Exposure to the Natural Compound Climacostol Induces Cell Damage and Oxidative Stress in the Fruit Fly Drosophila melanogaster
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Fly Husbandry and Treatments
2.3. Mating Procedure and Developmental Assays
2.4. Fluorescence Confocal Microscopy
2.5. Mitotic Index
2.6. RNA Extraction and PCR
2.7. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium Bromide (MTT) Assay
2.8. Survival and Climbing
2.9. Scanning Electron Microscopy (SEM)
2.10. Statistical Analysis
3. Results
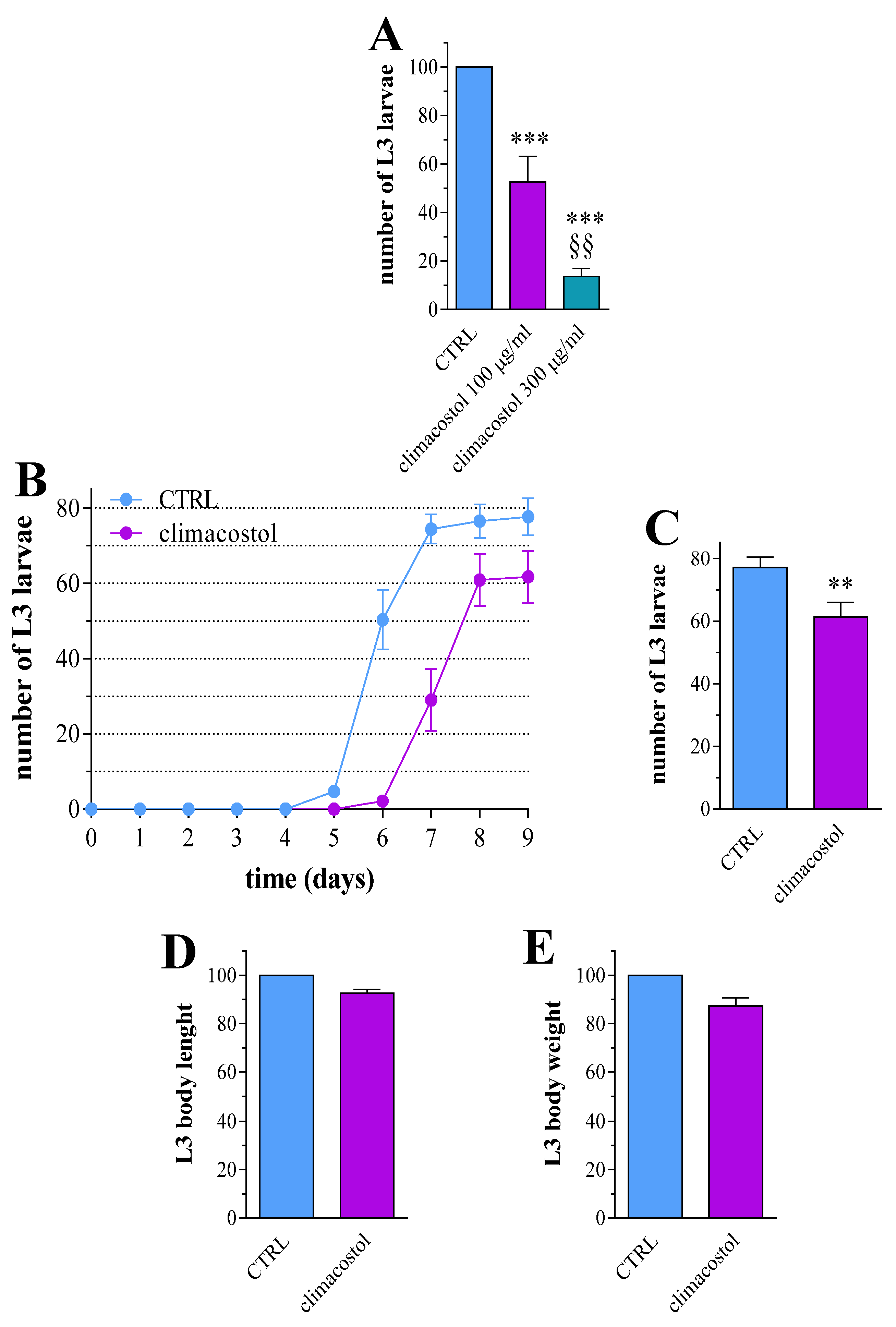
3.1. Effects on Development
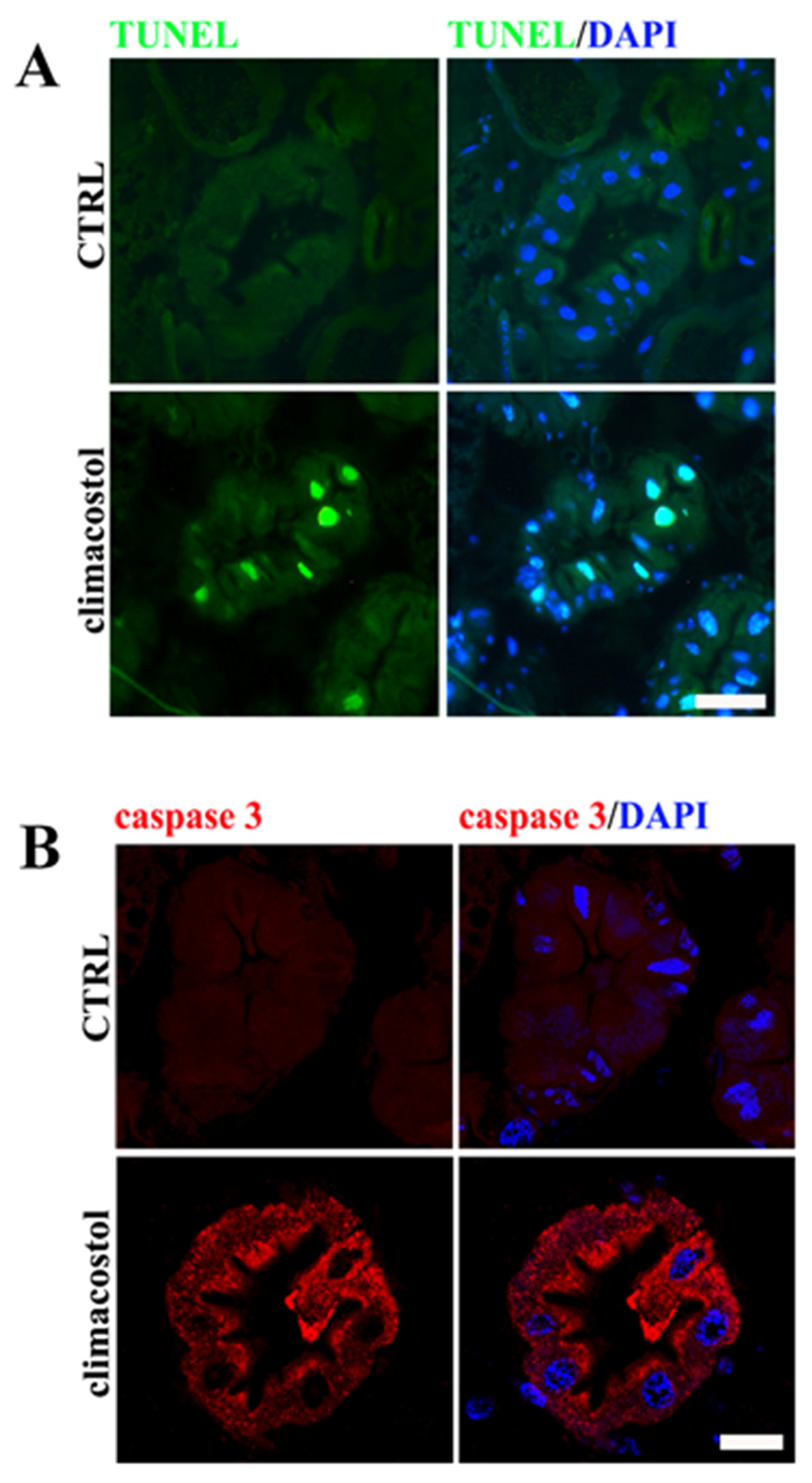
3.2. Cell Damage
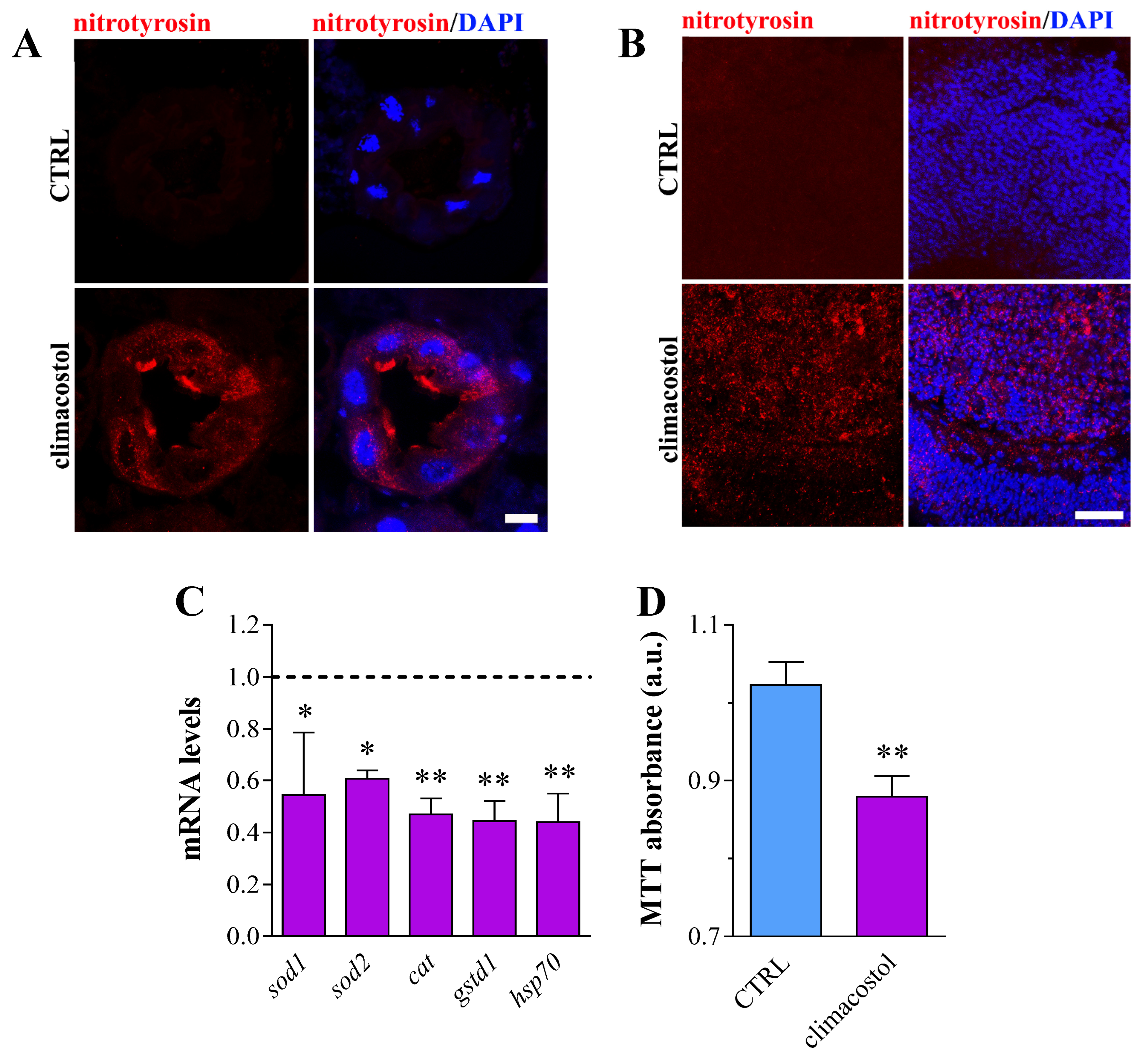
3.3. Redox State
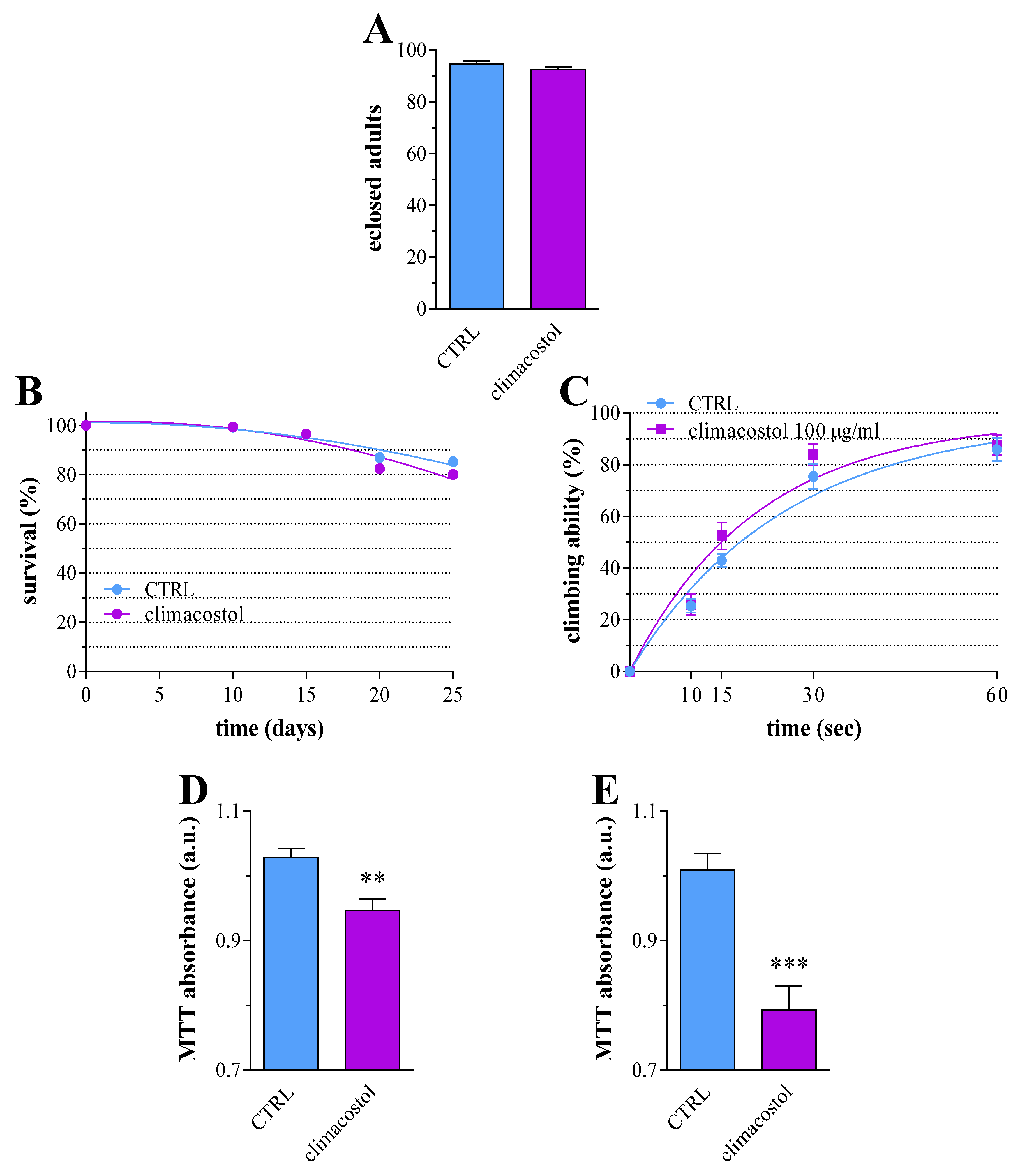
3.4. Long-Term Toxicity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abe, Y.; Mori, K. Simple synthesis of climacostol, a defensive secretion by the ciliate Climacostomum virens. Biosci. Biotechnol. Biochem. 2001, 65, 2110–2112. [Google Scholar] [CrossRef] [PubMed]
- Masaki, M.E.; Harumoto, T.; Terazima, M.N.; Miyake, A.; Usuki, Y.; Iio, H. Climacostol, a defense toxin of the heterotrich ciliate Climacostomum virens against predators. Tetrahedron Lett. 1999, 40, 8227–8229. [Google Scholar] [CrossRef]
- Rosati, G.; Modeo, L. Extrusomes in ciliates: Diversification, distribution, and phylogenetic implications. J. Eukaryot. Microbiol. 2003, 50, 383–402. [Google Scholar] [CrossRef]
- Buonanno, F.; Catalani, E.; Cervia, D.; Cimarelli, C.; Marcantoni, E.; Ortenzi, C. Natural Function and Structural Modification of Climacostol, a Ciliate Secondary Metabolite. Microorganisms 2020, 8, 809. [Google Scholar] [CrossRef]
- Petrelli, D.; Buonanno, F.; Vitali, L.A.; Ortenzi, C. Antimicrobial activity of the protozoan toxin climacostol and its derivatives. Biologia 2012, 67, 525–529. [Google Scholar] [CrossRef]
- Verani, M.; Di Giuseppe, G.; Federigi, I.; Buonanno, F.; Ortenzi, C.; Carducci, A. Preliminary Data Related to the Effect of Climacostol Produced by the Freshwater Ciliate Climacostomum virens on Human Adenovirus. Viruses 2020, 12, 658. [Google Scholar] [CrossRef]
- Perrotta, C.; Buonanno, F.; Zecchini, S.; Giavazzi, A.; Proietti Serafini, F.; Catalani, E.; Guerra, L.; Belardinelli, M.C.; Picchietti, S.; Fausto, A.M.; et al. Climacostol reduces tumour progression in a mouse model of melanoma via the p53-dependent intrinsic apoptotic programme. Sci. Rep. 2016, 6, 27281. [Google Scholar] [CrossRef]
