Influence and Mechanism of Solid-Phase Particle Factors on Oil–Solid Separation of Oily Sludge Treated by Flotation Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Materials
2.1.1. Experimental Materials
2.1.2. OS Preparation
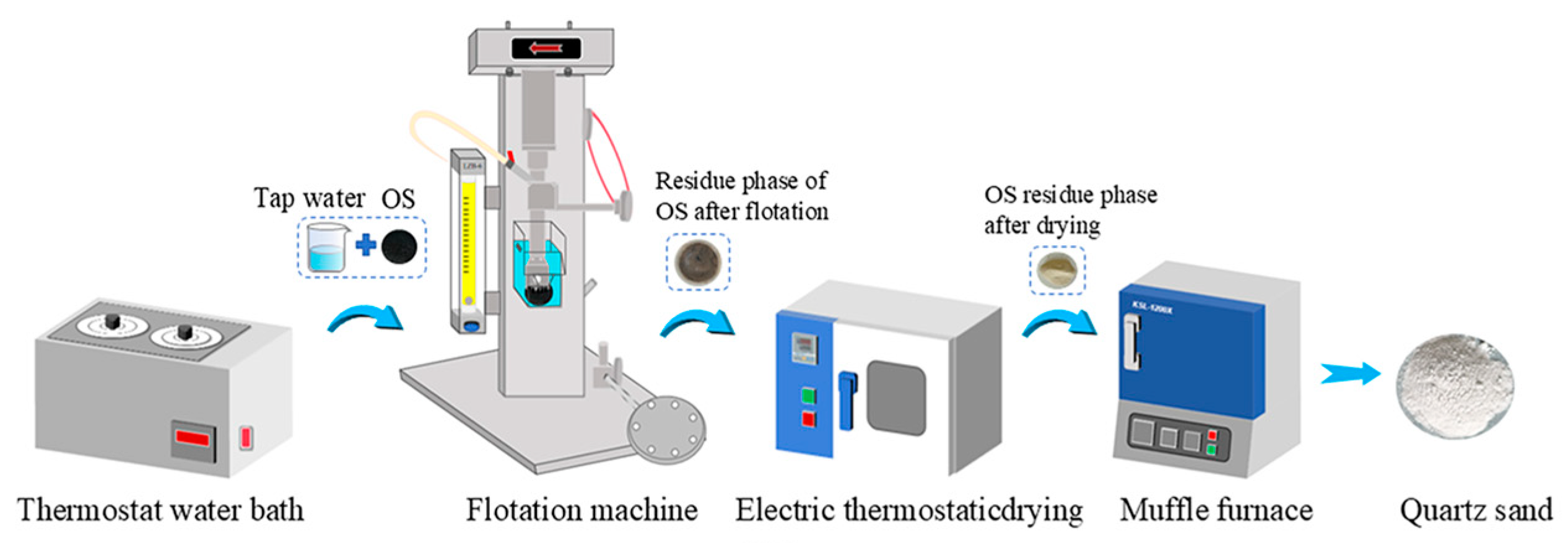
2.2. Flotation Experiments
2.2.1. Single-Size Sand OS Flotation Experiment
2.2.2. Mixed-Size Sand OS Flotation Experiment
2.2.3. Optimisation Experiments for Oil–Solid Separation of Mixed-Size Sand OS
2.2.4. Flotation Kinetics Experiments
2.2.5. Analysis Methods
3. Results
3.1. Flotation of Single-Size Sand OS
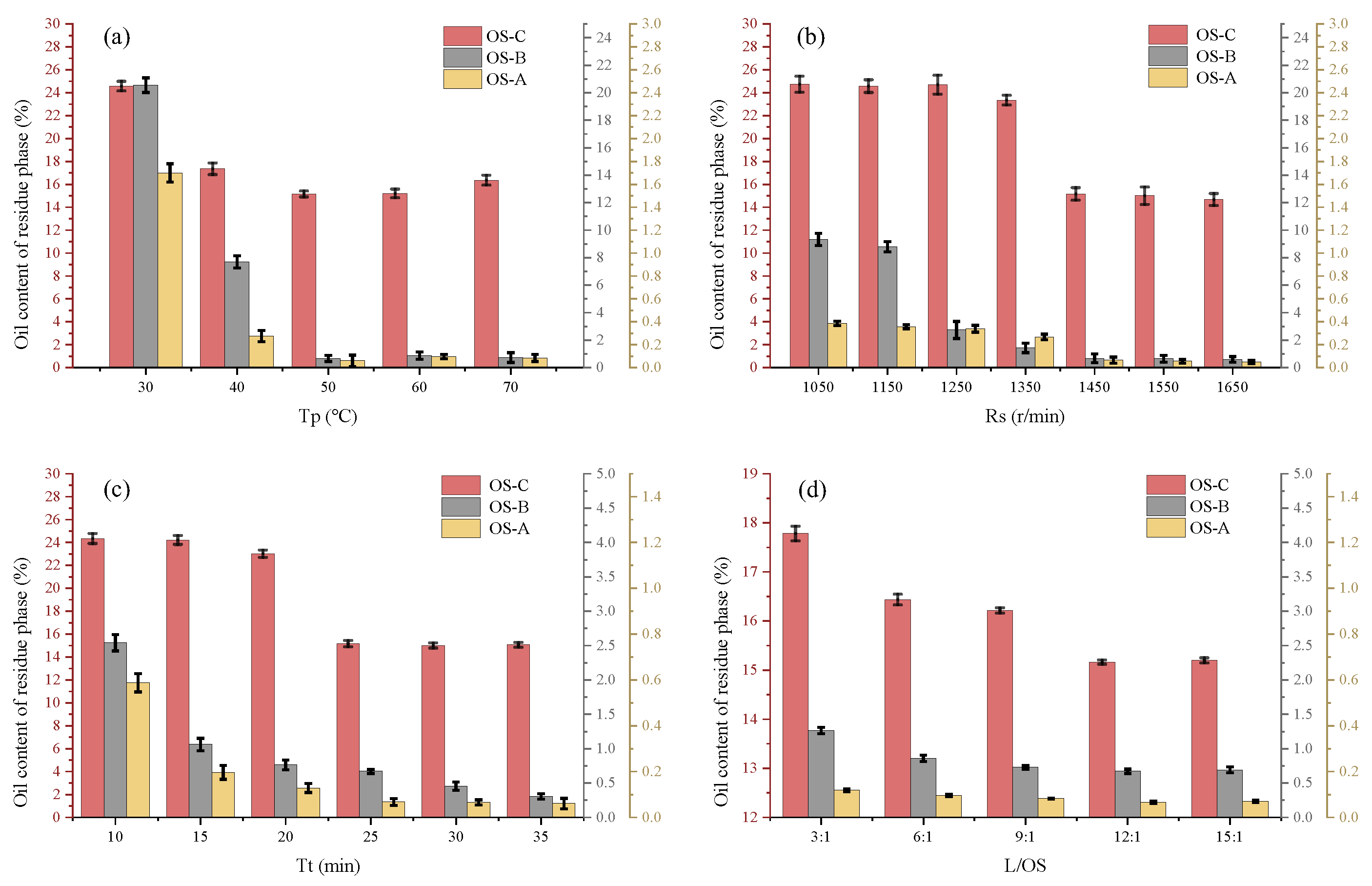
3.1.1. Effect of Flotation Temperature
3.1.2. Effect of Impeller Speed
3.1.3. Effect of Flotation Time
3.1.4. Effect of L/OS
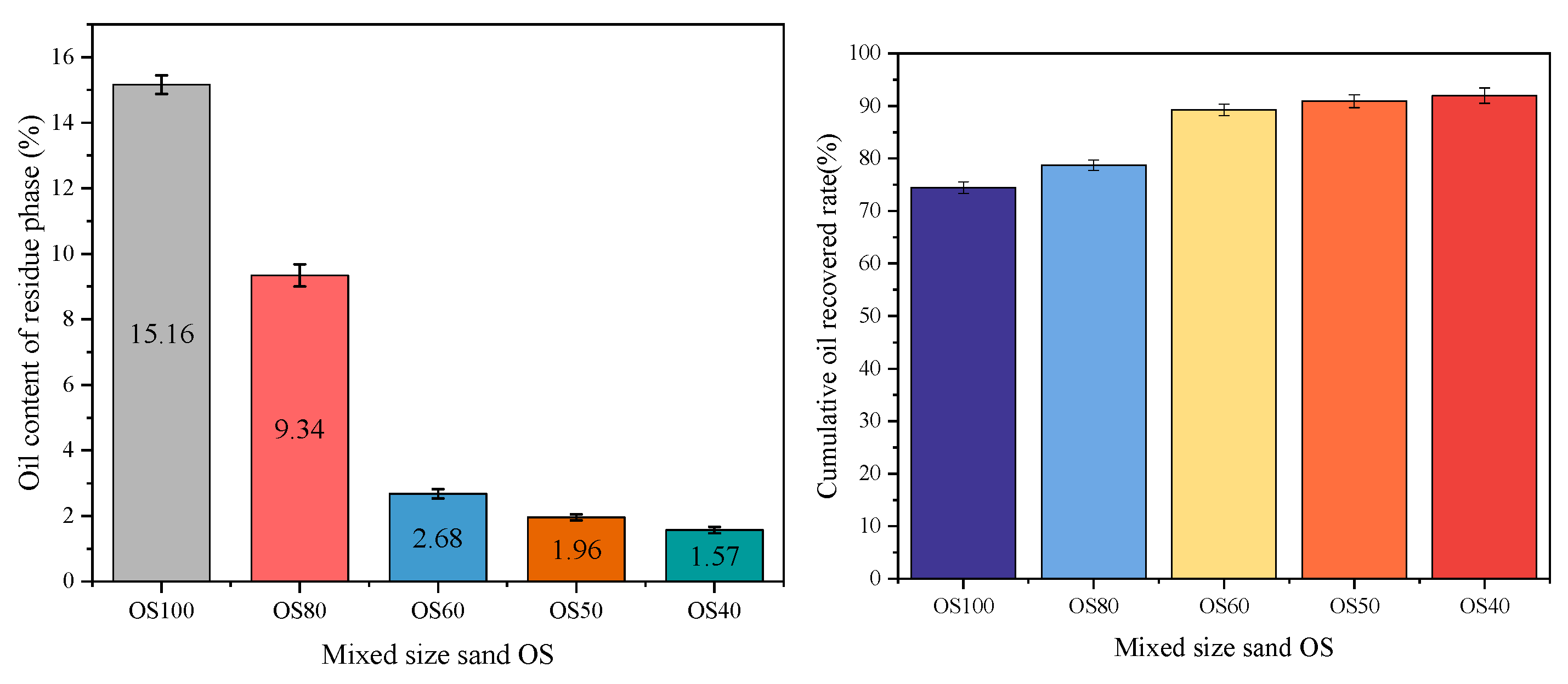
3.2. Flotation of Mixed-Size Sand OS
3.3. Flotation Kinetics of Single-Size Sand and Mixed-Size Sand OS
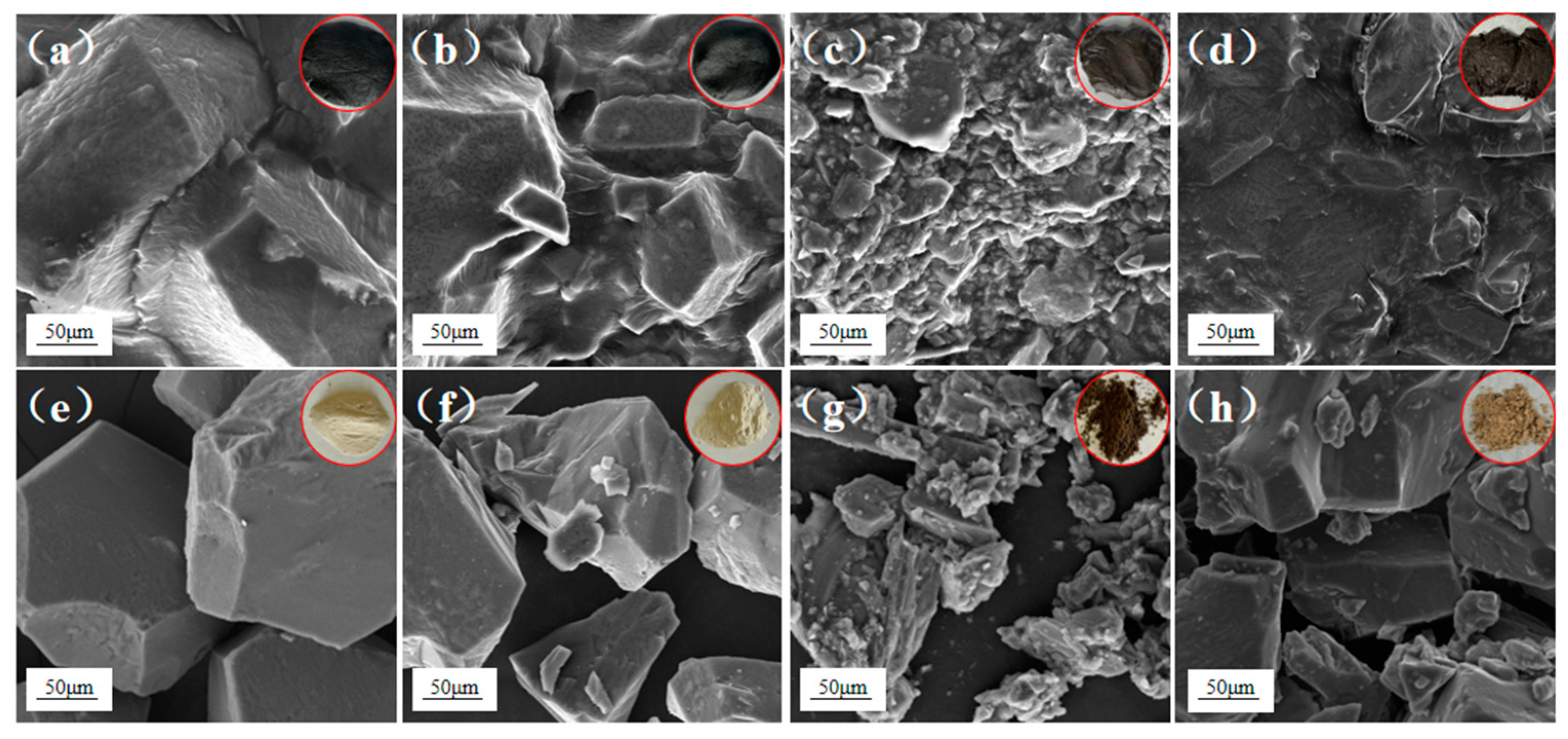
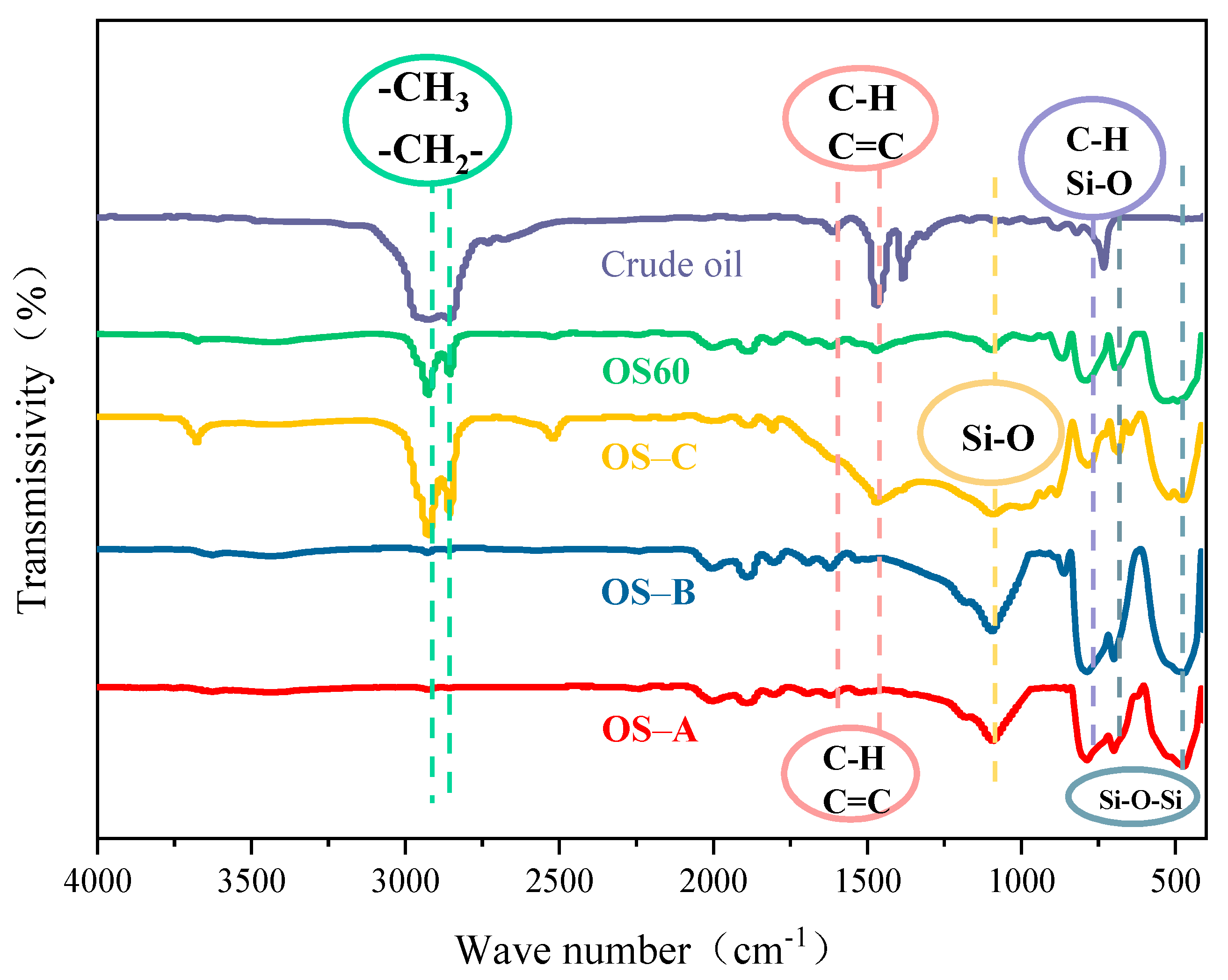
3.4. SEM and FT-IR of OS and Residue
3.5. Optimisation of the Oil–Solid Separation Flotation Process by the RSM Method
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yan, Y.; Tong, K.; Li, C.; Pan, L. The methods for improving the biodegradability of oily sludge: A critical review. Environ. Sci. Pollut. Res. 2024, 31, 41844–41853. [Google Scholar] [CrossRef] [PubMed]
- Gou, X.; Zheng, G.; Tang, J.; Tao, C.; Liu, R.; Liu, Z. Electric field efficiently enhanced thermochemical cleaning for oil recovery from oily sludge. Chem. Eng. Process. 2023, 185, 109314. [Google Scholar] [CrossRef]
- Qu, Y.; Li, A.; Wang, D.; Zhang, L.; Ji, G. Kinetic study of the effect of in-situ mineral solids on pyrolysis process of oil sludge. Chem. Eng. J. 2019, 374, 338–346. [Google Scholar] [CrossRef]
- Yin, Q.; Nie, H.; Nie, M.; Guo, Y.; Zhang, B.; Wang, L.; Yan, W.; Bai, X. Rapid effective treatment of waxy oily sludge using a method of dispersion combined with biodegradation in a semi-fluid state. Environ. Pollut. 2023, 319, 120971. [Google Scholar] [CrossRef]
