Energy, Aromatic, and Medicinal Plants’ Potential and Prospects for the Remediation of Potentially Toxic Element-Contaminated Agricultural Soils: A Critical Meta-Analysis
Abstract
1. Introduction
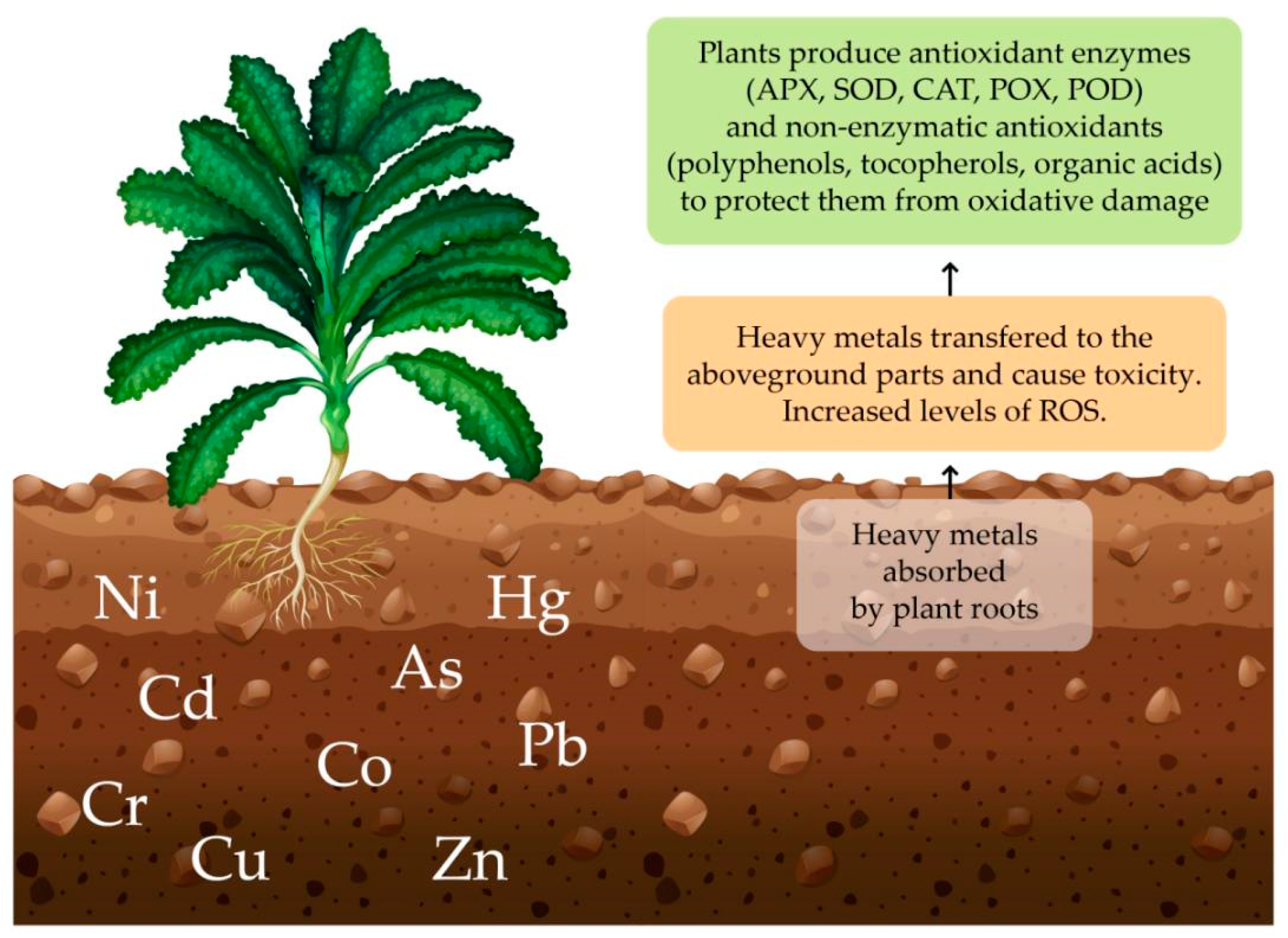
1.1. Mechanisms and Methods of Phytoremediation
1.2. Plant Categories Used for Phytoremediation Purposes
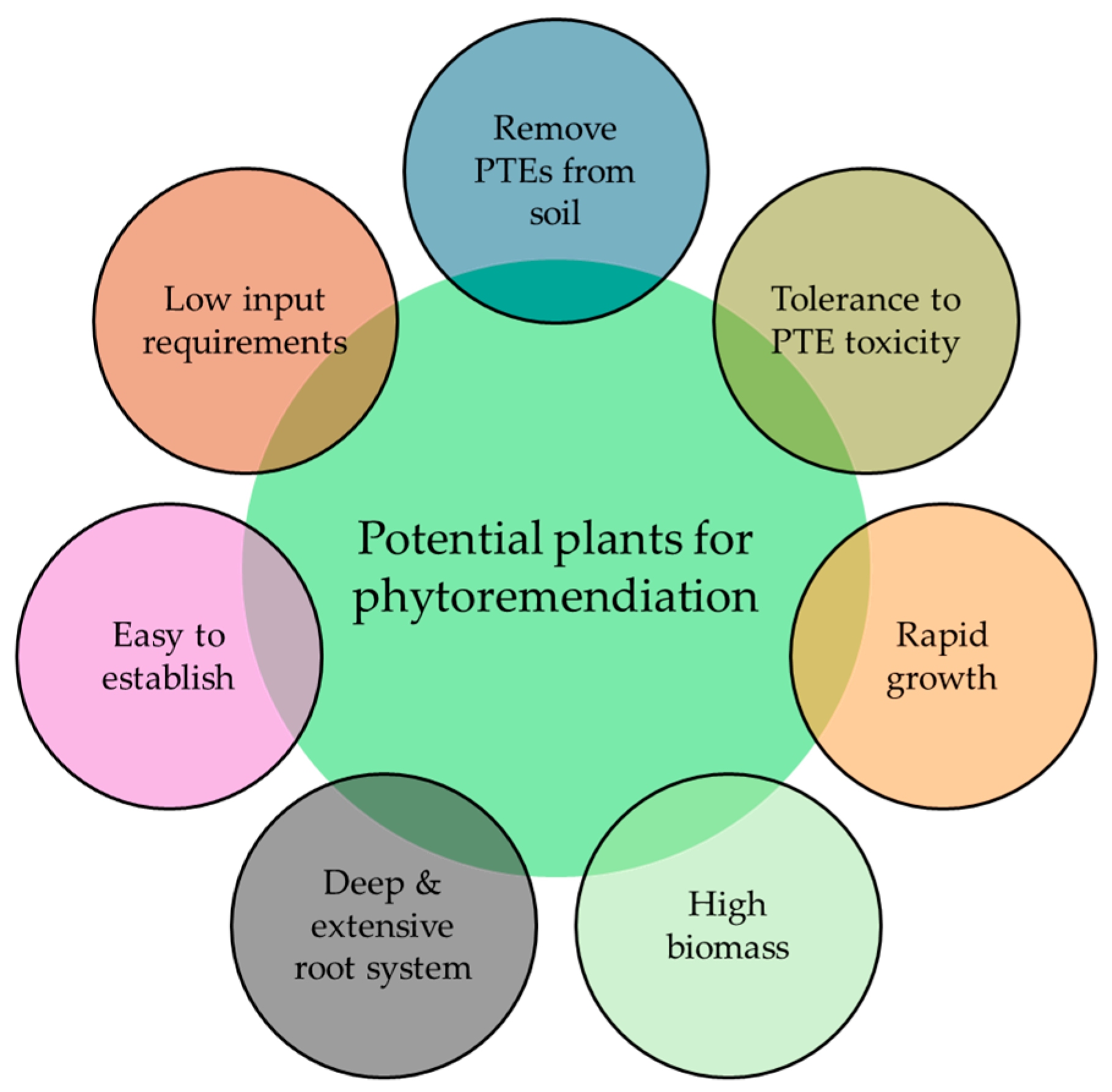
1.3. Plant Selection for Phytoremediation
2. Materials and Methods
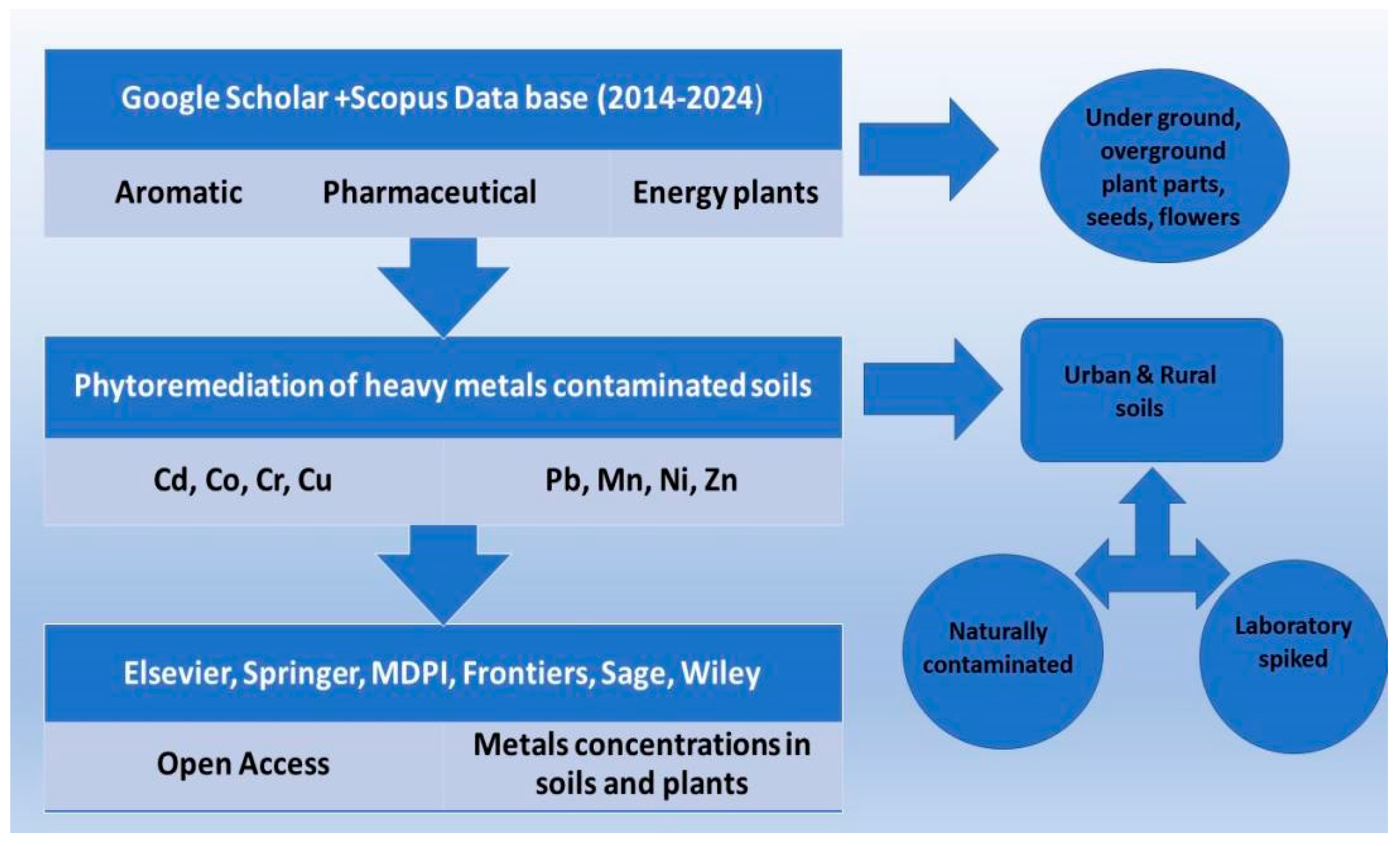
2.1. Methodology for Data Collection
2.2. PTEs Determination
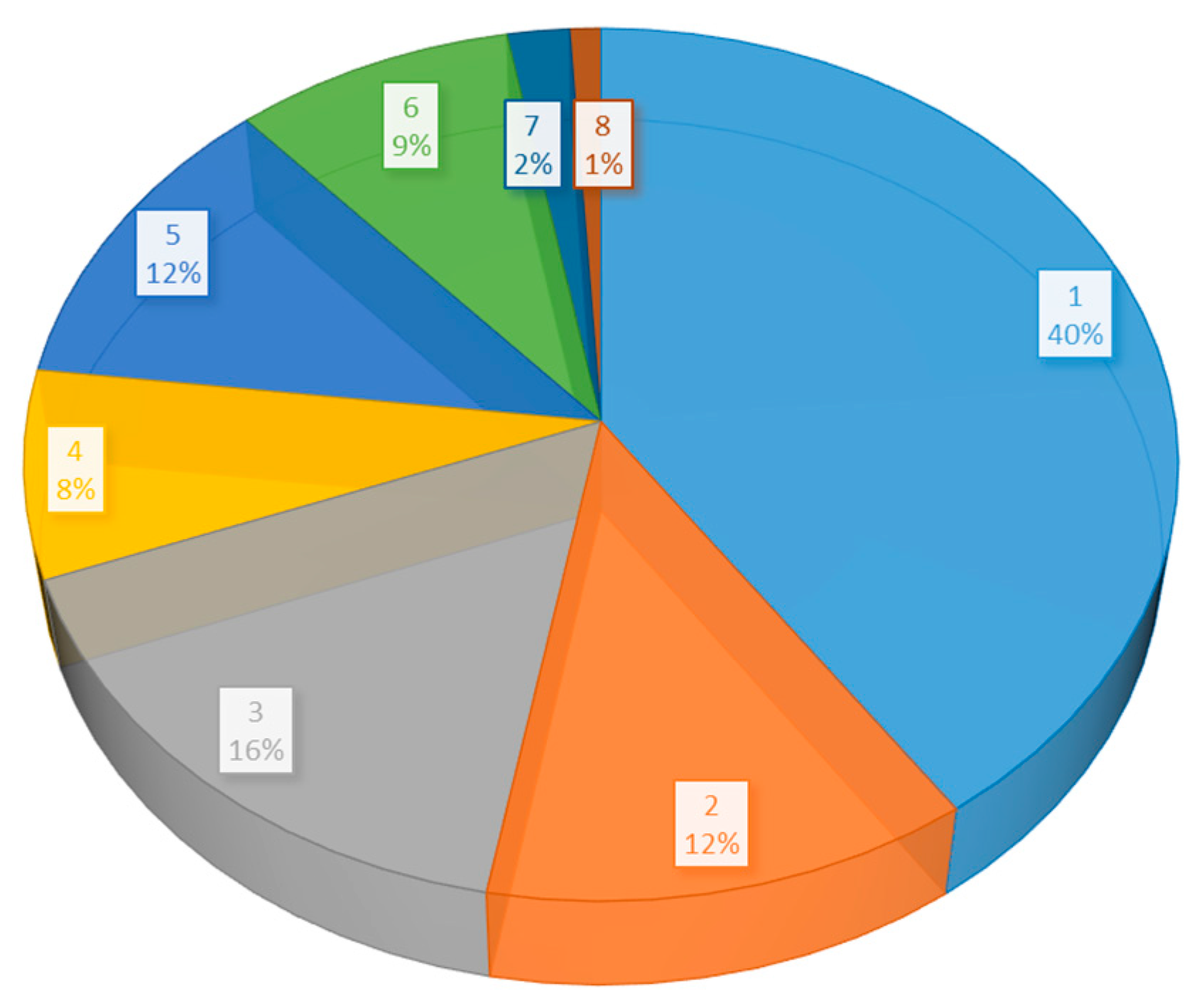
3. Results
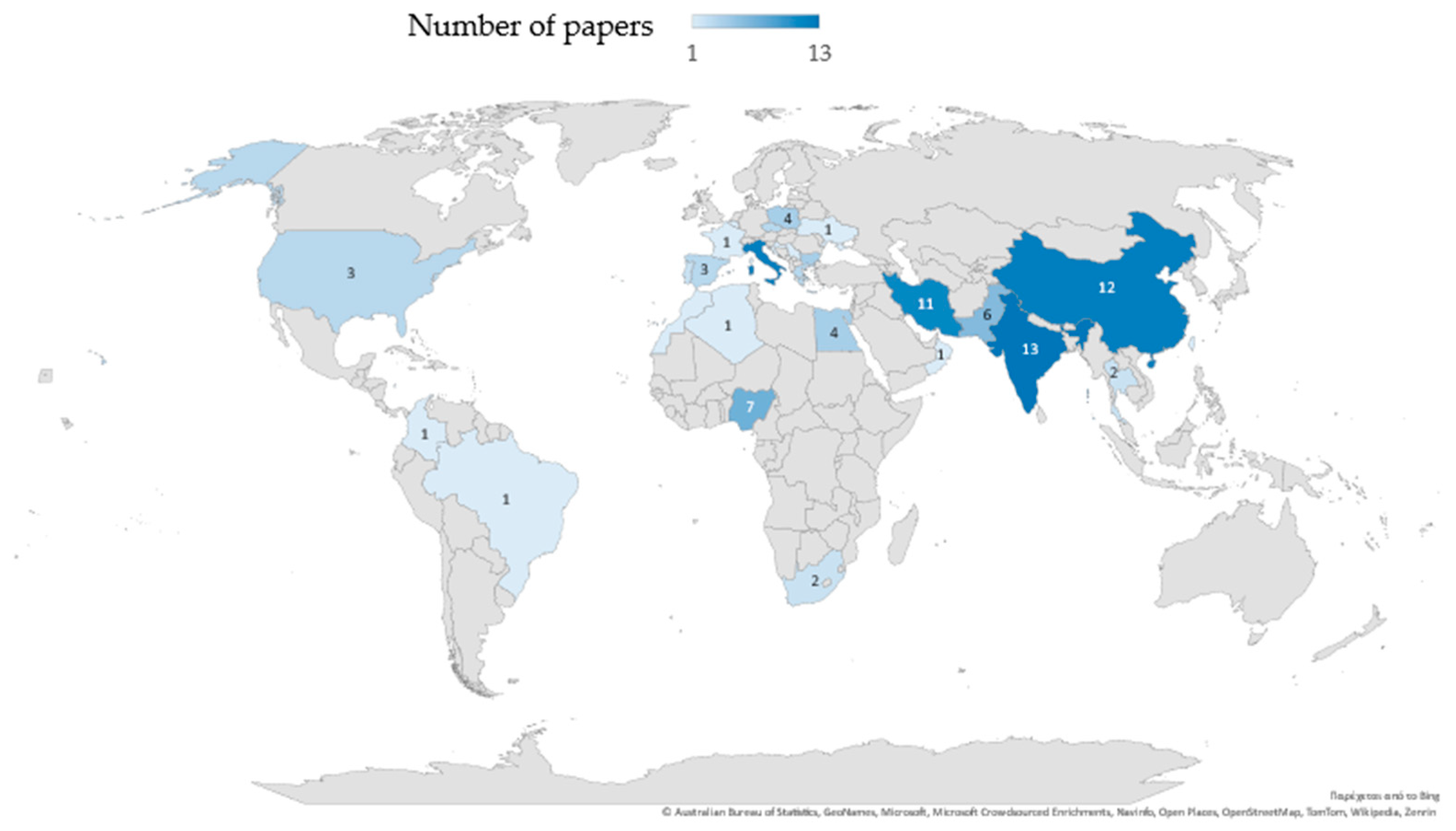
3.1. Overview of Selected Published Studies
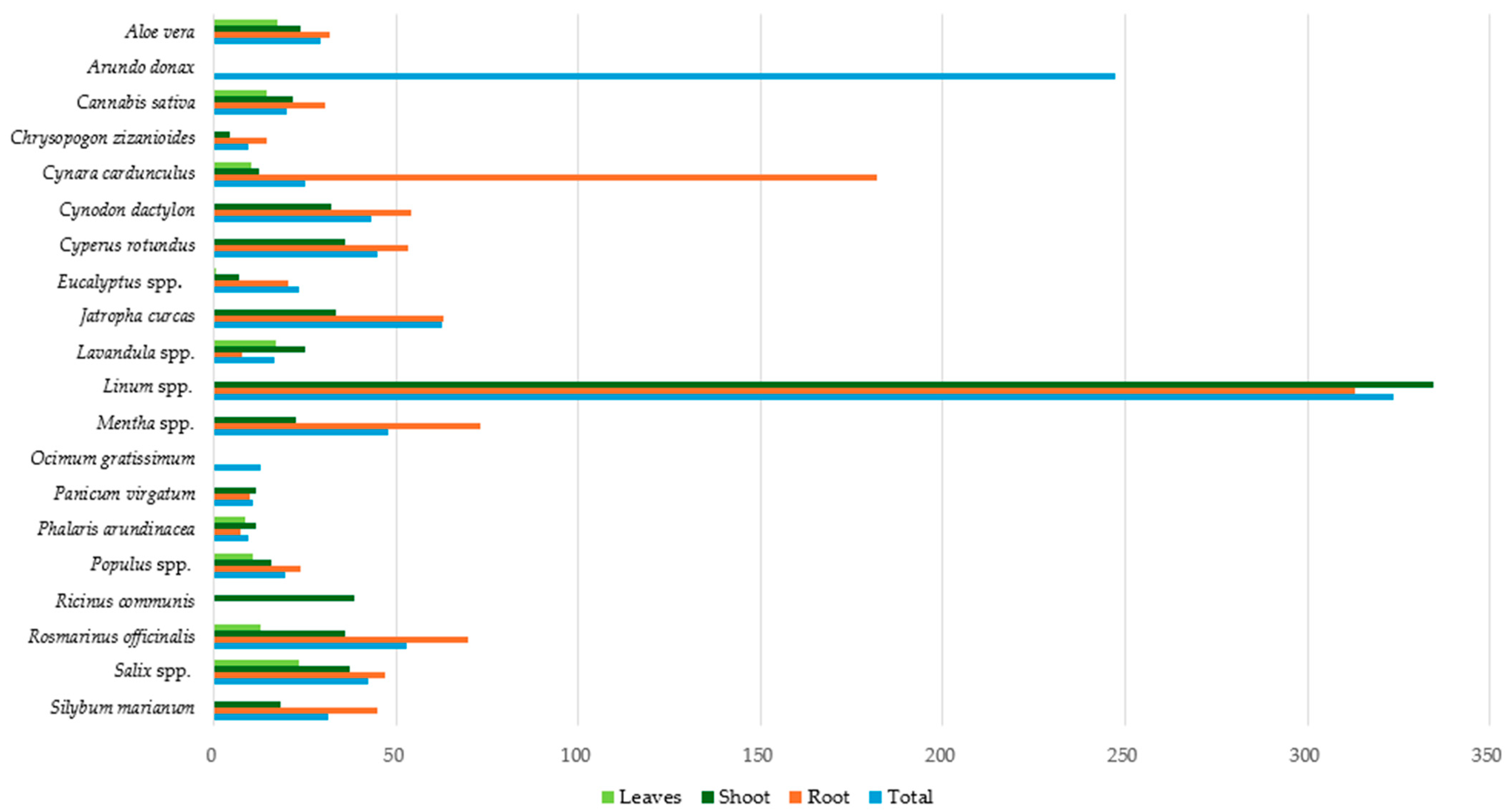
3.2. Pb Accumulation in Plant Parts
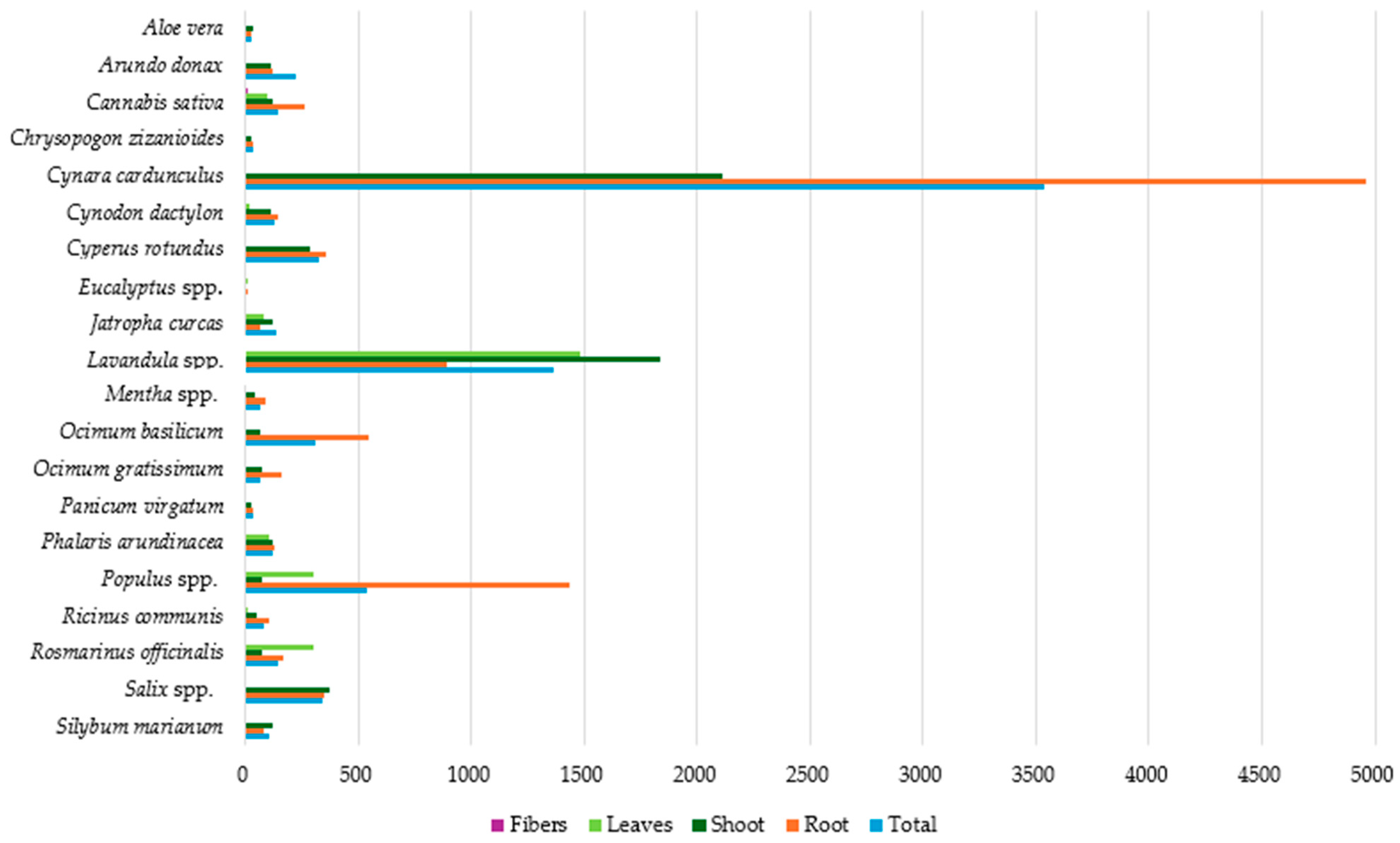
3.3. Cu Accumulation in Plant Parts
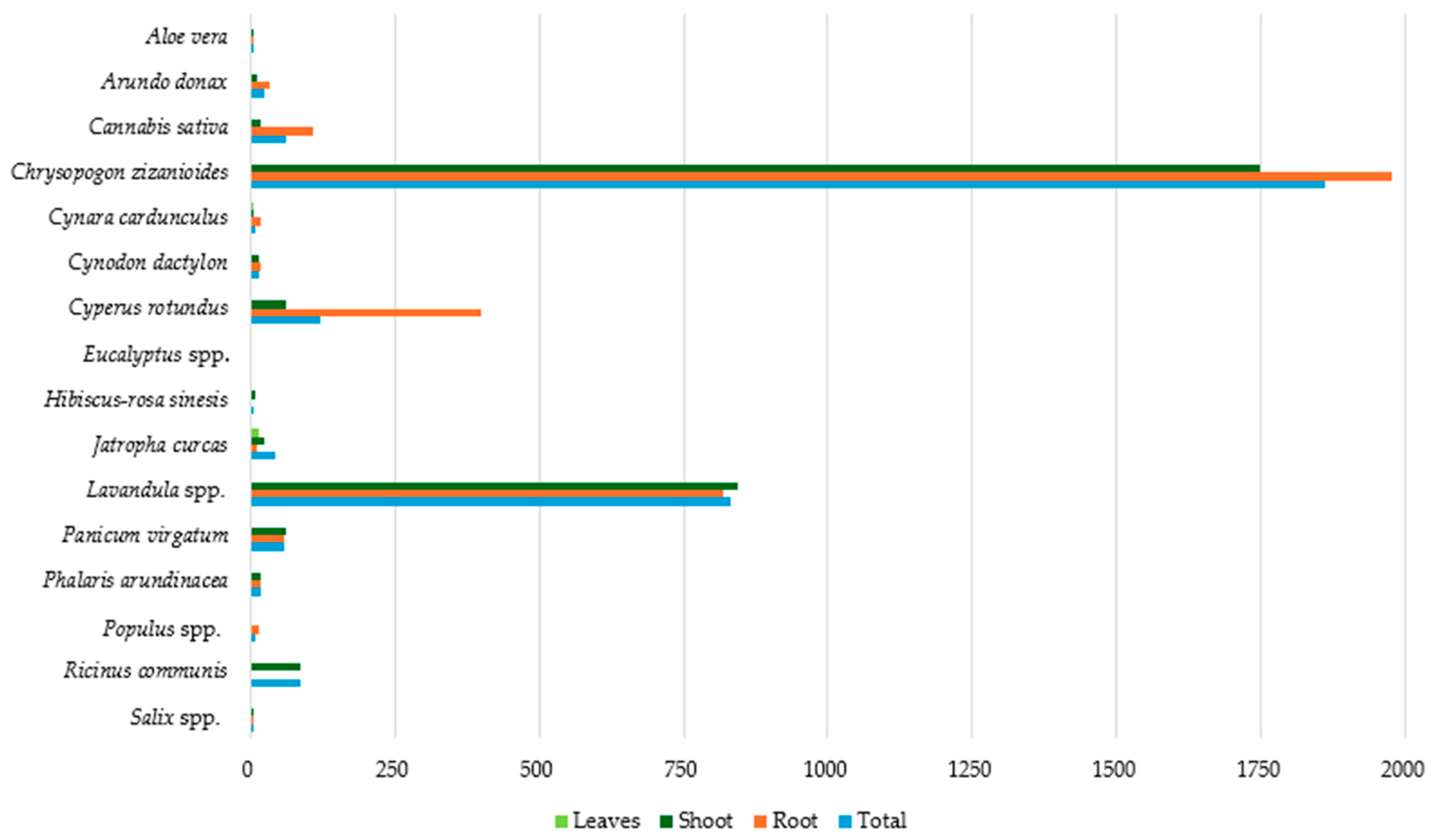
3.4. Cd Accumulation in Plant Parts
3.5. Zn Accumulation in Plant Parts
3.6. Cr Accumulation in Plant Parts
3.7. Ni Accumulation in Plant Parts
3.8. Co Accumulation in Plant Parts
3.9. Hg Accumulation in Plant Parts
3.10. As Accumulation in Plant Parts
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, Y.; Qiu, C.; Long, S.; Wang, H.; Wang, Y. Cadmium Accumulation, Translocation, and Assessment of Eighteen Linum usitatissimum L. Cultivars Growing in Heavy Metal Contaminated Soil. Int. J. Phytoremediat. 2020, 22, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Qi, S.; Peng, L.; Xie, X. Enhanced Phytoremediation Capacity of a Mixed-Species Plantation of Eucalyptus Globulus and Chickpeas. J. Geochem. Explor. 2017, 182, 201–205. [Google Scholar] [CrossRef]
- Álvarez-Mateos, P.; Alés-Álvarez, F.-J.; García-Martín, J.F. Phytoremediation of Highly Contaminated Mining Soils by Jatropha curcas L. and Production of Catalytic Carbons from the Generated Biomass. J. Environ. Manag. 2019, 231, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Chem, E.O. Accumulation of Heavy Metal in Soil and Their Transfer to Leafy Vegetables with Phytoremediation Potential. Am. J. Chem. 2015, 5, 125–131. [Google Scholar]
- Arena, C.; Figlioli, F.; Sorrentino, M.C.; Izzo, L.G.; Capozzi, F.; Giordano, S.; Spagnuolo, V. Ultrastructural, Protein and Photosynthetic Alterations Induced by Pb and Cd in Cynara cardunculus L., and Its Potential for Phytoremediation. Ecotoxicol. Environ. Saf. 2017, 145, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Angelova, V.R.; Grekov, D.F.; Kisyov, V.K.; Ivanov, K.I. Potential of Lavender (Lavandula vera L.) for Phytoremediation of Soils Contaminated with Heavy Metals. Int. J. Agric. Eng. 2015, 9, 522–529. [Google Scholar]
- Sakakibara, M.; Watanabe, A.; Inoue, M.; Sano, S.; Kaise, T. Phytoextraction and Phytovolatilization of Arsenic from As-Contaminated Soils by Pteris Vittata. Proc. Annu. Int. Conf. Soils 2010, 12, 26. [Google Scholar]
- Saleem, M.H.; Kamran, M.; Zhou, Y.; Parveen, A.; Rehman, M.; Ahmar, S.; Malik, Z.; Mustafa, A.; Ahmad Anjum, R.M.; Wang, B.; et al. Appraising Growth, Oxidative Stress and Copper Phytoextraction Potential of Flax (Linum usitatissimum L.) Grown in Soil Differentially Spiked with Copper. J. Environ. Manag. 2020, 257, 109994. [Google Scholar] [CrossRef]
- Aderholt, M.; Vogelien, D.L.; Koether, M.; Greipsson, S. Phytoextraction of Contaminated Urban Soils by Panicum virgatum L. Enhanced with Application of a Plant Growth Regulator (BAP) and Citric Acid. Chemosphere 2017, 175, 85–96. [Google Scholar] [CrossRef]
- Ng, C.C. Phytoaccumulation of Heavy Metals from Contaminated Soils by Vetiver Grass (Vetiveria zizanioides) in Malaysia. Ph.D. Thesis, University of Malaya, Kuala Lumpur, Malaysia, 2017. [Google Scholar]
- Suman, J.; Uhlik, O.; Viktorova, J.; Macek, T. Phytoextraction of Heavy Metals: A Promising Tool for Clean-Up of Polluted Environment? Front. Plant Sci. 2018, 9, 1476. [Google Scholar] [CrossRef] [PubMed]
- Jeguirim, M.; Dorge, S.; Trouvé, G. Thermogravimetric Analysis and Emission Characteristics of Two Energy Crops in Air Atmosphere: Arundo donax and Miscanthus giganthus. Bioresour. Technol. 2010, 101, 788–793. [Google Scholar] [CrossRef]
- Nokande, S.E.; Razavi, S.M.; Mohammadian, M.A. The Capacity of Heavy Metal Remediation by Cyperus alternifolius, Chrysopogon zizanioides (L.) Roberty, and Aloe vera (L.) Burm. f. under Industrial and Urban Wastewater Treatment. Chiang Mai Univ. J. Nat. Sci. 2022, 21, e2022057. [Google Scholar] [CrossRef]