- Zecchini, S.; Proietti Serafini, F.; Catalani, E.; Giovarelli, M.; Coazzoli, M.; Di Renzo, I.; De Palma, C.; Perrotta, C.; Clementi, E.; Buonanno, F.; et al. Dysfunctional autophagy induced by the pro-apoptotic natural compound climacostol in tumour cells. Cell Death Dis. 2019, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Buonanno, F.; Quassinti, L.; Bramucci, M.; Amantini, C.; Lucciarini, R.; Santoni, G.; Iio, H.; Ortenzi, C. The protozoan toxin climacostol inhibits growth and induces apoptosis of human tumor cell lines. Chem. Biol. Interact. 2008, 176, 151–164. [Google Scholar] [CrossRef]
- Muto, Y.; Tanabe, Y.; Kawai, K.; Okano, Y.; Iio, H. Climacostol inhibits Tetrahymena motility and mitochondrial respiration. Cent. Eur. J. Biol. 2011, 6, 99–104. [Google Scholar] [CrossRef]
- Quassinti, L.; Ortenzi, F.; Marcantoni, E.; Ricciutelli, M.; Lupidi, G.; Ortenzi, C.; Buonanno, F.; Bramucci, M. DNA binding and oxidative DNA damage induced by climacostol-copper(II) complexes: Implications for anticancer properties. Chem. Biol. Interact. 2013, 206, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Klionsky, D.J.; Abdel-Aziz, A.K.; Abdelfatah, S.; Abdellatif, M.; Abdoli, A.; Abel, S.; Abeliovich, H.; Abildgaard, M.H.; Abudu, Y.P.; Acevedo-Arozena, A.; et al. Guidelines for the use and interpretation of assays for monitoring autophagy (4th edition). Autophagy 2021, 17, 1–382. [Google Scholar] [CrossRef] [PubMed]
- Catalani, E.; Buonanno, F.; Lupidi, G.; Bongiorni, S.; Belardi, R.; Zecchini, S.; Giovarelli, M.; Coazzoli, M.; De Palma, C.; Perrotta, C.; et al. The Natural Compound Climacostol as a Prodrug Strategy Based on pH Activation for Efficient Delivery of Cytotoxic Small Agents. Front. Chem. 2019, 7, 463. [Google Scholar] [CrossRef] [PubMed]
- Xiu, M.; Wang, Y.; Yang, D.; Zhang, X.; Dai, Y.; Liu, Y.; Lin, X.; Li, B.; He, J. Using Drosophila melanogaster as a suitable platform for drug discovery from natural products in inflammatory bowel disease. Front. Pharmacol. 2022, 13, 1072715. [Google Scholar] [CrossRef]
- Su, T.T. Drug screening in Drosophila; why, when, and when not? Wiley Interdiscip. Rev. Dev. Biol. 2019, 8, e346. [Google Scholar] [CrossRef] [PubMed]
- Pandey, U.B.; Nichols, C.D. Human disease models in Drosophila melanogaster and the role of the fly in therapeutic drug discovery. Pharmacol. Rev. 2011, 63, 411–436. [Google Scholar] [CrossRef] [PubMed]
