Applications of Tert-Butyl-Phenolic Antioxidants in Consumer Products and Their Potential Toxicities in Humans
Abstract
:1. Introduction
2. Chemical Structure and Properties
2.1. Phenol Group
2.2. Tert-Butyl Group
2.3. Tert-Butyl Phenol
2.4. Classifications
2.5. Physical Properties
3. Applications in Consumer Products
3.1. Adhesives and Sealants
3.2. Lubricants and Greases
3.3. Plastics, Polymers, and Rubber
3.4. Cosmetics and Personal Care Products
3.5. Food and Coatings
3.6. Other Applications
4. Human Exposure
4.1. Dermal Contact
4.2. Inhalation Exposure
4.3. Oral Exposure
4.4. Gestational Exposure
4.5. Environmental Exposure
4.6. Occupational Exposure
5. Human Health Effects
5.1. Skin Toxicity
5.2. Liver Toxicity
5.3. Lung Toxicity
5.4. Endocrine Disruption
6. Prediction of Toxicities Using ADMET
7. Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yehye, W.A.; Rahman, N.A.; Ariffin, A.; Abd Hamid, S.B.; Alhadi, A.A.; Kadir, F.A.; Yaeghoobi, M. Understanding the Chemistry behind the Antioxidant Activities of Butylated Hydroxytoluene (BHT): A Review. Eur. J. Med. Chem. 2015, 101, 295–312. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Mabury, S.A. Synthetic Phenolic Antioxidants: A Review of Environmental Occurrence, Fate, Human Exposure, and Toxicity. Environ. Sci. Technol. 2020, 54, 11706–11719. [Google Scholar] [CrossRef] [PubMed]
- Fiege, H.; Voges, H.-W.; Hamamoto, T.; Umemura, S.; Iwata, T.; Miki, H.; Fujita, Y.; Buysch, H.-J.; Garbe, D.; Paulus, W. Phenol Derivatives. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Hoboken, NJ, USA, 2000; ISBN 9783527306732. [Google Scholar]
- Jacques, M.T.; de Souza, V.; Barbosa, F.A.R.; Faria Santos Canto, R.; Lopes, S.C.; Prediger, R.D.; Braga, A.L.; Aschner, M.; Farina, M. Novel Probucol Analogue, 4,4′-Diselanediylbis (2,6-Di-Tert-Butylphenol), Prevents Oxidative Glutamate Neurotoxicity In Vitro and Confers Neuroprotection in a Rodent Model of Ischemic Stroke. ACS Chem. Neurosci. 2023, 14, 2857–2867. [Google Scholar] [CrossRef] [PubMed]
- FUJITA, T.; FUJIMOTO, Y. Effect of various diuretics on lipid peroxidation in rat renal cortical mitochondria and in the supernatant. Jpn. J. Pharmacol. 1981, 31, 795–800. [Google Scholar] [CrossRef]
- Eleazu, C.O.; Obeten, U.N.; Ozor, G.; Njemanze, C.C.; Eleazu, K.C.; Egedigwe-Ekeleme, A.C.; Okorie, U.C.; Ogunwa, S.C.; Adeolu, A.I.; Okoh, P.-F.N.; et al. Tert-Butylhydroquinone Abrogates Fructose-Induced Insulin Resistance in Rats via Mitigation of Oxidant Stress, NFkB-Mediated Inflammation in the Liver but Not the Skeletal Muscle of High Fructose Drinking Rats. J. Food Biochem. 2022, 46, e14473. [Google Scholar] [CrossRef]
- Yang, B.; Huang, H.; He, Q.; Lu, W.; Zheng, L.; Cui, L. Tert-Butylhydroquinone Prevents Oxidative Stress-Mediated Apoptosis and Extracellular Matrix Degradation in Rat Chondrocytes. Evidence-Based Complement. Altern. Med. 2021, 2021, 1905995. [Google Scholar] [CrossRef]
- Zhang, H.; Li, J.; Xiang, X.; Zhou, B.; Zhao, C.; Wei, Q.; Sun, Y.; Chen, J.; Lai, B.; Luo, Z.; et al. Tert-Butylhydroquinone Attenuates Osteoarthritis by Protecting Chondrocytes and Inhibiting Macrophage Polarization. Bone Joint Res. 2021, 10, 704–713. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, J.; Wei, F.; Gu, Q.; Tian, M.; Lv, H.-B. Tert-Butylhydroquinone Protects the Retina from Oxidative Stress in STZ-Induced Diabetic Rats via the PI3K/Akt/ENOS Pathway. Eur. J. Pharmacol. 2022, 935, 175297. [Google Scholar] [CrossRef]
- Chen, Y.; Cao, H.; He, W.; Zhang, X.; Xu, R. Tert-Butylhydroquinone-Induced Formation of High-Molecular-Weight P62: A Novel Mechanism in the Activation of Nrf2-Keap1. Cell Biol. Int. 2022, 46, 1345–1354. [Google Scholar] [CrossRef]
- Wang, M.; Hu, S.; Yang, J.; Yuan, L.; Han, L.; Liang, F.; Zhang, F.; Zhao, H.; Liu, Y.; Gao, N. Arenobufagin Inhibits Lung Metastasis of Colorectal Cancer by Targeting C-MYC/Nrf2 Axis. Phytomedicine 2024, 127, 155391. [Google Scholar] [CrossRef]
- Duan, X.; Li, J.; Li, W.; Xing, X.; Zhang, Y.; Li, W.; Zhao, L.; Sun, G.; Gao, X.; Li, B. Antioxidant Tert-Butylhydroquinone Ameliorates Arsenic-Induced Intracellular Damages and Apoptosis through Induction of Nrf2-Dependent Antioxidant Responses as Well as Stabilization of Anti-Apoptotic Factor Bcl-2 in Human Keratinocytes. Free Radic. Biol. Med. 2016, 94, 74–87. [Google Scholar] [CrossRef] [PubMed]
- Saha, P.; Hegde, M.; Chakraborty, K.; Singha, A.; Mukerjee, N.; Ghosh, D.; Kunnumakkara, A.B.; Khan, M.S.; Ahmad, M.I.; Ghosh, A.; et al. Targeted Inhibition of Colorectal Cancer Proliferation: The Dual-Modulatory Role of 2,4-DTBP on Anti-Apoptotic Bcl-2 and Survivin Proteins. J. Cell Mol. Med. 2024, 28, e18150. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.; Kushveer, J.S.; Khan, M.I.K.; Pagal, S.; Meena, C.K.; Murali, A.; Dhayalan, A.; Venkateswara Sarma, V. 2,4-Di-Tert-Butylphenol Isolated from an Endophytic Fungus, Daldinia Eschscholtzii, Reduces Virulence and Quorum Sensing in Pseudomonas Aeruginosa. Front. Microbiol. 2020, 11, 1668. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.W.; Lim, Y.; Cho, S.K. 2,4-Di-tert-butylphenol, a Potential HDAC6 Inhibitor, Induces Senescence and Mitotic Catastrophe in Human Gastric Adenocarcinoma AGS Cells. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 675–683. [Google Scholar] [CrossRef]
- Reshma, V.R.; Nair Devi Velayudhan Jayasree, P.G.B.; Baby, S. Anti-Inflammatory and Anticancer Activities of Erythrodiol-3-Acetate and 2,4-Di-Tert-Butylphenol Isolated from Humboldtia Unijuga. Nat. Prod. Res. 2020, 34, 2319–2322. [Google Scholar] [CrossRef]
- Yang, L.; Wang, R.; Lin, W.; Li, B.; Jin, T.; Weng, Q.; Zhang, M.; Liu, P. Efficacy of 2,4-Di-Tert-Butylphenol in Reducing Ralstonia Solanacearum Virulence: Insights into the Underlying Mechanisms. ACS Omega 2024, 9, 4647–4655. [Google Scholar] [CrossRef]
- Choi, S.J.; Kim, J.K.; Kim, H.K.; Harris, K.; Kim, C.-J.; Park, G.G.; Park, C.-S.; Shin, D.-H. 2,4-Di-Tert-Butylphenol from Sweet Potato Protects Against Oxidative Stress in PC12 Cells and in Mice. J. Med. Food 2013, 16, 977–983. [Google Scholar] [CrossRef]
- Guo, M.; Peng, J.; Guo, P.; Wang, Q.; Zhang, L.; Shen, H.; Chen, F.; Zhang, P.; Lin, S.; Gao, H.; et al. Inhalation of 2,4-Di-Tert-Butylphenol-Loaded Micelles Suppresses Respiratory Syncytial Virus Infection in Mice. Antiviral Res. 2024, 226, 105880. [Google Scholar] [CrossRef]
- Kruk, J.; Aboul-Enein, B.H.; Duchnik, E.; Marchlewicz, M. Antioxidative Properties of Phenolic Compounds and Their Effect on Oxidative Stress Induced by Severe Physical Exercise. J. Physiol. Sci. 2022, 72, 19. [Google Scholar] [CrossRef]
- Bisel, P.; Al-Momani, L.; Müller, M. The Tert-Butyl Group in Chemistry and Biology. Org. Biomol. Chem. 2008, 6, 2655–2665. [Google Scholar] [CrossRef]
- Mamari, H.H. Al Phenolic Compounds: Classification, Chemistry, and Updated Techniques of Analysis and Synthesis. In Phenolic Compounds; Badria, F.A., Ed.; IntechOpen: Rijeka, Croatia, 2021; p. 4. ISBN 978-1-83969-347-2. [Google Scholar]
- de Araújo, F.F.; de Paulo Farias, D.; Neri-Numa, I.A.; Pastore, G.M. Polyphenols and Their Applications: An Approach in Food Chemistry and Innovation Potential. Food Chem. 2021, 338, 127535. [Google Scholar] [CrossRef] [PubMed]
- de Lima Cherubim, D.J.; Buzanello Martins, C.V.; Oliveira Fariña, L.; da Silva de Lucca, R.A. Polyphenols as Natural Antioxidants in Cosmetics Applications. J. Cosmet. Dermatol. 2020, 19, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Gasmi, A.; Mujawdiya, P.K.; Noor, S.; Lysiuk, R.; Darmohray, R.; Piscopo, S.; Lenchyk, L.; Antonyak, H.; Dehtiarova, K.; Shanaida, M.; et al. Polyphenols in Metabolic Diseases. Molecules 2022, 27, 6280. [Google Scholar] [CrossRef] [PubMed]
