Evaluation of Cytotoxicity and Oxidative Stress of Whole Aerosol from Vuse Alto ENDS Products
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Products
2.2. Whole Aerosol Generation
2.3. Photometers
2.4. Chemical Analyses
2.4.1. Nicotine Determination
2.4.2. Carbonyl Determination
2.5. Transepithelial Electrical Resistance (TEER)
2.6. Determination of Cytotoxicity
2.6.1. NRU Assay
2.6.2. MTT Assay
2.7. EpiAirway™ Nrf2 Tissue Model
LDH Assay
2.8. Determination of Oxidative Stress in 3D EpiAirway™ Cultures
Data Analysis and Statistical Methods
3. Results
3.1. Whole Aerosol Studies
3.1.1. NRU Assay
3.1.2. MTT Assay
3.1.3. LDH Release Assay and Oxidative Stress
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- G. United States Public Health Service Office of the Surgeon, P. National Center for Chronic Disease, S. Health Promotion Office on, and Health. 2010. Available online: https://sbpt.org.br/portal/wp-content/uploads/2019/02/2010-SGR-COMO-TABACO-CAUSA-DOENCAS.pdf (accessed on 2 February 2024).
- G. United States Public Health Service Office of the Surgeon, P. National Center for Chronic Disease, S. Health Promotion Office on, and Health. Smoking Cessation: A Report of the Surgeon General; U.S. Department of Health and Human Services: Rockville, MD, USA, 2020. [Google Scholar]
- Zeller, M.; Hatsukami, D. The Strategic Dialogue on Tobacco Harm Reduction: A vision and blueprint for action in the US. Tob. Control 2009, 18, 324. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. Harmful and Potentially Harmful Constituents in Tobacco Products and Tobacco Smoke; U.S. Department of Health and Human Services, Ed.; Federal Register: Washington, DC, USA, 2012; p. 20034. [Google Scholar]
- Food and Drug Administration. Premarket Tobacco Product Applications and Recordkeeping Requirements; U.S. Department of Health and Human Services: Washington, DC, USA, 2021; pp. 55300–55439. [Google Scholar]
- Johnson, M.D.; Schilz, J.; Djordjevic, M.V.; Rice, J.R.; Shields, P.G. Evaluation of in vitro assays for assessing the toxicity of cigarette smoke and smokeless tobacco. Cancer Epidemiol. Biomark. Prev. 2009, 18, 3263–3304. [Google Scholar] [CrossRef] [PubMed]
- Manuppello, J.R.; Sullivan, K.M. Toxicity assessment of tobacco products in vitro. Altern. Lab. Anim. 2015, 43, 39–67. [Google Scholar] [CrossRef] [PubMed]
- Coggins, C.R. A review of chronic inhalation studies with mainstream cigarette smoke in rats and mice. Toxicol. Pathol. 1998, 26, 307. [Google Scholar] [CrossRef] [PubMed]
- Clippinger, A.J.; Allen, D.; Jarabek, A.M.; Corvaro, M.; Gaça, M.; Gehen, S.; Hotchkiss, J.A.; Patlewicz, G.; Melbourne, J.; Hinderliter, P.; et al. Alternative approaches for acute inhalation toxicity testing to address global regulatory and non-regulatory data requirements: An international workshop report. Toxicol. In Vitro 2018, 48, 53–70. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Coyle, J.P.; Xiong, R.; Wang, Y.; Heflich, R.H.; Ren, B.; Gwinn, W.M.; Hayden, P.; Rojanasakul, L. Invited review: Human air-liquid-interface organotypic airway tissue models derived from primary tracheobronchial epithelial cells-overview and perspectives. In Vitro Cell Dev. Biol. Anim. 2021, 57, 104–132. [Google Scholar] [CrossRef] [PubMed]
- Mertens, T.C.J.; Karmouty-Quintana, H.; Taube, C.; Hiemstra, P.S. Use of airway epithelial cell culture to unravel the pathogenesis and study treatment in obstructive airway diseases. Pulm. Pharmacol. Ther. 2017, 45, 101–113. [Google Scholar] [CrossRef]
- Upadhyay, S.; Palmberg, L. Air-Liquid Interface: Relevant In Vitro Models for Investigating Air Pollutant-Induced Pulmonary Toxicity. Toxicol. Sci. 2018, 164, 21–30. [Google Scholar] [CrossRef]
- Rayner, R.E.; Makena, P.; Liu, G.; Prasad, G.L.; Cormet-Boyaka, E. Differential gene expression of 3D primary human airway cultures exposed to cigarette smoke and electronic nicotine delivery system (ENDS) preparations. BMC Med. Genom. 2022, 15, 76. [Google Scholar] [CrossRef]