- Freires, I.A.; Morelo, D.F.C.; Soares, L.F.F.; Costa, I.S.; de Araujo, L.P.; Breseghello, I.; Abdalla, H.B.; Lazarini, J.G.; Rosalen, P.L.; Pigossi, S.C.; et al. Progress and promise of alternative animal and non-animal methods in biomedical research. Arch. Toxicol. 2023, 97, 2329–2342. [Google Scholar] [CrossRef]
- Koon, A.C.; Chan, H.Y. Drosophila melanogaster As a Model Organism to Study RNA Toxicity of Repeat Expansion-Associated Neurodegenerative and Neuromuscular Diseases. Front. Cell. Neurosci. 2017, 11, 70. [Google Scholar] [CrossRef]
- Adedara, A.O.; Otenaike, T.A.; Farodoye, O.M.; Abolaji, A.O. Ellagic acid mitigates rotenone-induced damage via modulating mitochondria function in Drosophila melanogaster. J. Biochem. Mol. Toxicol. 2023, 37, e23332. [Google Scholar] [CrossRef]
- Rand, M.D.; Tennessen, J.M.; Mackay, T.F.C.; Anholt, R.R.H. Perspectives on the Drosophila melanogaster Model for Advances in Toxicological Science. Curr. Protoc. 2023, 3, e870. [Google Scholar] [CrossRef]
- Zdraljevic, S.; Andersen, E.C. Natural diversity facilitates the discovery of conserved chemotherapeutic response mechanisms. Curr. Opin. Genet. Dev. 2017, 47, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Cha, S.J.; Do, H.A.; Choi, H.J.; Lee, M.; Kim, K. The Drosophila Model: Exploring Novel Therapeutic Compounds against Neurodegenerative Diseases. Antioxidants 2019, 8, 623. [Google Scholar] [CrossRef] [PubMed]
- Baenas, N.; Wagner, A.E. Drosophila melanogaster as an alternative model organism in nutrigenomics. Genes Nutr. 2019, 14, 14. [Google Scholar] [CrossRef] [PubMed]
- Demir, E.; Turna Demir, F. Drosophila melanogaster as a dynamic in vivo model organism reveals the hidden effects of interactions between microplastic/nanoplastic and heavy metals. J. Appl. Toxicol. 2023, 43, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Catalani, E.; Del Quondam, S.; Brunetti, K.; Cherubini, A.; Bongiorni, S.; Taddei, A.R.; Zecchini, S.; Giovarelli, M.; De Palma, C.; Perrotta, C.; et al. Neuroprotective role of plumbagin on eye damage induced by high-sucrose diet in adult fruit fly Drosophila melanogaster. Biomed. Pharmacother. 2023, 166, 115298. [Google Scholar] [CrossRef] [PubMed]
- Catalani, E.; Fanelli, G.; Silvestri, F.; Cherubini, A.; Del Quondam, S.; Bongiorni, S.; Taddei, A.R.; Ceci, M.; De Palma, C.; Perrotta, C.; et al. Nutraceutical Strategy to Counteract Eye Neurodegeneration and Oxidative Stress in Drosophila melanogaster Fed with High-Sugar Diet. Antioxidants 2021, 10, 1197. [Google Scholar] [CrossRef] [PubMed]
