Non-Genotoxic and Environmentally Relevant Lower Molecular Weight Polycyclic Aromatic Hydrocarbons Significantly Increase Tumorigenicity of Benzo[a]pyrene in a Lung Two-Stage Mouse Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Two-Stage Initiation Promotion Lung Model
2.2. BALF Analysis
2.3. Lung Tumor Endpoints: Multiplicity and Histopathology
2.4. RNA Isolation, cDNA Synthesis, and Quantitative PCR (qRT-PCR)
2.5. KC (CXCL1) ELISA
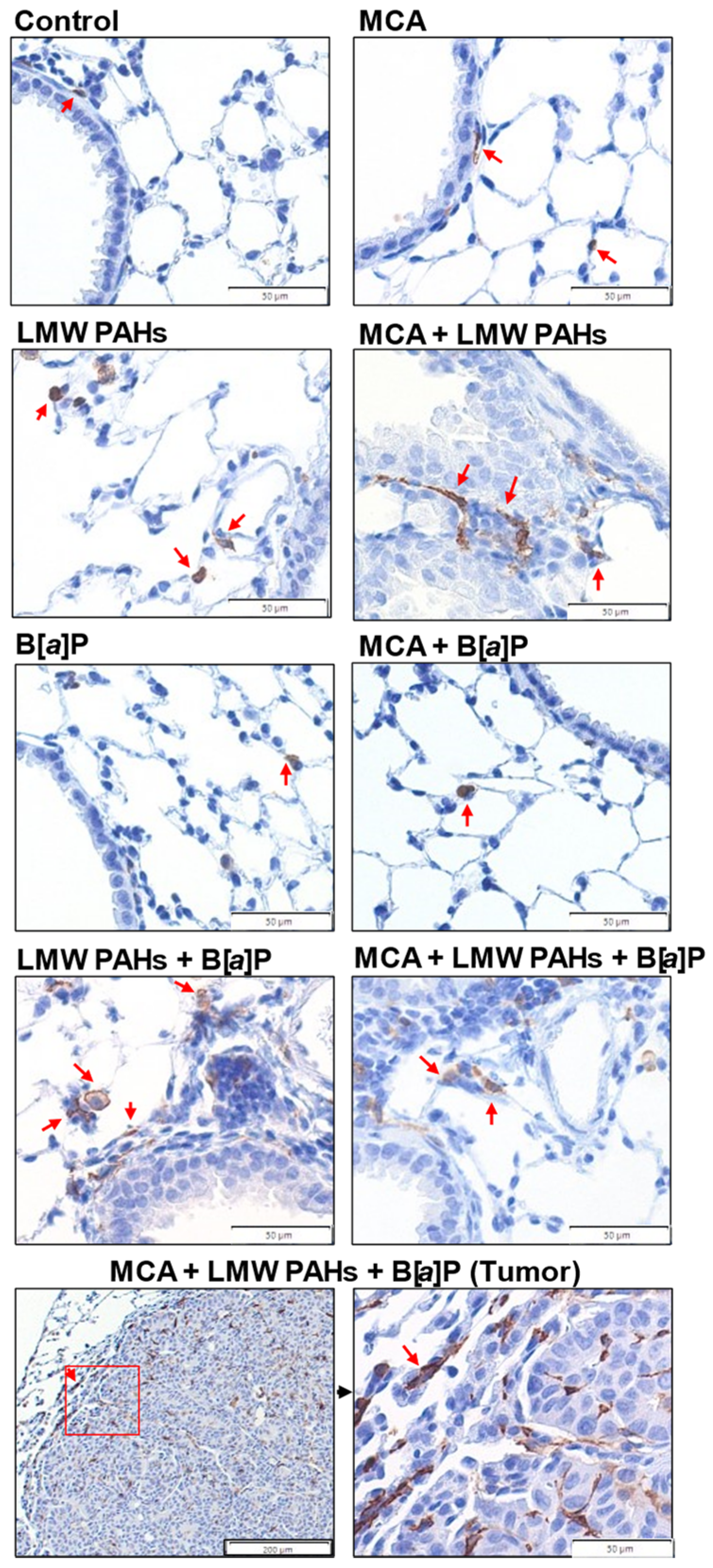
2.6. Immunohistochemistry on Lung Sections
2.7. Statistical Analysis
3. Results
3.1. Tumor Promotion Observed in Response to the Combined PAHs
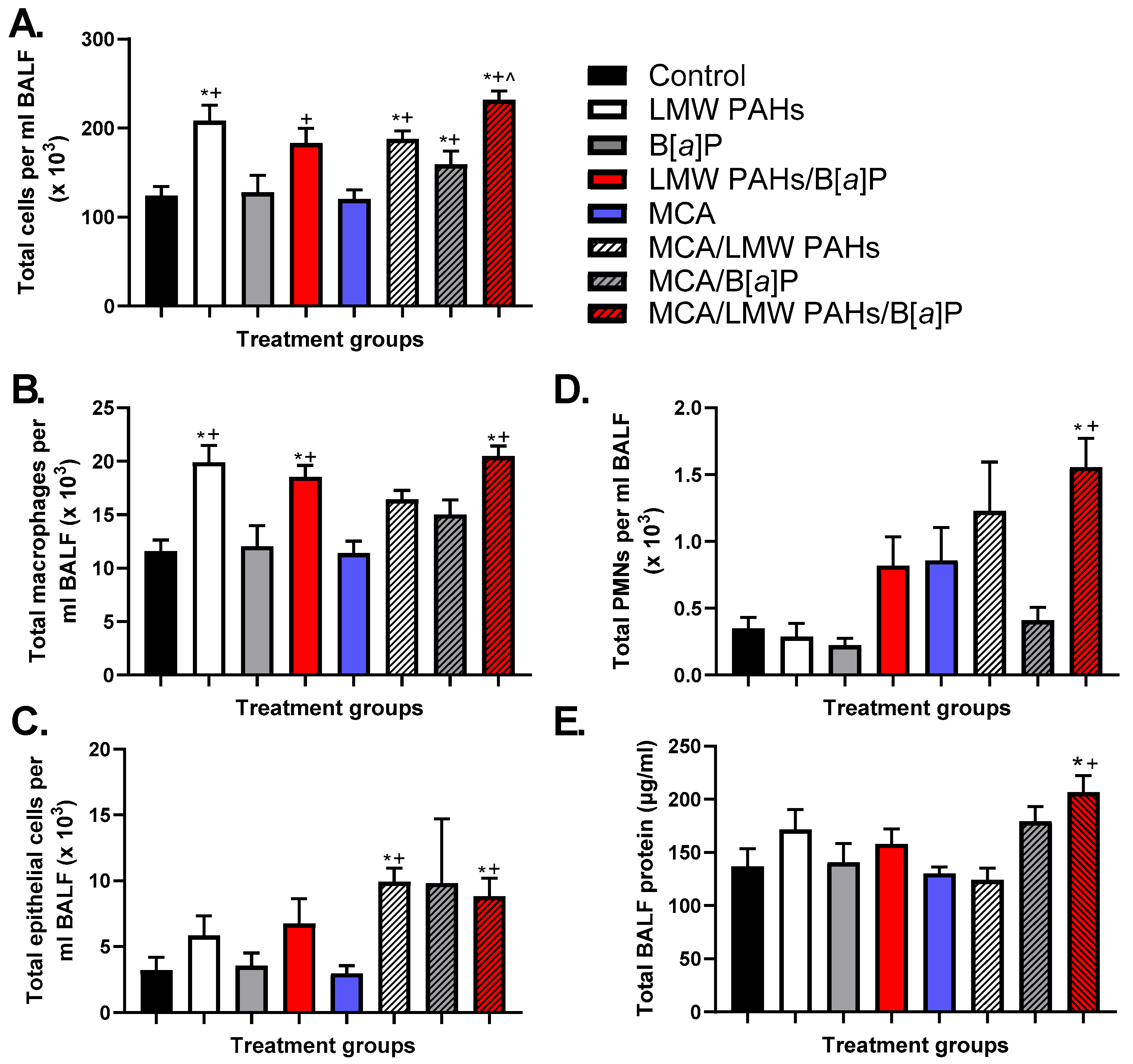
3.2. BALF Analysis in the Two-Stage Model Indicates Differences in Inflammatory Infiltrates Among Treatment Groups
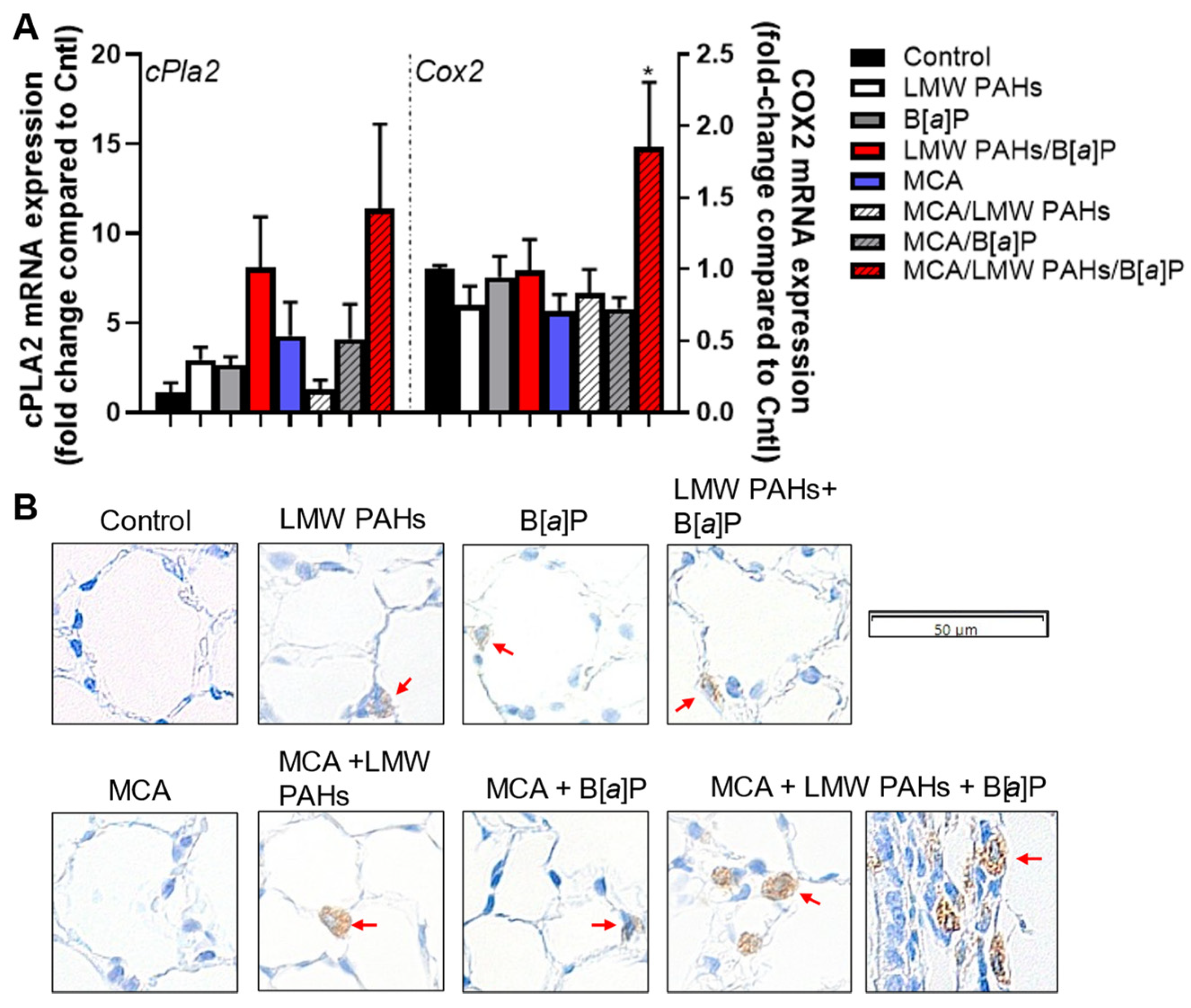
3.3. Secretion of KC and Increased mRNA Expression of Pro-Inflammatory Markers, Including a Bioactive Lipid Pathway
3.4. Additional Pathways Involved in Several Other Markers for Early-Stage LUAD Considered Hallmarks of Cancer
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kratzer, T.B.; Bandi, P.; Freedman, N.D.; Smith, R.A.; Travis, W.D.; Jemal, A.; Siegel, R.L. Lung cancer statistics, 2023. Cancer 2024, 130, 1330–1348. [Google Scholar] [CrossRef] [PubMed]
- Myers, R.; Brauer, M.; Dummer, T.; Atkar-Khattra, S.; Yee, J.; Melosky, B.; Ho, C.; McGuire, A.L.; Sun, S.; Grant, K.; et al. High-Ambient Air Pollution Exposure Among Never Smokers Versus Ever Smokers With Lung Cancer. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2021, 16, 1850–1858. [Google Scholar] [CrossRef]