- Gao, N.; Duan, Y.; Li, Z.; Quan, C.; Yoshikawa, K. Hydrothermal treatment combined with in-situ mechanical compression for floated oily sludge dewatering. J. Hazard. Mater. 2021, 402, 124173. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.Z.; Li, H.; Chow, R.S.; Liu, Q.; Xu, Z.; Masliyah, J. Role of mineral flotation technology in improving bitumen extraction from mined Athabasca oil sands—II. Flotation hydrodynamics of water-based oil sand extraction. Can. J. Chem. Eng. 2020, 98, 330–352. [Google Scholar] [CrossRef]
- da Silva, D.C.; dos Santos Lucas, C.R.; de Moraes Juviniano, H.B.; de Alencar Moura, M.C.P.; de Castro Dantas, T.N.; Neto, A.A.D. Analysis of the use of microemulsion systems to treat petroleum sludge from a water flotation unit. J. Environ. Chem. Eng. 2019, 7, 102934. [Google Scholar] [CrossRef]
- Gautam, P.; Bajagain, R.; Jeong, S.W. Combined effects of soil particle size with washing time and soil-to-water ratio on removal of total petroleum hydrocarbon from fuel contaminated soil. Chemosphere 2020, 250, 126206. [Google Scholar] [CrossRef]
- Li, Q.; Sun, D.; Chen, F.; Xu, H.; Xu, Z. New insights into interaction between oil and solid during hydrothermal treatment of oily sludge. J. Hazard. Mater. 2024, 471, 134358. [Google Scholar] [CrossRef]
- Lin, Z.; Xu, F.; Wang, L.; Hu, L.; Zhu, L.; Tan, J.; Li, Z.; Zhang, T. Characterization of oil component and solid particle of oily sludge treated by surfactant-assisted ultrasonication. Chin. J. Chem. Eng. 2021, 34, 53–60. [Google Scholar] [CrossRef]