- Goethel, G.; Augsten, L.V.; das Neves, G.M.; Goncalves, I.L.; de Souza, J.P.S.; Garcia, S.C.; Eifler-Lima, V.L. The Role of Alternative Toxicological Trials in Drug Discovery Programs. The Case of Caenorhabditis elegans and Other Methods. Curr. Med. Chem. 2022, 29, 5270–5288. [Google Scholar] [CrossRef]
- Tang, B.; More, V. Recent Advances in Drug Discovery Toxicology. Int. J. Toxicol. 2023, 42, 535–550. [Google Scholar] [CrossRef]
- Yi, Y.; Xu, W.; Fan, Y.; Wang, H.X. Drosophila as an emerging model organism for studies of food-derived antioxidants. Food Res. Int. 2021, 143, 110307. [Google Scholar] [CrossRef]
- Fiorini, D.; Giuli, S.; Marcantoni, E.; Quassinti, L.; Bramucci, M.; Amantini, C.; Santoni, G.; Buonanno, F.; Ortenzi, C. A Straightforward Diastereoselective Synthesis and Evaluation of Climacostol, A Natural Product with Anticancer Activities. Synthesis 2010, 9, 1550–1556. [Google Scholar]
- Kruger, L.; Denton, T.T. A standardized method for incorporation of drugs into food for use with Drosophila melanogaster. Anal. Biochem. 2020, 599, 113740. [Google Scholar] [CrossRef] [PubMed]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, RESEARCH0034. [Google Scholar] [CrossRef] [PubMed]
- Ecker, A.; Gonzaga, T.; Seeger, R.L.; Santos, M.M.D.; Loreto, J.S.; Boligon, A.A.; Meinerz, D.F.; Lugokenski, T.H.; Rocha, J.; Barbosa, N.V. High-sucrose diet induces diabetic-like phenotypes and oxidative stress in Drosophila melanogaster: Protective role of Syzygium cumini and Bauhinia forficata. Biomed. Pharmacother. 2017, 89, 605–616. [Google Scholar] [CrossRef]
- Fernandez-Moreno, M.A.; Farr, C.L.; Kaguni, L.S.; Garesse, R. Drosophila melanogaster as a model system to study mitochondrial biology. Methods Mol. Biol. 2007, 372, 33–49. [Google Scholar] [PubMed]
- Miguel-Aliaga, I.; Jasper, H.; Lemaitre, B. Anatomy and Physiology of the Digestive Tract of Drosophila melanogaster. Genetics 2018, 210, 357–396. [Google Scholar] [CrossRef]
- Capo, F.; Wilson, A.; Di Cara, F. The Intestine of Drosophila melanogaster: An Emerging Versatile Model System to Study Intestinal Epithelial Homeostasis and Host-Microbial Interactions in Humans. Microorganisms 2019, 7, 336. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Hou, S.X. Regulation of intestinal stem cells in mammals and Drosophila. J. Cell Physiol. 2010, 222, 33–37. [Google Scholar] [CrossRef]
- Casali, A.; Batlle, E. Intestinal stem cells in mammals and Drosophila. Cell Stem Cell 2009, 4, 124–127. [Google Scholar] [CrossRef]
