Occurrence and Distribution of Organophosphate Flame Retardants in Tap Water System—Implications for Human Exposure from Shanghai, China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Sample Collection
2.3. Analytical Methods
2.4. Quality Control and Quantification
2.5. Data Handing and Analysis
2.6. Exposure and Risk Assessment Methods
3. Results and Discussion
3.1. Concentrations of OPFRs
3.1.1. Concentrations of the OPFRs in the before- and after-Treatment Plant Water
3.1.2. Concentrations of OPFRs in Tap Water
3.2. Compositional Profiles of OPFR Compounds
3.3. Exposure Assessment of the OPFRs through Tap Water Consumption
3.4. Risk Assessment of OPFRs through Tap Water Consumption
3.5. Risk Management
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ali, N.; Shahzad, K.; Rashid, M.I.; Shen, H.; Ismail, I.; Eqani, S. Currently used organophosphate and brominated flame retardants in the environment of China and other developing countries (2000–2016). Environ. Sci. Pollut. Res. Int. 2017, 24, 18721–18741. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Bi, Y.; Fu, M.; Zhang, X.; Lei, B.; Huang, X.; Zhao, Z. Organophosphate tri- and diesters in source water supply and drinking water treatment systems of a metropolitan city in China. Environ. Geochem. Health 2023, 45, 2401–2414. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.H.; Ma, W.L. A review on the occurrence of organophosphate flame retardants in the aquatic environment in China and implications for risk assessment. Sci. Total Environ. 2021, 783, 147064. [Google Scholar] [CrossRef] [PubMed]
- Kim, U.J.; Kannan, K. Occurrence and Distribution of Organophosphate Flame Retardants/Plasticizers in Surface Waters, Tap Water, and Rainwater: Implications for Human Exposure. Environ. Sci. Technol. 2018, 52, 5625–5633. [Google Scholar] [CrossRef]
- Stepien, D.K.; Regnery, J.; Merz, C.; Puttmann, W. Behavior of organophosphates and hydrophilic ethers during bank filtration and their potential application as organic tracers. A field study from the Oderbruch, Germany. Sci. Total Environ. 2013, 458–460, 150–159. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Y.; Yang, C.; Meng, X.Z.; Zheng, H.; Gao, Y.; Cai, M. Application of Hi-throat/Hi-volume SPE technique in analyzing occurrence, influencing factors and human health risk of organophosphate esters (OPEs) in drinking water of China. J. Environ. Manage. 2021, 291, 112714. [Google Scholar] [CrossRef]
- Li, J.; Yu, N.; Zhang, B.; Jin, L.; Li, M.; Hu, M.; Zhang, X.; Wei, S.; Yu, H. Occurrence of organophosphate flame retardants in drinking water from China. Water Res. 2014, 54, 53–61. [Google Scholar] [CrossRef]
- Ding, J.; Shen, X.; Liu, W.; Covaci, A.; Yang, F. Occurrence and risk assessment of organophosphate esters in drinking water from Eastern China. Sci. Total Environ. 2015, 538, 959–965. [Google Scholar] [CrossRef]
- Liu, Y.; Song, N.; Guo, R.; Xu, H.; Zhang, Q.; Han, Z.; Feng, M.; Li, D.; Zhang, S.; Chen, J. Occurrence and partitioning behavior of organophosphate esters in surface water and sediment of a shallow Chinese freshwater lake (Taihu Lake): Implication for eco-toxicity risk. Chemosphere 2018, 202, 255–263. [Google Scholar] [CrossRef]
- Zhang, Z.; Shao, H.; Wu, M.; Zhang, J.; Li, D.; Li, J.; Wang, H.; Shi, W.; Xu, G. Occurrence, Distribution, and Potential Sources of Organophosphate Esters in Urban and Rural Surface Water in Shanghai, China. Arch. Environ. Contam. Toxicol. 2019, 77, 115–126. [Google Scholar] [CrossRef]
- GB/T 5750.2-2006; The Standard Examination Methods for Drinking Water—Part 2: Collection and Preservation of Water Samples. SAC: Beijing, China, 2006. Available online: http://www.nhc.gov.cn/cmsresources/zwgkzt/wsbz/new/20070628143525.pdf (accessed on 18 September 2024).