- Rayner, R.E.; Makena, P.; Prasad, G.L.; Cormet-Boyaka, E. Cigarette and ENDS preparations differentially regulate ion channels and mucociliary clearance in primary normal human bronchial 3D cultures. Am. J. Physiol. Lung Cell Mol. Physiol. 2019, 317, L295–L302. [Google Scholar] [CrossRef]
- Rayner, R.E.; Makena, P.; Prasad, G.L.; Cormet-Boyaka, E. Optimization of Normal Human Bronchial Epithelial (NHBE) Cell 3D Cultures for in vitro Lung Model Studies. Sci. Rep. 2019, 9, 500. [Google Scholar] [CrossRef] [PubMed]
- Rayner, R.E.; Makena, P.; Prasad, G.L.; Cormet-Boyaka, E. Cigarette smoke preparations, not electronic nicotine delivery system preparations, induce features of lung disease in a 3D lung repeat-dose model. Am. J. Physiol. Lung Cell Mol. Physiol. 2021, 320, L276–L287. [Google Scholar] [CrossRef] [PubMed]
- Rayner, R.E.; Wellmerling, J.; Makena, P.; Zhao, J.; Prasad, G.L.; Cormet-Boyaka, E. Transcriptomic Response of Primary Human Bronchial Cells to Repeated Exposures of Cigarette and ENDS Preparations. Cell Biochem. Biophys. 2022, 80, 217–228. [Google Scholar] [CrossRef]
- Miller-Holt, J.; Behrsing, H.; Crooks, I.; Curren, R.; Demir, K.; Gafner, J.; Gillman, G.; Hollings, M.; Leverette, R.; Oldham, M.; et al. Key challenges for in vitro testing of tobacco products for regulatory applications: Recommendations for dosimetry. Drug Test. Anal. 2022, 15, 1175–1188. [Google Scholar] [CrossRef]
- Lauterstein, D.; Savidge, M.; Chen, Y.; Weil, R.; Yeager, R.P. Nonanimal toxicology testing approaches for traditional and deemed tobacco products in a complex regulatory environment: Limitations, possibilities, and future directions. Toxicol. In Vitro 2020, 62, 104684. [Google Scholar] [CrossRef]
- Czekala, L.; Wieczorek, R.; Simms, L.; Yu, F.; Budde, J.; Trelles Sticken, E.; Rudd, K.; Verron, T.; Brinster, O.; Stevenson, M.; et al. Multi-endpoint analysis of human 3D airway epithelium following repeated exposure to whole electronic vapor product aerosol or cigarette smoke. Curr. Res. Toxicol. 2021, 2, 99–115. [Google Scholar] [CrossRef]
- Fields, W.; Maione, A.G.; Keyser, B.; Bombick, B. Characterization and Application of the VITROCELL VC1 Smoke Exposure System and 3D EpiAirway Models for Toxicological and e-Cigarette Evaluations. Appl. In Vitro Toxicol. 2017, 3, 68–83. [Google Scholar] [CrossRef]
- Neilson, L.; Mankus, C.; Thorne, D.; Jackson, G.; DeBay, J.; Meredith, C. Development of an in vitro cytotoxicity model for aerosol exposure using 3D reconstructed human airway tissue; application for assessment of e-cigarette aerosol. Toxicol. Vitr. Int. J. Publ. Assoc. BIBRA 2015, 29, 1952–1962. [Google Scholar] [CrossRef]
- Wieczorek, R.; Phillips, G.; Czekala, L.; Trelles Sticken, E.; O’Connell, G.; Simms, L.; Rudd, K.; Stevenson, M.; Walele, T. A comparative in vitro toxicity assessment of electronic vaping product e-liquids and aerosols with tobacco cigarette smoke. Toxicol. In Vitro 2020, 66, 104866. [Google Scholar] [CrossRef]
- Abrams, D.B.; Glasser, A.M.; Pearson, J.L.; Villanti, A.C.; Collins, L.K.; Niaura, R.S. Harm Minimization and Tobacco Control: Reframing Societal Views of Nicotine Use to Rapidly Save Lives. Annu. Rev. Public Health 2018, 39, 193–213. [Google Scholar] [CrossRef]
- Gottlieb, S.; Zeller, M. A Nicotine-Focused Framework for Public Health. N. Engl. J. Med. 2017, 377, 1111–1114. [Google Scholar] [CrossRef]
- FDA Denies Marketing of Six Flavored Vuse Alto E-Cigarette Products Following Determination They Do Not Meet Public Health Standard. Available online: https://www.fda.gov/news-events/press-announcements/fda-denies-marketing-six-flavored-vuse-alto-e-cigarette-products-following-determination-they-do-not (accessed on 2 February 2024).
- Center for Disease Control and Prevention, Centers for Disease Control and Prevention Website (2020). 2017. Available online: https://www.cdc.gov/tobacco/data_statistics/fact_sheets/tobacco_industry/brand_preference/ (accessed on 2 February 2024).
