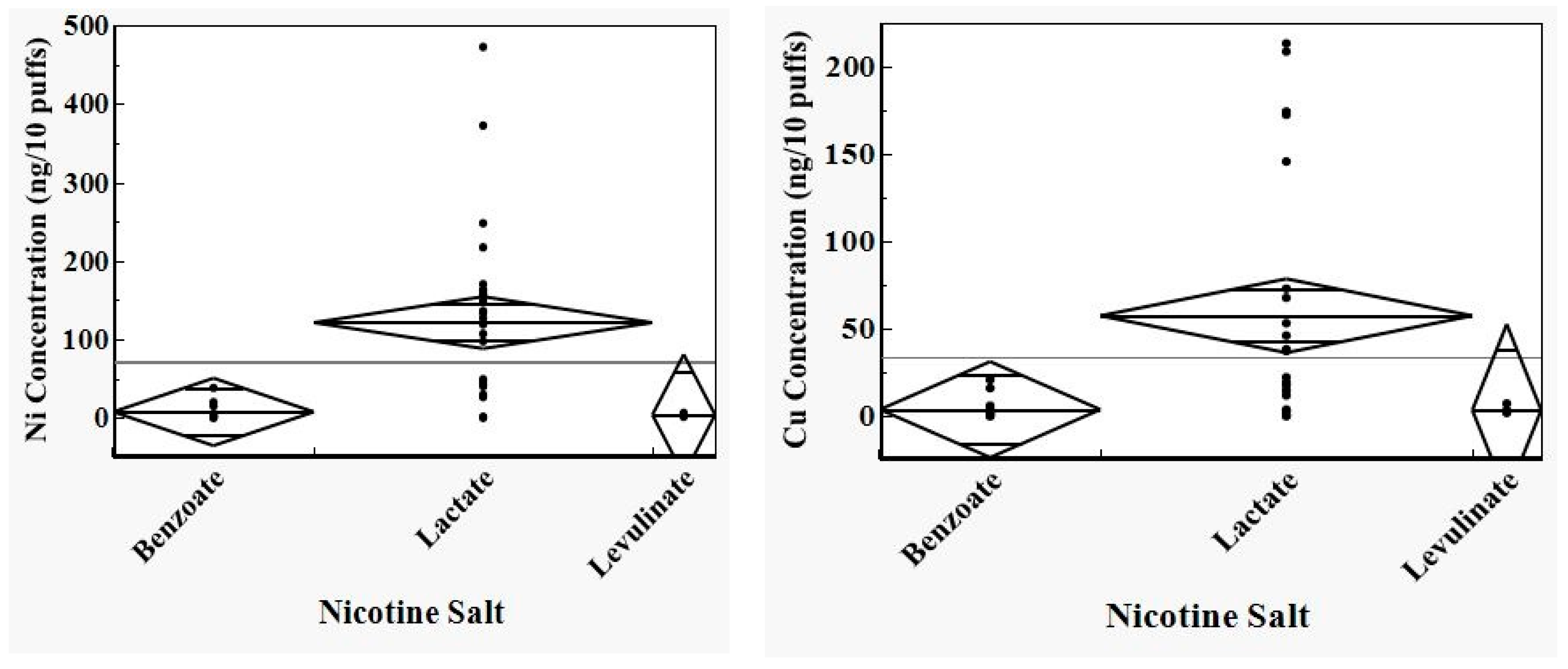
3.1. ICP-MS Results
3.2. SEM-EDS Results
3.3. Analysis of ICP-MS Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Disclaimers
References
- Farsalinos, K.E.; Spyrou, A.; Tsimopoulou, K.; Stefopoulos, C.; Romagna, G.; Voudris, V. Nicotine absorption from electronic cigarette use: Comparison between first and new-generation devices. Sci. Rep. 2014, 4, 4133. [Google Scholar] [CrossRef] [PubMed]
- Noël, A.; Verret, C.M.; Hasan, F.; Lomnicki, S.; Morse, J.; Robichaud, A.; Penn, A.L. Generation of electronic cigarette aerosol by a third-generation machine-vaping device: Application to toxicological studies. J. Vis. Exp. 2018, 25, 58095. [Google Scholar] [CrossRef]
- Gray, N.; Halstead, M.; Valentin-Blasini, L.; Watson, C.; Pappas, R.S. Toxic metals in liquid and aerosol from pod-type electronic cigarettes. J. Anal. Toxicol. 2021, 45, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Burch, S.G.; Gann, L.P.; Olsen, K.M.; Anderson, P.J.; Hiller, F.C.; Erbland, M.L. Effect of pH on nicotine absorption and side effects produced by aerosolized nicotine. J. Aerosol Med. 1993, 6, 45–52. [Google Scholar]
- Duell, A.K.; Pankow, J.F.; Peyton, D.H. Nicotine in tobacco product aerosols: ‘It’s déjà vu all over again’. Tob. Control 2019, 29, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Gray, N.; Halstead, M.; Gonzalez-Jimenez, N.; Valentin-Blasini, L.; Watson, C.; Pappas, R.S. Analysis of toxic metals in liquid from electronic cigarettes. Int. J. Environ. Res. Pub. Health 2019, 16, 4450. [Google Scholar] [CrossRef] [PubMed]
- Halstead, M.; Gray, N.; Gonzalez-Jimenez, N.; Fresquez, M.; Valentin-Blasini, L.; Watson, C.; Pappas, R.S. Analysis of toxic metals in electronic cigarette aerosols using a novel trap design. J. Anal. Toxicol. 2020, 44, 149–155. [Google Scholar] [CrossRef] [PubMed]
- ISO Standard 20768; Vapour Products—Routine Analytical Vaping Machine—Definitions and Standard Conditions. International Standards Organization: Geneva, Switzerland, 2018.