- Moghaddam, S.J.; Savai, R.; Salehi-Rad, R.; Sengupta, S.; Kammer, M.N.; Massion, P.; Beane, J.E.; Ostrin, E.J.; Priolo, C.; Tennis, M.A.; et al. Premalignant Progression in the Lung: Knowledge Gaps and Novel Opportunities for Interception of Non-Small Cell Lung Cancer. An Official American Thoracic Society Research Statement. Am. J. Respir. Crit. Care Med. 2024, 210, 548–571. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer (IARC). Outdoor air pollution. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 2015; Volume 109. [Google Scholar]
- Hill, W.; Lim, E.L.; Weeden, C.E.; Lee, C.; Augustine, M.; Chen, K.; Kuan, F.C.; Marongiu, F.; Evans, E.J., Jr.; Moore, D.A.; et al. Lung adenocarcinoma promotion by air pollutants. Nature 2023, 616, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Bilsback, K.R.; Dahlke, J.; Fedak, K.M.; Good, N.; Hecobian, A.; Herckes, P.; L’Orange, C.; Mehaffy, J.; Sullivan, A.; Tryner, J.; et al. A Laboratory Assessment of 120 Air Pollutant Emissions from Biomass and Fossil Fuel Cookstoves. Environ. Sci. Technol. 2019, 53, 7114–7125. [Google Scholar] [CrossRef] [PubMed]
- Hawley, B.; L’Orange, C.; Olsen, D.B.; Marchese, A.J.; Volckens, J. Oxidative stress and aromatic hydrocarbon response of human bronchial epithelial cells exposed to petro- or biodiesel exhaust treated with a diesel particulate filter. Toxicol. Sci. Off. J. Soc. Toxicol. 2014, 141, 505–514. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer (IARC). Diesel and Gasoline Engine Exhausts and Some Nitroarenes. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 2013; Volume 105. [Google Scholar]
- Landvik, N.E.; Gorria, M.; Arlt, V.M.; Asare, N.; Solhaug, A.; Lagadic-Gossmann, D.; Holme, J.A. Effects of nitrated-polycyclic aromatic hydrocarbons and diesel exhaust particle extracts on cell signalling related to apoptosis: Possible implications for their mutagenic and carcinogenic effects. Toxicology 2007, 231, 159–174. [Google Scholar] [CrossRef]