- MEPC. Exposure Factors Handbook of Chinese Population for Shanghai Adult Residents; China Environmental Science Press: Beijing, China, 2013. [Google Scholar]
- MEPC. Exposure Factors Handbook of Chinese Population for Shanghai Residents (0–5 Years Old); China Environmental Science Press: Beijing, China, 2016. [Google Scholar]
- MEPC. Exposure Factors Handbook of Chinese Population for Shanghai Residents (6–17 Years Old); China Environmental Science Press: Beijing, China, 2016. [Google Scholar]
- USEPA. Mid-Atlantic risk assessment: Washington, D.C, 2015. Available online: https://archive.epa.gov/region9/superfund/web/html/whatsnew.html (accessed on 18 September 2024).
- Li, J.; He, J.; Li, Y.; Liu, Y.; Li, W.; Wu, N.; Zhang, L.; Zhang, Y.; Niu, Z. Assessing the threats of organophosphate esters (flame retardants and plasticizers) to drinking water safety based on USEPA oral reference dose (RfD) and oral cancer slope factor (SFO). Water Res. 2019, 154, 84–93. [Google Scholar] [CrossRef] [PubMed]
- USEPA. Mid Atlantic Risk Assessment, Regional Screening Levels (RSLs)—Generic Tables: Washington, D.C, 2017. Available online: http://www.epa.gov/region9/superfund/prg (accessed on 10 September 2024).
- Zhang, Q.; Li, J.; Lin, S.; Ying, Z.; Hu, S.; Wang, Y.; Mo, X. Organophosphate flame retardants in Hangzhou tap water system: Occurrence, distribution, and exposure risk assessment. Sci. Total Environ. 2022, 849, 157644. [Google Scholar] [CrossRef] [PubMed]
- Bollmann, U.E.; Moller, A.; Xie, Z.; Ebinghaus, R.; Einax, J.W. Occurrence and fate of organophosphorus flame retardants and plasticizers in coastal and marine surface waters. Water Res. 2012, 46, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; He, Y.; Lin, L.; Zeng, F.; Luan, T. Application of fully automatic hollow fiber liquid phase microextraction to assess the distribution of organophosphate esters in the Pearl River Estuaries. Sci. Total Environ. 2014, 470–471, 263–269. [Google Scholar] [CrossRef]
