Annual and Seasonal Variability of Trichloromethane in Drinking Water of Kunshan City 2016–2022 and Associated Health Risks
Abstract
:1. Introduction
2. Materials and Methods
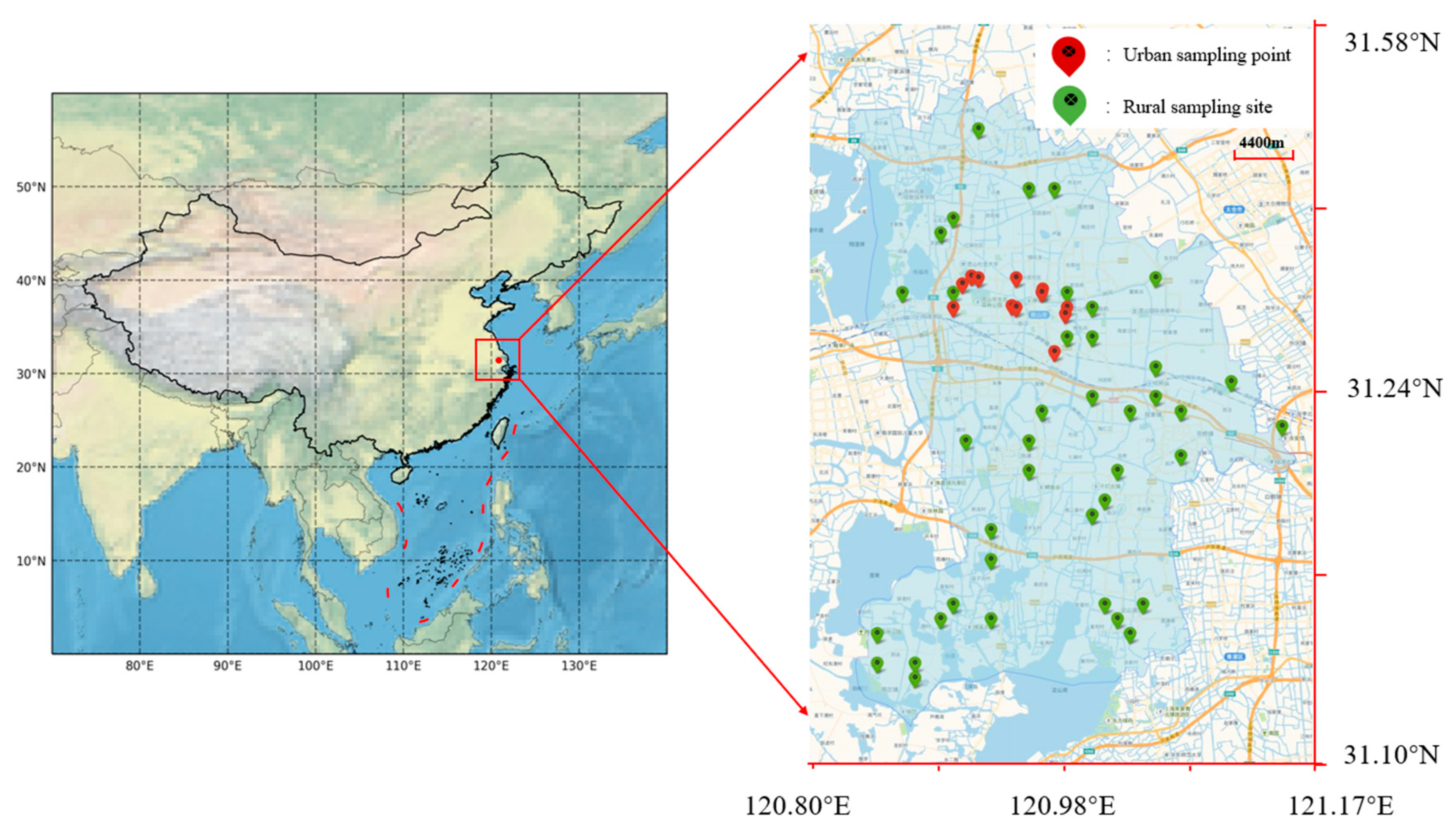
2.1. Water Plant Status and Sample Collection
2.2. Methods
2.2.1. Detection Method
2.2.2. Analysis Method
3. Results and Discussion
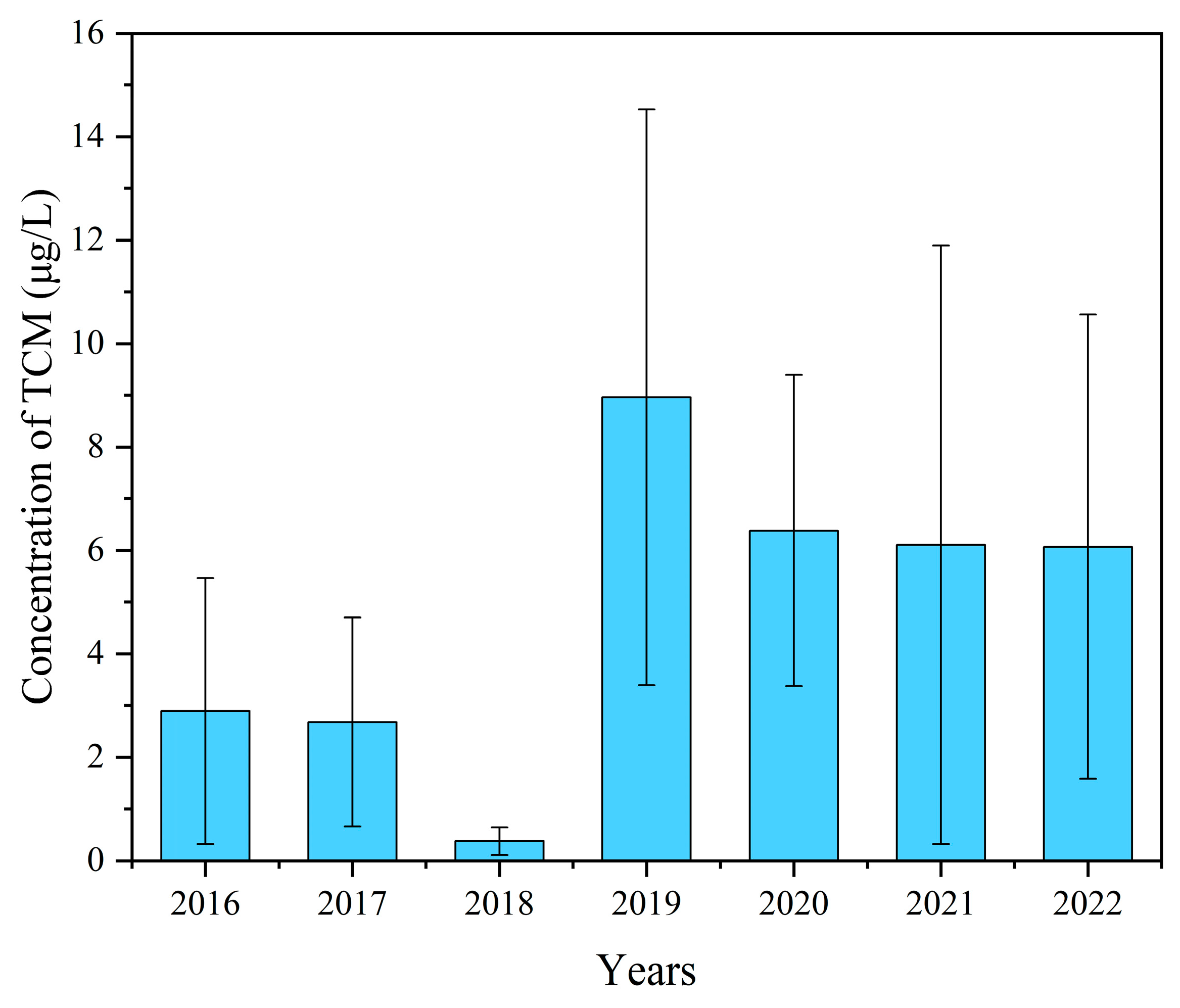
3.1. Annual Variation Characteristics of Trichloromethane
3.2. Seasonal Variation Characteristics of Trichloromethane
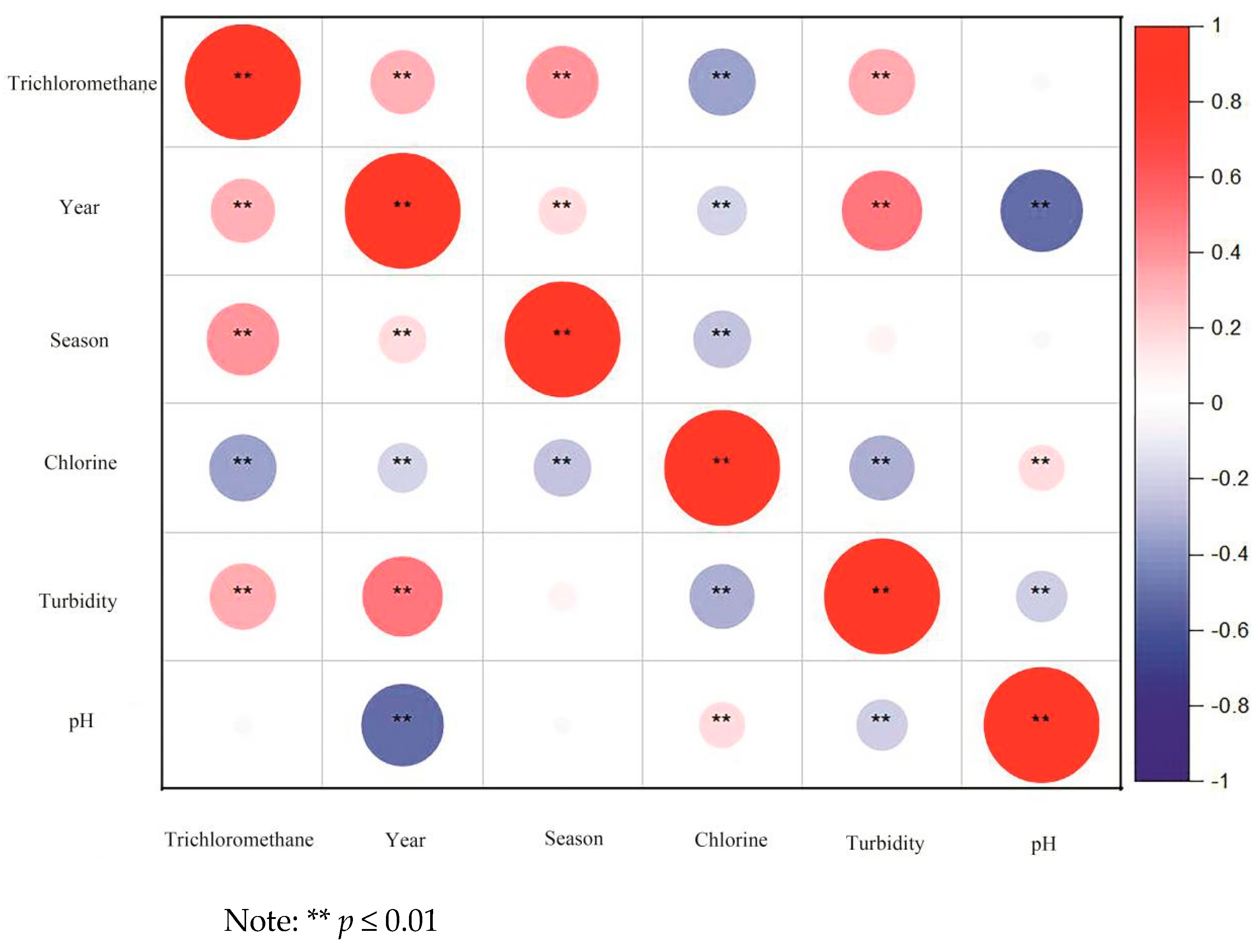
3.3. Influence of Water Components on Trichloromethane Content
3.3.1. Chloride
3.3.2. Turbidity
3.3.3. pH
3.4. Health Risk Assessment of Chloroform in Drinking Wate
3.4.1. Hazard Identification
3.4.2. Risk Characterization
3.4.3. Multiple Pathway Assessment of TCM Cancer Risk
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Srivastav, A.L.; Patel, N.; Chaudhary, V.K. Disinfection by-products in drinking water: Occurrence, toxicity and abatement. Environ. Pollut. 2020, 267, 115474. [Google Scholar] [CrossRef] [PubMed]
- Krasner, S.W.; Mitch, W.A.; McCurry, D.L.; Hanigan, D.; Westerhoff, P. Formation, precursors, control, and occurrence of nitrosamines in drinking water: A review. Water Res. 2013, 47, 4433–4450. [Google Scholar] [CrossRef] [PubMed]
- Yee, L.F.; Abdullah, M.P.; Abdullah, A.; Ishak, B.; Abidin, K.N.Z. Hydrophobicity characteristics of natural organic matter and the formation of THM. Malays. J. Anal. Sci. 2009, 13, 94–99. [Google Scholar]