- Herzog, K.P.B. Nielsen: Tobacco “All Channel” Data April 21 2018. Wells Fargo Securities, LLC: Charlotte, NC, USA, 2018. Available online: https://www.fda.gov/TobaccoProducts/GuidanceComplianceRegulatoryInformation/ucm246129.htm (accessed on 2 February 2024).
- Fields, W.; Fowler, K.; Hargreaves, V.; Reeve, L.; Bombick, B. Development, qualification, validation and application of the neutral red uptake assay in Chinese Hamster Ovary (CHO) cells using a VITROCELL® VC10® smoke exposure system. Toxicol. Vitr. Int. J. Publ. Assoc. BIBRA 2017, 40, 144–152. [Google Scholar] [CrossRef]
- Fowler, K.; Fields, W.; Hargreaves, V.; Reeve, L.; Bombick, B. Development, qualification, validation and application of the Ames test using a VITROCELL(®) VC10(®) smoke exposure system. Toxicol. Rep. 2018, 5, 542–551. [Google Scholar] [CrossRef]
- Keyser, B.M.; Leverette, R.; Hollings, M.; Seymour, A.; Weidman, R.A.; Bequette, C.J.; Jordan, K. Characterization of smoke and aerosol deliveries from combustible cigarettes, heated tobacco products and electronic nicotine delivery systems in the Vitrocell® Mammalian 6/48 exposure module. Toxicol. Rep. 2022, 9, 1985–1992. [Google Scholar] [CrossRef]
- Keyser, B.M.; Leverette, R.; Hollings, M.; Seymour, A.; Weidman, R.A.; Bequette, C.J.; Jordan, K. Characterization of Aerosol Deliveries from Combustible Cigarettes, Heated Tobacco Products, and Electronic Nicotine Delivery Systems Using the Vitrocell Ames 48. Appl. Vitr. Toxicol. 2022, 8, 39–49. [Google Scholar] [CrossRef]
- Keyser, B.M.; Leverette, R.; Hollings, M.; Rothwell, E.; Weidman, R.A.; Bequette, C.J.; Jordan, K. Validation of the VITROCELL Mammalian 48 Exposure Module Using In Vitro Cytotoxicity via the Neutral Red Uptake Assay in Chinese Hamster Ovary Cells Following Exposure to Whole Smoke from Combustible Cigarettes. Appl. Vitr. Toxicol. 2023, 9, 143–154. [Google Scholar] [CrossRef]
- Keyser, B.M.; Leverette, R.; Fowler, K.; Fields, W.; Hargreaves, V.; Reeve, L.; Bombick, B. Development of a quantitative method for assessment of dose in in vitro evaluations using a VITROCELL(R) VC10(R) smoke exposure system. Toxicol. Vitr. Int. J. Publ. Assoc. BIBRA 2019, 56, 19–29. [Google Scholar] [CrossRef] [PubMed]