- Mabilia, R.; Cecinato, A.; Tomasi Sciano, M.C.; Di Palo, V.; Possanzini, M. Characterization of polycyclic aromatic hydrocarbons and carbonyl compounds in diesel exhaust emissions. Ann. Chim. 2004, 94, 733–740. [Google Scholar] [CrossRef]
- Talaska, G.; Thoroman, J.; Schuman, B.; Kafferlein, H.U. Biomarkers of polycyclic aromatic hydrocarbon exposure in European coke oven workers. Toxicol. Lett. 2014, 231, 213–216. [Google Scholar] [CrossRef]
- Navarro, K.M.; Cisneros, R.; Noth, E.M.; Balmes, J.R.; Hammond, S.K. Occupational Exposure to Polycyclic Aromatic Hydrocarbon of Wildland Firefighters at Prescribed and Wildland Fires. Environ. Sci. Technol. 2017, 51, 6461–6469. [Google Scholar] [CrossRef]
- Navarro, K.M.; Cisneros, R.; Schweizer, D.; Chowdhary, P.; Noth, E.M.; Balmes, J.R.; Hammond, S.K. Incident command post exposure to polycyclic aromatic hydrocarbons and particulate matter during a wildfire. J. Occup. Envron. Hyg. 2019, 16, 735–744. [Google Scholar] [CrossRef] [PubMed]
- Jen, C.N.; Hatch, L.E.; Selimovic, V.; Yokelson, R.J.; Weber, R.; Fernandez, A.E.; Kreisberg, N.M.; Barsanti, K.C.; Goldstein, A.H. Speciated and total emission factors of particulate organics from burning western US wildland fuels and their dependence on combustion efficiency. Atmos. Chem. Phys. 2019, 19, 1013–1026. [Google Scholar] [CrossRef]
- Kim, Y.H.; Warren, S.H.; Kooter, I.; Williams, W.C.; George, I.J.; Vance, S.A.; Hays, M.D.; Higuchi, M.A.; Gavett, S.H.; DeMarini, D.M.; et al. Chemistry, lung toxicity and mutagenicity of burn pit smoke-related particulate matter. Part. Fibre Toxicol. 2021, 18, 45. [Google Scholar] [CrossRef] [PubMed]