- Zhou, J.; Boutros, M. Intestinal stem cells and their niches in homeostasis and disease. Cells Dev. 2023, 175, 203862. [Google Scholar] [CrossRef]
- Medina, A.; Bellec, K.; Polcownuk, S.; Cordero, J.B. Investigating local and systemic intestinal signalling in health and disease with Drosophila. Dis. Models Mech. 2022, 15, dmm049332. [Google Scholar] [CrossRef]
- Zeng, X.; Hou, S.X. Enteroendocrine cells are generated from stem cells through a distinct progenitor in the adult Drosophila posterior midgut. Development 2015, 142, 644–653. [Google Scholar] [CrossRef] [PubMed]
- Bayliak, M.M.; Abrat, O.B.; Storey, J.M.; Storey, K.B.; Lushchak, V.I. Interplay between diet-induced obesity and oxidative stress: Comparison between Drosophila and mammals. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2019, 228, 18–28. [Google Scholar] [CrossRef]
- Lennicke, C.; Cocheme, H.M. Redox signalling and ageing: Insights from Drosophila. Biochem. Soc. Trans. 2020, 48, 367–377. [Google Scholar] [CrossRef]
- Gong, W.J.; Golic, K.G. Loss of Hsp70 in Drosophila is pleiotropic, with effects on thermotolerance, recovery from heat shock and neurodegeneration. Genetics 2006, 172, 275–286. [Google Scholar] [CrossRef]
- Tower, J. Heat shock proteins and Drosophila aging. Exp. Gerontol. 2011, 46, 355–362. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; The International Natural Product Sciences Taskforce; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Coy-Barrera, E.; Ogungbe, I.V.; Schmidt, T.J. Natural Products for Drug Discovery in the 21st Century: Innovations for Novel Therapeutics. Molecules 2023, 28, 3690. [Google Scholar] [CrossRef] [PubMed]
- Santos-Cruz, L.F.; Ponciano-Gomez, A.; Torres-Gregorio, J.T.; Ramirez-Cruz, B.G.; Vazquez-Gomez, G.; Hernandez-Portilla, L.B.; Flores-Ortiz, C.M.; Duenas-Garcia, I.E.; Heres-Pulido, M.E.; Castaneda-Partida, L.; et al. Zearalenone Does Not Show Genotoxic Effects in the Drosophila melanogaster Wing Spot Test, but It Induces Oxidative Imbalance, Development, and Fecundity Alterations. Toxins 2023, 15, 358. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, F.; Wen, D.; Mu, R. Exposure to bisphenol A induced oxidative stress, cell death and impaired epithelial homeostasis in the adult Drosophila melanogaster midgut. Ecotoxicol. Environ. Saf. 2022, 248, 114285. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, C.; Zhang, X.; Song, Y.; Wang, B.; Zhang, K.; Sun, M. Developmental neurotoxic effects of bisphenol A and its derivatives in Drosophila melanogaster. Ecotoxicol. Environ. Saf. 2023, 260, 115098. [Google Scholar] [CrossRef]