- Moller, A.; Xie, Z.; Caba, A.; Sturm, R.; Ebinghaus, R. Organophosphorus flame retardants and plasticizers in the atmosphere of the North Sea. Environ. Pollut. 2011, 159, 3660–3665. [Google Scholar] [CrossRef]
- Han, X.; Li, W.; Zhao, Y.; Zhuang, Y.; Jia, Q.; Guan, H.; Liu, J.; Wu, C. Organophosphate Esters in Building Materials from China: Levels, Sources, Emissions, and Preliminary Assessment of Human Exposure. Environ. Sci. Technol. 2024, 58, 2434–2445. [Google Scholar] [CrossRef]
- Andresen, J.; Bester, K. Elimination of organophosphate ester flame retardants and plasticizers in drinking water purification. Water Res. 2006, 40, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Choo, G.; Oh, J.E. Seasonal occurrence and removal of organophosphate esters in conventional and advanced drinking water treatment plants. Water Res. 2020, 186, 116359. [Google Scholar] [CrossRef]
- Lee, S.; Jeong, W.; Kannan, K.; Moon, H.B. Occurrence and exposure assessment of organophosphate flame retardants (OPFRs) through the consumption of drinking water in Korea. Water Res. 2016, 103, 182–188. [Google Scholar] [CrossRef]
- Rodil, R.; Quintana, J.B.; Concha-Grana, E.; Lopez-Mahia, P.; Muniategui-Lorenzo, S.; Prada-Rodriguez, D. Emerging pollutants in sewage, surface and drinking water in Galicia (NW Spain). Chemosphere 2012, 86, 1040–1049. [Google Scholar] [CrossRef]
- Benotti, M.J.; Trenholm, R.A.; Vanderford, B.J.; Holady, J.C.; Stanford, B.D.; Snyder, S.A. Pharmaceuticals and endocrine disrupting compounds in U.S. drinking water. Environ. Sci. Technol. 2009, 43, 597–603. [Google Scholar] [CrossRef]
- Bach, C.; Dauchy, X.; Chagnon, M.C.; Etienne, S. Chemical compounds and toxicological assessments of drinking water stored in polyethylene terephthalate (PET) bottles: A source of controversy reviewed. Water Res. 2012, 46, 571–583. [Google Scholar] [CrossRef] [PubMed]