- Moir, D.; Rickert, W.S.; Levasseur, G.; Larose, Y.; Maertens, R.; White, P.; Desjardins, S. A comparison of mainstream and sidestream marijuana and tobacco cigarette smoke produced under two machine smoking conditions. Chem. Res. Toxicol. 2008, 21, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.L.; Hsieh, D.P.; Li, L.A. Polycyclic aromatic hydrocarbons in cigarette sidestream smoke particulates from a Taiwanese brand and their carcinogenic relevance. Chemosphere 2011, 82, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Marczynski, B.; Pesch, B.; Wilhelm, M.; Rossbach, B.; Preuss, R.; Hahn, J.U.; Rabstein, S.; Raulf-Heimsoth, M.; Seidel, A.; Rihs, H.P.; et al. Occupational exposure to polycyclic aromatic hydrocarbons and DNA damage by industry: A nationwide study in Germany. Arch. Toxicol. 2009, 83, 947–957. [Google Scholar] [CrossRef]
- Pesch, B.; Kappler, M.; Straif, K.; Marczynski, B.; Preuss, R.; Rossbach, B.; Rihs, H.P.; Weiss, T.; Rabstein, S.; Pierl, C.; et al. Dose-response modeling of occupational exposure to polycyclic aromatic hydrocarbons with biomarkers of exposure and effect. Cancer Epidemiol. Biomark. Prev. 2007, 16, 1863–1873. [Google Scholar] [CrossRef]
- Serdar, B.; Brindley, S.; Dooley, G.; Volckens, J.; Juarez-Colunga, E.; Gan, R. Short-term markers of DNA damage among roofers who work with hot asphalt. Environ. Health 2016, 15, 99. [Google Scholar] [CrossRef]
- Olsen, T.; Caruana, D.; Cheslack-Postava, K.; Szema, A.; Thieme, J.; Kiss, A.; Singh, M.; Smith, G.; McClain, S.; Glotch, T.; et al. Iraq/Afghanistan war lung injury reflects burn pits exposure. Sci. Rep. 2022, 12, 14671. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer (IARC). Some Non-Heterocyclic Polycyclic Aromatic Hydrocarbons and Some Related Exposures; IARC: Lyon, France, 2010; Volume 92.