- Duan, M.; Li, C.; Wang, X.; Fang, S.; Xiong, Y.; Shi, P. Solid separation from the heavy oil sludge produced from Liaohe Oilfield. J. Pet. Sci. Eng. 2019, 172, 1112–1119. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, X.; Bu, X.; Wang, M.; An, B.; Shao, H.; Ni, C.; Peng, Y.; Xie, G. A novel flotation technique combining carrier flotation and cavitation bubbles to enhance separation efficiency of ultra-fine particles. Ultrason. Sonochem. 2020, 64, 105005. [Google Scholar] [CrossRef] [PubMed]
- Fong, N.; Ng, S.; Chung, K.H.; Tu, Y.; Li, Z.; Sparks, B.D.; Kotlyar, L.S. A two level fractional factorial design to test the effect of oil sands composition and process water chemistry on bitumen recovery from model systems. Can. J. Chem. Eng. 2004, 82, 782–793. [Google Scholar] [CrossRef]
- Chong, J.; Ng, S.; Chung, K.H.; Sparks, B.D.; Kotlyar, L.S. Impact of fines content on a warm slurry extraction process using model oilsands. Fuel 2003, 82, 425–438. [Google Scholar] [CrossRef]
- Masliyah, J.H.; Czarnecki, J.; Xu, Z. Handbook on Theory and Practice on Bitumen Recovery from Athabasca Oil Sands; University of Alberta: Edmonton, AB, USA, 2011. [Google Scholar]
- Fong, N.; Ng, S.; Chung, K.H.; Tu, Y.; Li, Z.; Sparks, B.D.; Kotlyar, L.S. Bitumen recovery from model systems using a warm slurry extraction process: Effects of oilsands components and process water chemistry. Fuel 2004, 83, 1865–1880. [Google Scholar] [CrossRef]
- Gonçalves, M.A.; dos Santos, H.C.L.; da Silva, M.A.R.; da Cas Viegas, A.; da Rocha Filho, G.N.; da Conceição, L.R.V. Biodiesel production from waste cooking oil using an innovative magnetic solid acid catalyst based on Ni–Fe ferrite: RSM-BBD optimization approach. J. Ind. Eng. Chem. 2024, 135, 270–285. [Google Scholar] [CrossRef]
- Farahani, G.T.; Azari, P.Y. Improving the oil yield of Iranian Jatropha curcas seeds by optimising ultrasound-assisted ethanolic extraction process: A response surface method. Qual. Assur. Saf. Crops Foods 2016, 8, 95–104. [Google Scholar] [CrossRef]
- Bu, X.; Wang, X.; Zhou, S.; Li, B.; Zhan, H.; Xie, G. Discrimination of six flotation kinetic models used In the conventional flotation and carrier flotation of −74 μm coal fines. Acs Omega 2020, 5, 13813–13821. [Google Scholar] [CrossRef]