- ISO20778:2018; Cigarettes—Routine Analytical Cigarette Smoking Machine—Definitions and Standard Conditions with an Intense Smoking Regime. International Standards Organization: Geneva, Switzerland, 2018.
- ISO20768:2018; Vapour products—Routine Analytical Vaping Machine—Definitions and Standard Conditions. International Standards Organization: Geneva, Switzerland, 2018.
- Keyser, B.M.; Leverette, R.; Hollings, M.; Seymour, A.; Reeve, L.; Fields, W. Investigation of multiple whole smoke dosimetry techniques using a VITROCELL(R)VC10(R) smoke exposure system. Toxicol. Rep. 2019, 6, 1281–1288. [Google Scholar] [CrossRef] [PubMed]
- Kanobe, M.N.; Jones, B.A.; Nelson, P.; Brown, B.G.; Chen, P.; Makena, P.; Schmidt, E.; Darnell, J.; Caraway, J.W.; Prasad, G.L.; et al. Part three: A randomized study to assess biomarker changes in cigarette smokers switched to Vuse Solo or Abstinence. Sci. Rep. 2022, 12, 20658. [Google Scholar] [CrossRef] [PubMed]
- Gades, M.S.; Alcheva, A.; Riegelman, A.L.; Hatsukami, D.K. The Role of Nicotine and Flavor in the Abuse Potential and Appeal of Electronic Cigarettes for Adult Current and Former Cigarette and Electronic Cigarette Users: A Systematic Review. Nicotine Tob. Res. Off. J. Soc. Res. Nicotine Tob. 2022, 24, 1332–1343. [Google Scholar] [CrossRef]
- Miller, J.H.; Gardner, W.P.; Gonzalez, R.R. UHPLC separation with MS analysis for eight carbonyl compounds in mainstream tobacco smoke. J. Chromatogr. Sci. 2010, 48, 12–17. [Google Scholar] [CrossRef]
- ICCVAM. In Vitro Cytotoxicity Test methods for Estimating Starting Doses for Acute oral Systemic Toxicity Testing; National Toxicology Program: Research Triangle Park, NC, 2006. [Google Scholar]
- Mozaheb, N.; Arefian, E.; Amoozegar, M.A. Designing a whole cell bioreporter to show antioxidant activities of agents that work by promotion of the KEAP1-NRF2 signaling pathway. Sci. Rep. 2019, 9, 3248. [Google Scholar] [CrossRef]
- Azzopardi, D.; Patel, K.; Jaunky, T.; Santopietro, S.; Camacho, O.M.; McAughey, J.; Gaça, M. Electronic cigarette aerosol induces significantly less cytotoxicity than tobacco smoke. Toxicol. Mech. Methods 2016, 26, 477–491. [Google Scholar] [CrossRef]