- Metz, J.T.; Hajduk, P.J. Rational approaches to targeted polypharmacology: Creating and navigating protein-ligand interaction networks. Curr. Opin. Chem. Biol. 2010, 14, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Peon, A.; Naulaerts, S.; Ballester, P.J. Predicting the Reliability of Drug-target Interaction Predictions with Maximum Coverage of Target Space. Sci. Rep. 2017, 7, 3820. [Google Scholar] [CrossRef] [PubMed]
- Bantscheff, M.; Scholten, A.; Heck, A.J. Revealing promiscuous drug-target interactions by chemical proteomics. Drug Discov. Today 2009, 14, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Scheiber, J.; Chen, B.; Milik, M.; Sukuru, S.C.; Bender, A.; Mikhailov, D.; Whitebread, S.; Hamon, J.; Azzaoui, K.; Urban, L.; et al. Gaining insight into off-target mediated effects of drug candidates with a comprehensive systems chemical biology analysis. J. Chem. Inf. Model. 2009, 49, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.J., 3rd; Van Vleet, T.R.; Mittelstadt, S.W.; Blomme, E.A.G. Potential functional and pathological side effects related to off-target pharmacological activity. J. Pharmacol. Toxicol. Methods 2017, 87, 108–126. [Google Scholar] [CrossRef]
- Beck, H.; Harter, M.; Hass, B.; Schmeck, C.; Baerfacker, L. Small molecules and their impact in drug discovery: A perspective on the occasion of the 125th anniversary of the Bayer Chemical Research Laboratory. Drug Discov. Today 2022, 27, 1560–1574. [Google Scholar] [CrossRef]
- Deshpande, S.A.; Yamada, R.; Mak, C.M.; Hunter, B.; Soto Obando, A.; Hoxha, S.; Ja, W.W. Acidic Food pH Increases Palatability and Consumption and Extends Drosophila Lifespan. J. Nutr. 2015, 145, 2789–2796. [Google Scholar] [CrossRef]
- Ormerod, K.G.; LePine, O.K.; Abbineni, P.S.; Bridgeman, J.M.; Coorssen, J.R.; Mercier, A.J.; Tattersall, G.J. Drosophila development, physiology, behavior, and lifespan are influenced by altered dietary composition. Fly 2017, 11, 153–170. [Google Scholar] [CrossRef]
- Perez, S.; Talens-Visconti, R.; Rius-Perez, S.; Finamor, I.; Sastre, J. Redox signaling in the gastrointestinal tract. Free Radic. Biol. Med. 2017, 104, 75–103. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, F.; Zhang, W.; Zhou, S.; Wen, D.; Mu, R. Chronic exposure to zearalenone induces intestinal inflammation and oxidative injury in adult Drosophila melanogaster midgut. Ecotoxicol. Environ. Saf. 2023, 251, 114555. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Yang, X.; Sun, L.; Han, X.; Xu, L.; Gu, W.; Zhang, M. Effects of cadmium on oxidative stress and cell apoptosis in Drosophila melanogaster larvae. Sci. Rep. 2022, 12, 4762. [Google Scholar] [CrossRef]