- Bond, T.; Templeton, M.R.; Graham, N. Precursors of nitrogenous disinfection by-products in drinking water—A critical review and analysis. J. Hazard. Mater. 2012, 235, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Hua, G.; Reckhow, D.A. Characterization of disinfection byproduct precursors based on hydrophobicity and molecular size. Environ. Sci. Technol. 2007, 41, 3309–3315. [Google Scholar] [CrossRef]
- Mishra, B.K.; Priya, T.; Gupta, S.K.; Sinha, A. Modeling and characterization of natural organic matter and its relationship with the THMs formation. Glob. Nest J 2016, 18, 803–816. [Google Scholar]
- Golea, D.M.; Upton, A.; Jarvis, P.; Moore, G.; Sutherland, S.; Parsons, S.A.; Judd, S.J. THM and HAA formation from NOM in raw and treated surface waters. Water Res. 2017, 112, 226–235. [Google Scholar] [CrossRef]
- Assefa, E.; Jabasingh, A.; Mulugeta, E.; Dessalegne, M.; Teju, E. Impact of source water quality on total organic carbon and trihalomethane removal efficiency in a water treatment plant: A case study of Upper Awash, Ethiopia. J. Water Health 2024, 22, 337–349. [Google Scholar] [CrossRef]
- Ramavandi, B.; Farjadfard, S.; Ardjmand, M.; Dobaradaran, S. Effect of water quality and operational parameters on trihalomethanes formation potential in Dez River water, Iran. Water Resour. Ind. 2015, 11, 1–12. [Google Scholar] [CrossRef]