- Westphal, M.V.; Wolfstädter, B.T.; Plancher, J.-M.; Gatfield, J.; Carreira, E.M. Evaluation of Tert-Butyl Isosteres: Case Studies of Physicochemical and Pharmacokinetic Properties, Efficacies, and Activities. ChemMedChem 2015, 10, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.-C.; Palone, A.; Bietti, M.; Costas, M. Tert-Butyl as a Functional Group: Non-Directed Catalytic Hydroxylation of Sterically Congested Primary C−H Bonds. Angew. Chem. Int. Ed. 2024, 63, e202402858. [Google Scholar] [CrossRef]
- Charlton, N.C.; Mastyugin, M.; Török, B.; Török, M. Structural Features of Small Molecule Antioxidants and Strategic Modifications to Improve Potential Bioactivity. Molecules 2023, 28, 1057. [Google Scholar] [CrossRef]
- Ouellette, R.J.; Rawn, J.D. 5—Aromatic Compounds. In Principles of Organic Chemistry; Ouellette, R.J., Rawn, J.D., Eds.; Elsevier: Boston, MA, USA, 2015; pp. 133–162. ISBN 978-0-12-802444-7. [Google Scholar]
- Mtashobya, L. Insights on Tert-Butyl Alkylation Effects on Fluorobenzene. Sci. Afr. 2021, 11, e00659. [Google Scholar] [CrossRef]
- Nemeth, T.; de Wild, T.; Gubler, L.; Nauser, T. Impact of Substitution on Reactions and Stability of One-Electron Oxidised Phenyl Sulfonates in Aqueous Solution. Phys. Chem. Chem. Phys. 2022, 24, 895–901. [Google Scholar] [CrossRef]
- Hollande, L.; Domenek, S.; Allais, F. Chemo-Enzymatic Synthesis of Renewable Sterically-Hindered Phenolic Antioxidants with Tunable Polarity from Lignocellulose and Vegetal Oil Components. Int. J. Mol. Sci. 2018, 19, 3358. [Google Scholar] [CrossRef]
- Liszewski, W.; Owen, B.; Fournier, E.; Kerchinsky, L.; Wei, J.; Scheman, A. Assessment of Contact Allergens in “Hypoallergenic” Athletic Shoes by Mass Spectrometry. Dermatitis 2023, 34, 532–535. [Google Scholar] [CrossRef]
- Kaniwa, M.-A.; Isama, K.; Nakamura, A.; Kantoh, H.; Itoh, M.; Miyoshi, K.; Saito, S.; Shono, M. Identification of Causative Chemicals of Allergic Contact Dermatitis Using a Combination of Patch Testing in Patients and Chemical Analysis. Contact Dermat. 1994, 30, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Murru, C.; Badía-Laíño, R.; Díaz-García, M.E. Oxidative Stability of Vegetal Oil-Based Lubricants. ACS Sustain. Chem. Eng. 2021, 9, 1459–1476. [Google Scholar] [CrossRef] [PubMed]
- Xia, D.; Wang, Y.; Liu, H.; Yan, J.; Lin, H.; Han, S. Research Progress of Antioxidant Additives for Lubricating Oils. Lubricants 2024, 12, 115. [Google Scholar] [CrossRef]
- Hermabessiere, L.; Receveur, J.; Himber, C.; Mazurais, D.; Huvet, A.; Lagarde, F.; Lambert, C.; Paul-Pont, I.; Dehaut, A.; Jezequel, R.; et al. An Irgafos® 168 Story: When the Ubiquity of an Additive Prevents Studying Its Leaching from Plastics. Sci. Total Environ. 2020, 749, 141651. [Google Scholar] [CrossRef]
- Cui, S.; Yu, Y.; Zhan, T.; Zhang, C.; Zhuang, S. 2,6-Di-Tert-Butylphenol and Its Quinone Metabolite Trigger Aberrant Transcriptional Responses in C57BL/6 Mice Liver. Sci. Total Environ. 2021, 778, 146322. [Google Scholar] [CrossRef]
- Akhmadullin, R.M.; Gatiyatullin, D.R.; Vasil’ev, L.A.; Akhmadullina, A.G.; Mukmeneva, N.A.; Cherezova, E.N.; Yang, M. Performance of 4,4’-Bis(2,6-Di-Tert-Butylphenol) in Stabilization of Isoprene Rubber and Polypropylene. Russ. J. Appl. Chem. 2015, 88, 833–838. [Google Scholar] [CrossRef]
- Thörnblom, K.; Palmlöf, M.; Hjertberg, T. The Extractability of Phenolic Antioxidants into Water and Organic Solvents from Polyethylene Pipe Materials—Part I. Polym. Degrad. Stab. 2011, 96, 1751–1760. [Google Scholar] [CrossRef]
- Elena Dreassi, M.B.; Corti, P. Evaluation of Release of Antioxidant from High Density Polyethylene by Planar Chromatography. Food Addit. Contam. 1998, 15, 466–472. [Google Scholar] [CrossRef]
- Xu, J.; Hao, Y.; Yang, Z.; Li, W.; Xie, W.; Huang, Y.; Wang, D.; He, Y.; Liang, Y.; Matsiko, J.; et al. Rubber Antioxidants and Their Transformation Products: Environmental Occurrence and Potential Impact. Int. J. Environ. Res. Public Health 2022, 19, 14595. [Google Scholar] [CrossRef]
- Manteghi, A.; Ahmadi, S.; Arabi, H. Enhanced Thermo-Oxidative Stability through Covalent Attachment of Hindered Phenolic Antioxidant on Surface Functionalized Polypropylene. Polymer 2018, 138, 41–48. [Google Scholar] [CrossRef]
- Almeida, S.; Ozkan, S.; Gonçalves, D.; Paulo, I.; Queirós, C.S.G.P.; Ferreira, O.; Bordado, J.; dos Santos, R. A Brief Evaluation of Antioxidants, Antistatics, and Plasticizers Additives from Natural Sources for Polymers Formulation. Polymers 2023, 15, 6. [Google Scholar] [CrossRef] [PubMed]
- Barret, J.; Gijsman, P.; Swagten, J.; Lange, R.F.M. The Interaction of a Phenolic Anti-Oxidant and an Aromatic Amine in a Thermo-Oxidative Ageing Process. Polym. Degrad. Stab. 2002, 75, 367–374. [Google Scholar] [CrossRef]
- Siritham, C.; Thammakhet-Buranachai, C.; Thavarungkul, P.; Kanatharana, P. A Stir Foam Composed of Graphene Oxide, Poly(Ethylene Glycol) and Natural Latex for the Extraction of Preservatives and Antioxidant. Microchimica Acta 2018, 185, 148. [Google Scholar] [CrossRef] [PubMed]
- Le Coz, C.J.; Schneider, G.-A. Contact Dermatitis from Tertiary-Butylhydroquinone in a Hair Dye, with Cross-Sensitivity to BHA and BHT. Contact Dermat. 1998, 39, 39–40. [Google Scholar] [CrossRef]
- White, I.R.; Lovell, C.R.; Cronin, E. Antioxidants in Cosmetics. Contact Dermat. 1984, 11, 265–267. [Google Scholar] [CrossRef]
- Guan, Y.; Chu, Q.; Fu, L.; Ye, J. Determination of Antioxidants in Cosmetics by Micellar Electrokinetic Capillary Chromatography with Electrochemical Detection. J. Chromatogr. A 2005, 1074, 201–204. [Google Scholar] [CrossRef]
- Patrick, E.; Juberg, D.R.; O’Donoghue, J.; Maibach, H.I. Depigmentation with Tert-Butyl Hydroquinone Using Black Guinea Pigs. Food Chem. Toxicol. 1999, 37, 169–175. [Google Scholar] [CrossRef]
- Thörneby-Andersson, K.; Sterner, O.; Hansson, C. Tyrosinase-Mediated Formation of a Reactive Quinone from the Depigmenting Agents, 4-Tert-Butylphenol and 4-Tert-Butylcatechol. Pigment. Cell Res. 2000, 13, 33–38. [Google Scholar] [CrossRef]
- Yonemoto, K.; Gellin, G.A.; Epstein, W.L.; Fukuyama, K. Glutathione Reductase Activity in Skin Exposed to 4-Tertiary Butyl Catechol. Int. Arch. Occup. Environ. Health 1983, 51, 341–345. [Google Scholar] [CrossRef]
- Mansur, J.D.; Fukuyama, K.; Gellin, G.A.; Epstein, W.L. Effects of 4-Tertiary Butyl Catechol on Tissue Cultured Melanocytes. J. Investig. Dermatol. 1978, 70, 275–279. [Google Scholar] [CrossRef]
- Yonemoto, K.; Gellin, G.A.; Epstein, W.L.; Fukuyama, K. Reduction in Eumelanin by the Activation of Glutathione Reductase and γ-Glutamyl Transpeptidase after Exposure to a Depigmenting Chemical. Biochem. Pharmacol. 1983, 32, 1379–1382. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Kim, J.Y.; Lee, Y.S. Phenolic Compounds in Active Packaging and Edible Films/Coatings: Natural Bioactive Molecules and Novel Packaging Ingredients. Molecules 2022, 27, 7513. [Google Scholar] [CrossRef] [PubMed]
- Heiserman, W.M.; Can, S.Z.; Walker, R.A.; Begley, T.H.; Limm, W. Interfacial Behavior of Common Food Contact Polymer Additives. J. Colloid. Interface Sci. 2007, 311, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Rani, M.; Shim, W.J.; Han, G.M.; Jang, M.; Song, Y.K.; Hong, S.H. Benzotriazole-Type Ultraviolet Stabilizers and Antioxidants in Plastic Marine Debris and Their New Products. Sci. Total Environ. 2017, 579, 745–754. [Google Scholar] [CrossRef]
- Markley, L.C.; González Bonet, A.M.; Ogungbesan, A.; Bandele, O.J.; Bailey, A.B.; Patton, G.W. Safety Assessment for Tris(2,4-Di-Tert-Butylphenyl) Phosphite (Irgafos 168) Used as an Antioxidant and Stabilizer in Food Contact Applications. Food Chem. Toxicol. 2023, 178, 113877. [Google Scholar] [CrossRef]
- Wu, X.; Liu, P.; Shi, H.; Wang, H.; Huang, H.; Shi, Y.; Gao, S. Photo Aging and Fragmentation of Polypropylene Food Packaging Materials in Artificial Seawater. Water Res. 2021, 188, 116456. [Google Scholar] [CrossRef]