- Ye, L.-L.; Chen, Y.-S.; Chen, Y.-D.; Qian, L.-W.; Xiong, W.-L.; Xu, J.-H.; Jiang, J.-P. Phytomanagement of a Chromium-Contaminated Soil by a High-Value Plant: Phytostabilization of Heavy Metal Contaminated Site. BioResources 2020, 15, 3545–3565. [Google Scholar] [CrossRef]
- Papadimou, S.G.; Barbayiannis, Ν.; Golia, E.E. Preliminary Investigation of the Use of Silybum marianum (L.) Gaertn. as a Cd Accumulator in Contaminated Mediterranean Soils: The Relationships among Cadmium (Cd) Soil Fractions and Plant Cd Content. Euro Mediterr. J. Environ. Integr. 2024, 9, 405–417. [Google Scholar] [CrossRef]
- Epelde, L.; Becerril, J.M.; Mijangos, I.; Garbisu, C. Evaluation of the Efficiency of a Phytostabilization Process with Biological Indicators of Soil Health. J. Environ. Qual. 2009, 38, 2041–2049. [Google Scholar] [CrossRef] [PubMed]
- Frérot, H.; Lefèbvre, C.; Gruber, W.; Collin, C.; Dos Santos, A.; Escarré, J. Specific Interactions between Local Metallicolous Plants Improve the Phytostabilization of Mine Soils. Plant Soil 2006, 282, 53–65. [Google Scholar] [CrossRef]
- Zine, H.; Midhat, L.; Hakkou, R.; El Adnani, M.; Ouhammou, A. Guidelines for a Phytomanagement Plan by the Phytostabilization of Mining Wastes. Sci. Afr. 2020, 10, e00654. [Google Scholar] [CrossRef]
- Abhilash, P.C.; Pandey, V.C.; Srivastava, P.; Rakesh, P.S.; Chandran, S.; Singh, N.; Thomas, A.P. Phytofiltration of Cadmium from Water by Limnocharis flava (L.) Buchenau Grown in Free-Floating Culture System. J. Hazard. Mater. 2009, 170, 791–797. [Google Scholar] [CrossRef]
- Huang, J.W.; Poynton, C.Y.; Kochian, L.V.; Elless, M.P. Phytofiltration of Arsenic from Drinking Water Using Arsenic-Hyperaccumulating Ferns. Environ. Sci. Technol. 2004, 38, 3412–3417. [Google Scholar] [CrossRef]
- Moreno, F.N.; Anderson, C.W.N.; Stewart, R.B.; Robinson, B.H. Phytofiltration of Mercury-Contaminated Water: Volatilisation and Plant-Accumulation Aspects. Environ. Exp. Bot. 2008, 62, 78–85. [Google Scholar] [CrossRef]
- Gardea-Torresdey, J.L.; de la Rosa, G.; Peralta-Videa, J.R. Use of Phytofiltration Technologies in the Removal of Heavy Metals: A Review. Pure Appl. Chem. 2004, 76, 801–813. [Google Scholar] [CrossRef]
- Islam, S.; Ueno, Y.; Sikder, T.; Kurasaki, M. Phytofiltration of Arsenic and Cadmium from the Water Environment Using Micranthemum umbrosum (J.F. Gmel) S.F. Blake as a Hyperaccumulator. Int. J. Phytoremediat. 2013, 15, 1010–1021. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Dilawar, S.; Hassan, S.; Ullah, A.; Yasmin, H.; Ayaz, T.; Akhtar, F.; Gaafar, A.-R.Z.; Sekar, S.; Butt, S. Phytoremediation of Cu and Mn from Industrially Polluted Soil: An Eco-Friendly and Sustainable Approach. Water 2023, 15, 3439. [Google Scholar] [CrossRef]
- Fattahi, B.; Arzani, K.; Souri, M.K.; Barzegar, M. Effects of Cadmium and Lead on Seed Germination, Morphological Traits, and Essential Oil Composition of Sweet Basil (Ocimum basilicum L.). Ind. Crops Prod. 2019, 138, 111584. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Y.; Yuan, X.; Jiang, Y.; Zhu, Y.; Kang, X.; He, J.; Xiao, Y. Comparative Transcriptomic Analysis Provides Key Genetic Resources in Clove Basil (Ocimum gratissimum) under Cadmium Stress. Front. Genet. 2023, 14, 1224140. [Google Scholar] [CrossRef]
- Papadimou, S.G.; Golia, E.E.; Barbayiannis, N.; Tsiropoulos, N.G. Dual Role of the Hyperaccumulator Silybum marianum (L.) Gaertn. in Circular Economy: Production of Silymarin, a Valuable Secondary Metabolite, and Remediation of Heavy Metal Contaminated Soils. Sustain. Chem. Pharm. 2024, 38, 101454. [Google Scholar] [CrossRef]
- Vasilou, C.; Tsiropoulos, N.G.; Golia, E.E. Phytoremediation & Valorization of Cu-Contaminated Soils Through Cannabis sativa (L.) Cultivation: A Smart Way to Produce Cannabidiol (CBD) in Mediterranean Soils. Waste Biomass Valorization 2024, 15, 1711–1724. [Google Scholar] [CrossRef]
- Thanh, N.C.; Narayanan, M.; Saravanan, M.; Chinnathambi, A.; Alahmadi, T.A.; Brindhadevi, K.; Sharma, A.; Pugazhendhi, A. Hibiscus Rosa-Sinensis as a Potential Hyperaccumulator in Metal Contaminated Magnesite Mine Tailings. Chemosphere 2023, 339, 139738. [Google Scholar] [CrossRef]
- Rasouli, F.; Hassanpouraghdam, M.B.; Pirsarandib, Y.; Aazami, M.A.; Asadi, M.; Ercisli, S.; Mehrabani, L.V.; Puglisi, I.; Baglieri, A. Improvements in the Biochemical Responses and Pb and Ni Phytoremediation of Lavender (Lavandula angustifolia L.) Plants through Funneliformis mosseae Inoculation. BMC Plant Biol. 2023, 23, 252. [Google Scholar] [CrossRef] [PubMed]