- Pearce, K.; Gray, N.; Gaur, P.; Jeon, J.; Suarez, A.; Shannahan, J.; Pappas, R.S.; Watson-Wright, C. Toxicological analysis of aerosols derived from three electronic nicotine delivery systems using normal human bronchial epithelial cells. Toxicol. In Vitro 2020, 69, 104997. [Google Scholar] [CrossRef]
- Stabbert, R.; Dempsey, R.; Diekmann, J.; Euchenhofer, C.; Hagemeister, T.; Haussmann, H.J.; Knorr, A.; Mueller, B.P.; Pospisil, P.; Reininghaus, W.; et al. Studies on the contributions of smoke constituents, individually and in mixtures, in a range of in vitro bioactivity assays. Toxicol. In Vitro 2017, 42, 222–246. [Google Scholar] [CrossRef]
- Thorne, D.; Dalrymple, A.; Dillon, D.; Duke, M.; Meredith, C. A comparative assessment of cigarette smoke aerosols using an in vitro air-liquid interface cytotoxicity test. Inhal. Toxicol. 2015, 27, 629–640. [Google Scholar] [CrossRef]
- Hikisz, P.; Jacenik, D. The Tobacco Smoke Component, Acrolein, as a Major Culprit in Lung Diseases and Respiratory Cancers: Molecular Mechanisms of Acrolein Cytotoxic Activity. Cells 2023, 12, 897. [Google Scholar] [CrossRef] [PubMed]
- Horinouchi, T.; Higashi, T.; Mazaki, Y.; Miwa, S. Carbonyl Compounds in the Gas Phase of Cigarette Mainstream Smoke and Their Pharmacological Properties. Biol. Pharm. Bull. 2016, 39, 909–914. [Google Scholar] [CrossRef] [PubMed]
- Talih, S.; Karaoghlanian, N.; Salman, R.; Fallah, S.; Helal, A.; El-Hage, R.; Saliba, N.; Breland, A.; Eissenberg, T.; Shihadeh, A. Comparison of design characteristics and toxicant emissions from Vuse Solo and Alto electronic nicotine delivery systems. Tob. Control 2023, 10. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.; Bhattarai, C.; Samburova, V.; Khlystov, A. Carbonyls and Carbon Monoxide Emissions from Electronic Cigarettes Affected by Device Type and Use Patterns. Int. J. Environ. Res. Public Health 2020, 17, 2767. [Google Scholar] [CrossRef]
- Talih, S.; Salman, R.; Karaoghlanian, N.; El-Hellani, A.; Shihadeh, A. Carbonyl Emissions and Heating Temperatures across 75 Nominally Identical Electronic Nicotine Delivery System Products: Do Manufacturing Variations Drive Pulmonary Toxicant Exposure? Chem. Res. Toxicol. 2023, 36, 342–346. [Google Scholar] [CrossRef]