- Sundkvist, A.M.; Olofsson, U.; Haglund, P. Organophosphorus flame retardants and plasticizers in marine and fresh water biota and in human milk. J. Environ. Monit. 2010, 12, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Wu, R.; Ji, H.; Chen, X.; Xu, J.; Chen, Y.; Sun, M.; Pan, Y.; Zhou, L. Drinking water behavior and willingness to use filters by middle-aged and elderly residents in rural areas: A cross-sectional study in Tengchong, China. Front. Public. Health 2022, 10, 961870. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, Y.; Asimakopoulos, A.G.; Covaci, A.; Gevao, B.; Johnson-Restrepo, B.; Kumosani, T.A.; Malarvannan, G.; Moon, H.B.; Nakata, H.; et al. Organophosphate esters in indoor dust from 12 countries: Concentrations, composition profiles, and human exposure. Environ. Int. 2019, 133, 105178. [Google Scholar] [CrossRef]
- Ma, Y.; Xie, Z.; Lohmann, R.; Mi, W.; Gao, G. Organophosphate Ester Flame Retardants and Plasticizers in Ocean Sediments from the North Pacific to the Arctic Ocean. Environ. Sci. Technol. 2017, 51, 3809–3815. [Google Scholar] [CrossRef]
- He, J.; Wang, Z.; Zhao, L.; Ma, H.; Huang, J.; Li, H.; Mao, X.; Huang, T.; Gao, H.; Ma, J. Gridded emission inventory of organophosphorus flame retardants in China and inventory validation. Environ. Pollut. 2021, 290, 118071. [Google Scholar] [CrossRef]
- Hou, H.; Zeng, X.; Zhang, X. Production of Hydrogen Peroxide by Photocatalytic Processes. Angew. Chem. Int. Ed. Engl. 2020, 59, 17356–17376. [Google Scholar] [CrossRef]
- Orta, D.V.M.; Yanez-Noguez, I.; Jimenez-Cisneros, B.; Luna, P.V. Adding silver and copper to hydrogen peroxide and peracetic acid in the disinfection of an advanced primary treatment effluent. Environ. Technol. 2008, 29, 1209–1217. [Google Scholar] [CrossRef]