- Golia, E.E.; Bethanis, J.; Ntinopoulos, N.; Kaffe, G.G.; Komnou, A.A.; Vasilou, C. Investigating the Potential of Heavy Metal Accumulation from Hemp. The Use of Industrial Hemp (Cannabis sativa L.) for Phytoremediation of Heavily And and Moderated Polluted Soils. Sustain. Chem. Pharm. 2023, 31, 100961. [Google Scholar] [CrossRef]
- Golia, E.E.; Angelaki, A.; Giannoulis, K.D.; Skoufogianni, E.; Bartzialis, D.; Cavalaris, C.; Vleioras, S. Evaluation of Soil Properties, Irrigation and Solid Waste Application Levels on Cu and Zn Uptake by Industrial Hemp. Agron. Res. 2021, 19, 92–99. [Google Scholar] [CrossRef]
- Pandey, V.C.; Bajpai, O.; Singh, N. Energy Crops in Sustainable Phytoremediation. Renew. Sustain. Energy Rev. 2016, 54, 58–73. [Google Scholar] [CrossRef]
- Parra, C.R.; Ramirez, A.D.; Navas-Gracia, L.M.; Gonzales, D.; Correa-Guimaraes, A. Prospects for Bioenergy Development Potential from Dedicated Energy Crops in Ecuador: An Agroecological Zoning Study. Agriculture 2023, 13, 186. [Google Scholar] [CrossRef]
- Bona, E.; Marsano, F.; Cavaletto, M.; Berta, G. Proteomic Characterization of Copper Stress Response in Cannabis sativa Roots. Proteomics 2007, 7, 1121–1130. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, B.; Boléo, S.; Sidella, S.; Costa, J.; Duarte, M.P.; Mendes, B.; Cosentino, S.L.; Fernando, A.L. Phytoremediation of Heavy Metal-Contaminated Soils Using the Perennial Energy Crops Miscanthus spp. and Arundo donax L. BioEnergy Res. 2015, 8, 1500–1511. [Google Scholar] [CrossRef]
- Korzeniowska, J.; Stanislawska-Glubiak, E. Phytoremediation Potential of Phalaris arundinacea, Salix viminalis and Zea mays for Nickel-Contaminated Soils. Int. J. Environ. Sci. Technol. 2019, 16, 1999–2008. [Google Scholar] [CrossRef]
- Garau, M.; Castaldi, P.; Patteri, G.; Roggero, P.P.; Garau, G. Evaluation of Cynara cardunculus L. and Municipal Solid Waste Compost for Aided Phytoremediation of Multi Potentially Toxic Element–Contaminated Soils. Environ. Sci. Pollut. Res. 2021, 28, 3253–3265. [Google Scholar] [CrossRef] [PubMed]
- Gabriele, I.; Bianco, F.; Race, M.; Papirio, S.; Esposito, G. Phytoremediation of PAH- and Cu-Contaminated Soil by Cannabis sativa L.: Preliminary Experiments on a Laboratory Scale. Sustainability 2023, 15, 1852. [Google Scholar] [CrossRef]
- Ţîţei, V.; Gadibadi, M.; Guţu, A.; Daraduda, N.; Mazăre, V.; Armaş, A.; Cerempei, V. Biomass Quality of Hemp, Cannabis sativa L., and Prospects of Its Use for Various Energy Purposes; Institutional Repository of the Technical University of Moldova: Chisinau, Moldova, 2020. [Google Scholar]
- Sawicka, B.; Skiba, D.; Kiełtyka-Dadasiewicz, A.; Danilčenko, H. Jerusalem Artichoke (Helianthus tuberosus L.) as Energy Raw Material. In Rural Development: Proceedings of the International Scientific Conference; Latvia University of Life Sciences and Technologies: Jelgava, Latvia, 2019; pp. 336–342. https://doi.org/10.15544/RD.2019.042.Material. In Rural Development: Proceedings of the International Scientific Conference; Latvia University of Life Sciences and Technologies: Jelgava, Latvia, 2019; pp. 336–342. [Google Scholar] [CrossRef]
- Gao, K.; Zhang, Z.; Zhu, T.; Coulter, J.A. The Influence of Flower Removal on Tuber Yield and Biomass Characteristics of Helianthus tuberosus L. in a Semi-Arid Area. Ind. Crops Prod. 2020, 150, 112374. [Google Scholar] [CrossRef]
- Prapagdee, B.; Khonsue, N. Bacterial-Assisted Cadmium Phytoremediation by Ocimum gratissimum L. in Polluted Agricultural Soil: A Field Trial Experiment. Int. J. Environ. Sci. Technol. 2015, 12, 3843–3852. [Google Scholar] [CrossRef]
- Mishra, B.; Chandra, M. Evaluation of Phytoremediation Potential of Aromatic Plants: A Systematic Review. J. Appl. Res. Med. Aromat. Plants 2022, 31, 100405. [Google Scholar] [CrossRef]
- Youssef, N.A. Changes in the Morphological Traits and the Essential Oil Content of Sweet Basil (Ocimum basilicum L.) as Induced by Cadmium and Lead Treatments. Int. J. Phytoremediat. 2021, 23, 291–299. [Google Scholar] [CrossRef]
- Capozzi, F.; Sorrentino, M.C.; Caporale, A.G.; Fiorentino, N.; Giordano, S.; Spagnuolo, V. Exploring the Phytoremediation Potential of Cynara cardunculus: A Trial on an Industrial Soil Highly Contaminated by Heavy Metals. Environ. Sci. Pollut. Res. 2020, 27, 9075–9084. [Google Scholar] [CrossRef] [PubMed]