- Talih, S.; Salman, R.; El-Hage, R.; Karam, E.; Karaoghlanian, N.; El-Hellani, A.; Saliba, N.; Shihadeh, A. Characteristics and toxicant emissions of JUUL electronic cigarettes. Tob. Control 2019, 28, 678–680. [Google Scholar] [CrossRef]
- Margham, J.; McAdam, K.; Cunningham, A.; Porter, A.; Fiebelkorn, S.; Mariner, D.; Digard, H.; Proctor, C. The Chemical Complexity of e-Cigarette Aerosols Compared With the Smoke From a Tobacco Burning Cigarette. Front. Chem. 2021, 9, 743060. [Google Scholar] [CrossRef]
- Margham, J.; McAdam, K.; Forster, M.; Liu, C.; Wright, C.; Mariner, D.; Proctor, C. Chemical Composition of Aerosol from an E-Cigarette: A Quantitative Comparison with Cigarette Smoke. Chem. Res. Toxicol. 2016, 29, 1662–1678. [Google Scholar] [CrossRef]
- Putnam, K.P.; Bombick, D.W.; Doolittle, D.J. Evaluation of eight in vitro assays for assessing the cytotoxicity of cigarette smoke condensate. Toxicol. In Vitro 2002, 16, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Jackson, G.R., Jr.; Maione, A.G.; Klausner, M.; Hayden, P.J. Prevalidation of an Acute Inhalation Toxicity Test Using the EpiAirway In Vitro Human Airway Model. Appl. Vitr. Toxicol. 2018, 4, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Cipollina, C.; Bruno, A.; Fasola, S.; Cristaldi, M.; Patella, B.; Inguanta, R.; Vilasi, A.; Aiello, G.; La Grutta, S.; Torino, C.; et al. Cellular and Molecular Signatures of Oxidative Stress in Bronchial Epithelial Cell Models Injured by Cigarette Smoke Extract. Int. J. Mol. Sci. 2022, 23, 1770. [Google Scholar] [CrossRef] [PubMed]
- Czekala, L.; Simms, L.; Stevenson, M.; Tschierske, N.; Maione, A.G.; Walele, T. Toxicological comparison of cigarette smoke and e-cigarette aerosol using a 3D in vitro human respiratory model. Regul. Toxicol. Pharmacol. RTP 2019, 103, 314–324. [Google Scholar] [CrossRef]
- Espinoza-Derout, J.; Shao, X.M.; Lao, C.J.; Hasan, K.M.; Rivera, J.C.; Jordan, M.C.; Echeverria, V.; Roos, K.P.; Sinha-Hikim, A.P.; Friedman, T.C. Electronic Cigarette Use and the Risk of Cardiovascular Diseases. Front. Cardiovasc. Med. 2022, 9, 879726. [Google Scholar] [CrossRef] [PubMed]