- Ni, C.; Xie, G.; Xi, M.; Peng, Y.; Xia, W. The difference in flotation kinetics of various size fractions of bituminous coal between rougher and cleaner flotation processes. Powder Technol. 2016, 292, 210–216. [Google Scholar] [CrossRef]
- Xu, M.; Xing, Y.; Gui, X.; Cao, Y. Flotation kinetics of easy-to-float fine coal. Energy Sources Part A 2017, 39, 1978–1983. [Google Scholar] [CrossRef]
- Zhen, K.; Zheng, C.; Li, C.; Zhang, H. Flotation performance of low-rank coal in the presence of cetyltrimethyl ammonium bromide. Int. J. Coal Prep. Util. 2020, 40, 860–875. [Google Scholar] [CrossRef]
- Chen, G.; Cheng, C.; Zhang, J.; Sun, Y.; Hu, Q.; Qu, C.; Dong, S. Synergistic effect of surfactant and alkali on the treatment of oil sludge. J. Pet. Sci. Eng. 2019, 183, 106420. [Google Scholar] [CrossRef]
- Zhao, S.; Li, Y.J.; Qiao, N.; Xu, F.J.; Chu, G.W.; Zou, H.K.; Sun, B.C. Study on the oil-sludge separation by thermochemical method in rotating packed bed. Chem. Eng. Process 2022, 174, 108878. [Google Scholar] [CrossRef]
- Bao, Q.; Huang, L.; Xiu, J.; Yi, L.; Ma, Y. Study on the treatment of oily sludge in oil fields with lipopeptide/sophorolipid complex bio-surfactant. Ecotoxicol. Environ. Saf. 2021, 212, 111964. [Google Scholar] [CrossRef]
- Bao, Q.; Huang, L.; Xiu, J.; Yi, L.; Zhang, Y.; Wu, B. Study on the thermal washing of oily sludge used by rhamnolipid/sophorolipid binary mixed bio-surfactant systems. Ecotoxicol. Environ. Saf. 2022, 240, 113696. [Google Scholar] [CrossRef]
- Deglon, D.A. The effect of agitation on the flotation of platinum ores. Miner. Eng. 2005, 18, 839–844. [Google Scholar] [CrossRef]
- Amini, E.; Bradshaw, D.J.; Finch, J.A.; Brennan, M. Influence of turbulence kinetic energy on bubble size in different scale flotation cells. Miner. Eng. 2013, 45, 146–150. [Google Scholar] [CrossRef]