- International Agency for Research on Cancer (IARC). Benzo[a]pyrene. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 2012; Volume 100F. [Google Scholar]
- Bojes, H.K.; Pope, P.G. Characterization of EPA’s 16 priority pollutant polycyclic aromatic hydrocarbons (PAHs) in tank bottom solids and associated contaminated soils at oil exploration and production sites in Texas. Regul. Toxicol. Pharmacol. 2007, 47, 288–295. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer (IARC). Napthalene. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 2002; Volume 82. [Google Scholar]
- Cattley, R.C.; Kromhout, H.; Sun, M.; Tokar, E.J.; Abdallah, M.A.; Bauer, A.K.; Broadwater, K.R.; Campo, L.; Corsini, E.; Houck, K.A.; et al. Carcinogenicity of anthracene, 2-bromopropane, butyl methacrylate, and dimethyl hydrogen phosphite. Lancet Oncol. 2023, 24, 431–432. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.K.; Siegrist, K.J.; Wolff, M.; Nield, L.; Bruning, T.; Upham, B.L.; Kafferlein, H.U.; Plottner, S. The Carcinogenic Properties of Overlooked yet Prevalent Polycyclic Aromatic Hydrocarbons in Human Lung Epithelial Cells. Toxics 2022, 10, 28. [Google Scholar] [CrossRef] [PubMed]
- Osgood, R.S.; Upham, B.L.; Bushel, P.R.; Velmurugan, K.; Xiong, K.N.; Bauer, A.K. Secondhand Smoke-Prevalent Polycyclic Aromatic Hydrocarbon Binary Mixture-Induced Specific Mitogenic and Pro-inflammatory Cell Signaling Events in Lung Epithelial Cells. Toxicol. Sci. Off. J. Soc. Toxicol. 2017, 157, 156–171. [Google Scholar] [CrossRef]
- Romo, D.; Velmurugan, K.; Upham, B.L.; Dwyer-Nield, L.D.; Bauer, A.K. Dysregulation of Gap Junction Function and Cytokine Production in Response to Non-Genotoxic Polycyclic Aromatic Hydrocarbons in an In Vitro Lung Cell Model. Cancers 2019, 11, 572. [Google Scholar] [CrossRef] [PubMed]
- Siegrist, K.J.; Romo, D.; Upham, B.L.; Armstrong, M.; Quinn, K.; Vanderlinden, L.; Osgood, R.S.; Velmurugan, K.; Elie, M.; Manke, J.; et al. Early Mechanistic Events Induced by Low Molecular Weight Polycyclic Aromatic Hydrocarbons in Mouse Lung Epithelial Cells: A Role for Eicosanoid Signaling. Toxicol. Sci. Off. J. Soc. Toxicol. 2019, 169, 180–193. [Google Scholar] [CrossRef] [PubMed]
- Upham, B.L.; Blaha, L.; Babica, P.; Park, J.S.; Sovadinova, I.; Pudrith, C.; Rummel, A.M.; Weis, L.M.; Sai, K.; Tithof, P.K.; et al. Tumor promoting properties of a cigarette smoke prevalent polycyclic aromatic hydrocarbon as indicated by the inhibition of gap junctional intercellular communication via phosphatidylcholine-specific phospholipase C. Cancer Sci. 2008, 99, 696–705. [Google Scholar] [CrossRef]
- Weis, L.M.; Rummel, A.M.; Masten, S.J.; Trosko, J.E.; Upham, B.L. Bay or baylike regions of polycyclic aromatic hydrocarbons were potent inhibitors of Gap junctional intercellular communication. Environ. Health Perspect. 1998, 106, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Nahta, R.; Al-Mulla, F.; Al-Temaimi, R.; Amedei, A.; Andrade-Vieira, R.; Bay, S.N.; Brown, D.G.; Calaf, G.M.; Castellino, R.C.; Cohen-Solal, K.A.; et al. Mechanisms of environmental chemicals that enable the cancer hallmark of evasion of growth suppression. Carcinogenesis 2015, 36 (Suppl. S1), S2–S18. [Google Scholar] [CrossRef]
- Bauer, A.K.; Velmurugan, K.; Plottner, S.; Siegrist, K.J.; Romo, D.; Welge, P.; Bruning, T.; Xiong, K.N.; Kafferlein, H.U. Environmentally prevalent polycyclic aromatic hydrocarbons can elicit co-carcinogenic properties in an in vitro murine lung epithelial cell model. Arch. Toxicol. 2018, 92, 1311–1322. [Google Scholar] [CrossRef]
- Avanzo, J.L.; Mesnil, M.; Hernandez-Blazquez, F.J.; da Silva, T.C.; Fukumasu, H.; Mori, C.M.; Yamasaki, H.; Dagli, M.L. Altered expression of connexins in urethane-induced mouse lung adenomas. Life Sci. 2006, 79, 2202–2208. [Google Scholar] [CrossRef]
- Rosenkranz, M.; Rosenkranz, H.S.; Klopman, G. Intercellular communication, tumor promotion and non-genotoxic carcinogenesis: Relationships based upon structural considerations. Mutat. Res. 1997, 381, 171–188. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.S.; Busby, W.F., Jr. Induction of lung and liver tumors by fluoranthene in a preweanling CD-1 mouse bioassay. Carcinogenesis 1993, 14, 1871–1874. [Google Scholar] [CrossRef] [PubMed]
- Busby, W.F., Jr.; Goldman, M.E.; Newberne, P.M.; Wogan, G.N. Tumorigenicity of fluoranthene in a newborn mouse lung adenoma bioassay. Carcinogenesis 1984, 5, 1311–1316. [Google Scholar] [CrossRef] [PubMed]
- LaVoie, E.J.; Cai, Z.W.; Meschter, C.L.; Weyand, E.H. Tumorigenic activity of fluoranthene, 2-methylfluoranthene and 3-methylfluoranthene in newborn CD-1 mice. Carcinogenesis 1994, 15, 2131–2135. [Google Scholar] [CrossRef] [PubMed]
- Klaunig, J.E.; Kamendulis, L.M.; Xu, Y. Epigenetic mechanisms of chemical carcinogenesis. Hum. Exp. Toxicol. 2000, 19, 543–555. [Google Scholar] [CrossRef] [PubMed]
- Malkinson, A.M. Molecular comparison of human and mouse pulmonary adenocarcinomas. Exp. Lung Res. 1998, 24, 541–555. [Google Scholar] [CrossRef] [PubMed]
- Alexander, C.M.; Xiong, K.N.; Velmurugan, K.; Xiong, J.; Osgood, R.S.; Bauer, A.K. Differential innate immune cell signatures and effects regulated by toll-like receptor 4 during murine lung tumor promotion. Exp. Lung Res. 2016, 42, 154–173. [Google Scholar] [CrossRef][Green Version]