- National Academies of Sciences, Engineering, and Medicine. Public Health Consequences of E-Cigarettes; National Academies Press: Washington, DC, USA, 2018. [Google Scholar]
- Munakata, S.; Watanabe, T.; Takahashi, T.; Kimuro, S.; Ishimori, K.; Hashizume, T. Development of a micronucleus test using the EpiAirway™ organotypic human airway model. Genes Environ. 2023, 45, 14. [Google Scholar] [CrossRef] [PubMed]
- Bishop, E.; Gaça, M.; Thorne, D. Advances in whole aerosol approaches for in vitro e-cigarette testing. Drug Test. Anal. 2023, 15, 1133–1144. [Google Scholar] [CrossRef]
- Lacroix, G.; Koch, W.; Ritter, D.; Gutleb, A.C.; Larsen, S.T.; Loret, T.; Zanetti, F.; Constant, S.; Chortarea, S.; Rothen-Rutishauser, B.; et al. Air-Liquid Interface In Vitro Models for Respiratory Toxicology Research: Consensus Workshop and Recommendations. Appl. In Vitro Toxicol. 2018, 4, 91–106. [Google Scholar] [CrossRef]
- Cao, X.; Wang, Y.; Xiong, R.; Muskhelishvili, L.; Davis, K.; Richter, P.A.; Heflich, R.H. Cigarette whole smoke solutions disturb mucin homeostasis in a human in vitro airway tissue model. Toxicology 2018, 409, 119–128. [Google Scholar] [CrossRef]
- Xiong, R.; Wu, Q.; Muskhelishvili, L.; Davis, K.; Shemansky, J.M.; Bryant, M.; Rosenfeldt, H.; Healy, S.M.; Cao, X. Evaluating Mode of Action of Acrolein Toxicity in an In Vitro Human Airway Tissue Model. Toxicol. Sci. 2018, 166, 451–464. [Google Scholar] [CrossRef]
- Xiong, R.; Wu, Q.; Trbojevich, R.; Muskhelishvili, L.; Davis, K.; Bryant, M.; Richter, P.; Cao, X. Disease-related responses induced by cadmium in an in vitro human airway tissue model. Toxicol. Lett. 2019, 303, 16–27. [Google Scholar] [CrossRef]
- Xiong, R.; Wu, Y.; Wu, Q.; Muskhelishvili, L.; Davis, K.; Tripathi, P.; Chen, Y.; Chen, T.; Bryant, M.; Rosenfeldt, H.; et al. Integration of transcriptome analysis with pathophysiological endpoints to evaluate cigarette smoke toxicity in an in vitro human airway tissue model. Arch. Toxicol. 2021, 95, 1739–1761. [Google Scholar] [CrossRef]
- Ren, B.; Wu, Q.; Muskhelishvili, L.; Davis, K.; Wang, Y.; Rua, D.; Cao, X. Evaluating the Sub-Acute Toxicity of Formaldehyde Fumes in an In Vitro Human Airway Epithelial Tissue Model. Int. J. Mol. Sci. 2022, 23, 2593. [Google Scholar] [CrossRef] [PubMed]