- Duan, J.; Fornasiero, D.; Ralston, J. Calculation of the flotation rate constant of halcopyrite particles in an ore. Int. J. Miner. Process. 2003, 72, 227–237. [Google Scholar] [CrossRef]
- Li, G.; Guo, S.; Hu, J. The influence of clay minerals and surfactants on hydrocarbon removal during the washing of petroleum-contaminated soil. Chem. Eng. J. 2016, 286, 191–197. [Google Scholar] [CrossRef]
- Darabi, H.; Koleini, S.J.; Deglon, D.; Rezai, B.; Abdollahy, M. Investigation of bubble-particle attachment, detachment and collection efficiencies in a mechanical flotation cell. Powder Technol. 2020, 375, 109–123. [Google Scholar] [CrossRef]
- Liang, J.; Zhao, L.; Du, N.; Li, H.; Hou, W. Solid effect in solvent extraction treatment of pre-treated oily sludge. Sep. Purif. Technol. 2014, 130, 28–33. [Google Scholar] [CrossRef]
- Liang, J.; Zhao, L.; Hou, W. Solid effect in chemical cleaning treatment of oily sludge. Colloids Surf. A 2017, 522, 38–42. [Google Scholar] [CrossRef]
- Liu, J.; Xu, Z.; Masliyah, J. Interaction forces in bitumen extraction from oil sands. J. Colloid Interface Sci. 2005, 287, 507–520. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, D.R.; Oliveira, B.R.; Saide, V.G.P.; Magalhães, S.C.; Scheid, C.M.; Calçada, L.A. Effects of particle-size distribution and solid additives in the apparent viscosity of drilling fluids. J. Pet. Sci. Eng. 2019, 182, 106275. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, Y.; Li, G.; Liao, Y.; Xing, Y.; Gui, X. Combined effect of chemical composition and spreading velocity of collector on flotation performance of oxidized coal. Powder Technol. 2018, 325, 1–10. [Google Scholar] [CrossRef]
- Bu, X.; Xie, G.; Chen, Y.; Ni, C. The order of kinetic models in coal fines flotation. Int. J. Coal Prep. Util. 2017, 37, 113–123. [Google Scholar] [CrossRef]