- Saunier, J.; Mazel, V.; Paris, C.; Yagoubi, N. Polymorphism of Irganox 1076®: Discovery of New Forms and Direct Characterization of the Polymorphs on a Medical Device by Raman Microspectroscopy. Eur. J. Pharm. Biopharm. 2010, 75, 443–450. [Google Scholar] [CrossRef]
- Saunier, J.; Herry, J.-M.; Marlière, C.; Renault, M.; Bellon-Fontaine, M.-N.; Yagoubi, N. Modification of the Bacterial Adhesion of Staphylococcus Aureus by Antioxidant Blooming on Polyurethane Films. Mater. Sci. Eng. C 2015, 56, 522–531. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, Q.; Anderson, J.M.; Hiltner, A.; Lodoen, G.A.; Payet, C.R. Effect of Some Additives on the Biostability of a Poly(Etherurethane) Elastomer. J. Biomed. Mater. Res. 1991, 25, 725–739. [Google Scholar] [CrossRef]
- Parris, P.; Martin, E.A.; Stanard, B.; Glowienke, S.; Dolan, D.G.; Li, K.; Binazon, O.; Giddings, A.; Whelan, G.; Masuda-Herrera, M.; et al. Considerations When Deriving Compound-Specific Limits for Extractables and Leachables from Pharmaceutical Products: Four Case Studies. Regul. Toxicol. Pharmacol. 2020, 118, 104802. [Google Scholar] [CrossRef]
- Bartsch, N.; Girard, M.; Schneider, L.; Van De Weijgert, V.; Wilde, A.; Kappenstein, O.; Vieth, B.; Hutzler, C.; Luch, A. Chemical Stabilization of Polymers: Implications for Dermal Exposure to Additives. J. Environ. Sci. Health Part. A 2018, 53, 405–420. [Google Scholar] [CrossRef] [PubMed]
- Birnbaum, L.S.; Heaney, S.M. Dermal Absorption of the Antioxidant 4,4′-Thiobis(6-Tert-Butyl-m-Cresol) in Sencar Mice and Fischer Rats. Toxicol. Lett. 1987, 37, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Final Report on the Safety Assessment of BHT. Int. J. Toxicol. 2002, 21, 19–94. [CrossRef] [PubMed]
- Ji, X.; Liu, J.; Liang, J.; Feng, X.; Liu, X.; Wang, Y.; Chen, X.; Qu, G.; Yan, B.; Liu, R. The Hidden Diet: Synthetic Antioxidants in Packaged Food and Their Impact on Human Exposure and Health. Environ. Int. 2024, 186, 108613. [Google Scholar] [CrossRef]
- Liu, R.; Mabury, S.A. Synthetic Phenolic Antioxidants in Personal Care Products in Toronto, Canada: Occurrence, Human Exposure, and Discharge via Greywater. Environ. Sci. Technol. 2019, 53, 13440–13448. [Google Scholar] [CrossRef]
- Angelini, E.; Marinaro, C.; Carrozzo, A.M.; Bianchi, L.; Delogu, A.; Giannelo, G.; Nini, G. Allergic Contact Dermatitis of the Lip Margins from Para-Tertiary-Butylphenol in a Lip Liner. Contact Dermat. 1993, 28, 146–148. [Google Scholar] [CrossRef]
- Rich, P.; Belozer, M.L.; Norris, P.; Storrs, F.J. Allergic Contact Dermatitis to Two Antioxidants in Latex Gloves: 4,4′-Thiobis(6-Tert-Butyl-Meta-Cresol) (Lowinox 44S36) and Butylhydroxyanisole: Allergen Alternatives for Glove-Allergic Patients. J. Am. Acad. Dermatol. 1991, 24, 37–43. [Google Scholar] [CrossRef]
- Myers, L.P.; Law, B.F.; Fedorowicz, A.; Siegel, P.D.; Butterworth, L.F.; Anderson, S.E.; Sussman, G.; Shapiro, M.; Meade, B.J.; Beezhold, D. Identification of Phenolic Dermal Sensitizers in a Wound Closure Tape. J. Immunotoxicol. 2007, 4, 303–310. [Google Scholar] [CrossRef]
- Antelmi, A.; Lejding, T.; Bruze, M.; Mowitz, M.; Dahlin, J. 4,4′-Thiobis(2-Tert-Butyl-5-Methylphenol), an Antioxidant in Medical Devices That May Cause Allergic Contact Dermatitis. Contact Dermatitis 2023, 89, 103–106. [Google Scholar] [CrossRef]
- Mowitz, M.; Lejding, T.; Ulriksdotter, J.; Antelmi, A.; Bruze, M.; Svedman, C. Further Evidence of Allergic Contact Dermatitis Caused by 2,2′-Methylenebis(6-Tert-Butyl-4-Methylphenol) Monoacrylate, a New Sensitizer in the Dexcom G6 Glucose Sensor. Dermatitis 2022, 33, 287–292. [Google Scholar] [CrossRef]
- Sherson, D.L.; Jacobsen, I.B.; Thomsen, G.F. The Antioxidant, Tert-Butylhydroquinone: A New Cause of Asthma. Occup. Med. 2023, 73, 109–111. [Google Scholar] [CrossRef] [PubMed]
- Simoneit, B.R.T.; Medeiros, P.M.; Didyk, B.M. Combustion Products of Plastics as Indicators for Refuse Burning in the Atmosphere. Environ. Sci. Technol. 2005, 39, 6961–6970. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Gu, Y.; Wei, Y. Determination of Polymer Additives–Antioxidants and Ultraviolet (UV) Absorbers by High-Performance Liquid Chromatography Coupled with UV Photodiode Array Detection in Food Simulants. J. Agric. Food Chem. 2011, 59, 12982–12989. [Google Scholar] [CrossRef] [PubMed]
- Gisele, C.; Maziero, C.B.; Toledo, M.C.F. Estimates of the Theoretical Maximum Daily Intake of Phenolic Antioxidants BHA, BHT and TBHQ in Brazil. Food Addit. Contam. 2001, 18, 365–373. [Google Scholar] [CrossRef]
- Kirkpatrick, D.C.; Lauer, B.H. Intake of Phenolic Antioxidants from Foods in Canada. Food Chem. Toxicol. 1986, 24, 1035–1037. [Google Scholar] [CrossRef]
- Suh, H.-J.; Chung, M.-S.; Cho, Y.-H.; Kim, J.-W.; Kim, D.-H.; Han, K.-W.; Kim, C.-J. Estimated Daily Intakes of Butylated Hydroxyanisole (BHA), Butylated Hydroxytoluene (BHT) and Tert-Butyl Hydroquinone (TBHQ) Antioxidants in Korea. Food Addit. Contam. 2005, 22, 1176–1188. [Google Scholar] [CrossRef]
- Nawel Bemrah, J.-C.L.; Volatier, J.-L. Assessment of Dietary Exposure in the French Population to 13 Selected Food Colours, Preservatives, Antioxidants, Stabilizers, Emulsifiers and Sweeteners. Food Addit. Contam. Part. B 2008, 1, 2–14. [Google Scholar] [CrossRef]
- Mancini, F.R.; Paul, D.; Gauvreau, J.; Volatier, J.L.; Vin, K.; Hulin, M. Dietary Exposure to Benzoates (E210–E213), Parabens (E214–E219), Nitrites (E249–E250), Nitrates (E251–E252), BHA (E320), BHT (E321) and Aspartame (E951) in Children Less than 3 Years Old in France. Food Addit. Contam. Part. A 2015, 32, 293–306. [Google Scholar] [CrossRef]
- Suh, H.-J.; Choi, S.-H. Safety Assessment of Estimated Daily Intakes of Antioxidants in Korean Using Dietary Survey Approach and Food Supply Survey Approach. Korean J. Food Sci. Technol. 2010, 42, 762–767. [Google Scholar]
- Soubra, L.; Sarkis, D.; Hilan, C.; Verger, P. Dietary Exposure of Children and Teenagers to Benzoates, Sulphites, Butylhydroxyanisol (BHA) and Butylhydroxytoluen (BHT) in Beirut (Lebanon). Regul. Toxicol. Pharmacol. 2007, 47, 68–77. [Google Scholar] [CrossRef]
- Verhagen, H.; Deerenberg, I.; Marx, A.; ten Hoor, F.; Henderson, P.T.; Kleinjans, J.C.S. Estimate of the Maximal Daily Dietary Intake of Butylated Hydroxyanisole and Butylated Hydroxytoluene in The Netherlands. Food Chem. Toxicol. 1990, 28, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Vin, K.; Connolly, A.; McCaffrey, T.; McKevitt, A.; O’Mahony, C.; Prieto, M.; Tennant, D.; Hearty, A.; Volatier, J.L. Estimation of the Dietary Intake of 13 Priority Additives in France, Italy, the UK and Ireland as Part of the FACET Project. Food Addit. Contam. Part. A 2013, 30, 2050–2080. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS). Scientific Opinion on the Re-Evaluation of Butylated Hydroxytoluene BHT (E 321) as a Food Additive. EFSA J. 2012, 10, 2588. [Google Scholar] [CrossRef]
- Lestido-Cardama, A.; de Quirós, A.; Bustos, J.; Lomo, M.L.; Paseiro Losada, P.; Sendón, R. Estimation of Dietary Exposure to Contaminants Transferred from the Packaging in Fatty Dry Foods Based on Cereals. Foods 2020, 9, 1038. [Google Scholar] [CrossRef]
- Wang, X.; Xu, M.; Yang, A.; Wang, Y.; Hou, S.; Zheng, N.; Liang, D.; Hua, X.; Dong, D. Health Risks of Population Exposure to Phthalic Acid Esters through the Use of Plastic Containers for Takeaway Food in China. Sci. Total Environ. 2021, 785, 147347. [Google Scholar] [CrossRef]
- Tang, S.; Sun, X.; Qiao, X.; Cui, W.; Yu, F.; Zeng, X.; Covaci, A.; Chen, D. Prenatal Exposure to Emerging Plasticizers and Synthetic Antioxidants and Their Potency to Cross Human Placenta. Environ. Sci. Technol. 2022, 56, 8507–8517. [Google Scholar] [CrossRef]
- Du, B.; Zhang, Y.; Lam, J.C.W.; Pan, S.; Huang, Y.; Chen, B.; Lan, S.; Li, J.; Luo, D.; Zeng, L. Prevalence, Biotransformation, and Maternal Transfer of Synthetic Phenolic Antioxidants in Pregnant Women from South China. Environ. Sci. Technol. 2019, 53, 13959–13969. [Google Scholar] [CrossRef]