- Gray, N.; Pappas, R.S. Desolvating introduction system temperature optimization for linear zinc, cadmium, and tin calibrations with triple quad icp-ms for e-cigarette aerosol analysis. Abstract. In Proceedings of the 76th Tobacco Science Research Conference, Norfolk, VA, USA, 24 September 2023; Available online: www.tsrcinfo.com (accessed on 21 November 2023).
- Caudill, S.P.; Schleicher, R.L.; Pirkle, J.L. Multi-rule quality control for the age-related eye disease study. Stat. Med. 2008, 27, 4094–4106. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Jimenez, N.; Gray, N.; Pappas, R.S.; Halstead, M.; Lewis, E.; Valentin-Blasini, L.; Watson, C.; Blount, B. Analysis of toxic metals in aerosols from devices associated with electronic cigarette, or vaping, product use associated lung injury. Toxics 2021, 9, 240. [Google Scholar] [CrossRef] [PubMed]
- Saffari, A.; Daher, N.; Ruprecht, A.; De Marco, C.; Pozzi, P.; Boffi, R.; Hamad, S.H.; Shafer, M.M.; Schauer, J.J.; Westerdahle, D.; et al. Particulate metals and organic compounds from electronic and tobacco-containing cigarettes: Comparison of emission rates and secondhand exposure. Environ. Sci. Processes Impacts 2014, 16, 2259–2267. [Google Scholar] [CrossRef] [PubMed]
- Goniewicz, M.L.; Knysak, J.; Gawron, M.; Kosmider, L.; Sobczak, A.; Kurek, J.; Prokopowicz, A.; Jablonska-Czapla, M.; Rosik-Dulewska, C.; Havel, C.; et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob. Control 2014, 23, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.; Li, J.; Talbot, P. Effects of model, method of collection, and topography on chemical elements and metals in the aerosol of tank-style electronic cigarettes. Sci. Rep. 2019, 9, 13969. [Google Scholar] [CrossRef] [PubMed]
- Crook, P. A Guide to the Metallurgical, Corrosion, and Wear Characteristics of the Wrought Nickel and Cobalt Alloys. Haynes International, Inc. 2022. Available online: https://www.haynesintl.com/docs/default-source/pdfs/new-alloy-brochures/a-guide-to-the-metallurgical-corrosion-and-wear-characteristics.pdf?sfvrsn=413606d4_8 (accessed on 25 October 2023).
- Harvanko, A.M.; Havel, C.M.; Jacob, P.; Benowitz, N.L. Characterization of nicotine salts in 23 electronic cigarette refill liquids. Nicotine Tob. Res. 2020, 22, 1239–1243. [Google Scholar] [CrossRef] [PubMed]
- Weast, R.C.; Astle, M.J.; Beyer, W.H. (Eds.) CRC Handbook of Chemistry and Physics; CRC Press, Inc.: Boca Raton, FL, USA, 1984; pp. D-165–D-166. [Google Scholar]
- Counts, M.E.; Hsu, F.S.; Laffoon, S.W.; Dwyer, R.W.; Cox, R.H. Mainstream smoke constituent yields and predicting relationships from a worldwide market sample of cigarette brands: ISO smoking conditions. Reg. Toxicol. Pharmacol. 2004, 39, 111–134. [Google Scholar] [CrossRef] [PubMed]
- Pappas, R.S.; Fresquez, M.R.; Martone, N.; Watson, C.H. Toxic metal concentrations in mainstream smoke from cigarettes available in the USA. J. Anal. Toxicol. 2014, 38, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Pappas, R.S.; Gray, N.; Halstead, M.; Valentin-Blasini, L.; Watson, C. Toxic metal-containing particles in aerosols from pod-type electronic cigarettes. J. Anal. Toxicol. 2021, 45, 337–347. [Google Scholar] [CrossRef] [PubMed]
- U.S. Environmental Protection Agency. Nickel Compounds. National Center for Environmental Assessment; Office of Research and Development: Washington, DC, USA, 2000. Available online: https://www.epa.gov/sites/default/files/2016-09/documents/nickle-compounds.pdf (accessed on 19 October 2023).