- Thomas, G.; Sheridan, C.; Holm, P.E. Co-Cropping Vetiver Grass and Legume for the Phytoremediation of an Acid Mine Drainage (AMD) Impacted Soil. Environ. Pollut. 2024, 341, 122873. [Google Scholar] [CrossRef] [PubMed]
- Cristaldi, A.; Oliveri Conti, G.; Cosentino, S.L.; Mauromicale, G.; Copat, C.; Grasso, A.; Zuccarello, P.; Fiore, M.; Restuccia, C.; Ferrante, M. Phytoremediation Potential of Arundo donax (Giant Reed) in Contaminated Soil by Heavy Metals. Environ. Res. 2020, 185, 109427. [Google Scholar] [CrossRef] [PubMed]
- Pietrini, F.; Passatore, L.; Patti, V.; Francocci, F.; Giovannozzi, A.; Zacchini, M. Morpho-Physiological and Metal Accumulation Responses of Hemp Plants (Cannabis sativa L.) Grown on Soil from an Agro-Industrial Contaminated Area. Water 2019, 11, 808. [Google Scholar] [CrossRef]
- Manikandan, R.; Sahi, S.V.; Venkatachalam, P. Impact Assessment of Mercury Accumulation and Biochemical and Molecular Response of Mentha arvensis: A Potential Hyperaccumulator Plant. Sci. World J. 2015, 2015, 715217. [Google Scholar] [CrossRef]
- Rajendran, M.; An, W.; Li, W.; Perumal, V.; Wu, C.; Sahi, S.V.; Sarkar, S.K. Chromium Detoxification Mechanism Induced Growth and Antioxidant Responses in Vetiver (Chrysopogon zizanioides (L.) Roberty). J. Cent. South Univ. 2019, 26, 489–500. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, L.; Xu, J.; Ding, S.; Feng, X.; Xiao, H. Effects of Different Concentrations of Mercury on Accumulation of Mercury by Five Plant Species. Ecol. Eng. 2017, 106, 273–278. [Google Scholar] [CrossRef]
- El Rasafi, T.; Pereira, R.; Pinto, G.; Gonçalves, F.J.M.; Haddioui, A.; Ksibi, M.; Römbke, J.; Sousa, J.P.; Marques, C.R. Potential of Eucalyptus globulus for the Phytoremediation of Metals in a Moroccan Iron Mine Soil—A Case Study. Environ. Sci. Pollut. Res. 2021, 28, 15782–15793. [Google Scholar] [CrossRef] [PubMed]
- Pistelli, L.; D’Angiolillo, F.; Morelli, E.; Basso, B.; Rosellini, I.; Posarelli, M.; Barbafieri, M. Response of Spontaneous Plants from an Ex-Mining Site of Elba Island (Tuscany, Italy) to Metal (Loid) Contamination. Environ. Sci. Pollut. Res. 2017, 24, 7809–7820. [Google Scholar] [CrossRef] [PubMed]
- Atma, W.; Larouci, M.; Meddah, B.; Benabdeli, K.; Sonnet, P. Evaluation of the Phytoremediation Potential of Arundo donax L. for Nickel-Contaminated Soil. Int. J. Phytoremediat. 2017, 19, 377–386. [Google Scholar] [CrossRef]
- Zemiani, A.; Boldarini, M.T.B.; Anami, M.H.; de Oliveira, E.F.; da Silva, A.F. Tolerance of Mentha crispa L. (Garden Mint) Cultivated in Cadmium-Contaminated Oxisol. Environ. Sci. Pollut. Res. 2021, 28, 42107–42120. [Google Scholar] [CrossRef] [PubMed]
- Marrugo-Negrete, J.; Durango-Hernández, J.; Pinedo-Hernández, J.; Olivero-Verbel, J.; Díez, S. Phytoremediation of Mercury-Contaminated Soils by Jatropha curcas. Chemosphere 2015, 127, 58–63. [Google Scholar] [CrossRef]
- Zhang, C.; Guo, J.; Lee, D.K.; Anderson, E.; Huang, H. Growth Responses and Accumulation of Cadmium in Switchgrass (Panicum virgatum L.) and Prairie Cordgrass (Spartina pectinata Link). RSC Adv. 2015, 5, 83700–83706. [Google Scholar] [CrossRef]
- Charvalas, G.; Solomou, A.D.; Giannoulis, K.D.; Skoufogianni, E.; Bartzialis, D.; Emmanouil, C.; Danalatos, N.G. Determination of Heavy Metals in the Territory of Contaminated Areas of Greece and Their Restoration through Hyperaccumulators. Environ. Sci. Pollut. Res. 2021, 28, 3858–3863. [Google Scholar] [CrossRef] [PubMed]
- Golia, E.E.; Aslanidis, P.S.C.; Papadimou, S.G.; Kantzou, O.D.; Chartodiplomenou, M.A.; Lakiotis, K.; Androudi, M.; Tsiropoulos, N.G. Assessment of Remediation of Soils, Moderately Contaminated by Potentially Toxic Metals, Using Different Forms of Carbon (Charcoal, Biochar, Activated Carbon), Impacts on contamination, metals availability and soil indices. Sustain. Chem. Pharm. 2022, 28, 100724. [Google Scholar] [CrossRef]
- Alloway, B.J. Sources of Heavy Metals and Metalloids in Soils. In Heavy Metals in Soils; Springer: Dordrecht, The Netherlands, 2013; pp. 11–50. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants; CRC Press: Abingdon, UK, 2010; ISBN 9780429192036. [Google Scholar]
- Golia, E.E.; Diakoloukas, V. Soil Parameters Affecting the Levels of Potentially Harmful Metals in Thessaly Area, Greece: A Robust Quadratic Regression Approach of Soil Pollution Prediction. Environ. Sci. Pollut. Res. 2022, 29, 29544–29561. [Google Scholar] [CrossRef]