- Chen, Q.; Stricek, I.; Gray, M.R.; Liu, Q. Influence of hydrophobicity distribution of particle mixtures on emulsion stabilization. J. Colloid Interface Sci. 2017, 491, 179–189. [Google Scholar] [CrossRef]
- Chen, Q.; Stricek, I.; Cao, M.; Gray, M.R.; Liu, Q. Influence of hydrothermal treatment on filterability of fine solids in bitumen froth. Fuel 2016, 180, 314–323. [Google Scholar] [CrossRef]
- Sedghi, M.; Piri, M.; Goual, L. Atomistic molecular dynamics simulations of crude oil/brine displacement in calcite mesopores. Langmuir 2016, 32, 3375–3384. [Google Scholar] [CrossRef]
- Dong, X.; Liu, H.; Hou, J.; Wu, K.; Chen, Z. Phase equilibria of confined fluids in nanopores of tight and shale rocks considering the effect of capillary pressure and adsorption film. Ind. Eng. Chem. Res. 2016, 55, 798–811. [Google Scholar] [CrossRef]
- Taheri-Shakib, J.; Saadati, N.; Esfandiarian, A.; Hosseini, S.A.; Rajabi-Kochi, M. Characterizing the wax-asphaltene interaction and surface morphology using analytical spectroscopy and microscopy techniques. J. Mol. Liq. 2020, 302, 112506. [Google Scholar] [CrossRef]
- Zojaji, I.; Esfandiarian, A.; Taheri-Shakib, J. Toward molecular characterization of asphaltene from different origins under different conditions by means of FT-IR spectroscopy. Adv. Colloid Interface Sci. 2021, 289, 102314. [Google Scholar] [CrossRef] [PubMed]
- Cannane, N.O.A.; Rajendran, M.; Selvaraju, R. FT-IR spectral studies on polluted soils from industrial area at Karaikal, Puducherry State, South India. Spectrochim. Acta Part A 2013, 110, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Gao, N.; Sipra, A.T.; Tong, K.; Quan, C. Characterization of heavy metals and oil components in the products of oily sludge after hydrothermal treatment. J. Hazard. Mater. 2022, 424, 127293. [Google Scholar] [CrossRef]