- Tan, H.; Yang, L.; Huang, Y.; Tao, L.; Chen, D. “Novel” Synthetic Antioxidants in House Dust from Multiple Locations in the Asia-Pacific Region and the United States. Environ. Sci. Technol. 2021, 55, 8675–8682. [Google Scholar] [CrossRef]
- Wang, W.; Asimakopoulos, A.G.; Abualnaja, K.O.; Covaci, A.; Gevao, B.; Johnson-Restrepo, B.; Kumosani, T.A.; Malarvannan, G.; Minh, T.B.; Moon, H.-B.; et al. Synthetic Phenolic Antioxidants and Their Metabolites in Indoor Dust from Homes and Microenvironments. Environ. Sci. Technol. 2016, 50, 428–434. [Google Scholar] [CrossRef]
- Liu, Z.; Yin, H.; Dang, Z. Do Estrogenic Compounds in Drinking Water Migrating from Plastic Pipe Distribution System Pose Adverse Effects to Human? An Analysis of Scientific Literature. Environ. Sci. Pollut. Res. 2017, 24, 2126–2134. [Google Scholar] [CrossRef]
- Bell, A.M.; Baier, R.; Kocher, B.; Reifferscheid, G.; Buchinger, S.; Ternes, T. Ecotoxicological Characterization of Emissions from Steel Coatings in Contact with Water. Water Res. 2020, 173, 115525. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Zhang, X.; Liu, J.; Zhou, L.; Shang, Y.; Zhang, C.; Liu, W.; Zhuang, S. Natural Sunlight-Driven Aquatic Toxicity Enhancement of 2,6-Di-Tert-Butylphenol toward Photobacterium Phosphoreum. Environ. Pollut. 2019, 251, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Oztas, P.; Polat, M.; Cinar, L.; Alli, N. Shoe Dermatitis from Para-Tertiary Butylphenol Formaldehyde. Contact Dermat. 2007, 56, 294–295. [Google Scholar] [CrossRef] [PubMed]
- Sadakane, K.; Ichinose, T.; Takano, H.; Yanagisawa, R.; Koike, E.; Inoue, K. The Alkylphenols 4-Nonylphenol, 4-Tert-Octylphenol and 4-Tert-Butylphenol Aggravate Atopic Dermatitis-like Skin Lesions in NC/Nga Mice. J. Appl. Toxicol. 2014, 34, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Sarangarajan, R.; Caroline Le Poole, I.; Boissy, R.E.; Medrano, E.E. The Cytotoxicity and Apoptosis Induced by 4-Tertiary Butylphenol in Human Melanocytes Are Independent of Tyrosinase Activity. J. Investig. Dermatol. 2000, 114, 157–164. [Google Scholar] [CrossRef]
- Manga, P.; Sheyn, D.; Yang, F.; Sarangarajan, R.; Boissy, R.E. A Role for Tyrosinase-Related Protein 1 in 4-Tert-Butylphenol-Induced Toxicity in Melanocytes: Implications for Vitiligo. Am. J. Pathol. 2006, 169, 1652–1662. [Google Scholar] [CrossRef]
- Nair, X.; Tramposch, K.M. The Yucatan Miniature Swine as an in Vivo Model for Screening Skin Depigmentation. J. Dermatol. Sci. 1991, 2, 428–433. [Google Scholar] [CrossRef]
- Kawashima, T.; Yonemoto, K.; Gellin, G.A.; Epstein, W.L.; Fukuyama, K. Effects of 4-Tertiary Butyl Catechol on Glutathione-Metabolizing Enzymes In Vivo and In Vitro. J. Investig. Dermatol. 1984, 82, 53–56. [Google Scholar] [CrossRef]
- Harris, J.E. Chemical-Induced Vitiligo. Dermatol. Clin. 2017, 35, 151–161. [Google Scholar] [CrossRef]
- Wang, H.; Liu, J.; Qiang, S.; Che, Y.; Hu, T. 4-Tert-Butylphenol Impairs the Liver by Inducing Excess Liver Lipid Accumulation via Disrupting the Lipid Metabolism Pathway in Zebrafish. Environ. Pollut. 2024, 356, 124385. [Google Scholar] [CrossRef]
- Cui, J.; Qiu, M.; Liu, Y.; Liu, Y.; Tang, Y.; Teng, X.; Li, S. Nano-Selenium Protects Grass Carp Hepatocytes against 4-Tert-Butylphenol-Induced Mitochondrial Apoptosis and Necroptosis via Suppressing ROS-PARP1 Axis. Fish. Shellfish. Immunol. 2023, 135, 108682. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Zhou, Q.; Yu, M.; Liu, Y.; Teng, X.; Gu, X. 4-Tert-Butylphenol Triggers Common Carp Hepatocytes Ferroptosis via Oxidative Stress, Iron Overload, SLC7A11/GSH/GPX4 Axis, and ATF4/HSPA5/GPX4 Axis. Ecotoxicol. Environ. Saf. 2022, 242, 113944. [Google Scholar] [CrossRef] [PubMed]
- Ansar, S.; Tabassum, H.; Jameil, N. Al Protective Effect of Butylated Hydroxytoluene on Ferric Nitrilotriacetate Induced Hepatotoxicity and Oxidative Stress in Mice. Hum. Exp. Toxicol. 2013, 32, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Fahim, S.A.; Ibrahim, S.; Tadros, S.A.; Badary, O.A. Protective Effects of Butylated Hydroxytoluene on the Initiation of N-Nitrosodiethylamine-Induced Hepatocellular Carcinoma in Albino Rats. Hum. Exp. Toxicol. 2023, 42, 09603271231165664. [Google Scholar] [CrossRef]
- Al-Qahtani, W.H.; Binobead, M.A. Anti-Inflammatory, Antioxidant and Antihepatotoxic Effects of Spirulina Platensis against d-Galactosamine Induced Hepatotoxicity in Rats. Saudi J. Biol. Sci. 2019, 26, 647–652. [Google Scholar] [CrossRef]
- Guarisco, J.A.; Hall, J.O.; Coulombe, R.A. Mechanisms of Butylated Hydroxytoluene Chemoprevention of Aflatoxicosis—Inhibition of Aflatoxin B1 Metabolism. Toxicol. Appl. Pharmacol. 2008, 227, 339–346. [Google Scholar] [CrossRef]
- Guarisco, J.A.; Hall, J.O.; Coulombe, R.A. Butylated Hydroxytoluene Chemoprevention of Aflatoxicosis—Effects on Aflatoxin B1 Bioavailability, Hepatic DNA Adduct Formation, and Biliary Excretion. Food Chem. Toxicol. 2008, 46, 3727–3731. [Google Scholar] [CrossRef]
- Abou-Hadeed, A.H.; Mohamed, A.T.; Hegab, D.Y.; Ghoneim, M.H. Ethoxyquin and Butylated Hydroxy Toluene Distrub the Hematological Parameters and Induce Structural and Functional Alterations in Liver of Rats. Arch. Razi Inst. 2021, 76, 1765–1776. [Google Scholar] [CrossRef]
- Stierum, R.; Conesa, A.; Heijne, W.; van Ommen, B.; Junker, K.; Scott, M.P.; Price, R.J.; Meredith, C.; Lake, B.G.; Groten, J. Transcriptome Analysis Provides New Insights into Liver Changes Induced in the Rat upon Dietary Administration of the Food Additives Butylated Hydroxytoluene, Curcumin, Propyl Gallate and Thiabendazole. Food Chem. Toxicol. 2008, 46, 2616–2628. [Google Scholar] [CrossRef]
- Engin, A.B.; Bukan, N.; Kurukahvecioglu, O.; Memis, L.; Engin, A. Effect of Butylated Hydroxytoluene (E321) Pretreatment versus l-Arginine on Liver Injury after Sub-Lethal Dose of Endotoxin Administration. Environ. Toxicol. Pharmacol. 2011, 32, 457–464. [Google Scholar] [CrossRef]
- Sun, Z.; Tang, Z.; Yang, X.; Liu, Q.S.; Zhang, J.; Zhou, Q.; Jiang, G. 3-Tert-Butyl-4-Hydroxyanisole Impairs Hepatic Lipid Metabolism in Male Mice Fed with a High-Fat Diet. Environ. Sci. Technol. 2022, 56, 3204–3213. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, O.; Hiraga, K. Effects of Four Bisphenolic Antioxidants on Lipid Contents of Rat Liver. Toxicol. Lett. 1981, 8, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Takagi, A.; Kawasaki, N.; Momma, J.; Aida, Y.; Ohno, Y.; Hasegawa, R.; Kurokawa, Y. Toxicity Studies of a Synthetic Antioxidant, 2, 2′-Methylenebis (4-ethyl-6-tert-butylphenol) in Rats 2. Uncoupling Effect on Oxidative Phosphorylation of Liver Mitochondria. J. Toxicol. Sci. 1993, 18, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Ochiai, T.; Sekita, K.; Uchida, O.; Furuya, T.; Kurokawa, Y. Chronic Toxicity of 2, 4, 6-Tri-tert-butylphenol in Rats. J. Toxicol. Sci. 1991, 16, 167–179. [Google Scholar] [CrossRef]
- Yoshifumi, M.; Michihito, T.; Fumio, F.; Kazuhiro, T.; Hidetaka, S.; Yuzo, H. Pneumotoxicity of Butylated Hydroxytoluene Applied Dermally to CD-1 Mice. Toxicol. Lett. 1986, 34, 99–105. [Google Scholar] [CrossRef]
- Thompson, D.C.; Trush, M.A. Studies on the Mechanism of Enhancement of Butylated Hydroxytoluene-Induced Mouse Lung Toxicity by Butylated Hydroxyanisole. Toxicol. Appl. Pharmacol. 1988, 96, 122–131. [Google Scholar] [CrossRef]
- Bauer, A.K.; Dwyer-Nield, L.D. Chapter 10—Two-Stage 3-Methylcholanthrene and Butylated Hydroxytoluene-Induced Lung Carcinogenesis in Mice. In Carcinogen-Driven Mouse Models of Oncogenesis; Galluzzi, L., Buqué, A., Eds.; Methods in Cell Biology; Academic Press: Cambridge, MA, USA, 2021; Volume 163, pp. 153–173. [Google Scholar]