- Bauer, A.K.; Velmurugan, K.; Xiong, K.N.; Alexander, C.M.; Xiong, J.; Brooks, R. Epiregulin is required for lung tumor promotion in a murine two-stage carcinogenesis model. Mol. Carcinog. 2016, 56, 94–105. [Google Scholar] [CrossRef]
- Rondini, E.A.; Walters, D.M.; Bauer, A.K. Vanadium pentoxide induces pulmonary inflammation and tumor promotion in a strain-dependent manner. Part. Fibre Toxicol. 2010, 7, 9. [Google Scholar] [CrossRef]
- Fritz, J.M.; Dwyer-Nield, L.D.; Russell, B.M.; Malkinson, A.M. The Kras mutational spectra of chemically induced lung tumors in different inbred mice mimics the spectra of KRAS mutations in adenocarcinomas in smokers versus nonsmokers. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2010, 5, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Duan, S.; Shao, H.; Zhang, A.; Chen, S.; Zhang, P.; Wang, N.; Wang, W.; Wu, Y.; Wang, J.; et al. NLRP3 deletion inhibits inflammation-driven mouse lung tumorigenesis induced by benzo(a)pyrene and lipopolysaccharide. Respir. Res. 2019, 20, 20. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.K.; Fostel, J.; Degraff, L.M.; Rondini, E.A.; Walker, C.; Grissom, S.F.; Foley, J.; Kleeberger, S.R. Transcriptomic analysis of pathways regulated by toll-like receptor 4 in a murine model of chronic pulmonary inflammation and carcinogenesis. Mol. Cancer 2009, 8, 107. [Google Scholar] [CrossRef]
- Hill, T.; Osgood, R.S.; Velmurugan, K.; Alexander, C.M.; Upham, B.L.; Bauer, A.K. Bronchoalveolar Lavage Fluid Utilized Ex Vivo to Validate In Vivo Findings: Inhibition of Gap Junction Activity in Lung Tumor Promotion is Toll-Like Receptor 4-Dependent. J. Mol. Biomark. Diagn. 2013, 5, 160. [Google Scholar] [CrossRef]
- Osgood, R.S.; Upham, B.L.; Hill, T., 3rd; Helms, K.L.; Velmurugan, K.; Babica, P.; Bauer, A.K. Polycyclic aromatic hydrocarbon-induced signaling events relevant to inflammation and tumorigenesis in lung cells are dependent on molecular structure. PLoS ONE 2013, 8, e65150. [Google Scholar] [CrossRef]
- Gong, L.; Cumpian, A.M.; Caetano, M.S.; Ochoa, C.E.; De la Garza, M.M.; Lapid, D.J.; Mirabolfathinejad, S.G.; Dickey, B.F.; Zhou, Q.; Moghaddam, S.J. Promoting effect of neutrophils on lung tumorigenesis is mediated by CXCR2 and neutrophil elastase. Mol. Cancer 2013, 12, 154. [Google Scholar] [CrossRef]
- Vikis, H.G.; Gelman, A.E.; Franklin, A.; Stein, L.; Rymaszewski, A.; Zhu, J.; Liu, P.; Tichelaar, J.W.; Krupnick, A.S.; You, M. Neutrophils are required for 3-methylcholanthrene-initiated, butylated hydroxytoluene-promoted lung carcinogenesis. Mol. Carcinog. 2012, 51, 993–1002. [Google Scholar] [CrossRef]
- Parra, E.R.; Zhang, J.; Jiang, M.; Tamegnon, A.; Pandurengan, R.K.; Behrens, C.; Solis, L.; Haymaker, C.; Heymach, J.V.; Moran, C.; et al. Immune cellular patterns of distribution affect outcomes of patients with non-small cell lung cancer. Nat. Commun. 2023, 14, 2364. [Google Scholar] [CrossRef]
- Norris, P.C.; Dennis, E.A. A lipidomic perspective on inflammatory macrophage eicosanoid signaling. Adv. Biol. Regul. 2014, 54, 99–110. [Google Scholar] [CrossRef]
- Komurasaki, T.; Toyoda, H.; Uchida, D.; Morimoto, S. Epiregulin binds to epidermal growth factor receptor and ErbB-4 and induces tyrosine phosphorylation of epidermal growth factor receptor, ErbB-2, ErbB-3 and ErbB-4. Oncogene 1997, 15, 2841–2848. [Google Scholar] [CrossRef]
- Riese, D.J., 2nd; Komurasaki, T.; Plowman, G.D.; Stern, D.F. Activation of ErbB4 by the bifunctional epidermal growth factor family hormone epiregulin is regulated by ErbB2. J. Biol. Chem. 1998, 273, 11288–11294. [Google Scholar] [CrossRef] [PubMed]
- Shelly, M.; Pinkas-Kramarski, R.; Guarino, B.C.; Waterman, H.; Wang, L.M.; Lyass, L.; Alimandi, M.; Kuo, A.; Bacus, S.S.; Pierce, J.H.; et al. Epiregulin is a potent pan-ErbB ligand that preferentially activates heterodimeric receptor complexes. J. Biol. Chem. 1998, 273, 10496–10505. [Google Scholar] [CrossRef] [PubMed]
- Roskoski, R., Jr. The ErbB/HER family of protein-tyrosine kinases and cancer. Pharmacol. Res. 2014, 79, 34–74. [Google Scholar] [CrossRef]
- Sunaga, N.; Kaira, K.; Imai, H.; Shimizu, K.; Nakano, T.; Shames, D.S.; Girard, L.; Soh, J.; Sato, M.; Iwasaki, Y.; et al. Oncogenic KRAS-induced epiregulin overexpression contributes to aggressive phenotype and is a promising therapeutic target in non-small-cell lung cancer. Oncogene 2013, 32, 4034–4042. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Iwanaga, K.; Choi, K.C.; Wislez, M.; Raso, M.G.; Wei, W.; Wistuba, I.I.; Kurie, J.M. Intratumoral epiregulin is a marker of advanced disease in non-small cell lung cancer patients and confers invasive properties on EGFR-mutant cells. Cancer Prev. Res. 2008, 1, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Gyorffy, B. Transcriptome-level discovery of survival-associated biomarkers and therapy targets in non-small-cell lung cancer. Br. J. Pharmacol. 2024, 181, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Prüss-Üstün, A.; Wolf, J.; Corvalán, C.; Bos, R.; Neira, M. Preventing Disease Through Healthy Environments: A Global Assessment of the Burden of Disease from Environmental Risks; World Health Organization: Geneva, Switzerland, 2016.