- Fatima, A.; Shabaan, M.; Ali, Q.; Malik, M.; Asghar, H.N.; Aslam, M.; Zulfiqar, U.; Hameed, A.; Nazim, M.; Mustafa, A.E.-Z.M.A.; et al. Integrated Application of Metal tolerant P. Fluorescens and Press Mud for Conferring Heavy Metal Tolerance to Aloe Vera (Aloe barbadensis). Plant Stress 2024, 11, 100333. [Google Scholar] [CrossRef]
- Shokri, F.; Ziarati, P.; Mousavi, Z. Removal of Selected Heavy Metals from Pharmaceutical Effluent by Aloe vera L. Biomed. Pharmacol. J. 2016, 9, 705–713. [Google Scholar] [CrossRef]
- Khankhane, P.J.; Tabassum, A.; Patel, A. Cadmium Tolerance and Its Enhanced Accumulation Potential of Arundo donax by EDTA. J. Environ. Biol. 2017, 38, 327–334. [Google Scholar] [CrossRef]
- Parihar, J.K.; Parihar, P.K.; Pakade, Y.B.; Katnoria, J.K. Bioaccumulation Potential of Indigenous Plants for Heavy Metal Phytoremediation in Rural Areas of Shaheed Bhagat Singh Nagar, Punjab (India). Environ. Sci. Pollut. Res. 2021, 28, 2426–2442. [Google Scholar] [CrossRef]
- De Vos, B.; Souza, M.F.; Michels, E.; Meers, E. Industrial Hemp (Cannabis sativa L.) in a Phytoattenuation Strategy: Remediation Potential of a Cd, Pb and Zn Contaminated Soil and Valorization Potential of the Fibers for Textile Production. Ind. Crops Prod. 2022, 178, 114592. [Google Scholar] [CrossRef]
- Wielgusz, K.; Praczyk, M.; Irzykowska, L.; Świerk, D. Fertilization and Soil PH Affect Seed and Biomass Yield, Plant Morphology, and Cadmium Uptake in Hemp (Cannabis sativa L.). Ind. Crops Prod. 2022, 175, 114245. [Google Scholar] [CrossRef]
- Angelini, L.; Tavarini, S.; Cestone, B.; Agrochimica, C.B. Variation in Mineral Composition in Three Different Plant Organs of Five Fibre Hemp (Cannabis sativa L.) Cultivars. Agrochimica 2014, 58, 1–18. [Google Scholar]
- Ćaćić, M.; Perčin, A.; Zgorelec, Ž.; Kisić, I. Evaluation of Heavy Metals Accumulation Potential of Hemp (Cannabis sativa L.). J. Cen. Eur. Agric. 2019, 20, 700–711. [Google Scholar] [CrossRef]
- Canu, M.; Mulè, P.; Spanu, E.; Fanni, S.; Marrone, A.; Carboni, G. Hemp Cultivation in Soils Polluted by Cd, Pb and Zn in the Mediterranean Area: Sites Characterization and Phytoremediation in Real Scale Settlement. Appl. Sci. 2022, 12, 3548. [Google Scholar] [CrossRef]
- Ferrarini, A.; Fracasso, A.; Spini, G.; Fornasier, F.; Taskin, E.; Fontanella, M.C.; Beone, G.M.; Amaducci, S.; Puglisi, E. Bioaugmented Phytoremediation of Metal-Contaminated Soils and Sediments by Hemp and Giant Reed. Front. Microbiol. 2021, 12, 645893. [Google Scholar] [CrossRef]
- Otunola, B.; Aghoghovwia, M.P.; Thwala, M.; Ololade, O.O. A Mesocosm Study on the Use of Clay Minerals to Improve Heavy Metal Phytoremediation Capacity of Vetiver Grass (Chrysopogon zizanioides L. Roberty). S. Afr. J. Sci. 2023, 119, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Delgado, M.; Miralles de Imperial, R.; Gonzalez, I.; Lobo, C.; Plaza, A.; Martinez, S.; Martin, J.V. Phytoremediation Potential of Thistle (Cynara cardunculus L.) and Its Ability to Remove Heavy Metals from Polluted Soils with High Rates of Sewage Sludge. Pol. J. Environ. Stud. 2017, 26, 1935–1941. [Google Scholar] [CrossRef] [PubMed]
- Sabo, A.; Ladan, M. Phytoremediation Potential of Some Indigenous Herbaceous Plant Species Growing on Metalliferous Mining Site at Nahuta, Bauchi State, Nigeria. IOSR J. Environ. Sci. 2014, 12, 747–755. [Google Scholar]
- Mazumdar, K.; Das, S. Phytoremediation of Pb, Zn, Fe, and Mg with 25 Wetland Plant Species from a Paper Mill Contaminated Site in North East India. Environ. Sci. Pollut. Res. 2015, 22, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Li, C.; Chen, W. Phytoremediation Potential of Bermuda Grass (Cynodon dactylon (L.) Pers.) in Soils Co-Contaminated with Polycyclic Aromatic Hydrocarbons and Cadmium. Ecotoxicol. Environ. Saf. 2022, 234, 113389. [Google Scholar] [CrossRef] [PubMed]
- Shuaibu, L.; Abdullahi, U.; Yaradua, A.I.; Bungudu, J.I. Phytoremediation Potentials of Cynodon dactylon on Heavy Metal Contaminated Soils from Challawa Industrial Estate, Kano-Nigeria. Asian J. Appl. Chem. Res. 2021, 9, 25–36. [Google Scholar] [CrossRef]
- Garba, S.T.; Gudusu, M.; Inuwa, L.B. Accumulation Ability of the Native Grass Species, Cyperus rotundus for the Heavy Metals; Zinc (Zn), Cadmium (Cd), Nickel (Ni) and Lead (Pb). Int. Res. J. Pure Appl. Chem. 2018, 17, 1–15. [Google Scholar] [CrossRef]
- Ilyas, S.; Sabo, A.; Akomolafe, G.F. Assessment of Metal Accumulation in Plant and Soil at Eggon Community Mining Site, Nasarawa, Nigeria. J. Res. For. Wildl. Environ. 2021, 13, 11–21. [Google Scholar]