- International Agency for Research on Cancer. Nickel and Nickel Compounds, Monograph 100C; International Agency for Research on Cancer: Geneva, Switzerland, 2018. [Google Scholar]
- Agency for Toxic Substances Disease Registry. Toxicological Profile for Nickel. Agency for Toxic Substances and Disease Registry; Agency for Toxic Substances Disease Registry: Atlanta, GA, USA, 2023; p. 11. Available online: https://www.atsdr.cdc.gov/ToxProfiles/tp15.pdf (accessed on 12 January 2024).
- FEELM. NJOY Ace Becomes the First FDA-Authorized Closed Pod Vape Equipped with FEELM Tech. Available online: https://www.feelmtech.com/index.php/OfficialReports/44.html (accessed on 1 September 2023).
- Business Wire. Bluehole Publishes an Industry Comment on Vuse Overtaking Juul and Becoming the U.S Vaping Market Champion Again. Available online: https://www.businesswire.com/news/home/20220512005478/en/Bluehole-Publishes-an-Industry-Comment-on-Vuse-Overtaking-Juul-and-Becoming-the-U.S-Vaping-Market-Champion-Again#:~:text=Equipped%20with%20an%20industry-leading%20ceramic%20coil%2C%20Vuse%20Alto,a%20full-bodied%2C%20rich%20taste%20of%20tobacco%20and%20menthol (accessed on 1 September 2023).
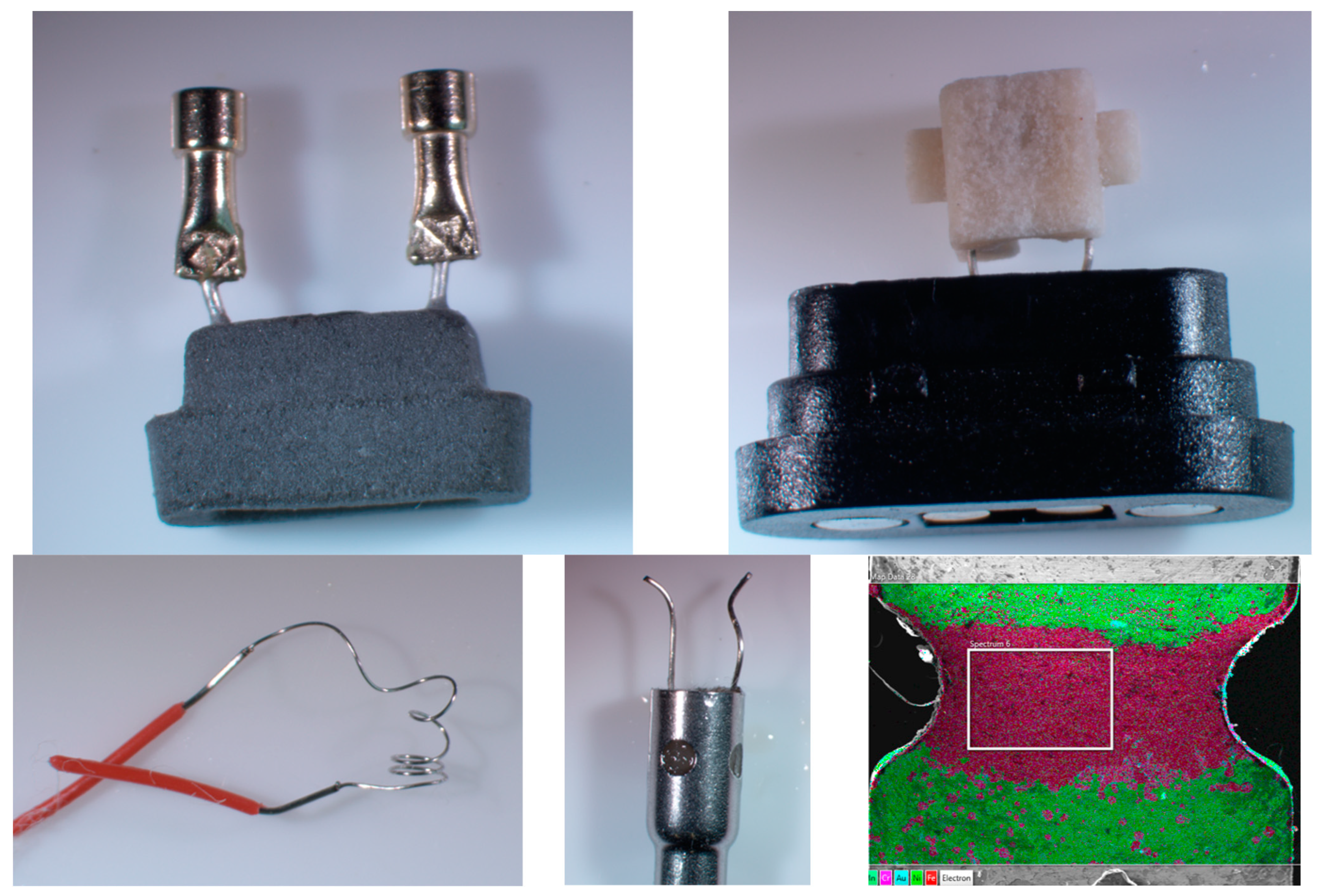
| Cr (ng/10 Puffs) | Ni (ng/10 Puffs) | Cu (ng/10 Puffs) | Zn (ng/10 Puffs) | Cd (ng/10 Puffs) | Sn (ng/10 Puffs) | Pb (ng/10 Puffs) | |
|---|---|---|---|---|---|---|---|
| LOD 1 | 0.125 | 0.250 | 0.200 | 5.00 | 0.050 | 0.100 | 0.050 |
| LRL 2 | 0.500 | 0.500 | 1.00 | 10.0 | 0.200 | 0.200 | 0.500 |
| LACTIC ACID | |||||||
| 2022 NJOY ACE® Classic Tobacco, 5% nicotine pod 3 | 5.35 ± 1.22 | 265 ± 182 | 51.4 ± 24.1 | 279 ± 114 | <LOD | 1.19 ± 0.73 | <LRL |
| 2023 NJOY ACE® Classic Tobacco 5% nicotine pod 4 | 15.0 ± 3.1 | 226 ± 142 | 23.9 ± 28.6 | 353 ± 272 | <LOD | 1.58 ± 1.94 | <LRL |