- He, M.J.; Lu, J.F.; Wei, S.Q. Organophosphate esters in biota, water, and air from an agricultural area of Chongqing, western China: Concentrations, composition profiles, partition and human exposure. Environ. Pollut. 2019, 244, 388–397. [Google Scholar] [CrossRef]
- Wu, M.; Yu, G.; Cao, Z.; Wu, D.; Liu, K.; Deng, S.; Huang, J.; Wang, B.; Wang, Y. Characterization and human exposure assessment of organophosphate flame retardants in indoor dust from several microenvironments of Beijing, China. Chemosphere 2016, 150, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yao, Y.; Han, X.; Li, W.; Zhu, H.; Wang, L.; Sun, H.; Kannan, K. Organophosphate di- and tri-esters in indoor and outdoor dust from China and its implications for human exposure. Sci. Total Environ. 2020, 700, 134502. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Li, J.; Zhang, B.; Cui, Q.; Wei, S.; Yu, H. Regional distribution of halogenated organophosphate flame retardants in seawater samples from three coastal cities in China. Mar. Pollut. Bull. 2014, 86, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Deng, T.; Xu, M.; Wang, S.; Yang, F. Residuals of organophosphate esters in foodstuffs and implication for human exposure. Environ. Pollut. 2018, 233, 986–991. [Google Scholar] [CrossRef]
- Zhao, L.; Jian, K.; Su, H.; Zhang, Y.; Li, J.; Letcher, R.J.; Su, G. Organophosphate esters (OPEs) in Chinese foodstuffs: Dietary intake estimation via a market basket method, and suspect screening using high-resolution mass spectrometry. Environ. Int. 2019, 128, 343–352. [Google Scholar] [CrossRef]
- Goralczyk, K.; Strucinski, P.; Hernik, A.; Czaja, K.; Korcz, W.; Minorczyk, M.; Ludwicki, J.K. Indoor dust as a pathway of human exposure to polybrominated diphenyl ethers (PBDEs). Rocz. Panstw. Zakl. Hig. 2012, 63, 1–8. [Google Scholar]