- Vikis, H.G.; Gelman, A.E.; Franklin, A.; Stein, L.; Rymaszewski, A.; Zhu, J.; Liu, P.; Tichelaar, J.W.; Krupnick, A.S.; You, M. Neutrophils Are Required for 3-Methylcholanthrene-Initiated, Butylated Hydroxytoluene-Promoted Lung Carcinogenesis. Mol. Carcinog. 2012, 51, 993–1002. [Google Scholar] [CrossRef]
- Shearn, C.T.; Fritz, K.S.; Thompson, J.A. Protein Damage from Electrophiles and Oxidants in Lungs of Mice Chronically Exposed to the Tumor Promoter Butylated Hydroxytoluene. Chem. Biol. Interact. 2011, 192, 278–286. [Google Scholar] [CrossRef]
- Bauer, A.K.; Dixon, D.; DeGraff, L.M.; Cho, H.-Y.; Walker, C.R.; Malkinson, A.M.; Kleeberger, S.R. Toll-Like Receptor 4 in Butylated Hydroxytoluene–Induced Mouse Pulmonary Inflammation and Tumorigenesis. JNCI J. Natl. Cancer Inst. 2005, 97, 1778–1781. [Google Scholar] [CrossRef]
- Eskandani, M.; Hamishehkar, H.; Ezzati Nazhad Dolatabadi, J. Cytotoxicity and DNA Damage Properties of Tert-Butylhydroquinone (TBHQ) Food Additive. Food Chem. 2014, 153, 315–320. [Google Scholar] [CrossRef]
- Endo, S.; Nishiyama, A.; Suyama, M.; Takemura, M.; Soda, M.; Chen, H.; Tajima, K.; El-Kabbani, O.; Bunai, Y.; Hara, A.; et al. Protective Roles of Aldo-Keto Reductase 1B10 and Autophagy against Toxicity Induced by p-Quinone Metabolites of Tert-Butylhydroquinone in Lung Cancer A549 Cells. Chem. Biol. Interact. 2015, 234, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Olsen, C.M.; Meussen-Elholm, E.T.M.; Holme, J.A.; Hongslo, J.K. Brominated Phenols: Characterization of Estrogen-like Activity in the Human Breast Cancer Cell-Line MCF-7. Toxicol. Lett. 2002, 129, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Myllymäki, S.; Haavisto, T.; Vainio, M.; Toppari, J.; Paranko, J. In Vitro Effects of Diethylstilbestrol, Genistein, 4-Tert-Butylphenol, and 4-Tert-Octylphenol on Steroidogenic Activity of Isolated Immature Rat Ovarian Follicles. Toxicol. Appl. Pharmacol. 2005, 204, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Haavisto, T.E.; Adamsson, N.A.; Myllymäki, S.A.; Toppari, J.; Paranko, J. Effects of 4-Tert-Octylphenol, 4-Tert-Butylphenol, and Diethylstilbestrol on Prenatal Testosterone Surge in the Rat. Reprod. Toxicol. 2003, 17, 593–605. [Google Scholar] [CrossRef]
- Pérez-Albaladejo, E.; Lacorte, S.; Porte, C. Differential Toxicity of Alkylphenols in JEG-3 Human Placental Cells: Alteration of P450 Aromatase and Cell Lipid Composition. Toxicol. Sci. 2019, 167, 336–346. [Google Scholar] [CrossRef]
- Pop, A.; Drugan, T.; Gutleb, A.C.; Lupu, D.; Cherfan, J.; Loghin, F.; Kiss, B. Estrogenic and Anti-Estrogenic Activity of Butylparaben, Butylated Hydroxyanisole, Butylated Hydroxytoluene and Propyl Gallate and Their Binary Mixtures on Two Estrogen Responsive Cell Lines (T47D-Kbluc, MCF-7). J. Appl. Toxicol. 2018, 38, 944–957. [Google Scholar] [CrossRef]
- Sun, Z.; Gao, R.; Chen, X.; Liu, X.; Ding, Y.; Geng, Y.; Mu, X.; Liu, T.; Li, F.; Wang, Y.; et al. Exposure to Butylated Hydroxytoluene Compromises Endometrial Decidualization during Early Pregnancy. Environ. Sci. Pollut. Res. 2021, 28, 42024–42036. [Google Scholar] [CrossRef]
- Yang, X.; Song, W.; Liu, N.; Sun, Z.; Liu, R.; Liu, Q.S.; Zhou, Q.; Jiang, G. Synthetic Phenolic Antioxidants Cause Perturbation in Steroidogenesis in Vitro and in Vivo. Environ. Sci. Technol. 2018, 52, 850–858. [Google Scholar] [CrossRef]
- Pop, A.; Drugan, T.; Gutleb, A.C.; Lupu, D.; Cherfan, J.; Loghin, F.; Kiss, B. Individual and Combined in Vitro (Anti)Androgenic Effects of Certain Food Additives and Cosmetic Preservatives. Toxicol. Vitr. 2016, 32, 269–277. [Google Scholar] [CrossRef]
- Ham, J.; Lim, W.; Whang, K.-Y.; Song, G. Butylated Hydroxytoluene Induces Dysregulation of Calcium Homeostasis and Endoplasmic Reticulum Stress Resulting in Mouse Leydig Cell Death. Environ. Pollut. 2020, 256, 113421. [Google Scholar] [CrossRef]
- Takahashi, O.; Oishi, S. Male Reproductive Toxicity of Four Bisphenol Antioxidants in Mice and Rats and Their Estrogenic Effect. Arch. Toxicol. 2006, 80, 225–241. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.G.; Jeong, S.H.; Cho, J.H.; Kim, D.G.; Park, J.M.; Cho, M.H. Evaluation of Estrogenic and Androgenic Activity of Butylated Hydroxyanisole in Immature Female and Castrated Rats. Toxicology 2005, 213, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Xiong, G.; Wu, Z.; Yi, J.; Fu, L.; Yang, Z.; Hsieh, C.; Yin, M.; Zeng, X.; Wu, C.; Lu, A.; et al. ADMETlab 2.0: An Integrated Online Platform for Accurate and Comprehensive Predictions of ADMET Properties. Nucleic Acids Res. 2021, 49, W5–W14. [Google Scholar] [CrossRef] [PubMed]
- Lamas Bervejillo, M.; Ferreira, A.M. Understanding Peroxisome Proliferator-Activated Receptors: From the Structure to the Regulatory Actions on Metabolism. In Bioactive Lipids in Health and Disease; Trostchansky, A., Rubbo, H., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 39–57. ISBN 978-3-030-11488-6. [Google Scholar]
- Christofides, A.; Konstantinidou, E.; Jani, C.; Boussiotis, V.A. The Role of Peroxisome Proliferator-Activated Receptors (PPAR) in Immune Responses. Metabolism 2021, 114, 154338. [Google Scholar] [CrossRef]
- Brewer, M.S. Natural Antioxidants: Sources, Compounds, Mechanisms of Action, and Potential Applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 221–247. [Google Scholar] [CrossRef]
- Vilchis-Landeros, M.M.; Vázquez-Meza, H.; Vázquez-Carrada, M.; Uribe-Ramírez, D.; Matuz-Mares, D. Antioxidant Enzymes and Their Potential Use in Breast Cancer Treatment. Int. J. Mol. Sci. 2024, 25, 5675. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Novel Antioxidants in Food Quality Preservation and Health Promotion. Eur. J. Lipid Sci. Technol. 2010, 112, 930–940. [Google Scholar] [CrossRef]
| Group | No. | Chemicals | Structure | Common Name | Abbreviation | CAS. No |
|---|---|---|---|---|---|---|
| Mono-TBP | 1 | 4-Tert-butylphenol | 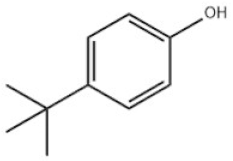 | PTBP | PTBP | 98-54-4 |
| 2 | 2-Tert-Butyl-4,6-dimethylphenol | 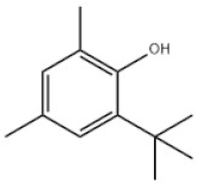 | Antioxidant AO30 | AO30 | 1879-09-0 | |
| 3 | 2,6-Di-tert-butylphenol | 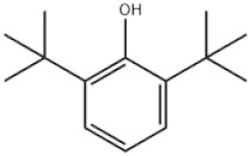 | Ethanox 701 | AO701 | 128-39-2 | |
| 4 | Butylated Hydroxytoluene | 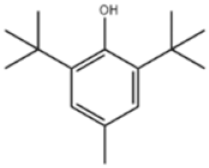 | BHT | BHT | 128-37-0 | |
| 5 | 2,6-Di-tert-Butyl-4-(dimethylaminomethyl)phenol |  | Antioxidant 703 | AO703 | 88-27-7 | |
| 6 | Diethyl 3,5-di-tert-butyl-4-hydroxybenzylphosphonate | 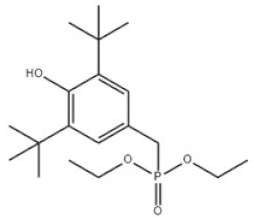 | Antioxidant 1222 | AO1222 | 976-56-7 | |
| 7 | Octadecyl 3-(3,5-ditert-butyl-4-hydroxyphenyl)propanoate |  | Antioxidant 1076 | AO1076 | 2082-79-3 | |
| 8 | 4-((4,6-Bis(octylthio)-1,3,5-triazin-2-yl)amino)-2,6-di-tert-butylphenol | 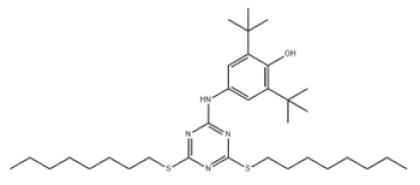 | Antioxidant 565 | AO565 | 991-84-4 | |
| 9 | Calcium bis(ethyl 3,5-di-tert-butyl-4-hydroxybenzylphosphonate) | 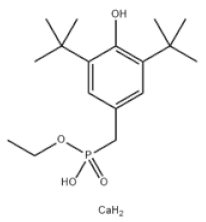 | Antioxidant 1425 | AO1425 | 65140-91-2 | |
| 10 | 2,4,6-Tri-tert-butylphenol | 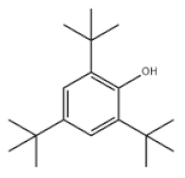 | Antioxidant 246 | AO246 | 732-26-3 | |
| 11 | 2-Tert-Butyl-4-methoxyphenol |  | 3-BHA | BHA | 121-00-6 | |
| 12 | 2,4-Di-tert-butylphenol | 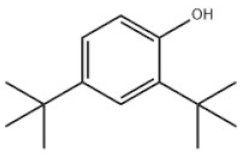 | Antioxidant 33 | AO33 | 96-76-4 | |
| 13 | Tert-Butylhydroquinone | 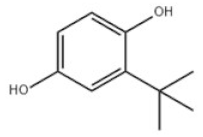 | TBHQ | TBHQ | 1948-33-0 | |
| 14 | 3,5-Di-tert-butyl-4-hydroxybenzyl alcohol |  | Antioxidant 754 | AO754 | 88-26-6 | |
| 15 | Octyl-3,5-di-tert-butyl-4-hydroxy-hydrocinnamate |  | Antioxidant 1135 | AO1135 | 125643-61-0 | |
| 16 | 11-Methyldodecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propanoate | 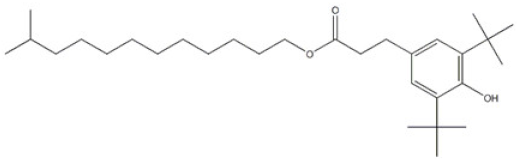 | Antioxidant 1077 | AO1077 | 847488-62-4 | |
| 17 | 4-Sec-Butyl-2,6-di-tert-butylphenol |  | ISONOX 132 | AO132 | 17540-75-9 | |
| 18 | Methyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate |  | Ralox 35 | AO35 | 6386-38-5 | |