| 2023 Vuse Alto® Rich Tobacco 5% nicotine pod 4 | 3.75 ± 2.91 | 61.8 ± 36.8 | 104 ± 85 | 719 ± 672 | <LOD | 3.06 ± 2.86 | <LRL |
| 2023 blu® Rich Tobacco 3.6% nicotine pod 4 | <LRL | 151 ± 16 | <LRL | <LOD | <LOD | <LRL | <LOD |
| BENZOIC ACID | |||||||
| 2022 bidiStick® Gold Fresh Mango 6% nicotine disposable 3 | 0.609 ± 0.360 | 18.3 ± 6.1 | 4.57 ± 8.26 | 13.8 ± 8.7 | <LOD | 1.27 ± 1.40 | <LRL |
| 2023 bidiStick® Gold Fresh Mango 6% nicotine disposable 4 | 1.38 ± 1.48 | 20.4 ± 11.8 | <LRL | 16.3 ± 14.6 | <LOD | 5.59 ± 6.47 | <LRL |
| 2023 Brez® Tobacco 5% Nicotine pod 4 | <LOD | 3.58 ± 2.74 | 6.12 ± 8.46 | <LRL | <LOD | <LOD | 1.75 ± 3.48 |
| LEVULINIC ACID | |||||||
| 2023 glas® Signature Tobacco 5% nicotine pod 4 | 1.56 ± 0.52 | 4.38 ± 2.63 | 3.37 ± 2.19 | <LOD | <LOD | <LOD | <LRL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pappas, R.S.; Gray, N.; Halstead, M.; Watson, C.H. Lactic Acid Salts of Nicotine Potentiate the Transfer of Toxic Metals into Electronic Cigarette Aerosols. Toxics 2024, 12, 65. https://doi.org/10.3390/toxics12010065
Pappas RS, Gray N, Halstead M, Watson CH. Lactic Acid Salts of Nicotine Potentiate the Transfer of Toxic Metals into Electronic Cigarette Aerosols. Toxics. 2024; 12(1):65. https://doi.org/10.3390/toxics12010065
Chicago/Turabian StylePappas, R. Steven, Naudia Gray, Mary Halstead, and Clifford H. Watson. 2024. "Lactic Acid Salts of Nicotine Potentiate the Transfer of Toxic Metals into Electronic Cigarette Aerosols" Toxics 12, no. 1: 65. https://doi.org/10.3390/toxics12010065
APA StylePappas, R. S., Gray, N., Halstead, M., & Watson, C. H. (2024). Lactic Acid Salts of Nicotine Potentiate the Transfer of Toxic Metals into Electronic Cigarette Aerosols. Toxics, 12(1), 65. https://doi.org/10.3390/toxics12010065