- Chupeau, Z.; Bonvallot, N.; Mercier, F.; Le Bot, B.; Chevrier, C.; Glorennec, P. Organophosphorus Flame Retardants: A Global Review of Indoor Contamination and Human Exposure in Europe and Epidemiological Evidence. Int. J. Environ. Res. Public Health 2020, 17, 6713. [Google Scholar] [CrossRef]
- Lyu, Y.; Yang, L. Environmental monitoring and enforcement in China: An economic perspective review. China Econ. J. 2024, 17, 3–25. [Google Scholar] [CrossRef]
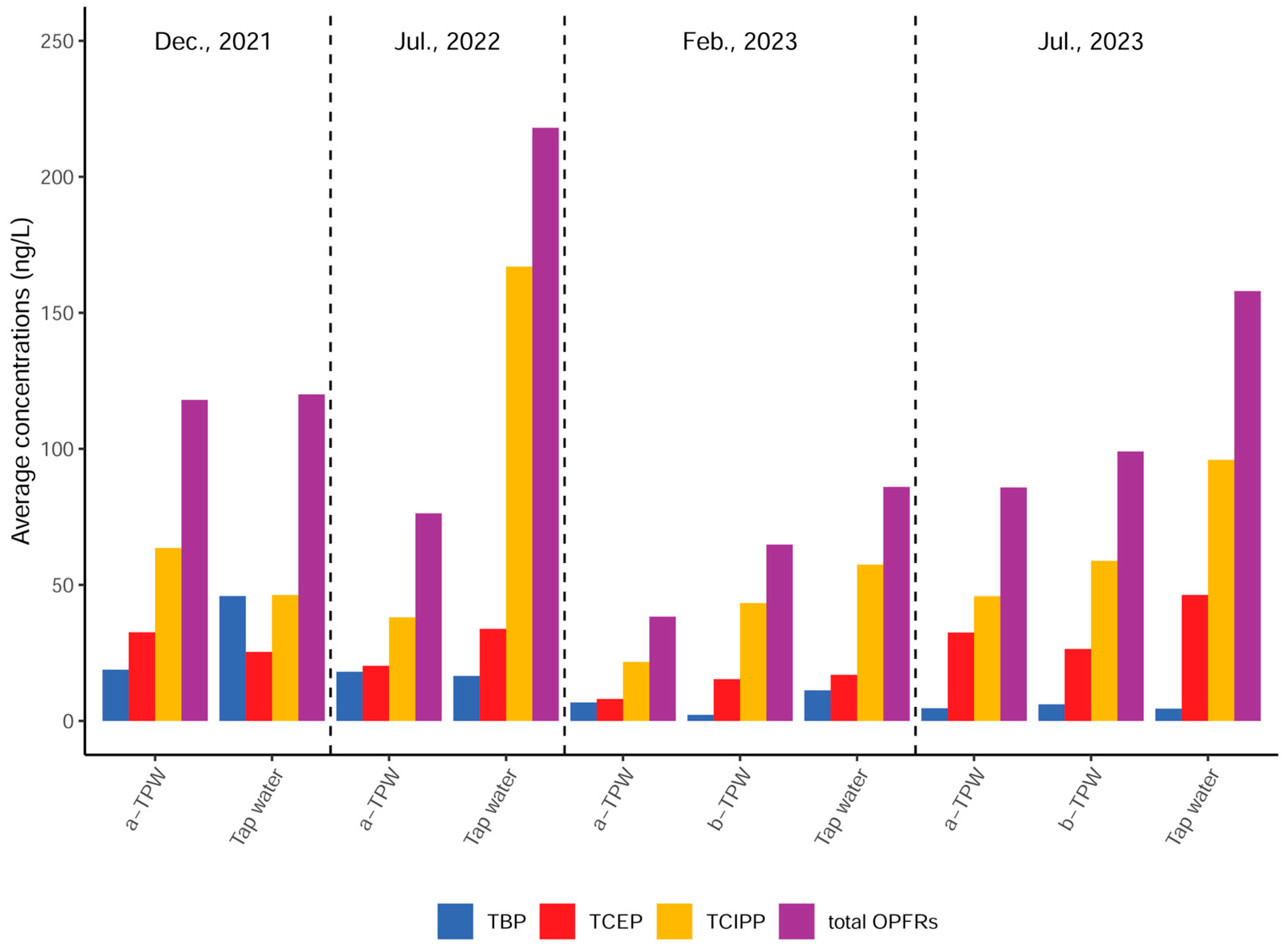

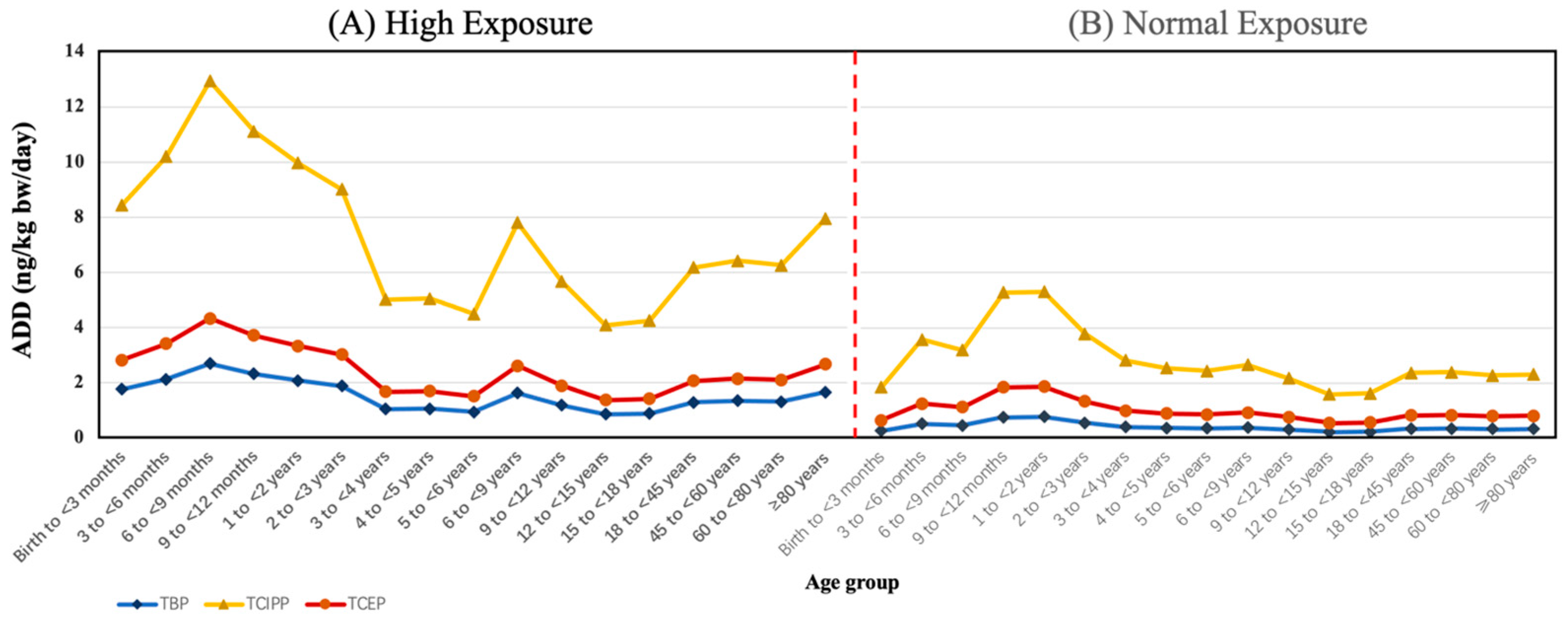
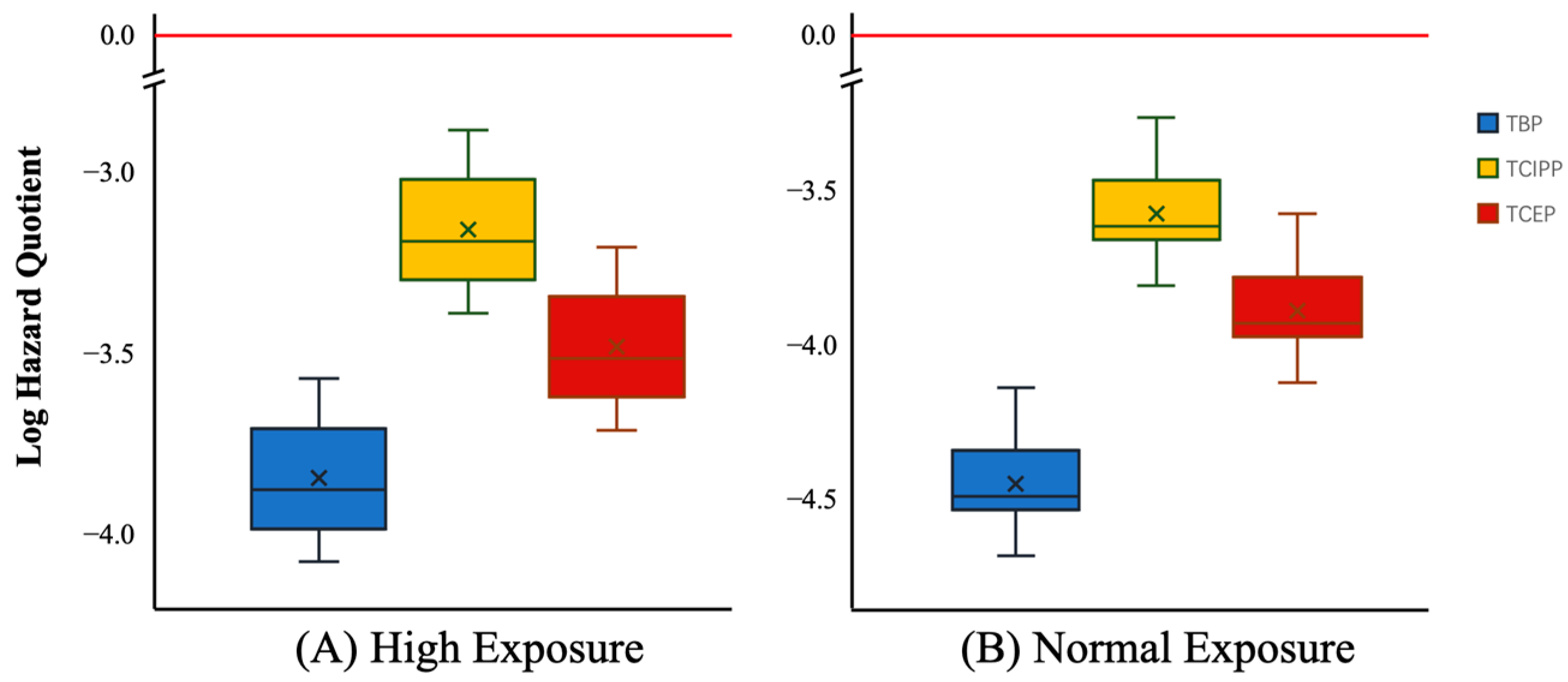
| Conc. Unit (ng/L) | TBP | TCIPP | TCEP | TEP | TPrP | TPhP | TBEP | TDBPP | TDCPP | TCP | ∑10OPFRs | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2021.12 | MIN | 8.96 | 20.9 | <LOQ | - | - | - | <LOQ | - | - | - | 53.0 |
| (Dry season) | MAX | 162 | 81.4 | 49.6 | - | - | - | 12.5 | - | - | - | 251 |
| n = 15 | MEAN | 45.9 | 46.3 | 25.3 | - | - | - | 2.03 | - | - | - | 120 |
| MEDIAN | 21.1 | 42.7 | 24.5 | - | - | - | - | - | - | - | 95.0 | |
| D.F. | 100% | 100% | 93% | 0% | 0% | 0% | 20% | 0% | 0% | 0% | 100% | |
| 2022.07 | MIN | <LOQ | 66.1 | 17.0 | - | - | - | - | - | - | - | 83.0 |
| (Wet season) | MAX | 31.3 | 348 | 61.0 | - | - | - | - | - | - | - | 425 |
| n = 15 | MEAN | 16.5 | 167 | 33.8 | - | - | - | - | - | - | - | 218 |
| MEDIAN | 18.1 | 173 | 33.5 | - | - | - | - | - | - | - | 223 | |
| D.F. | 93% | 100% | 100% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 100% | |
| 2023.02 | MIN | <LOQ | <LOQ | <LOQ | - | - | <LOQ | - | - | - | - | 11.0 |
| (Dry season) | MAX | 25.3 | 137 | 44.6 | - | - | 5.47 | - | - | - | - | 197 |
| n = 15 | MEAN | 11.2 | 57.4 | 16.9 | - | - | 0.36 | - | - | - | - | 86.0 |
| MEDIAN | 11.7 | 47.4 | 18.2 | - | - | - | - | - | - | - | 79.5 | |
| D.F. | 80% | 73% | 67% | 0% | 0% | 7% | 0% | 0% | 0% | 0% | 100% | |
| 2023.07 | MIN | <LOQ | 60.4 | 15.7 | - | <LOQ | <LOQ | - | - | <LOQ | <LOQ | 93.8 |