- He, J.; Han, S.; Wang, Y.; Kang, Q.; Wang, X.; Su, Y.; Li, Y.; Liu, Y.; Cai, H.; Xiu, M. Irinotecan cause the side effects on development and adult physiology, and induces intestinal damage via innate immune response and oxidative damage in Drosophila. Biomed. Pharmacother. 2023, 169, 115906. [Google Scholar] [CrossRef]
- Zhang, P.; Edgar, B.A. Insect Gut Regeneration. Cold Spring Harb. Perspect. Biol. 2022, 14, a040915. [Google Scholar] [CrossRef] [PubMed]
- Morris, O.; Jasper, H. Reactive Oxygen Species in intestinal stem cell metabolism, fate and function. Free Radic. Biol. Med. 2021, 166, 140–146. [Google Scholar] [CrossRef]
- Chen, F.; Su, R.; Ni, S.; Liu, Y.; Huang, J.; Li, G.; Wang, Q.; Zhang, X.; Yang, Y. Context-dependent responses of Drosophila intestinal stem cells to intracellular reactive oxygen species. Redox Biol. 2021, 39, 101835. [Google Scholar] [CrossRef]
- Sawicki, R.; Singh, S.P.; Mondal, A.K.; Benes, H.; Zimniak, P. Cloning, expression and biochemical characterization of one Epsilon-class (GST-3) and ten Delta-class (GST-1) glutathione S-transferases from Drosophila melanogaster, and identification of additional nine members of the Epsilon class. Biochem. J. 2003, 370, 661–669. [Google Scholar] [CrossRef]
- Wang, M.; Kaufman, R.J. Protein misfolding in the endoplasmic reticulum as a conduit to human disease. Nature 2016, 529, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Walter, P.; Ron, D. The unfolded protein response: From stress pathway to homeostatic regulation. Science 2011, 334, 1081–1086. [Google Scholar] [CrossRef]
- Gupta, S.C.; Siddique, H.R.; Mathur, N.; Vishwakarma, A.L.; Mishra, R.K.; Saxena, D.K.; Chowdhuri, D.K. Induction of hsp70, alterations in oxidative stress markers and apoptosis against dichlorvos exposure in transgenic Drosophila melanogaster: Modulation by reactive oxygen species. Biochim. Biophys. Acta 2007, 1770, 1382–1394. [Google Scholar] [CrossRef] [PubMed]
- Hetz, C. The unfolded protein response: Controlling cell fate decisions under ER stress and beyond. Nat. Rev. Mol. Cell Biol. 2012, 13, 89–102. [Google Scholar] [CrossRef]
- Mitra, S.; Ryoo, H.D. The unfolded protein response in metazoan development. J. Cell Sci. 2019, 132, jcs217216. [Google Scholar] [CrossRef] [PubMed]