- Omer, N.H. Water Quality Parameters. In Water Quality-Science, Assessments and Policy; Summers, J.K., Ed.; Books on Demand: Norderstedt, Germany, 2019; pp. 1–34. [Google Scholar]
- Hong, H.; Xiong, Y.; Ruan, M.; Liao, F.; Lin, H.; Liang, Y. Factors affecting THMs, HAAs and HNMs formation of Jin Lan Reservoir water exposed to chlorine and monochloramine. Sci. Total Environ. 2013, 444, 196–204. [Google Scholar] [CrossRef]
- Guo, Z.B.; Lin, Y.L.; Xu, B.; Hu, C.Y.; Huang, H.; Zhang, T.Y.; Chu, W.H.; Gao, N.Y. Factors affecting THM, HAN and HNM formation during UV-chlor (am) ination of drinking water. Chem. Eng. J. 2016, 306, 1180–1188. [Google Scholar] [CrossRef]
- Duan, X.; Liao, X.; Chen, J.; Xie, S.; Qi, H.; Li, F.; Yuan, B. THMs, HAAs and NAs production from culturable microorganisms in pipeline network by ozonation, chlorination, chloramination and joint disinfection strategies. Sci. Total Environ. 2020, 744, 140833. [Google Scholar] [CrossRef] [PubMed]
- Gallard, H.; Von Gunten, U. Chlorination of natural organic matter: Kinetics of chlorination and of THM formation. Water Res. 2002, 36, 65–74. [Google Scholar] [CrossRef]
- Wu, T.; Karimi-Maleh, H.; Dragoi, E.N.; Puri, P.; Zhang, D.; Zhang, Z. Traditional methods and biosensors for detecting disinfection by-products in water: A review. Environ. Res. 2023, 237, 116935. [Google Scholar] [CrossRef] [PubMed]