- Mohammadi, L.; Zafar, M.N.; Bashir, M.; Sumrra, S.H.; Shafqat, S.S.; Zarei, A.A.; Dahmardeh, H.; Ahmad, I.; Halawa, M.I. Modeling of phenol removal from water by NiFe2O4 nanocomposite using response surface methodology and artificial neural network techniques. J. Environ. Chem. Eng. 2021, 9, 105576. [Google Scholar] [CrossRef]
- Kashyap, S.S.; Gogate, P.R.; Joshi, S.M. Ultrasound assisted synthesis of biodiesel from karanja oil by interesterification: Intensification studies and optimization using SM. Ultrason. Sonochem. 2019, 50, 36–45. [Google Scholar] [CrossRef]



| Number | Name | Formulas |
|---|---|---|
| Model 1 | classical first-order model | |
| Model 2 | fully mixed factor model | |
| Model 3 | second-order with rectangular distribution |
| OS | Rs | Model 1 | Model 2 | Model 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| K | R2 | ε∞ | K | R2 | ε∞ | K | R2 | ε∞ | ||
| OS-A | 1250 r/min | 0.185 | 0.996 | 95.316 | 4.313 | 0.980 | 110.053 | 0.524 | 0.968 | 117.509 |
| 1450 r/min | 0.213 | 0.993 | 97.661 | 3.131 | 0.968 | 109.415 | 0.773 | 0.950 | 115.120 | |
| 1650 r/min | 0.241 | 0.998 | 98.026 | 2.517 | 0.926 | 107.896 | 1.013 | 0.899 | 112.503 | |
| OS-B | 1250 r/min | 0.179 | 0.991 | 81.980 | 3.246 | 0.978 | 92.167 | 0.739 | 0.964 | 97.143 |
| 1450 r/min | 0.195 | 0.991 | 94.679 | 3.645 | 0.982 | 107.569 | 0.642 | 0.969 | 113.963 | |
| 1650 r/min | 0.229 | 0.981 | 94.525 | 2.782 | 0.984 | 104.949 | 0.891 | 0.971 | 109.939 | |
| OS-C | 1250 r/min | 0.175 | 0.965 | 68.720 | 3.168 | 0.995 | 77.143 | 0.759 | 0.988 | 81.265 |
| 1450 r/min | 0.190 | 0.990 | 74.677 | 3.645 | 0.984 | 85.118 | 0.615 | 0.972 | 90.315 | |
| 1650 r/min | 0.200 | 0.974 | 84.239 | 3.506 | 0.993 | 95.409 | 0.671 | 0.984 | 100.941 | |
| OS60 | 1250 r/min | 0.200 | 0.992 | 75.180 | 3.496 | 0.980 | 85.105 | 0.675 | 0.967 | 89.998 |
| 1450 r/min | 0.208 | 0.996 | 89.769 | 3.259 | 0.971 | 100.952 | 0.736 | 0.954 | 106.407 | |
| 1650 r/min | 0.219 | 0.998 | 93.986 | 2.971 | 0.953 | 104.829 | 0.825 | 0.932 | 110.039 | |