- Thorne, D.; Adamson, J.; Sticken, E.T.; Wieczorek, R.; Behrsing, H.; Steiner, S.; Majeed, S.; Frentzel, S.; Ishikawa, S.; Ito, S.; et al. An interlaboratory in vitro aerosol exposure system reference study. Toxicol. Res. Appl. 2021, 5, 2397847321992752. [Google Scholar] [CrossRef]
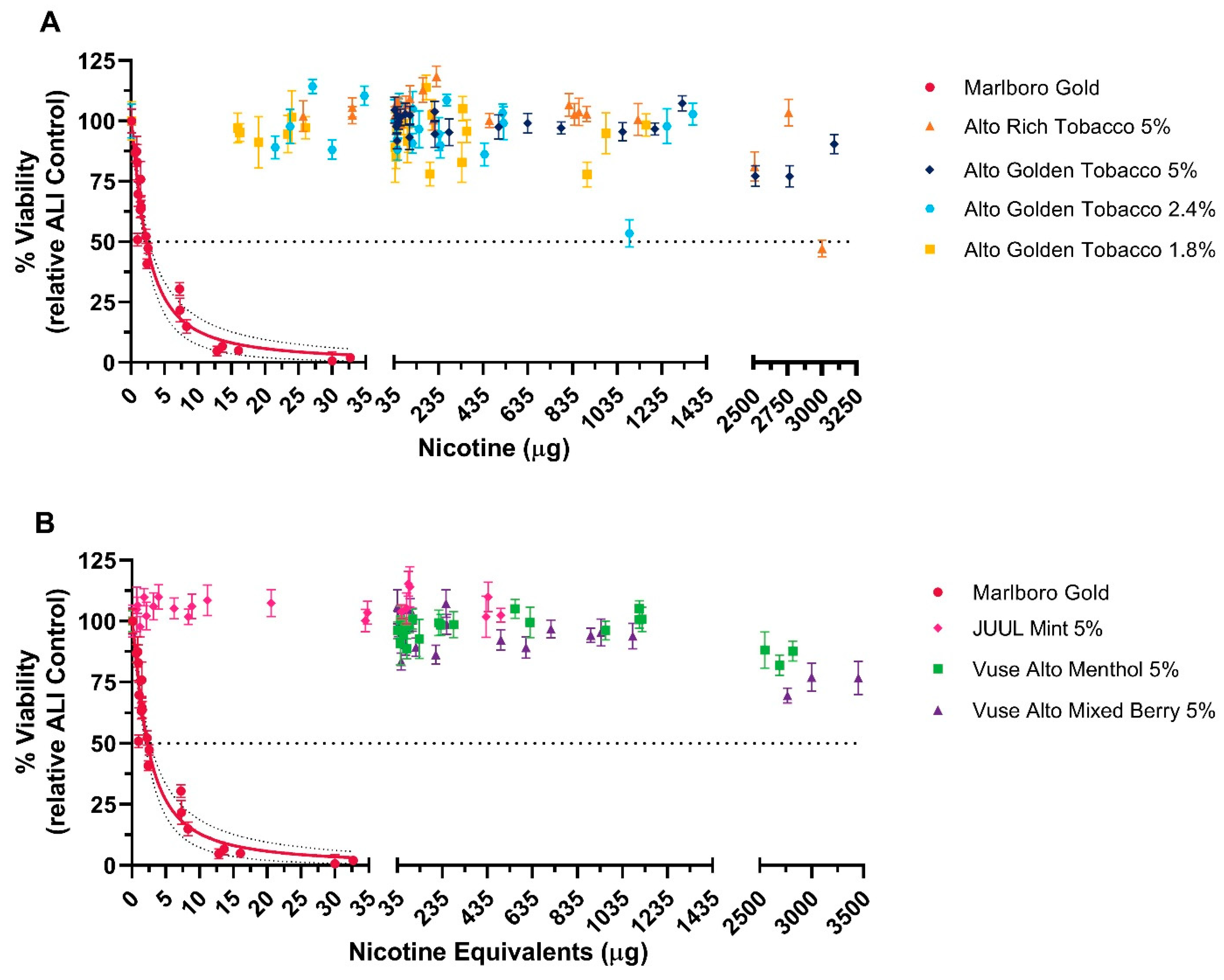

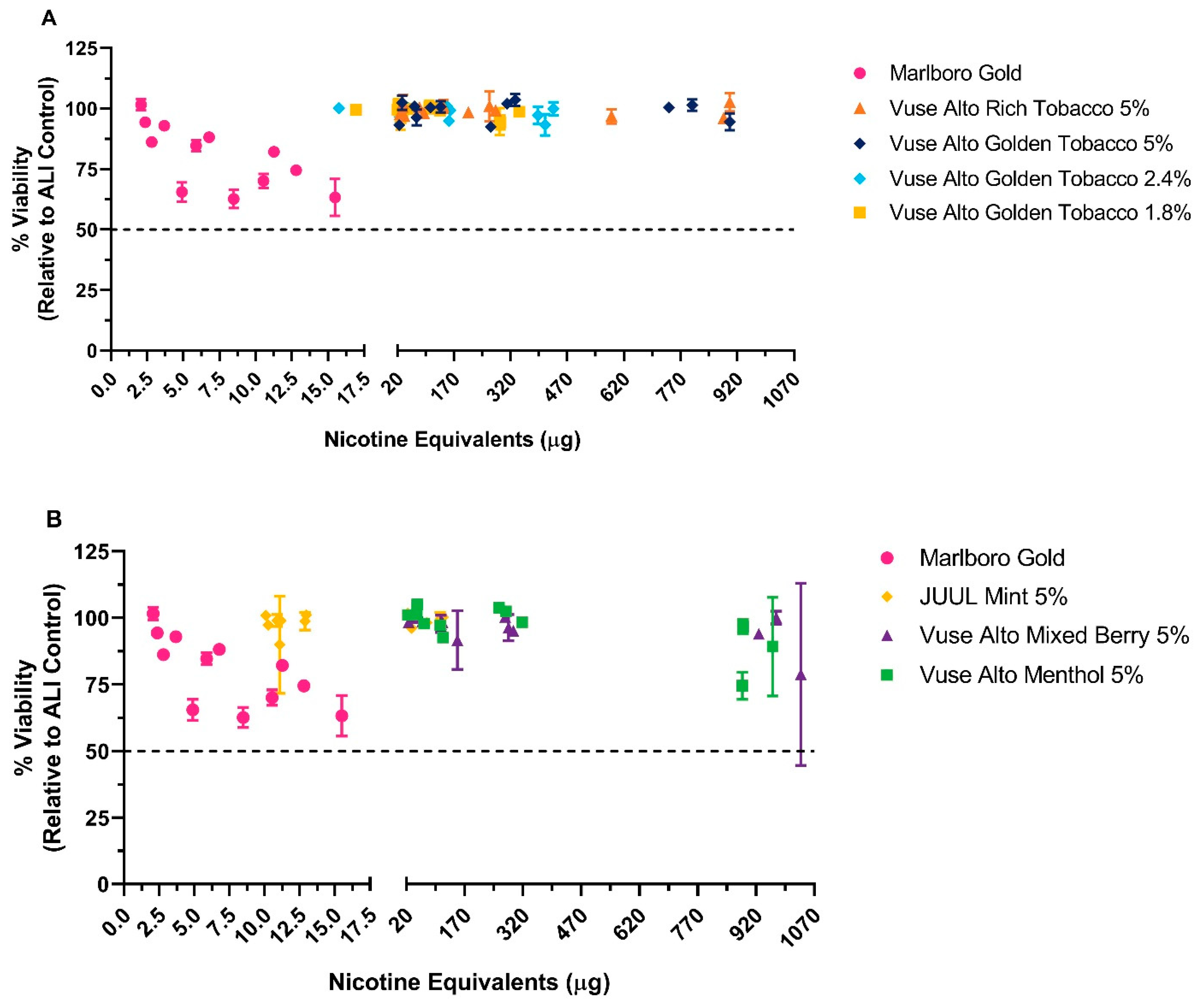
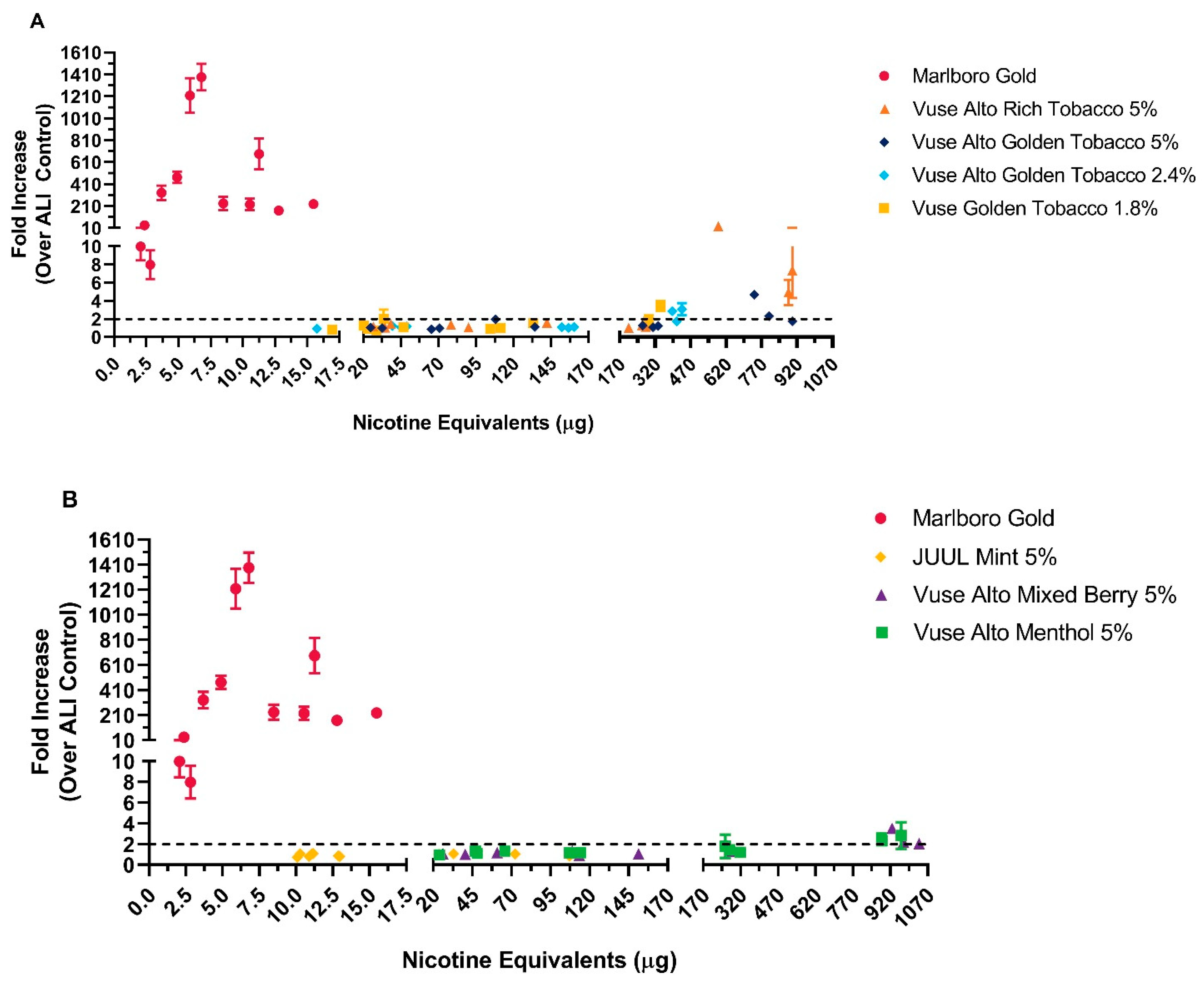
| Test Product | Nicotine (µg/mL) | Formaldehyde | Acetaldehyde | Acrolein | Crotonaldehyde |
|---|---|---|---|---|---|
| (µg/mL) | (µg/mL) | (µg/mL) | (µg/mL) | ||
| Marlboro Gold | 14.37 ± 2.25 | 1.52 ± 0.23 | 6.59 ± 0.65 | 0.56 ± 0.08 | 0.73 ± 0.06 |
| Vuse Alto Golden Tobacco 5% | 903.67 ± 73.71 | 0.51 ± 0.10 | <LOQ | <LOQ | <LOQ |
| Vuse Alto Golden Tobacco 2.4% | 458 ± 18.38 | 0.37 ± 0.04 | <LOQ | <LOQ | <LOQ |
| Vuse Alto Golden Tobacco 1.8% | 343 ± 26.88 | 0.4 ± 0.13 | <LOQ | <LOQ | <LOQ |
| Vuse Alto Menthol 5% | 1012.67 ± 40.55 | 0.28 ± 0.09 | <LOQ | <LOQ | <LOQ |
| Vuse Alto Mixed Berry 5% | 1086.67 ± 49.22 | 0.40 ± 0.08 | 0.325 ± 0.09 | <LOQ | <LOQ |
| Vuse Alto Rich Tobacco 5% | 878 ± 159.98 | 0.34 ± 0.065 | 0.26 ± 0.04 | 0.07 ± 0.01 | <LOQ |
| JUUL Mint 5% | 108.77 ± 20.39 | 0.5 ± 0.06 | <LOQ | <LOQ | <LOQ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keyser, B.M.; Leverette, R.; Wertman, J.; Shutsky, T.; McRae, R.; Szeliga, K.; Makena, P.; Jordan, K. Evaluation of Cytotoxicity and Oxidative Stress of Whole Aerosol from Vuse Alto ENDS Products. Toxics 2024, 12, 129. https://doi.org/10.3390/toxics12020129
Keyser BM, Leverette R, Wertman J, Shutsky T, McRae R, Szeliga K, Makena P, Jordan K. Evaluation of Cytotoxicity and Oxidative Stress of Whole Aerosol from Vuse Alto ENDS Products. Toxics. 2024; 12(2):129. https://doi.org/10.3390/toxics12020129
Chicago/Turabian StyleKeyser, Brian M., Robert Leverette, John Wertman, Tom Shutsky, Reagan McRae, Ken Szeliga, Patrudu Makena, and Kristen Jordan. 2024. "Evaluation of Cytotoxicity and Oxidative Stress of Whole Aerosol from Vuse Alto ENDS Products" Toxics 12, no. 2: 129. https://doi.org/10.3390/toxics12020129