- Nobukawa, T.; Sanukida, S. Effect of bromide ions on genotoxicity of halogenated by-products from chlorination of humic acid in water. Water Res. 2001, 35, 4293–4298. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Chen, X.; Huang, C.; Ding, X. Trihalomethane content in tap water of Wuxi City and its carcinogenic risk to adults. Pract. Prev. Med. 2015, 22, 550–552. [Google Scholar]
- Florentin, A.; Hautemanière, A.; Hartemann, P. Health effects of disinfection by-products in chlorinated swimming pools. Int. J. Hyg. Environ. Health 2011, 214, 461–469. [Google Scholar] [CrossRef]
- Pang, Z.; Zhang, P.; Chen, X.; Dong, F.; Deng, J.; Li, C.; Liu, J.; Ma, X.; Dietrich, A.M. Occurrence and modeling of disinfection byproducts in distributed water of a megacity in China: Implications for human health. Sci. Total Environ. 2022, 848, 157674. [Google Scholar] [CrossRef]
- Semerjian, L.; Dennis, J. Multipathway risk assessment of trihalomethane exposure in drinking water of Lebanon. J. Water Health 2007, 5, 511–522. [Google Scholar] [CrossRef]
- Huang, J.; Zhong, G. Research progress on the health effects of chlorinated disinfection by-products in drinking water. Appl. Prev. Med. 2021, 27, 476–479. [Google Scholar]
- Legay, C.; Rodriguez, M.J.; Sadiq, R.; Sérodes, J.B.; Levallois, P.; Proulx, F. Spatial variations of human health risk associated with exposure to chlorination by-products occurring in drinking water. J. Environ. Manag. 2011, 92, 892–901. [Google Scholar] [CrossRef] [PubMed]
- GB 5749–2022; Standards for Drinking Water Quality. China Standard Press: Beijing, China, 2022.