| Source | Sum of Squares | Df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 1412.00 | 14 | 100.86 | 25.65 | <0.0001 |
| A | 1000.61 | 1 | 1000.61 | 254.51 | <0.0001 |
| B | 11.78 | 1 | 11.78 | 3.00 | 0.1054 |
| C | 80.02 | 1 | 80.02 | 20.35 | 0.0005 |
| D | 19.37 | 1 | 19.37 | 4.93 | 0.0435 |
| AB | 1.37 | 1 | 1.37 | 0.35 | 0.5640 |
| AC | 19.65 | 1 | 19.65 | 5.00 | 0.0422 |
| AD | 12.28 | 1 | 12.28 | 3.12 | 0.0989 |
| BC | 23.24 | 1 | 23.24 | 5.91 | 0.0291 |
| BD | 0.029 | 1 | 0.029 | 7.452 × 10−3 | 0.9324 |
| CD | 4.49 | 1 | 4.49 | 1.14 | 0.3031 |
| A2 | 209.85 | 1 | 209.85 | 53.38 | <0.0001 |
| B2 | 9.45 | 1 | 9.45 | 2.40 | 0.1433 |
| C2 | 2.25 | 1 | 2.25 | 0.57 | 0.4617 |
| D2 | 5.81 | 1 | 5.81 | 1.48 | 0.2442 |
| Residue | 55.04 | 14 | 3.93 | ||
| Lack of Fit | 49.45 | 10 | 4.94 | 3.53 | 0.1175 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.; Yao, X.; Wang, X.; Yuan, W.; Li, Q.; Niu, X.; Ma, Y. Influence and Mechanism of Solid-Phase Particle Factors on Oil–Solid Separation of Oily Sludge Treated by Flotation Method. Toxics 2024, 12, 880. https://doi.org/10.3390/toxics12120880
Wu S, Yao X, Wang X, Yuan W, Li Q, Niu X, Ma Y. Influence and Mechanism of Solid-Phase Particle Factors on Oil–Solid Separation of Oily Sludge Treated by Flotation Method. Toxics. 2024; 12(12):880. https://doi.org/10.3390/toxics12120880
Chicago/Turabian StyleWu, Shuhui, Xue Yao, Xiao Wang, Wenyan Yuan, Qiuhong Li, Xiaoyin Niu, and Yanfei Ma. 2024. "Influence and Mechanism of Solid-Phase Particle Factors on Oil–Solid Separation of Oily Sludge Treated by Flotation Method" Toxics 12, no. 12: 880. https://doi.org/10.3390/toxics12120880
APA StyleWu, S., Yao, X., Wang, X., Yuan, W., Li, Q., Niu, X., & Ma, Y. (2024). Influence and Mechanism of Solid-Phase Particle Factors on Oil–Solid Separation of Oily Sludge Treated by Flotation Method. Toxics, 12(12), 880. https://doi.org/10.3390/toxics12120880