| 19 | 6-Tert-Butyl-m-cresol |  | TBMC | 88-60-8 | ||
| Di-TBP | 20 | 2,2′-Methylenebis(4-methyl-6-tert-butylphenol) | 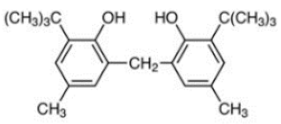 | Antioxidant 2246 | AO2246 | 119-47-1 |
| 21 | 2,2′-Thiobis(6-tert-butyl-p-cresol) | 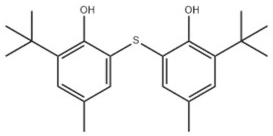 | Antioxidant 1081 | AO1081 | 90-66-4 | |
| 22 | 2,2′-Methylenebis(4-ethyl-6-tert-butylphenol) |  | Antioxidant 425 | AO425 | 88-24-4 | |
| 23 | Santowhite | 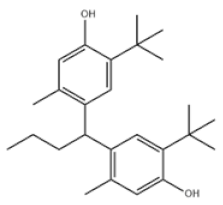 | Santowhite | STW | 85-60-9 | |
| 24 | 2-Tert-Butyl-6-(3-tert-butyl-2-hydroxy-5-methylbenzyl)-4-methylphenyl acrylate |  | Antioxidant 3052 | AO3052 | 61167-58-6 | |
| 25 | 4,4′-Methylenebis(2,6-Di-tert-butylphenol) | 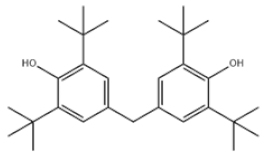 | Antioxidant 702 | AO702 | 118-82-1 | |
| 26 | 1,2-Bis(3,5-di-tert-butyl-4-hydroxyhydrocinnamoyl)hydrazine |  | Antioxidant 1024 | AO1024 | 32687-78-8 | |
| 27 | Triethylene glycol bis(3-tert-butyl-4-hydroxy-5-methylphenyl)propionate |  | Antioxidant 245 | AO245 | 36443-68-2 | |
| 28 | N,N’-Propane-1,3-diylbis[3-(3,5-DI-tert-butyl-4-hydroxyphenyl)propionamide] | 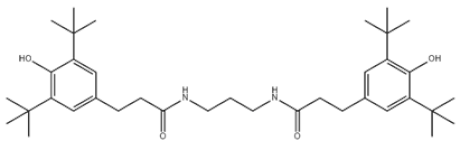 | Antioxidant 1019 | AO1019 | 69851-61-2 | |
| 29 | 3,9-Bis(2,4-di-tert-butylphenoxy)-2,4,8,10-tetraoxa-3,9-diphosphaspiro[5.5]undecane |  | Ultranox 626 | AO626 | 26741-53-7 | |
| 30 | Bis(2,6-di-tert-butyl-4-methylphenyl)pentaerythritol diphosphite |  | Antioxidant PEP-36 | AO36 | 80693-00-1 | |
| 31 | Hexamethylene bis[3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate] |  | Irganox 259 | AO259 | 35074-77-2 | |
| 32 | Irganox 1035 |  | Antioxidant 1035 | AO1035 | 41484-35-9 | |
| 33 | Sumilizer AG 80 |  | Antioxidant AO80 | AO80 | 90498-90-1 | |
| 34 | 2,2′-Ethylidenebis(4,6-di-tert-butylphenol) | 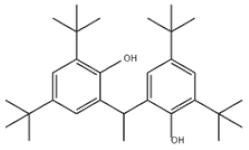 | Antioxidant 1290 | AO1290 | 35958-30-6 | |
| 35 | Benzenepropanamide, N,N’-1,6-hexanediylbis(3,5-bis(1,1-dimethylethyl)-4-hydroxy- | 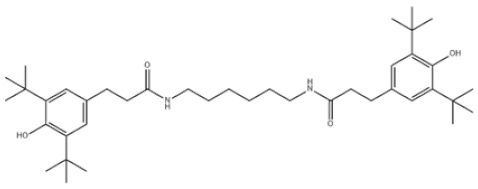 | Antioxidant 1098 | AO1098 | 23128-74-7 | |
| 36 | Naugard XL-1 |  | Antioxidant MD-697 | AO697 | 70331-94-1 | |
| 37 | 4,4′-Thiobis(6-tert-butyl-m-cresol) | 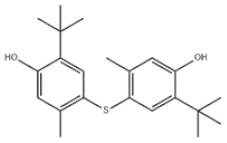 | Antioxidant 300 | AO300 | 96-69-5 | |
| Poly-TBP | 38 | 1,1,3-Tris(2-methyl-4-hydroxy-5-tert-butylphenyl)butane | 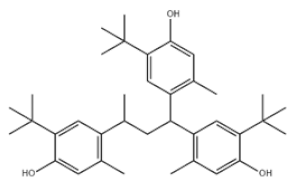 | Antioxidant CA | AOCA | 1843-03-4 |
| 39 | Tris(2,4-di-tert-butylphenyl) phosphite | 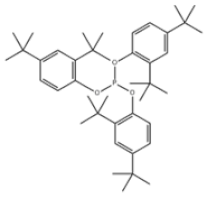 | Antioxidant 168 | AO168 | 31570-04-4 | |
| 40 | 1,3,5-Triazine-2,4,6(1H,3H,5H)-trione, 1,3,5-tris[[4-(1,1-dimethylethyl)-3-hydroxy-2,6-dimethylphenyl]methyl]- | 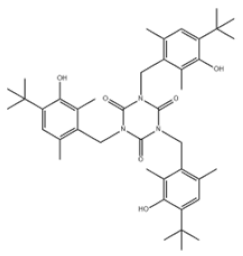 | Antioxidant 1790 | AO1790 | 40601-76-1 | |
| 41 | 1,3,5-Trimethyl-2,4,6-tris(3,5-di-tert-butyl-4-hydroxybenzyl)benzene | 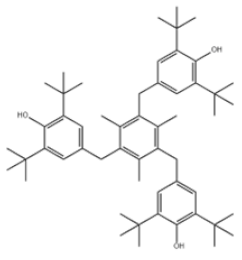 | Antioxidant 1330 | AO1330 | 1709-70-2 | |
| 42 | 1,3,5-Tris[(3,5-ditert-butyl-4-hydroxyphenyl)methyl]-1,3,5-triazinane-2,4,6-trione |  | Antioxidant 3114 | AO3114 | 27676-62-6 | |
| 43 | Pentaerythritol tetrakis(3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate) |  | Antioxidant 1010 | AO1010 | 6683-19-8 |
| No. | Compounds | MW (g/mol) | Melting Point | Boiling Point | Form | Color | LogP | pKa |
|---|---|---|---|---|---|---|---|---|
| 1 | PTBP | 150.22 | 96–101 °C | 236–238 °C | Flakes or pastilles | White to light beige | 3 at 23 °C | 10.23 at 25 °C |
| 2 | AO30 | 178.27 | 22 °C | 249 °C | Powder to lump to clear liquid | White or colorless to light yellow | 3.64 at 35 °C | 12.00 ± 0.23 |
| 3 | AO701 | 206.32 | 34–37 °C | 253 °C | Crystalline solid | White to light yellow | 4.5 at 24 °C | 12.16 ± 0.40 |
| 4 | BHT | 220.35 | 69–73 °C | 265 °C | Crystals | White | 5.2 | 14 (uncertain) |
| 5 | AO703 | 263.42 | 93–94 °C | 172 °C at 30 mm Hg | Powder to crystal | White to yellow to orange | 4.24 | 11.17 ± 0.70 |
| 6 | AO1222 | 356.44 | 122 °C | 417.0 ± 33.0 °C | Powder to crystal | White to almost white | 2.9 at 23 °C | 12.04 ± 0.40 |
| 7 | AO1076 | 530.86 | 50–52 °C | 568.1 ± 45.0 °C | - | White to off-white | 13.930 | 12.33 ± 0.40 |
| 8 | AO565 | 588.95 | 92–95 °C | 670.7 ± 65.0 °C | Solid | White to off-white | 13.2 at 25 °C | 12.27 ± 0.70 |
| 9 | AO1425 | 370.48 | - | - | - | - | −0.1 at 23 °C | - |
| 10 | AO246 | 262.43 | 125–130 °C | 277 °C | Buffered aqueous glycerol solution | Off-white to pale yellow | 7.1 at 35 °C | 12.61 ± 0.40 |
| 11 | BHA | 180.24 | 58–64 °C | 269 °C | Powder/waxy solid | White to off-white | 1–2.82 at 25–27 °C | 11.83 ± 0.18 |
| 12 | AO33 | 206.32 | 53–56 °C | 265 °C | Crystalline solid | White to yellow | 4.8 at 23 °C | 11.56 ± 0.18 |
| 13 | TBHQ | 166.22 | 127–129 °C | 295 °C | Crystalline powder | White to light tan; may contain black specks | 1.521 at 25 °C | 10.80 ± 0.18 |
| 14 | AO754 | 236.35 | 139–141 °C | 214 °C at 40 mm Hg | Solid: particulate/powder | White to yellow to orange | 3.16 at 20 °C | 12.01 ± 0.40 |
| 15 | AO1135 | 390.6 | - | 343–370.65 °C at 101 325 Pa | Oil | Colorless | 7.18–9.2 at 0–30 °C | - |
| 16 | AO1077 | 460.73 | - | 220–245 °C at 101.3 kPa | Viscous | - | 3.56 at 25 °C | - |
| 17 | AO132 | 262.43 | 25 °C | 141–142 °C at 10 mm Hg | Liquid | White or colorless to yellow | 7.2 at 20 °C | 11.85 ± 0.70 |
| 18 | AO35 | 292.41 | 60–67 °C | 125–130 °C (Press: 0.1 Torr) | Powder to crystal | White to almost white | 4.866 | 12.17 ± 0.40 |
| 19 | TBMC | 164.24 | 20 °C | 117–118 °C at 12 mm Hg | - | Colorless to red to green | 3.97 | 11.45 ± 0.10 |
| 20 | AO2246 | 340.5 | 123–127 °C | 187 °C | Solid | White to off-white | 6.25 at 20 °C | 11.32 ± 0.48 |
| 21 | AO1081 | 358.54 | 84–85 °C | 431.1 ± 45.0 °C | - | - | 6.5 at 25 °C | 10.30 ± 0.50 |
| 22 | AO425 | 368.55 | 119–122 °C | 458.86 °C | Solid | White to off-white | 8.95 at 20 °C | 11.37 ± 0.48 |
| 23 | STW | 382.58 | 211 °C | 469.7 °C | Powder to crystal | White to almost white | 6.4 at 20 °C | 10.44 ± 0.20 |
| 24 | AO3052 | 394.55 | 128–133 °C | 491 °C | Solid | White to off-white | - | 11.66 ± 0.48 |
| 25 | AO702 | 424.66 | 155–159 °C | 289 °C at 40 mm Hg | solid | White | 8.9 at 25 °C | 12.03 ± 0.40 |
| 26 | AO1024 | 552.79 | 60–67 °C | 652.6 ± 55.0 °C | Solid | White to off-white | 4.8 at 23 °C | 11.10 ± 0.50 |
| 27 | AO245 | 586.77 | 79–81 °C | 602.88 °C | Solid | White to off-white | 4.7 at 23 °C | 11.44 ± 0.25 |
| 28 | AO1019 | 594.87 | - | 711.0 ± 60.0 °C | - | - | 8.13 | 12.08 ± 0.40 |
| 29 | AO626 | 604.69 | 160–178 °C | 555.8 ± 50.0 °C | Solid | White to off-white | 10.9 at 25 °C | - |
| 30 | AO36 | 632.75 | 235–240 °C | 577.0 ± 50.0 °C | - | - | 6 at 25 °C | - |
| 31 | AO259 | 638.92 | 102–105 °C | 648.1 ± 55.0 °C | Solid | Off-white | - | 12.03 ± 0.40 |
| 32 | AO1035 | 642.94 | 78 °C | 659.4 ± 55.0 °C | Solid | White to off-white | - | 12.02 ± 0.40 |
| 33 | AO80 | 740.98 | 125 °C | 755.7 ± 55.0 °C | Solid | White to off-white | - | 11.44 ± 0.25 |
| 34 | AO1290 | 438.68 | 162–164 °C | 464.8 ± 40.0 °C | - | - | 9.840 | 11.28 ± 0.50 |
| 35 | AO1098 | 636.95 | 156–161 °C | 740.1 ± 60.0 °C | Solid | White to off-white | 9.6 at 25 °C | 12.08 ± 0.40 |
| 36 | AO697 | 696.91 | 178 °C | - | Solid | White to off-white | 8.1 at 35 °C | 11.48 ± 0.46 |
| 37 | AO300 | 358.54 | 160–165 °C | 460.94 °C | Powder | White to off-white | 5.24 at 25 °C | 10.76 ± 0.36 |
| 38 | AOCA | 544.81 | 183–190 °C | 578.54 °C | - | - | 12.7 at 25 °C | 10.38 ± 0.20 |
| 39 | AO168 | 646.94 | 181–184 °C | 594.2 ± 50.0 °C | Powder | White | 18 at 25 °C | - |
| 40 | AO1790 | 699.92 | 163–165 °C | 793.8 ± 60.0 °C | Solid | White to off-white | 15.281 at 20 °C | 11.36 ± 0.28 |
| 41 | AO1330 | 775.2 | 248–250 °C | 739.54 °C | Solid | White to off-white | 17.17 | 11.91 ± 0.40 |
| 42 | AO3114 | 784.08 | 218–220 °C | 757.9 ± 60.0 °C | - | White to off-white | 15.18 | 11.45 ± 0.40 |