- Cotruvo, J.A. Trihalomethanes (THMs) in drinking water. J. Environ. Sci. Technol. 1981, 15, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Ye, B.; Wang, W.; Yang, L.; Wei, J.; Xueli, E. Factors influencing disinfection by-products formation in drinking water of six cities in China. J. Hazard. Mater. 2009, 171, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.H.; Tung, H.H.; Chao, C.C.; Wang, G.S. Occurrence of haloacetic acids (HAAs) and trihalomethanes (THMs) in drinking water of Taiwan. Environ. Monit. Assess. 2010, 162, 237–250. [Google Scholar] [CrossRef]
- Gan, W.; Guo, W.; Mo, J.; He, Y.; Liu, Y.; Liu, W.; Liang, Y.; Yang, X. The occurrence of disinfection by-products in municipal drinking water in China’s Pearl River Delta and a multipathway cancer risk assessment. Sci. Total Environ. 2013, 447, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Meng, L.; Zhang, H.; Yu, J.; An, W.; Hu, J.; Yang, M. Occurrence, profiling and prioritization of halogenated disinfection by-products in drinking water of China. Environ. Sci. Process. Impacts 2013, 15, 1424–1429. [Google Scholar] [CrossRef]
- Pan, S.; An, W.; Li, H.; Su, M.; Zhang, J.; Yang, M. Cancer risk assessment on trihalomethanes and haloacetic acids in drinking water of China using disability-adjusted life years. J. Hazard. Mater. 2014, 280, 288–294. [Google Scholar] [CrossRef]
- Huang, H.; Zhu, H.; Gan, W.; Chen, X.; Yang, X. Occurrence of nitrogenous and carbonaceous disinfection byproducts in drinking water distributed in Shenzhen, China. Chemosphere 2017, 188, 257–264. [Google Scholar] [CrossRef]
- Yao, Z.; Sun, S.; Wang, M.; Zhao, Q.; Jia, R. The occurrence of THMs and AOX in drinking water of Shandong Province, China. Environ. Sci. Pollut. Res. 2019, 26, 18583–18592. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Ma, X.; Chen, R.; Li, G.; Tao, H.; Shi, B. The occurrence and transformation behaviors of disinfection byproducts in drinking water distribution systems in rural areas of eastern China. Chemosphere 2019, 228, 101–109. [Google Scholar] [CrossRef]
- Zhou, X.; Zheng, L.; Chen, S.; Du, H.; Raphael, B.M.G.; Song, Q.; Wu, F.; Chen, J.; Lin, H.; Hong, H. Factors influencing DBPs occurrence in tap water of Jinhua Region in Zhejiang Province, China. Ecotoxicol. Environ. Saf. 2019, 171, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Uden, P.C.; Miller, J.W. Chlorinated acids and chloral in drinking water. J. Am. Water Work. Assoc. 1983, 75, 524–527. [Google Scholar] [CrossRef]
- Krasner, S.W.; McGuire, M.J.; Jacangelo, J.G.; Patania, N.L.; Reagan, K.M.; Aieta, E.M. The occurrence of disinfection by-products in US drinking water. J. Am. Water Work. Assoc. 1989, 81, 41–53. [Google Scholar] [CrossRef]
- Singer, P.C.; Obolensky, A.; Greiner, A. DBPs in chlorinated North Carolina drinking waters. J. Am. Water Work. Assoc. 1995, 87, 83–92. [Google Scholar] [CrossRef]
- Krasner, S.W.; Weinberg, H.S.; Richardson, S.D.; Pastor, S.J.; Chinn, R.; Sclimenti, M.J.; Onstad, G.D.; Thruston, A.D. Occurrence of a new generation of disinfection byproducts. Environ. Sci. Technol. 2006, 40, 7175–7185. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.T.; LeBel, G.L.; Benoit, F.M. Disinfection by-products in Canadian drinking water. Chemosphere 1997, 34, 299–316. [Google Scholar] [CrossRef]
- Legay, C.; Rodriguez, M.J.; Sérodes, J.B.; Levallois, P. The assessment of population exposure to chlorination by-products: A study on the influence of the water distribution system. Environ. Health 2010, 9, 59. [Google Scholar] [CrossRef]
- Rodriguez, M.J.; Sérodes, J.B.; Levallois, P. Behavior of trihalomethanes and haloacetic acids in a drinking water distribution system. Water Res. 2004, 38, 4367–4382. [Google Scholar] [CrossRef]
- Norin, H.; Renberg, L. Determination of trihalomethanes (THM) in water using high efficiency solvent extraction. Water Res. 1980, 14, 1397–1402. [Google Scholar] [CrossRef]
- Attias, L.; Contu, A.; Loizzo, A.; Massiglia, M.; Valente, P.; Zapponi, G.A. Trihalomethanes in drinking water and cancer: Risk assessment and integrated evaluation of available data, in animals and humans. Sci. Total Environ. 1995, 171, 61–68. [Google Scholar] [CrossRef]
- Cancho, B.; Ventura, F.; Galceran, M.T. Behavior of halogenated disinfection by-products in the water treatment plant of Barcelona, Spain. Bull. Environ. Contam. Toxicol. 1999, 63, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Malliarou, E.; Collins, C.; Graham, N.; Nieuwenhuijsen, M.J. Haloacetic acids in drinking water in the United Kingdom. Water Res. 2005, 39, 2722–2730. [Google Scholar] [CrossRef] [PubMed]
- Krasner, S.W.; Kostopoulou, M.; Toledano, M.B.; Wright, J.; Patelarou, E.; Kogevinas, M.; Villanueva, C.M.; Carrasco-Turigas, G.; Santa Marina, L.; Fernández-Somoano, A.; et al. Occurrence of DBPs in drinking water of European regions for epidemiology studies. J. Am. Water Work. Assoc. 2016, 108, E501–E512. [Google Scholar] [CrossRef]