- Shingadgaon, S.S.; Chavan, B.L. Evaluation of Bioaccumulation Factor (BAF), Bioconcentration Factor (BCF), Translocation Factor (TF) and Metal Enrichment Factor (MEF) Abilities of aquatic macrophyte species exposed to metal contaminated wastewater. Int. J. Innov. Res. Sci. Eng. Technol. 2019, 8, 329–347. [Google Scholar] [CrossRef]
- Subhashini, V.; Swamy, A.V.V.S. Phytoremediation of Cadmium and Chromium Contaminated Soils by Cyperus rotundus L. Am. Int. J. Res. Sci. Technol. Eng. Math. 2014, 6, 97–101. [Google Scholar]
- de Oliveira Araújo, S.; Neiva, D.M.; de Cássia Carneiro, A.; Esteves, B.; Pereira, H. Potential of Mild Torrefaction for Upgrading the Wood Energy Value of Different Eucalyptus Species. Forests 2018, 9, 535. [Google Scholar] [CrossRef]
- Pietrini, F.; Iori, V.; Bianconi, D.; Mughini, G.; Massacci, A.; Zacchini, M. Assessment of Physiological and Biochemical Responses, Metal Tolerance and Accumulation in Two Eucalypt Hybrid Clones for Phytoremediation of Cadmium-Contaminated Waters. J. Environ. Manag. 2015, 162, 221–231. [Google Scholar] [CrossRef]
- Reboredo, F.H.; Pelica, J.; Lidon, F.C.; Pessoa, M.F.; Silva, M.M.; Guerra, M.; Leitão, R.; Ramalho, J.C. The Tolerance of Eucalyptus globulus to Soil Contamination with Arsenic. Plants 2021, 10, 627. [Google Scholar] [CrossRef]
- Shahin, S.; Mahmoud, A.; Said, R. To What Extent Can Rose of China (Hibiscus rosa-sinensis, L.) Transplants Tolerate Toxicity of Some Heavy Metals in Combinations? Egypt. J. Agric. Res. 2020, 98, 645–652. [Google Scholar] [CrossRef]
- Chang, F.C.; Ko, C.H.; Tsai, M.J.; Wang, Y.N.; Chung, C.Y. Phytoremediation of Heavy Metal Contaminated Soil by Jatropha curcas. Ecotoxicology 2014, 23, 1969–1978. [Google Scholar] [CrossRef] [PubMed]
- Awotedu, O.L.; Ogunbamowo, P.O. Comparative Heavy Metal Uptake and Phytoremediation Potential of Three Jatropha Species. Environ. Ecosyst. Sci. 2019, 3, 26–30. [Google Scholar] [CrossRef]
- Devanesan, S.; Mir, M.S.; AlSalhi, M.S.; Angulo-Bejarano, P.I. Phytoremediation and Genetic Adaptation Potential of Jatropha curcas on Heavy Metals Enriched Mine Tailings. J. Taiwan Inst. Chem. Eng. 2024, 166, 105325. [Google Scholar] [CrossRef]
- Khan, A.; Khan, A.A.; Irfan, M.; Sayeed Akhtar, M.; Hasan, S.A. Lead-Induced Modification of Growth and Yield of Linum usitatissimum L. and Its Soil Remediation Potential. Int. J. Phytoremediat. 2023, 25, 1067–1076. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, G.; Gao, S.; Zhang, Z.; Huang, L. Effect of Humic Acid on Phytoremediation of Heavy Metal Contaminated Sediment. J. Hazard. Mater. Adv. 2023, 9, 100235. [Google Scholar] [CrossRef]
- Mohseni, A.; Reyhanitabar, A.; Najafi, N.; Oustan, S.; Bazargan, K. Phytoremediation Potential and Essential Oil Quality of Peppermint Grown in Contaminated Soils as Affected by Sludge and Time. J. Agric. Sci. Techn. 2022, 24, 723–737. [Google Scholar]
- Alamo-Nole, L.; Su, Y.F. Translocation of Cadmium in Ocimum Basilicum at Low Concentration of CdSSe Nanoparticles. Appl. Mater. Today 2017, 9, 314–318. [Google Scholar] [CrossRef]
- Zahedifar, M.; Moosavi, A.A.; Zarei, Z.; Shafigh, M.; Karimian, F. Heavy Metals Content and Distribution in Basil (Ocimum basilicum L.) as Influenced by Cadmium and Different Potassium Sources. Int. J. Phytoremediat. 2019, 21, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Choden, D.; Pokethitiyook, P.; Poolpak, T.; Kruatrachue, M. Phytoremediation of Soil Co-Contaminated with Zinc and Crude Oil Using Ocimum gratissimum (L.) in Association with Pseudomonas putida MU02. Int. J. Phytoremediat. 2021, 23, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Bisht, M.; Pande, C.; Tewari, G.; Bhatt, S.; Triphati, S. Effect of Zinc on the Growth and Essential Oil Composition of Ocimum gratissimum L. J. Essent. Oil Bear. Plants 2019, 22, 441–454. [Google Scholar] [CrossRef]
- Guo, Z.; Gao, Y.; Cao, X.; Jiang, W.; Liu, X.; Liu, Q.; Chen, Z.; Zhou, W.; Cui, J.; Wang, Q. Phytoremediation of Cd and Pb Interactive Polluted Soils by Switchgrass (Panicum virgatum L.). Int. J. Phytoremediat. 2019, 21, 1486–1496. [Google Scholar] [CrossRef] [PubMed]
- Polechońska, L.; Klink, A. Trace Metal Bioindication and Phytoremediation Potentialities of Phalaris arundinacea L. (Reed Canary Grass). J. Geochem. Explor. 2014, 146, 27–33. [Google Scholar] [CrossRef]
- Xu, J.; Zheng, L.; Xu, L.; Wang, X. Uptake and Allocation of Selected Metals by Dominant Vegetation in Poyang Lake Wetland: From Rhizosphere to Plant Tissues. Catena 2020, 189, 104477. [Google Scholar] [CrossRef]