| 43 | AO1010 | 1177.66 | 115–118 °C | 779.1 °C | Solid | White to off-white | 18.832 | 11.71 ± 0.40 |
| No. | Compounds | Consumer Products and Materials Using TBP-AOs | ||
|---|---|---|---|---|
| ECHA | Chemicalbook | ChemBK | ||
| 1 | PTBP | Sealants, adhesives, and coatings. | Sealants, adhesives, coating products, polymers, and other materials. | |
| 2 | AO30 | Fuels, hydraulic fluids, metal working fluids, lubricants, and greases. | Jet and rocket fuels. | Fuel, acrylic acid polymerization, and pharmaceuticals. |
| 3 | AO701 | Fuels, lubricants, and greases. | Fuels, lubricants, plastics, rubber, and polymers. | Fuel, rubber and plastic, UV absorber, and pesticide and dye applications. |
| 4 | BHT | Cleaning products, plant protection, lubricants, greases, adhesives, sealants, polishes, waxes, coating, and fertilizers. | Oils and fat-containing foods. | Rubber, plastic, gasoline, oil, and food. |
| 5 | AO703 | Rubber, synthetic resin, gasoline, and oil. | ||
| 6 | AO1222 | Polyester, polycondensation, dimethyl terephthalate, polyamide, and UV absorber applications. | ||
| 7 | AO1076 | Coatings, lubricants, greases, adhesives, sealants, polishes, waxes, air care, and cleaning products. | Plastics, synthetic fibers, elastomers, adhesives, waxes, oils, fats, polyolefin, and other polymers. | Resin, rubber, petroleum, polyolefin, and polyvinyl chloride. |
| 8 | AO565 | Adhesives and sealants. | Resin, elastomers, adhesives, polystyrene, polyamide, and polyolefin. | Resins, elastomers, rubber, adhesives, and ABS plastic. |
| 9 | AO1425 | Rosin, resin, and polymers (*). | ||
| 10 | AO246 | Electromagnetic bushing. | Rubber. | |
| 11 | BHA | Cosmetics and personal care products. | Cosmetics, topical medications, foods, fuels, rubber, plastics, paints, and glues. | Organic raw materials, biochemical reagents, etc. |
| 12 | AO33 | Fuels. | Polyolefins, UV stabilizers, pharmaceuticals, and fragrances. | Used as a chemical intermediate, light stabilizer, UV absorber, and plasticizer. |
| 13 | TBHQ | Cosmetics, edible fats and vegetable oils, potato chips, and dry cereal. | Cosmetics, edible oils and fats, lard, frying foods, rubber, resin, plastics, pharmaceuticals, etc. | |
| 14 | AO754 | Gasoline and other hydrocarbons. | ||
| 15 | AO1135 | Polymers, food packaging, and textile staining. | Rubber, elastomer, metal deactivators, polyether, polyurethane, oil. | |
| 16 | AO1077 | Plastics, synthetic fiber, waxes, greases, elastomers, and rubber. | ||
| 17 | AO132 | Polyols, PVC, adhesives, and functional fluids. | ||
| 18 | AO35 | Cosmetics, biocides, fragrances, polishes, waxes, cleaning, air care, and personal care products. | Polyethylene. | |
| 19 | TBMC | Lubricating oil and others. | Organic synthesis intermediate. | |
| 20 | AO2246 | Fuels, adhesives, sealants, lubricants, greases, and hydraulic and metal working fluids. | Distilled biodiesel. | Rubber, latex, other materials, and petroleum products. |
| 21 | AO1081 | Rubber and polymer materials. | ||
| 22 | AO425 | Rubber and synthetic resin. | ||
| 23 | STW | Coatings, adhesives, and sealants. | Polyolefin and rubber. | |
| 24 | AO3052 | Adhesive, plastic, and elastomer materials. | Cosmetics, food processing, plastics, rubber, paint, drugs. | |
| 25 | AO702 | Coatings, lubricants and greases, and washing and cleaning products. | Polymers and resin. | |
| 26 | AO1024 | Packaging polymers, resins, adhesives, and food contact. | Polyolefin materials, wires, cables, and insulating materials. | |
| 27 | AO245 | Adhesives and polymers for food contact. | Rubber, latex, resins, and polymers. | |
| 28 | AO1019 | Wires and cables and metal-contact materials (*). | ||
| 29 | AO626 | Coatings, adhesives, sealants, inks, and toners. | Food contact, rubber, elastomers, coatings, adhesives, polymers, and plastics. | Polymer materials. |
| 30 | AO36 | Plastics. | ||
| 31 | AO259 | Lubricants and greases. | ||
| 32 | AO1035 | Wire, cable, polymers, resins, and adhesives. | Coatings, plastics, and rubber. | |
| 33 | AO80 | Polymers, plastics, rubber, adhesives, sealants, etc. | ||
| 34 | AO1290 | Act as antioxidant. | ||
| 35 | AO1098 | Plastics, adhesives, elastomers, polymers, and fibers. | Polymers, resin, and rubber. | |
| 36 | AO697 | Cables, polymers, resin, and other materials. | ||
| 37 | AO300 | Rubber, plastics, and food contact polymers. | Polyethylene packaging film, rubber, resin, etc. | |
| 38 | AOCA | Adhesives and sealants. | Polymers, resins, and light-colored rubber products. | |
| 39 | AO168 | Coatings, adhesives and sealants, inks, and toners. | Polymers, resin, plastics, binding agent, rubber, and petroleum. | Polymers, fiber, resin, and other plastics. |
| 40 | AO1790 | Polystyrene and rubber-modified polystyrene in food contact. | Nylon, pipes, agricultural films, household appliances, polymers, resin, and plastics. | |
| 41 | AO1330 | Adhesives, sealants, lubricants, and greases. | Polymers, elastomers, fibers, adhesives, waxes, oils, and fats | Polymers, plastics, resin, and rubber. |
| 42 | AO3114 | Adhesives and sealants. | Nonfatty food packaging and propylene copolymers. | Polymers. |
| 43 | AO1010 | Coatings, adhesives, sealants, lubricants, greases, polishes, waxes, and cleaning and air care products. | Plastics, fibers, elastomers, adhesives, waxes, oils, and fats. | Polymers, resin, and plastic products. |
| Compounds | Mean EDI (mg/kg bw/day) | Subjects | Country | Year | Ref. |
|---|---|---|---|---|---|
| BHA | 0.14–0.17 | 16,014 households | Brazil | 1997–1999, 2003 | [77] |
| 5.49–12.12 (mg/person/day) | 13,000 individuals of all ages | Canada | 1973 | [78] | |
| 0–0.04 | 11,525 individuals of all ages | Korea | 1998 | [79] | |
| 0.001–0.017 | 3003 individuals of all ages | France | 1998–1999 | [80] | |
| 0–0.72 | 706 children (aged 1–36 months) | France | 2005 | [81] | |
| 5.1 × 10−4–0.3 | 134 samples | Korea | 2005 | [82] | |
| 0.15 | 230 children (aged 9–18 years) | Lebanon | 2002–2003 | [83] | |
| BHA and/or BHT | 0.075 | 5898 individuals of all ages | The Netherlands | 1987/1988 | [84] |
| BHT | 0.09–0.11 | 17,014 households | Brazil | 1997–1999, 2003 | [77] |
| 0.13–0.39 | 13,000 individuals of all ages | Canada | 1973 | [78] | |
| 1.56 × 10−5–0.04 | 11,525 individuals of all ages | Korea | 1998 | [79] | |
| 0–0.013 | 3003 individuals of all ages | France | 1998–1999 | [80] | |
| 0–0.267 | 441–4079 individuals of all ages | France, Italy, the UK, Ireland | 1992–2007 | [85] | |
| 0.018–0.025 | 230 children (age 9–18 years) | Lebanon | 2002–2003 | [83] | |
| 7.5 × 10−4–0.29 | 131 samples | Korea | 2005 | [82] | |
| 0.003–0.087 | 32 different dietary surveys on peoples of all ages | Europe (22 countries) | 2010 | [86] | |
| 1.4 × 10−03 | Snack and cookie samples | China | 2019 | [67] | |
| 2.51 × 10−5–4.71 × 10−5 | 1780 individuals (aged 6 months–17 years) | Spain | 2012–2014 | [87] | |
| 6.61 × 10−3 | 952 individuals of all ages | China | 2021 | [88] | |
| TBHQ | 0.11–0.14 | 18 014 households | Brazil | 1997–1999, 2003 | [77] |
| 1.2 × 10−6–0.04 | 11,525 individuals of all ages | Korea | 1998 | [79] | |
| 2.5 × 10−4–0.28 | 104 samples | Korea | 2005 | [82] | |
| AO1076 | 1.07 × 10−3 | Snack and cookie samples | China | 2019 | [67] |
| AO2246 | 7.81 × 10−5 | Snack and cookie samples | China | 2019 | [67] |
| AO245 | 1.40 × 10−5 | Snack and cookie samples | China | 2019 | [67] |
| No. | Chemicals | EPA | ECHA | |
|---|---|---|---|---|
| Total Exposed Workers * | 2019 National Aggregated Production (lbs.) | Annual Manufactured and Import Volume (tons) | ||
| 1 | PTBP | 235–515 | 20,000,000–100,000,000 | ≥10,000 |
| 2 | AO30 | 25–60 | 1,000,000–20,000,000 | 100–1000 |
| 3 | AO701 | 75–220 | 100,000,000–1,000,000,000 | 1000–10,000 |
| 4 | BHT | 905–2265 | 1,000,000–10,000,000 | 10,000–100,000 |
| 5 | AO703 | 10–35 | <1,000,000 | 100–1000 |