- Cancer Stat Facts: Lung and Bronchus Cancer. Available online: https://seer.cancer.gov/statfacts/html/lungb.html (accessed on 13 October 2024).
- World Health Organization. WHO Global Report on Trends in Prevalence of Tobacco Use 2000–2030; World Health Organization: Geneva, Switzerland, 2024; p. 11.
- Navarro, K.M.; Kleinman, M.T.; Mackay, C.E.; Reinhardt, T.E.; Balmes, J.R.; Broyles, G.A.; Ottmar, R.D.; Naher, L.P.; Domitrovich, J.W. Wildland firefighter smoke exposure and risk of lung cancer and cardiovascular disease mortality. Envron. Res. 2019, 173, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Korsiak, J.; Pinault, L.; Christidis, T.; Burnett, R.T.; Abrahamowicz, M.; Weichenthal, S. Long-term exposure to wildfires and cancer incidence in Canada: A population-based observational cohort study. Lancet Planet Health 2022, 6, e400–e409. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Schick, S.F.; Glantz, S.A. Sidestream cigarette smoke toxicity increases with aging and exposure duration. Tob. Control 2006, 15, 424–429. [Google Scholar] [CrossRef]
- Schick, S.; Glantz, S. Philip Morris toxicological experiments with fresh sidestream smoke: More toxic than mainstream smoke. Tob. Control 2005, 14, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Schick, S.; Glantz, S. Scientific analysis of second-hand smoke by the tobacco industry, 1929–1972. Nicotine Tob. Res. Off. J. Soc. Res. Nicotine Tob. 2005, 7, 591–612. [Google Scholar] [CrossRef] [PubMed]
- Russell, P.A.; Barnett, S.A.; Walkiewicz, M.; Wainer, Z.; Conron, M.; Wright, G.M.; Gooi, J.; Knight, S.; Wynne, R.; Liew, D.; et al. Correlation of mutation status and survival with predominant histologic subtype according to the new IASLC/ATS/ERS lung adenocarcinoma classification in stage III (N2) patients. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2013, 8, 461–468. [Google Scholar] [CrossRef]
- Nicholson, A.G.; Tsao, M.S.; Beasley, M.B.; Borczuk, A.C.; Brambilla, E.; Cooper, W.A.; Dacic, S.; Jain, D.; Kerr, K.M.; Lantuejoul, S.; et al. The 2021 WHO Classification of Lung Tumors: Impact of Advances Since 2015. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2022, 17, 362–387. [Google Scholar] [CrossRef] [PubMed]
- Kauffman, S.L. Histogenesis of the papillary Clara cell adenoma. Am. J. Pathol. 1981, 103, 174–180. [Google Scholar]
- Redente, E.F.; Higgins, D.M.; Dwyer-Nield, L.D.; Orme, I.M.; Gonzalez-Juarrero, M.; Malkinson, A.M. Differential polarization of alveolar macrophages and bone marrow-derived monocytes following chemically and pathogen-induced chronic lung inflammation. J. Leukoc. Biol. 2010, 88, 159–168. [Google Scholar] [CrossRef]
- Keith, R.L.; Miller, Y.E. Lung cancer chemoprevention: Current status and future prospects. Nat. Rev. Clin. Oncol. 2013, 10, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Fritz, J.M.; Tennis, M.A.; Orlicky, D.J.; Lin, H.; Ju, C.; Redente, E.F.; Choo, K.S.; Staab, T.A.; Bouchard, R.J.; Merrick, D.T.; et al. Depletion of tumor-associated macrophages slows the growth of chemically induced mouse lung adenocarcinomas. Front. Immunol. 2014, 5, 587. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J. Macrophage Polarization. Annu. Rev. Physiol. 2017, 79, 541–566. [Google Scholar] [CrossRef]
- Keith, R.L.; Miller, Y.E.; Ghosh, M.; Franklin, W.A.; Nakachi, I.; Merrick, D.T. Lung cancer: Premalignant biology and medical prevention. Semin. Oncol. 2022, 49, 254–260. [Google Scholar] [CrossRef]
- Kisley, L.R.; Barrett, B.S.; Dwyer-Nield, L.D.; Bauer, A.K.; Thompson, D.C.; Malkinson, A.M. Celecoxib reduces pulmonary inflammation but not lung tumorigenesis in mice. Carcinogenesis 2002, 23, 1653–1660. [Google Scholar] [CrossRef] [PubMed]