- Mayerová, M.; Petrová, Š.; Madaras, M.; Lipavský, J.; Šimon, T.; Vaněk, T. Non-Enhanced Phytoextraction of Cadmium, Zinc, and Lead by High-Yielding Crops. Environ. Sci. Pollut. Res. 2017, 24, 14706–14716. [Google Scholar] [CrossRef] [PubMed]
- El-Mahrouk, E.-S.M.; Eisa, E.A.-H.; Hegazi, M.A.; Abdel-Gayed, M.E.-S.; Dewir, Y.H.; El-Mahrouk, M.E.; Naidoo, Y. Phytoremediation of Cadmium-, Copper-, and Lead-Contaminated Soil by Salix mucronata (Synonym Salix safsaf). HortScience 2019, 54, 1249–1257. [Google Scholar] [CrossRef]
- Kubátová, P.; Hejcman, M.; Száková, J.; Vondráčková, S.; Tlustoš, P. Effects of Sewage Sludge Application on Biomass Production and Concentrations of Cd, Pb and Zn in Shoots of Salix and Populus Clones: Improvement of Phytoremediation Efficiency in Contaminated Soils. BioEnergy Res. 2016, 9, 809–819. [Google Scholar] [CrossRef]
- Hussain, S.; Akram, M.; Abbas, G.; Murtaza, B.; Shahid, M.; Shah, N.S.; Bibi, I.; Niazi, N.K. Arsenic Tolerance and Phytoremediation Potential of Conocarpus erectus L. and Populus deltoides L. Int. J. Phytoremediat. 2017, 19, 985–991. [Google Scholar] [CrossRef] [PubMed]
- Romeo, S.; Francini, A.; Ariani, A.; Sebastiani, L. Phytoremediation of Zn: Identify the Diverging Resistance, Uptake and Biomass Production Behaviours of Poplar Clones under High Zinc Stress. Water Air Soil Pollut. 2014, 225, 1813. [Google Scholar] [CrossRef]
- Ancona, V.; Caracciolo, A.B.; Campanale, C.; Rascio, I.; Grenni, P.; Di Lenola, M.; Bagnuolo, G.; Uricchio, V.F. Heavy Metal Phytoremediation of a Poplar Clone in a Contaminated Soil in Southern Italy. J. Chem. Technol. Biotechnol. 2020, 95, 940–949. [Google Scholar] [CrossRef]
- Hadi, F.; Arifeen, M.Z.U.; Aziz, T.; Nawab, S.; Nabi, G. Phytoremediation of Cadmium by Ricinus communis L. in Hydrophonic Condition. Cell 2015, 92, 8112741. [Google Scholar] [CrossRef]
- Kİran, B.R.; Prasad, M.N.V. Responses of Ricinus communis L. (Castor Bean, Phytoremediation Crop) Seedlings to Lead (Pb) Toxicity in Hydroponics. Selcuk J. Agric. Food Sci. 2017, 31, 73–80. [Google Scholar] [CrossRef]
- Palanivel, T.M.; Pracejus, B.; Victor, R. Phytoremediation Potential of Castor (Ricinus communis L.) in the Soils of the Abandoned Copper Mine in Northern Oman: Implications for Arid Regions. Environ. Sci. Pollut. Res. 2020, 27, 17359–17369. [Google Scholar] [CrossRef] [PubMed]
- Ardalan, F.; Vakili, M.; Kourepaz, S. Lead Phytoremediation of Rosmarinus officinalis and Its Effect on the Plant Growth. Int. J. Biosci. 2014, 4, 75–79. [Google Scholar] [CrossRef]
- Ardalan, F.; Vakili, M.; Samadiyan-Sarbangholi, V.; Ardalan, M. Cadmium Uptake and Accumulation Ability of Rosmarinus officinalis and Its Growth Changes. J. Biodivers. Environ. Sci. 2015, 6, 83–87. [Google Scholar]
- Affholder, M.-C.; Laffont-Schwob, I.; Coulomb, B.; Rabier, J.; Borla, A.; Boudenne, J.-L.; Demelas, C.; Prudent, P. Implication of Phytometabolites on Metal Tolerance of the Pseudo-Metallophyte—Rosmarinus officinalis—in a Mediterranean Brownfield. Chemosphere 2020, 249, 126159. [Google Scholar] [CrossRef] [PubMed]
- Abbaslou, H.; Bakhtiari, S.; Hashemi, S.S. Rehabilitation of Iron Ore Mine Soil Contaminated with Heavy Metals Using Rosemary Phytoremediation-Assisted Mycorrhizal Arbuscular Fungi Bioaugmentation and Fibrous Clay Mineral Immobilization. Iran. J. Sci. Technol. Trans. A Sci. 2018, 42, 431–441. [Google Scholar] [CrossRef]
- Parra, A.; Zornoza, R.; Conesa, E.; Gómez-López, M.D.; Faz, A. Seedling Emergence, Growth and Trace Elements Tolerance and Accumulation by Lamiaceae Species in a Mine Soil. Chemosphere 2014, 113, 132–140. [Google Scholar] [CrossRef]
- Shi, X.; Wang, S.; Sun, H.; Chen, Y.; Wang, D.; Pan, H.; Zou, Y.; Liu, J.; Zheng, L.; Zhao, X.; et al. Comparative of Quercus spp. and Salix spp. for Phytoremediation of Pb/Zn Mine Tailings. Environ. Sci. Pollut. Res. 2017, 24, 3400–3411. [Google Scholar] [CrossRef]
- Urošević, J.; Stanković, D.; Jokanović, D.; Trivan, G.; Rodzkin, A.; Jović, Đ.; Jovanović, F. Phytoremediation Potential of Different Genotypes of Salix alba and S. Viminalis. Plants 2024, 13, 735. [Google Scholar] [CrossRef] [PubMed]
- El-Mahrouk, E.S.M.; Eisa, E.A.E.H.; Ali, H.M.; Hegazy, M.A.E.N.; El-Sayed, M.; Abd El-Gayed, M.E.S. Populus nigra as a Phytoremediator for Cd, Cu, and Pb in Contaminated Soil. BioResources 2020, 15, 869–893. [Google Scholar] [CrossRef]