| 6 | AO1222 | - | - | 10–100 |
| 7 | AO1076 | 970–2700 | 20,000,000–100,000,000 | ≥10,000 |
| 8 | AO565 | 25–80 | 100,000–<500,000 | 100–1000 |
| 9 | AO1425 | 10–45 | <1,000,000 | 100–1000 |
| 10 | AO246 | 75–160 | 20,000,000–100,000,000 | 100–1000 |
| 11 | BHA | - | - | ≥10 |
| 12 | AO33 | 125–260 | 20,000,000–100,000,000 | ≥1000 |
| 13 | TBHQ | - | <1,000,000 | 100–1000 |
| 14 | AO754 | - | - | 1–10 |
| 15 | AO1135 | 620–1390 | 10,000,000–50,000,000 | - |
| 16 | AO1077 | - | - | 10–100 |
| 17 | AO1320 | 25–49 | 1,000,000–20,000,000 | 10–100 |
| 18 | AO35 | 3000–5996 | 20,000,000–100,000,000 | 1–100 |
| 19 | TBMC | 25–49 | 1,000,000–20,000,000 | - |
| 20 | AO2246 | 700–1750 | 1,000,000–10,000,000 | 1000–10,000 |
| 21 | AO1081 | - | - | 10–100 |
| 22 | AO425 | <10 | <1,000,000 | 1–10 |
| 23 | STW | - | 100,000–500,000 | 100–1000 |
| 24 | AO3052 | - | - | 10–100 |
| 25 | AO702 | 50–110 | 1,000,000–20,000,000 | 100–1000 |
| 26 | AO1024 | 50–130 | 1,000,000–10,000,000 | 100–1000 |
| 27 | AO245 | 200–1040 | 1,000,000–10,000,000 | 1000–10,000 |
| 28 | AO1019 | - | - | 10–100 |
| 29 | AO626 | 50–120 | 1,000,000–20,000,000 | 1000–10,000 |
| 30 | AO36 | <10 | 138713 | 10 |
| 31 | AO259 | 500–1020 | <1,000,000 | 100–1000 |
| 32 | AO1035 | 500–1020 | 1,000,000–20,000,000 | 100–1000 |
| 33 | AO80 | <10 | <1,000,000 | - |
| 34 | AO1290 | 60–125 | <1,000,000 | - |
| 35 | AO1098 | 50–110 | <1,000,000 | 1000–10,000 |
| 36 | AO697 | 50–100 | <1,000,000 | 100–1000 |
| 37 | AO300 | 50–110 | 100,000–500,000 | 100–1000 |
| 38 | AOCA | 1050–10130 | 100,000–500,000 | 100–1000 |
| 39 | AO168 | 1985–4995 | 10,000,000–50,000,000 | 10,000–100,000 |
| 40 | AO1790 | 75–160 | 1,000,000–20,000,000 | 100–1000 |
| 41 | AO1330 | 100–240 | 1,000,000–20,000,000 | 1000–10,000 |
| 42 | AO3114 | 75–180 | 1,000,000–10,000,000 | 1000–10,000 |
| 43 | AO1010 | 2070–5490 | 50,000,000–100,000,000 | ≥10,000 |
| Compounds | Model | Treatment | Testosterone (LOEL) | Androgen | Aromatase | Progesterone (LOEL) | Estrogen (LOEL) | Ref. | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose | Period | AGO | ANT | AGO | ANT (IC50) | AGO | ANT (IC50) | AGO | ANT | AGO | ANT | |||
| PTBP | Isolated fetuses’ testes | 10–500 mg/L | 24 h | 100 mg/L | - | - | - | - | - | 100 mg/L | - | - | - | [128] |
| Isolated immature rat ovarian follicles | 0.01–1 μM | 3 days | 0.01 μM | 0.1 μM | - | - | - | - | - | - | - | 0.1 μM | [127] | |
| 5 days | - | 0.1 μM | - | - | N | - | - | - | - | 0.01 μM | ||||
| MCF-7 cells | 10 nM–10 μM | 24 h | - | - | - | - | - | - | - | - | - | 10 μM | [126] | |
| JEG-3 cells | 5–500 μM | 24 h | - | - | - | - | - | 283 μM | - | - | - | - | [129] | |
| BHT | T47D-Kbluc cells | 0.3–200 μM | 24 h | - | - | - | - | - | - | - | - | N | IC25 = 15,734 μM | [130] |
| MCF-7 cells | 0.3–200 μM | 24 h | - | - | - | - | - | - | - | - | 10 μM | N | ||
| MDA-kb2 cells | 0.3–300 μM | 24 h | - | - | N | 43.2 μM | - | - | - | - | - | - | [133] | |
| Pregnant CD1 mice | 200,400 mg/kg/d | 6 days | - | - | - | - | - | - | 200 mg/kg/d | - | 200 mg/kg/d | - | [131] | |
| H295R cells | 1–100 μM | 48 h | N | - | N | - | - | - | - | N | 100 μM | - | [132] | |
| BHA | T47D-Kbluc cells | 0.3–100 μM | 24 h | - | - | - | - | - | - | - | - | 100 μM | IC50 = 100.22 μM | [130] |
| MCF-7 cells | 0.3–200 μM | 24 h | - | - | - | - | - | - | - | - | EC25 = 5.53 μM, EC40 = 8.96 μM | IC50 = 116.83 μM | ||
| H295R cells | 1–100 μM | 48 h | N | - | N | - | - | - | - | N | 1 μM | - | [132] | |
| Zebrafish gonads | 1–5 μM | 21 days | 1 μM | - | - | - | - | - | - | - | 1 μM | - | ||
| MDA-kb2 cells | 0.3–300 μM | 24 h | - | - | N | 172.5 μM | - | - | - | - | - | - | [133] | |
| TBHQ | H295R cells | 0.01–1 μM | 48 h | N | - | N | - | - | - | - | N | N | - | [132] |
| AO2246 | H295R cells | 0.01–1 μM | 48 h | N | - | N | - | - | - | - | N | 0.1 μM | - | |
| AO701 | JEG-3 cells | 50–200 μM | 24 h | - | - | - | - | - | N | - | - | - | - | [129] |
| AO246 | JEG-3 cells | 3–320 μM | 24 h | - | - | - | - | - | 58 μM | - | - | - | - | |
| No. | Compounds | Skin Sensitivity | Eye Corrosion/ Eye Irritation | Respiratory | Endocrine Disruption | PPARs | ARE | HSE | MMP | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Androgen Receptor | Aromatase | Estrogen Receptor | ||||||||||||
| EC | EI | AR | AR-LBD | ER | ER-LBD | |||||||||
| 1 | PTBP | +++ | +++ | +++ | + | +++ | +++ | + | +++ | |||||
| 2 | AO30 | +++ | +++ | +++ | + | + | + | |||||||
| 3 | AO701 | +++ | +++ | +++ | + | + | +++ | +++ | ||||||
| 4 | BHT | +++ | +++ | +++ | + | +++ | +++ | |||||||
| 5 | AO703 | +++ | +++ | +++ | + | +++ | ||||||||
| 6 | AO1222 | + | + | +++ | +++ | +++ | + | +++ | ||||||
| 7 | AO1076 | +++ | +++ | + | + | + | + | + | ||||||
| 8 | AO565 | +++ | +++ | +++ | +++ | + | +++ | +++ | +++ | +++ | ||||
| 9 | AO1425 | +++ | +++ | + | +++ | +++ | +++ | |||||||
| 10 | AO246 | + | +++ | +++ | + | + | + | +++ | + | +++ | ||||
| 11 | BHA | +++ | +++ | +++ | +++ | + | + | +++ | ||||||
| 12 | AO33 | +++ | +++ | +++ | + | + | +++ | + | + | +++ | ||||
| 13 | TBHQ | +++ | +++ | +++ | + | +++ | +++ | +++ | +++ | +++ | ||||
| 14 | AO754 | +++ | +++ | +++ | + | + | +++ | |||||||
| 15 | AO1135 | +++ | +++ | +++ | + | + | +++ | + | +++ | |||||
| 16 | AO1077 | +++ | +++ | +++ | + | + | + | +++ | ||||||
| 17 | AO132 | + | +++ | + | + | +++ | +++ | |||||||
| 18 | AO35 | + | + | +++ | +++ | |||||||||
| 19 | TBMC | + | +++ | +++ | + | + | +++ | |||||||
| 20 | AO2246 | +++ | +++ | + | + | +++ | + | +++ | +++ | |||||
| 21 | AO1081 | +++ | +++ | +++ | + | + | +++ | + | +++ | +++ | ||||
| 22 | AO425 | +++ | +++ | + | +++ | +++ | +++ | +++ | +++ | |||||
| 23 | STW | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | |||
| 24 | AO3052 | +++ | +++ | +++ | + | + | + | +++ | +++ | +++ | +++ | |||
| 25 | AO702 | +++ | +++ | + | + | +++ | + | +++ | + | + | +++ | |||
| 26 | AO1024 | +++ | + | +++ | +++ | +++ | +++ | |||||||
| 27 | AO245 | +++ | + | + | + | +++ | +++ | +++ | ||||||
| 28 | AO1019 | +++ | + | +++ | +++ | + | +++ | |||||||
| 29 | AO626 | + | +++ | +++ | + | + | +++ | |||||||
| 30 | AO36 | +++ | +++ | + | + | |||||||||
| 31 | AO259 | +++ | +++ | + | + | + | +++ | + | + | +++ | ||||
| 32 | AO1035 | +++ | +++ | + | + | + | +++ | + | + | +++ | ||||
| 33 | AO80 | + | + | + | + | + | +++ | |||||||
| 34 | AO1290 | +++ | +++ | + | + | +++ | +++ | +++ | + | + | +++ | |||
| 35 | AO1098 | +++ | + | +++ | +++ | + | +++ | |||||||
| 36 | AO697 | +++ | +++ | + | +++ | +++ | + | +++ | ||||||
| 37 | AO300 | + | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | ||||
| 38 | AOCA | +++ | +++ | + | +++ | +++ | +++ | +++ | +++ | +++ | +++ | |||
| 39 | AO168 | +++ | +++ | + | + | + | +++ | |||||||
| 40 | AO1790 | +++ | +++ | + | +++ | +++ | +++ | |||||||
| 41 | AO1330 | +++ | +++ | + | +++ | + | + | + | +++ | +++ | ||||
| 42 | AO3114 | +++ | +++ | +++ | +++ | +++ | +++ | |||||||
| 43 | AO1010 | +++ | + | +++ | + | + | +++ | +++ | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoang, N.M.H.; Park, K. Applications of Tert-Butyl-Phenolic Antioxidants in Consumer Products and Their Potential Toxicities in Humans. Toxics 2024, 12, 869. https://doi.org/10.3390/toxics12120869
Hoang NMH, Park K. Applications of Tert-Butyl-Phenolic Antioxidants in Consumer Products and Their Potential Toxicities in Humans. Toxics. 2024; 12(12):869. https://doi.org/10.3390/toxics12120869
Chicago/Turabian StyleHoang, Ngoc M. H., and Kwangsik Park. 2024. "Applications of Tert-Butyl-Phenolic Antioxidants in Consumer Products and Their Potential Toxicities in Humans" Toxics 12, no. 12: 869. https://doi.org/10.3390/toxics12120869
APA StyleHoang, N. M. H., & Park, K. (2024). Applications of Tert-Butyl-Phenolic Antioxidants in Consumer Products and Their Potential Toxicities in Humans. Toxics, 12(12), 869. https://doi.org/10.3390/toxics12120869