- Mouly, D.; Joulin, E.; Rosin, C.; Beaudeau, P.; Zeghnoun, A.; Olszewski-Ortar, A.; Munoz, J.F.; Welte, B.; Joyeux, M.; Seux, R.; et al. Variations in trihalomethane levels in three French water distribution systems and the development of a predictive model. Water Res. 2010, 44, 5168–5179. [Google Scholar] [CrossRef]
- Kuivinen, J.; Johnsson, H. Determination of trihalomethanes and some chlorinated solvents in drinking water by headspace technique with capillary column gas-chromatography. Water Res. 1999, 33, 1201–1208. [Google Scholar] [CrossRef]
- Zhang, X.L.; Yang, H.W.; Wang, X.M.; Fu, J.; Xie, Y.F. Formation of disinfection by-products: Effect of temperature and kinetic modeling. Chemosphere 2013, 90, 634–639. [Google Scholar] [CrossRef]
- Pang, X.; Liu, Q.; Xu, H.; Lu, S.; Yang, H.; Zhao, M. Trichloromethane concentration analysis and health risk assessment for drinking water in Suzhou, China, 2018–2022. J. Environ. Hyg. 2024, 14, 438–442. [Google Scholar]
- Wang, J.J.; Chow, A.T.; Sweeney, J.M.; Mazet, J.A. Trihalomethanes in marine mammal aquaria: Occurrences, sources, and health risks. Water Res. 2014, 59, 219–228. [Google Scholar] [CrossRef]
- Nguyen, H.V.M.; Hur, J.; Shin, H.S. Humic acids and fulvic acids: Characteristics, sorption of hydrophobic organic contaminants, and formation of disinfection by-products during chlorination. In Humus and Humic Substances-Recent Advances; Makan, A., Ed.; IntechOpen: London, UK, 2022. [Google Scholar]
- Bond, T.; Goslan, E.H.; Parsons, S.A.; Jefferson, B. A critical review of trihalomethane and haloacetic acid formation from natural organic matter surrogates. Environ. Technol. Rev. 2012, 1, 93–113. [Google Scholar] [CrossRef]
- Wu, M. Study on the Formation and Removal of Disinfection By-Products in Typical Water Treatment Processes. Master’s Thesis, Suzhou University of Science and Technology, Suzhou, China, 2019. [Google Scholar]
- Song, J. Study on the Formation and Degradation Mechanism of Chloroform in Swimming Pool Water. Master’s Thesis, Chongqing University, Chongqing, China, 2015. [Google Scholar]
- Abdel Azeem, S.M.; Burham, N.; Borik, M.G.; El Shahat, M.F. Trihalomethanes formation in water treatment plants and distribution lines: A monitoring and modeling scheme. Toxicol. Environ. Chem. 2014, 96, 12–26. [Google Scholar] [CrossRef]
- Tsitsifli, S.; Kanakoudis, V. Developing THMs’ predictive models in two water supply systems in Greece. Water 2020, 12, 1422. [Google Scholar] [CrossRef]
- Sun, B.; Fan, G.; Zhang, Y. Analysis of trihalomethane formation and elimination in tap water from lake and reservoir sources. Environ. Bull. Heilongjiang 2018, 42, 22–24. [Google Scholar]
- Navalon, S.; Alvaro, M.; Garcia, H. Carbohydrates as trihalomethanes precursors. Influence of pH and the presence of Cl− and Br− on trihalomethane formation potential. Water Res. 2008, 42, 3990–4000. [Google Scholar] [CrossRef] [PubMed]
- Hua, G.; Reckhow, D.A. DBP formation during chlorination and chloramination: Effect of reaction time, pH, dosage, and temperature. J. Am. Water Work. Assoc. 2008, 100, 82–95. [Google Scholar] [CrossRef]
- Duan, X.L. Exposure Factors Handbook of Chinese Population; China Environmental Press: Beijing, China, 2013. [Google Scholar]
- Wang, L.; Li, J.; Zheng, J.; Liang, J.; Li, R.; Gong, Z. Source tracing and health risk assessment of phthalate esters in household tap-water: A case study of the urban area of Quanzhou, Southeast China. Ecotoxicol. Environ. Saf. 2022, 248, 114277. [Google Scholar] [CrossRef]
- Echigo, S.; Itoh, S.; Natsui, T.; Araki, T.; Ando, R. Contribution of brominated organic disinfection by-products to the mutagenicity of drinking water. Water Sci. Technol. 2004, 50, 321–328. [Google Scholar] [CrossRef]
- Li, B.; Qu, C.; Bi, J. Identification of trace organic pollutants in drinking water and the associated human health risks in Jiangsu Province, China. Bull. Environ. Contam. Toxicol. 2012, 88, 880–884. [Google Scholar] [CrossRef]
- Manasfi, T.; Coulomb, B.; Boudenne, J.L. Occurrence, origin, and toxicity of disinfection byproducts in chlorinated swimming pools: An overview. Int. J. Hyg. Environ. Health 2017, 220, 591–603. [Google Scholar] [CrossRef]
- Dong, F.; Chen, J.; Li, C.; Ma, X.; Jiang, J.; Lin, Q.; Lin, C.; Diao, H. Evidence-based analysis on the toxicity of disinfection byproducts in vivo and in vitro for disinfection selection. Water Res. 2019, 165, 114976. [Google Scholar] [CrossRef]
- World Health Organization. Disinfectants and disinfectant by-products. In Environmental Health Criteria 216; World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- Lee, S.C.; Guo, H.; Lam, S.M.J.; Lau, S.L.A. Multipathway risk assessment on disinfection by-products of drinking water in Hong Kong. Environ. Res. 2004, 94, 47–56. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency. Mid Atlantic Risk Assessment, Regional Screening Levels (RSLs)—Generic Tables. 2024. Available online: http://www.epa.gov/region9/superfund/prg (accessed on 18 October 2024).