- Papanikolopoulou, K.; Mudher, A.; Skoulakis, E. An assessment of the translational relevance of Drosophila in drug discovery. Expert Opin. Drug Discov. 2019, 14, 303–313. [Google Scholar] [CrossRef]
- Munnik, C.; Xaba, M.P.; Malindisa, S.T.; Russell, B.L.; Sooklal, S.A. Drosophila melanogaster: A platform for anticancer drug discovery and personalized therapies. Front. Genet. 2022, 13, 949241. [Google Scholar] [CrossRef]
- Tello, J.A.; Williams, H.E.; Eppler, R.M.; Steinhilb, M.L.; Khanna, M. Animal Models of Neurodegenerative Disease: Recent Advances in Fly Highlight Innovative Approaches to Drug Discovery. Front. Mol. Neurosci. 2022, 15, 883358. [Google Scholar] [CrossRef] [PubMed]


| Gene Name | FlyBase ID | Primer Sequence | Amplicon Size |
|---|---|---|---|
| sod1 | FBgn0003462 | F: 5’-ACCGACTCCAAGATTACGCTC-3’ R: 5’-CAGTGGCCGACATCGGAATA-3’ | 197 bp |
| sod2 | FBgn0010213 | F: 5’-AATCTAAATGCCGCCGAGGA-3’ R: 5’-CTCTTCCACTGCGACTCGAT-3’ | 197 bp |
| cat | FBgn0000261 | F: 5’-CTATGGCTCGCACACCTTCA-3’ R: 5’-TCGTCCAACTGGGGAACTTG-3’ | 194 bp |
| gstd1 | FBgn0001149 | F: 5’-CGCGCCATCCAGGTGTATTT-3’ R: 5’-CTGGTACAGCGTTCCCATGT-3’ | 123 bp |
| hsp70 | FBgn0013279 | F: 5’-CGGAGTCTCCATTCAGGTGT-3’ R: 5’-GCTGACGTTCAGGATTCCA-3’ | 160 bp |
| rpl32 | FBgn0002626 | F: 5’-GACCATCCGCCCAGCATAC-3’ R: 5’-CGGCGACGCACTCTGTT-3’ | 138 bp |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Catalani, E.; Brunetti, K.; Del Quondam, S.; Bongiorni, S.; Picchietti, S.; Fausto, A.M.; Lupidi, G.; Marcantoni, E.; Perrotta, C.; Achille, G.; et al. Exposure to the Natural Compound Climacostol Induces Cell Damage and Oxidative Stress in the Fruit Fly Drosophila melanogaster. Toxics 2024, 12, 102. https://doi.org/10.3390/toxics12020102
Catalani E, Brunetti K, Del Quondam S, Bongiorni S, Picchietti S, Fausto AM, Lupidi G, Marcantoni E, Perrotta C, Achille G, et al. Exposure to the Natural Compound Climacostol Induces Cell Damage and Oxidative Stress in the Fruit Fly Drosophila melanogaster. Toxics. 2024; 12(2):102. https://doi.org/10.3390/toxics12020102
Chicago/Turabian StyleCatalani, Elisabetta, Kashi Brunetti, Simona Del Quondam, Silvia Bongiorni, Simona Picchietti, Anna Maria Fausto, Gabriele Lupidi, Enrico Marcantoni, Cristiana Perrotta, Gabriele Achille, and et al. 2024. "Exposure to the Natural Compound Climacostol Induces Cell Damage and Oxidative Stress in the Fruit Fly Drosophila melanogaster" Toxics 12, no. 2: 102. https://doi.org/10.3390/toxics12020102