- Papadimou, S.G.; Golia, E.E. Green and Sustainable Practices for an Energy Plant Cultivation on Naturally Contaminated versus Spiked Soils. The Impact of Ageing Soil Pollution in the Circular Economy Framework. Environ. Res. 2024, 246, 118130. [Google Scholar] [CrossRef]
- Angelova, V.; Perifanova-Nemska, M.N.; Krustev, L.K.; Uzunova, G.P. Potential of Silybum marianum L. for Phytoremediation of Soils Contaminated with Heavy Metals. J. Int. Sci. Publ. Ecol. Saf. 2018, 12, 267–282. [Google Scholar]
- Hammami, H.; Alaie, E.; Dastgheib, S.M.M. The Ability of Silybum marianum to Phytoremediate Cadmium and/or Diesel Oil from the Soil. Int. J. Phytoremediat. 2018, 20, 756–763. [Google Scholar] [CrossRef]
- Razanov, S.F.; Tkachuk, O.P.; Razanova, A.M.; Bakhmat, M.I.; Bakhmat, O.M. Intensity of Heavy Metal Accumulation in Plants of Silybum marianum L. in Conditions of Field Rotation. Ukr. J. Ecol. 2020, 10, 131–136. [Google Scholar] [CrossRef]


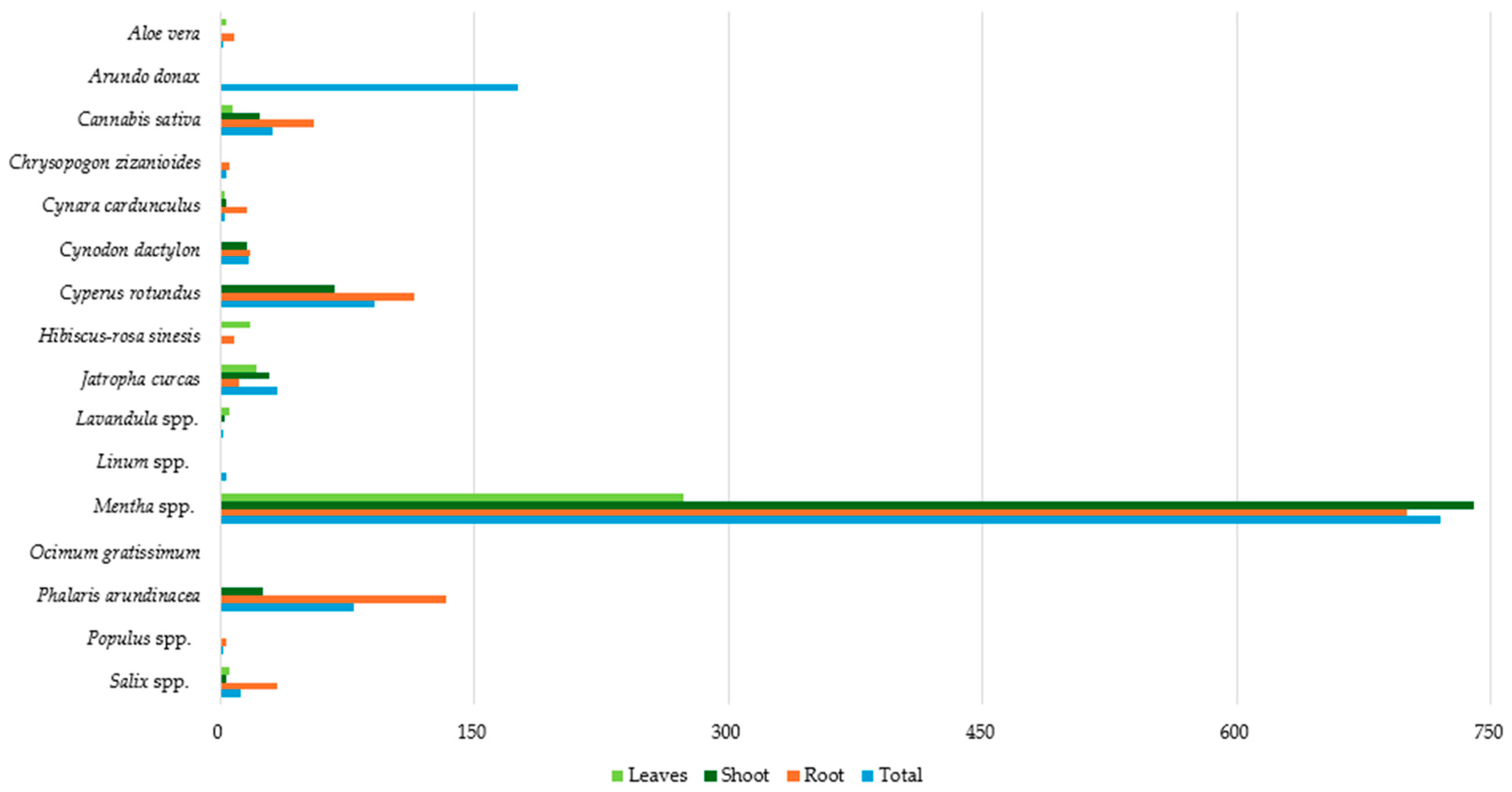
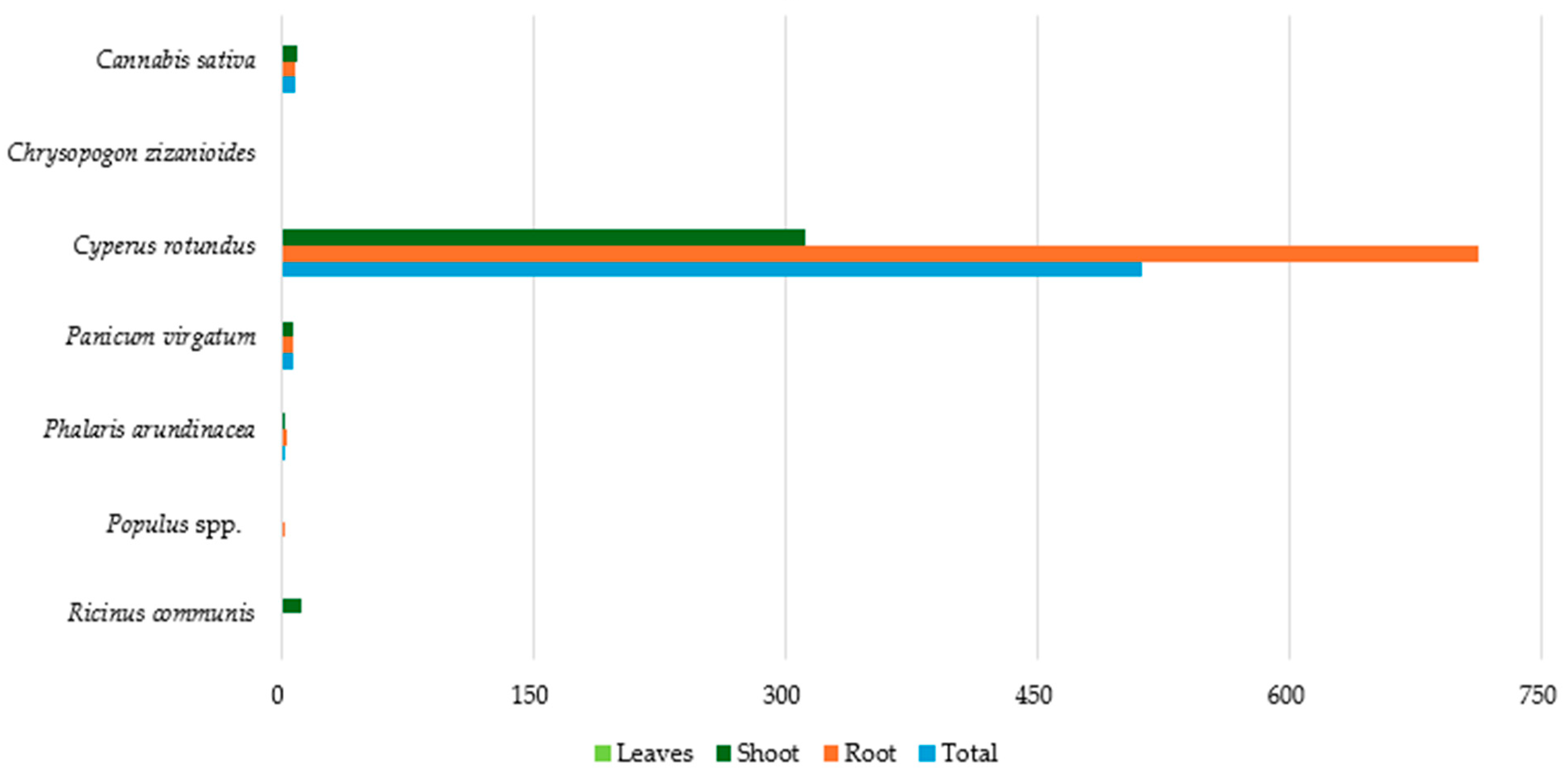
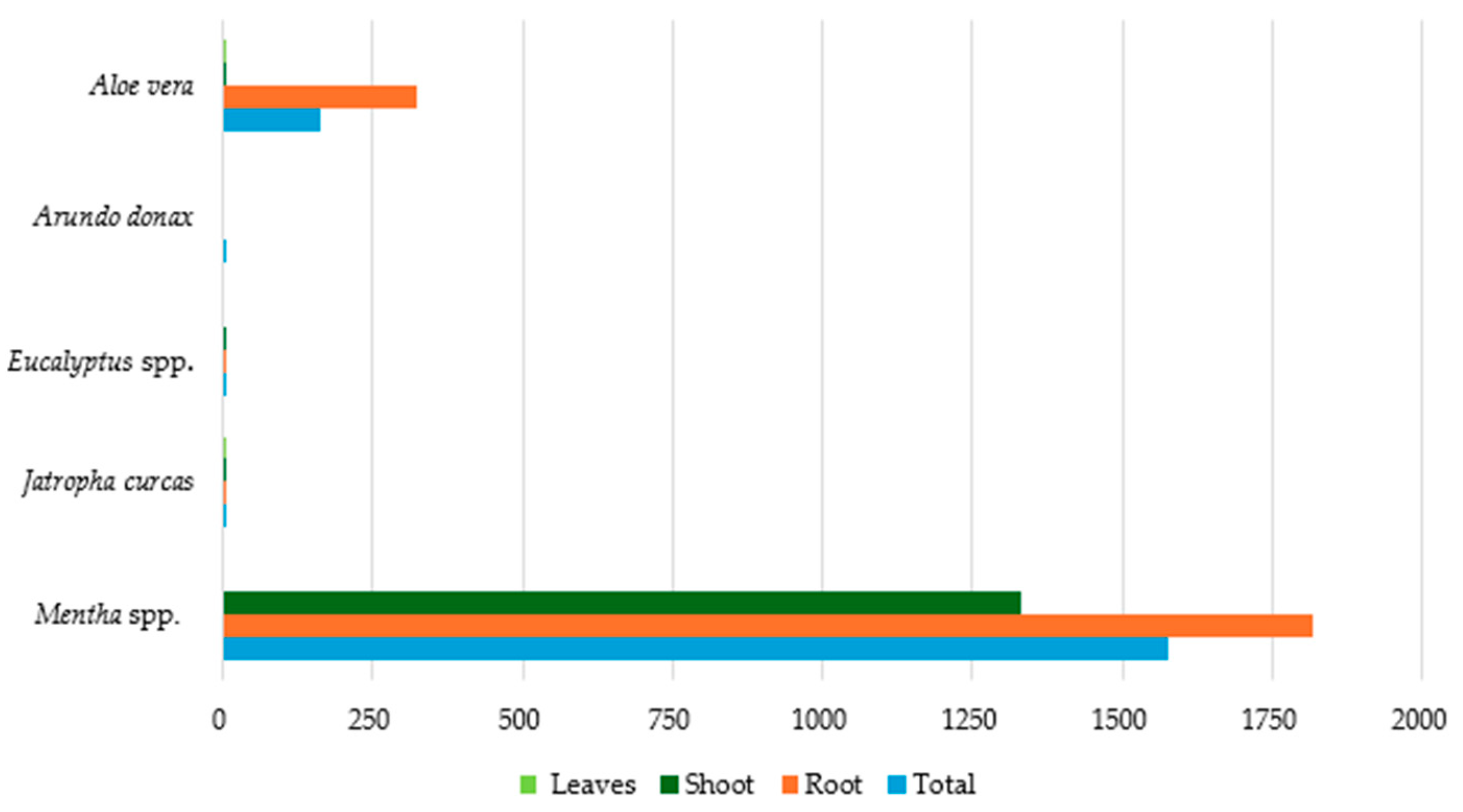

| Plant Species | PTEs | Countries | References |
|---|---|---|---|
| Aloe vera | Pb, Cu, Cd, Zn, Cr, Ni, Hg | Iran, Pakistan, China | [13,52,64,65] |
| Arundo donax | Pb, Cu, Cd, Zn, Cr, Ni, Hg, As | Algeria, Portugal, Italy, India | [12,36,48,55,66] |
| Cannabis sativa | Pb, Cu, Cd, Zn, Cr, Co, Ni | India, Belgium, Poland, Italy, Croatia | [39,40,49,67,68,69,70,71,72,73] |
| Chrysopogon zizanioides | Pb, Cu, Cd, Zn, Cr, Co, Ni, As | China, Iran, Malesia, South Africa | [8,10,13,47,74] |
| Cynara cardunculus | Pb, Cu, Cd, Zn, Cr, Ni, As | Italy, Spain | [5,38,46,75] |
| Cynodon dactylon | Pb, Cu, Cd, Zn, Cr, Ni | Nigeria, India, China, Pakistan | [76,77,78,79] |
| Cyperus rotundus | Pb, Cu, Cd, Zn, Cr, Co, Ni | Nigeria, India | [76,80,81,82,83] |
| Eucalyptus spp. | Cu, Cd, Zn, Cr, Hg, As | Morocco, China, Italy, Portugal | [2,53,84,85,86] |
| Hibiscus rosa-sinesis | Pb, Cd, Cr, Ni | Egypt | [29,87] |
| Jatropha curcas | Pb, Cu, Cd, Zn, Cr, Ni, Hg | Colombia, Taiwan, Spain, Nigeria, India | [3,57,88,89,90] |
| Lavandula spp. | Pb, Cu, Cd, Zn, Cr, Ni | Bulgaria, Iran, China, Italy | [6,14,30,54,86] |
| Linum spp. | Pb, Cu, Cd, Ni | India, China, Pakistan | [1,8,55,91] |
| Mentha spp. | Pb, Cu, Cd, Zn, Ni, Hg | India, Brazil, China, Iran, Pakistan | [50,56,66,92,93] |
| Ocimum basilicum | Pb, Cd, Zn | Bulgaria, USA, Iran, Egypt | [25,45,94,95] |
| Ocimum gratissimum | Pb, Cu, Cd, Zn, Ni, As | Thailand, Nigeria, India, China | [4,43,96,97] |
| Panicum virgatum | Pb, Cu, Cd, Zn, Cr, Co | India, China, USA | [9,58,67,98] |
| Phalaris arundinacea | Pb, Cu, Cd, Zn, Cr, Co, Ni | Poland, China, Czech Republic | [37,99,100,101] |
| Populus spp. | Pb, Cu, Cd, Zn, Cr, Co, Ni, As | Egypt, Czech Republic, Pakistan, Italy | [102,103,104,105,106] |
| Ricinus communis | Pb, Cu, Cd, Zn, Cr, Co, As | Oman, Pakistan, India | [67,77,107,108,109] |
| Rosmarinus officinalis | Pb, Cu, Cd, Zn, As | Iran, France, Spain | [110,111,112,113,114] |
| Salix spp. | Pb, Cu, Cd, Zn, Cr, Ni, As | Poland, Czech Republic, Serbia, China, Egypt | [37,103,115,116,117] |
| Silybum marianum | Pb, Cu, Cd, Zn | Greece, Iran, Bulgaria, Ukraine | [15,27,118,119,120,121] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golia, E.E.; Barbieri, E.; Papadimou, S.G.; Alexiadis, D. Energy, Aromatic, and Medicinal Plants’ Potential and Prospects for the Remediation of Potentially Toxic Element-Contaminated Agricultural Soils: A Critical Meta-Analysis. Toxics 2024, 12, 914. https://doi.org/10.3390/toxics12120914
Golia EE, Barbieri E, Papadimou SG, Alexiadis D. Energy, Aromatic, and Medicinal Plants’ Potential and Prospects for the Remediation of Potentially Toxic Element-Contaminated Agricultural Soils: A Critical Meta-Analysis. Toxics. 2024; 12(12):914. https://doi.org/10.3390/toxics12120914
Chicago/Turabian StyleGolia, Evangelia E., Edoardo Barbieri, Sotiria G. Papadimou, and Dimitrios Alexiadis. 2024. "Energy, Aromatic, and Medicinal Plants’ Potential and Prospects for the Remediation of Potentially Toxic Element-Contaminated Agricultural Soils: A Critical Meta-Analysis" Toxics 12, no. 12: 914. https://doi.org/10.3390/toxics12120914
APA StyleGolia, E. E., Barbieri, E., Papadimou, S. G., & Alexiadis, D. (2024). Energy, Aromatic, and Medicinal Plants’ Potential and Prospects for the Remediation of Potentially Toxic Element-Contaminated Agricultural Soils: A Critical Meta-Analysis. Toxics, 12(12), 914. https://doi.org/10.3390/toxics12120914