- Little, J.C. Applying the two-resistance theory to contaminant volatilization in showers. Environ. Sci. Technol. 1992, 26, 1341–1349. [Google Scholar] [CrossRef]
- Yang, L.; Teng, W.; Wang, Q.; Chen, Z.; Liu, W.; Xu, S.; Chen, S. Distribution and health risk of disinfection by-products in drinking water in Hangzhou main urban zone. J. Environ. Occup. Med. 2016, 33, 237–242. [Google Scholar]
- Li, J.; Chen, J.; Hu, Z.; Li, X.; Li, M.; Wang, Y.; Zhang, Z.; Liang, X. Overlooked inorganic DBPs in trichloroisocyanuric acid (TCCA) disinfected indoor swimming pool: Evidences from concentration, cytotoxicity, and human health risk. Chemosphere 2023, 335, 139061. [Google Scholar] [CrossRef] [PubMed]

| Area | Location | Total Concentration (µg/L) | Reference |
|---|---|---|---|
| China | six cities | Max: 92.8 | [25] |
| Taiwan | ND-133.2 | [26] | |
| Pearl River Delta | 0.7–62.7 | [27] | |
| 31 cities | 0.8–107 | [28] | |
| 35 cities | 1.5–94.9 | [29] | |
| Shenzhen | 1.3–74.8 | [30] | |
| Shandong | 2.1–105 | [31] | |
| Rural eastern regions | 3.3–69.3 | [32] | |
| Zhejiang | 9.1–40.9 | [33] | |
| North America | Amherst | 0–190 (TCM) | [34] |
| USA | 30–44 | [35] | |
| North Carolina | 39–82 | [36] | |
| USA | Max: 164 | [37] | |
| Canada | 0.3–342 | [38] | |
| Canada | 38.1–111 | [39] | |
| Canada | Max: 106 | [40] | |
| EU | Sweden | 0.2–25 | [41] |
| Italy | 168 | [42] | |
| Spain | 58–91 | [43] | |
| UK | 27.6–50.9 | [44] | |
| BDL-83 | [45] | ||
| France | Max: 88.6 | [46] |
| Item | Year | Sample Size | Range (μg/L) | Median (μg/L) | Interquartile Range | W | p | Mean Rank | H (K) |
|---|---|---|---|---|---|---|---|---|---|
| TCM | 2016 | 44 | 0.02~12.98 | 2.31 | 1.94 | 0.69 | <0.01 | 154 | 223 |
| 2017 | 44 | 0.44~8.83 | 2.01 | 2.23 | 0.85 | <0.01 | 153 | ||
| 2018 | 44 | 0.02~1.11 | 0.31 | 0.30 | 0.92 | <0.01 | 27.9 | ||
| 2019 | 134 | 1.68~25.3 | 0.05 | 9.78 | 0.90 | <0.01 | 372 | ||
| 2020 | 132 | 0.78~16.9 | 6.42 | 3.64 | 0.93 | <0.01 | 344 | ||
| 2021 | 116 | 2.03~43.5 | 4.40 | 1.83 | 0.53 | <0.01 | 303 | ||
| 2022 | 52 | 0.22~19.5 | 5.00 | 6.39 | 0.90 | <0.01 | 292 | ||
| Total | 566 | 0.02~43.5 | 4.26 | 5.11 | 0.84 | <0.01 |
| Indicator | Quarter | Sample Size | Range (μg/L) | Median (μg/L) | Interquartile Range | W | p | Mean Rank | H (K) |
|---|---|---|---|---|---|---|---|---|---|
| TCM | 1 | 243 | 0.02~8.83 | 3.53 | 3.42 | 0.96 | <0.01 | 216 | 94.1 |
| 2 | 70 | 0.32~19.5 | 3.76 | 2.72 | 0.78 | <0.01 | 256 | ||
| 3 | 162 | 0.02~25.3 | 7.99 | 9.13 | 0.95 | <0.01 | 363 | ||
| 4 | 91 | 1.69~43.5 | 5.91 | 9.88 | 0.77 | <0.01 | 344 |
| Item | Sample Size | Minimum | Maximum | Interquartile Range | Median |
|---|---|---|---|---|---|
| pH | 566 | 6.81 | 8.36 | 0.25 | 7.67 |
| Chloride (mg/L) | 566 | 1.00 | 41.00 | 9.90 | 28.60 |
| Turbidity (NTU) | 566 | 0.05 | 0.64 | 0.09 | 0.19 |
| Population | IRW (L/d) | BW (kg) | SA (cm2) | EF (Day/Year) | ED (Years) | Shower Time (min/Day) | IRa (m3/d) | |