- Gulyas, M.; Mattsson, J.S.M.; Lindgren, A.; Ek, L.; Lamberg Lundstrom, K.; Behndig, A.; Holmberg, E.; Micke, P.; Bergman, B.; Swedish Lung Cancer Study, G. COX-2 expression and effects of celecoxib in addition to standard chemotherapy in advanced non-small cell lung cancer. Acta Oncol. 2018, 57, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Liao, Z.M.; Fu, Y.; Wu, Y.P.; Zhang, Q. Pooled analysis of the clinical benefit of cyclooxygenase-2 inhibitors combined with chemotherapy in advanced non-small cell lung cancer. Transl. Cancer Res. 2019, 8, 1258–1267. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.L.; Feng, P.H.; Lee, K.Y.; Chen, K.Y.; Sun, W.L.; Van Hiep, N.; Luo, C.S.; Wu, S.M. The Role of EREG/EGFR Pathway in Tumor Progression. Int. J. Mol. Sci. 2021, 22, 2828. [Google Scholar] [CrossRef]
- Xu, X.; Chen, W.; Leng, S.; Padilla, M.T.; Saxton, B.; Hutt, J.; Tessema, M.; Kato, K.; Kim, K.C.; Belinsky, S.A.; et al. Muc1 knockout potentiates murine lung carcinogenesis involving an epiregulin-mediated EGFR activation feedback loop. Carcinogenesis 2017, 38, 604–614. [Google Scholar] [CrossRef]
- Iijima, M.; Anai, M.; Kodama, T.; Shibasaki, Y. Epiregulin-blocking antibody inhibits epiregulin-dependent EGFR signaling. Biochem. Biophys. Res. Commun. 2017, 489, 83–88. [Google Scholar] [CrossRef]
- Avanzo, J.L.; Mesnil, M.; Hernandez-Blazquez, F.J.; Mackowiak, I.I.; Mori, C.M.; da Silva, T.C.; Oloris, S.C.; Garate, A.P.; Massironi, S.M.; Yamasaki, H.; et al. Increased susceptibility to urethane-induced lung tumors in mice with decreased expression of connexin43. Carcinogenesis 2004, 25, 1973–1982. [Google Scholar] [CrossRef]
- Bauer, A.K.; Dwyer-Nield, L.D.; Hankin, J.A.; Murphy, R.C.; Malkinson, A.M. The lung tumor promoter, butylated hydroxytoluene (BHT), causes chronic inflammation in promotion-sensitive BALB/cByJ mice but not in promotion-resistant CXB4 mice. Toxicology 2001, 169, 1–15. [Google Scholar] [CrossRef]
- Sargent, L.M.; Porter, D.W.; Staska, L.M.; Hubbs, A.F.; Lowry, D.T.; Battelli, L.; Siegrist, K.J.; Kashon, M.L.; Mercer, R.R.; Bauer, A.K.; et al. Promotion of lung adenocarcinoma following inhalation exposure to multi-walled carbon nanotubes. Part. Fibre Toxicol. 2014, 11, 3. [Google Scholar] [CrossRef]
- Siegfried, J.M. Sex and Gender Differences in Lung Cancer and Chronic Obstructive Lung Disease. Endocrinology 2022, 163, bqab254. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bauer, A.K.; Romo, D.; Friday, F.; Cho, K.; Velmurugan, K.; Upham, B.L. Non-Genotoxic and Environmentally Relevant Lower Molecular Weight Polycyclic Aromatic Hydrocarbons Significantly Increase Tumorigenicity of Benzo[a]pyrene in a Lung Two-Stage Mouse Model. Toxics 2024, 12, 882. https://doi.org/10.3390/toxics12120882
Bauer AK, Romo D, Friday F, Cho K, Velmurugan K, Upham BL. Non-Genotoxic and Environmentally Relevant Lower Molecular Weight Polycyclic Aromatic Hydrocarbons Significantly Increase Tumorigenicity of Benzo[a]pyrene in a Lung Two-Stage Mouse Model. Toxics. 2024; 12(12):882. https://doi.org/10.3390/toxics12120882
Chicago/Turabian StyleBauer, Alison K., Deedee Romo, Finnegan Friday, Kaila Cho, Kalpana Velmurugan, and Brad L. Upham. 2024. "Non-Genotoxic and Environmentally Relevant Lower Molecular Weight Polycyclic Aromatic Hydrocarbons Significantly Increase Tumorigenicity of Benzo[a]pyrene in a Lung Two-Stage Mouse Model" Toxics 12, no. 12: 882. https://doi.org/10.3390/toxics12120882
APA StyleBauer, A. K., Romo, D., Friday, F., Cho, K., Velmurugan, K., & Upham, B. L. (2024). Non-Genotoxic and Environmentally Relevant Lower Molecular Weight Polycyclic Aromatic Hydrocarbons Significantly Increase Tumorigenicity of Benzo[a]pyrene in a Lung Two-Stage Mouse Model. Toxics, 12(12), 882. https://doi.org/10.3390/toxics12120882