| (Wet season) | MAX | 8.47 | 154 | 100 | - | 31.1 | 31.1 | - | - | 10.7 | 16.0 | 259 |
| n = 15 | MEAN | 4.51 | 95.9 | 46.3 | - | 4.90 | 4.17 | - | - | 4.27 | 2.07 | 158 |
| MEDIAN | 3.71 | 91.8 | 44.7 | - | - | - | - | - | 5.30 | - | 153 | |
| D.F. | 93% | 100% | 100% | 0% | 25% | 20% | 0% | 0% | 60% | 33% | 100% |
| High Exposure | Normal Exposure | ||||
|---|---|---|---|---|---|
| MIN 1 | MAX 2 | MIN 3 | MAX 4 | ||
| Hazard quotient (HQ) 5 | TBP | 8.5 × 10−5 | 2.7 × 10−4 | 2.3 × 10−5 | 7.8 × 10−5 |
| TCIPP | 4.1 × 10−4 | 1.3 × 10−3 | 1.6 × 10−4 | 5.3 × 10−4 | |
| TCEP | 2.0 × 10−4 | 6.2 × 10−4 | 8.0 × 10−5 | 2.7 × 10−4 | |
| Hazard index (HI) | ∑OPFRs | 6.9 × 10−4 | 2.2 × 10−3 | 2.6 × 10−4 | 8.8 × 10−4 |
| Carcinogenic risk (CR) 6 | TBP | 7.7 × 10−9 | 2.4 × 10−8 | 2.1 × 10−9 | 7.0 × 10−9 |
| TCEP | 2.7 × 10−8 | 8.7 × 10−8 | 1.1 × 10−8 | 3.7 × 10−8 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.-S.; Zheng, L.; Zheng, W.-W.; Zheng, R.; Wang, Y.-J.; Hu, B.-Q.; Yang, M.-J.; Zhao, Y.-J. Occurrence and Distribution of Organophosphate Flame Retardants in Tap Water System—Implications for Human Exposure from Shanghai, China. Toxics 2024, 12, 696. https://doi.org/10.3390/toxics12100696
Zhu Y-S, Zheng L, Zheng W-W, Zheng R, Wang Y-J, Hu B-Q, Yang M-J, Zhao Y-J. Occurrence and Distribution of Organophosphate Flame Retardants in Tap Water System—Implications for Human Exposure from Shanghai, China. Toxics. 2024; 12(10):696. https://doi.org/10.3390/toxics12100696
Chicago/Turabian StyleZhu, Yuan-Shen, Lei Zheng, Wei-Wei Zheng, Rong Zheng, Ya-Juan Wang, Bing-Qing Hu, Min-Juan Yang, and Yi-Jing Zhao. 2024. "Occurrence and Distribution of Organophosphate Flame Retardants in Tap Water System—Implications for Human Exposure from Shanghai, China" Toxics 12, no. 10: 696. https://doi.org/10.3390/toxics12100696