|---|---|---|---|---|---|---|---|---|
| Gender | Female | 2.1 | 57.8 | 15,000 | 350 | 77.4 | 8 | 17.7 |
| Male | 2.5 | 66.1 | 17,000 | 350 | 72.4 | 8 | 14.5 | |
| Region | Urban | 2.4 | 63.4 | 16,000 | 350 | 80 | 9 | 16.3 |
| Rural | 2.3 | 60.8 | 16,000 | 350 | 70 | 7 | 16 | |
| [18, 44) | 2.3 | 61.9 | 16,000 | 350 | 44 | 9 | 16.7 | |
| Age | [45, 59) | 2.3 | 63.5 | 16,000 | 350 | 59 | 8 | 16.7 |
| [60, 79) | 2.2 | 60.3 | 16,000 | 350 | 79 | 7 | 13.8 | |
| 80+ | 1.9 | 55.5 | 16,000 | 350 | 80 | 6 | 12 | |
| Variables | HQ_Oral | HQ_Der | HQ_Inh | HI-Total | Risk_Oral | Risk_Der | Risk_Inh | Risk-Total | |
|---|---|---|---|---|---|---|---|---|---|
| Gender | Female | 1.48 × 10−2 | 1.50 × 10−3 | 3.21 × 10−4 | 1.67 × 10−2 | 5.09 × 10−6 | 5.13 × 10−7 | 1.59 × 10−8 | 5.62 × 10−6 |
| Male | 1.54 × 10−2 | 1.48 × 10−3 | 2.30 × 10−4 | 1.72 × 10−2 | 4.95 × 10−6 | 4.76 × 10−7 | 1.07 × 10−8 | 5.44 × 10−6 | |
| Region | Urban | 1.55 × 10−2 | 1.46 × 10−3 | 2.69 × 10−4 | 1.72 × 10−2 | 5.48 × 10−6 | 5.16 × 10−7 | 1.38 × 10−8 | 6.01 × 10−6 |
| Rural | 1.55 × 10−2 | 1.52 × 10−3 | 2.76 × 10−4 | 1.72 × 10−2 | 4.79 × 10−6 | 4.70 × 10−7 | 1.24 × 10−8 | 5.27 × 10−6 | |
| [18, 44) | 1.52 × 10−2 | 1.49 × 10−3 | 2.83 × 10−4 | 1.70 × 10−2 | 2.96 × 10−6 | 2.90 × 10−7 | 7.97 × 10−9 | 3.26 × 10−6 | |
| Age | [45, 59) | 1.48 × 10−2 | 1.45 × 10−3 | 2.75 × 10−4 | 1.65 × 10−2 | 3.87 × 10−6 | 3.80 × 10−7 | 1.04 × 10−8 | 4.26 × 10−6 |
| [60, 79) | 1.49 × 10−2 | 1.53 × 10−3 | 2.40 × 10−4 | 1.67 × 10−2 | 5.21 × 10−6 | 5.35 × 10−7 | 1.21 × 10−8 | 5.76 × 10−6 | |
| 80+ | 1.40 × 10−2 | 1.66 × 10−3 | 2.26 × 10−4 | 1.59 × 10−2 | 4.95 × 10−6 | 5.89 × 10−7 | 1.16 × 10−8 | 5.56 × 10−6 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, X.; Qian, G.; Wang, Y.; Chen, M.; Liu, Y.; Zhao, P.; Li, J.; Wang, Y.; Liu, Y. Annual and Seasonal Variability of Trichloromethane in Drinking Water of Kunshan City 2016–2022 and Associated Health Risks. Toxics 2024, 12, 865. https://doi.org/10.3390/toxics12120865
Liang X, Qian G, Wang Y, Chen M, Liu Y, Zhao P, Li J, Wang Y, Liu Y. Annual and Seasonal Variability of Trichloromethane in Drinking Water of Kunshan City 2016–2022 and Associated Health Risks. Toxics. 2024; 12(12):865. https://doi.org/10.3390/toxics12120865
Chicago/Turabian StyleLiang, Xiaojun, Guohua Qian, Yihan Wang, Mengyao Chen, Yang Liu, Ping Zhao, Junling Li, Yuan Wang, and Yuyan Liu. 2024. "Annual and Seasonal Variability of Trichloromethane in Drinking Water of Kunshan City 2016–2022 and Associated Health Risks" Toxics 12, no. 12: 865. https://doi.org/10.3390/toxics12120865
APA StyleLiang, X., Qian, G., Wang, Y., Chen, M., Liu, Y., Zhao, P., Li, J., Wang, Y., & Liu, Y. (2024). Annual and Seasonal Variability of Trichloromethane in Drinking Water of Kunshan City 2016–2022 and Associated Health Risks. Toxics, 12(12), 865. https://doi.org/10.3390/toxics12120865







