Boron Compounds Mitigate 2,3,7,8-Tetrachlorodibenzo-p-dioxin-Induced Toxicity in Human Peripheral Blood Mononuclear Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Blood Collection
2.3. PBMC Isolation
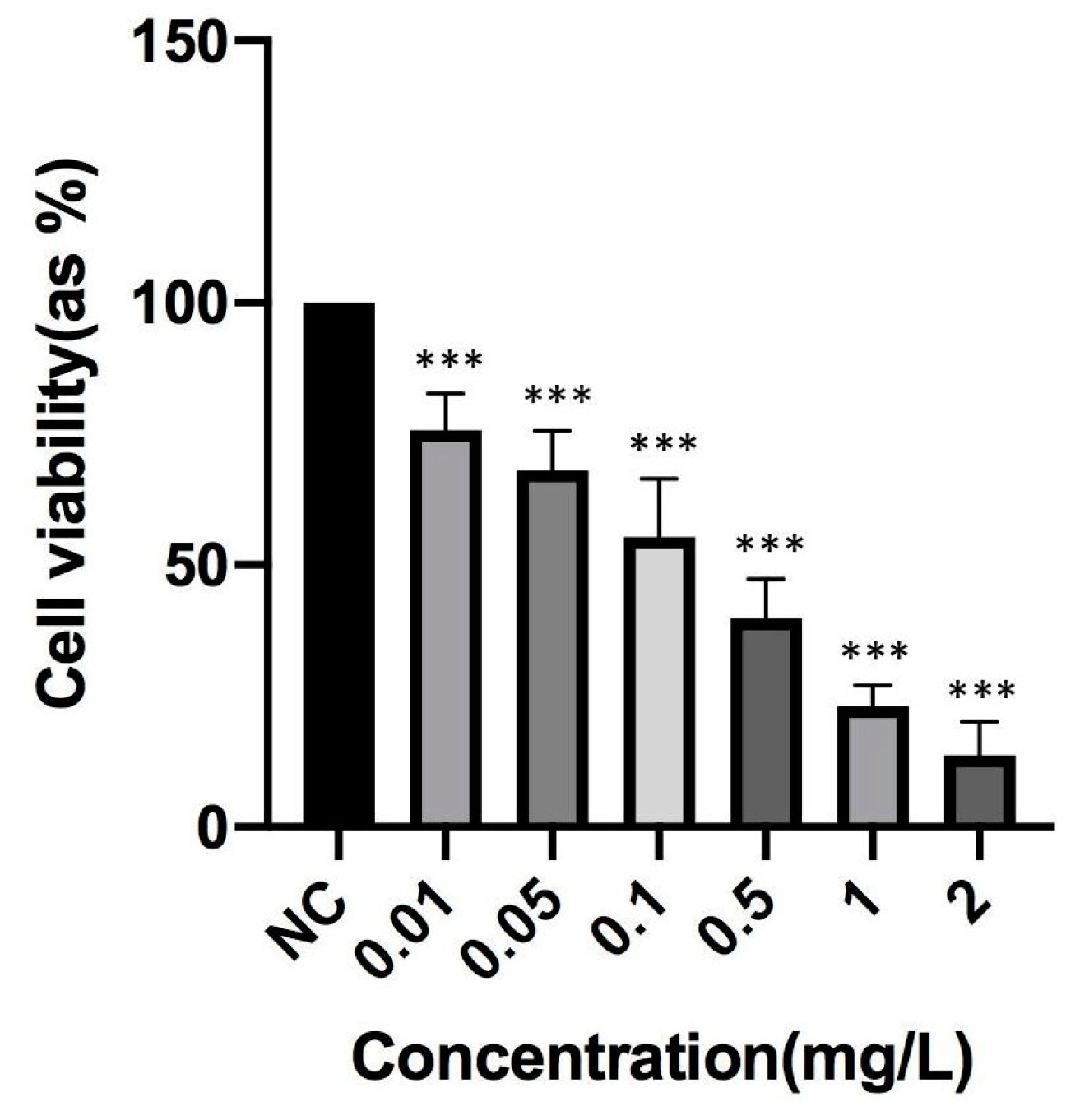
2.4. Determination of TCDD Toxicity
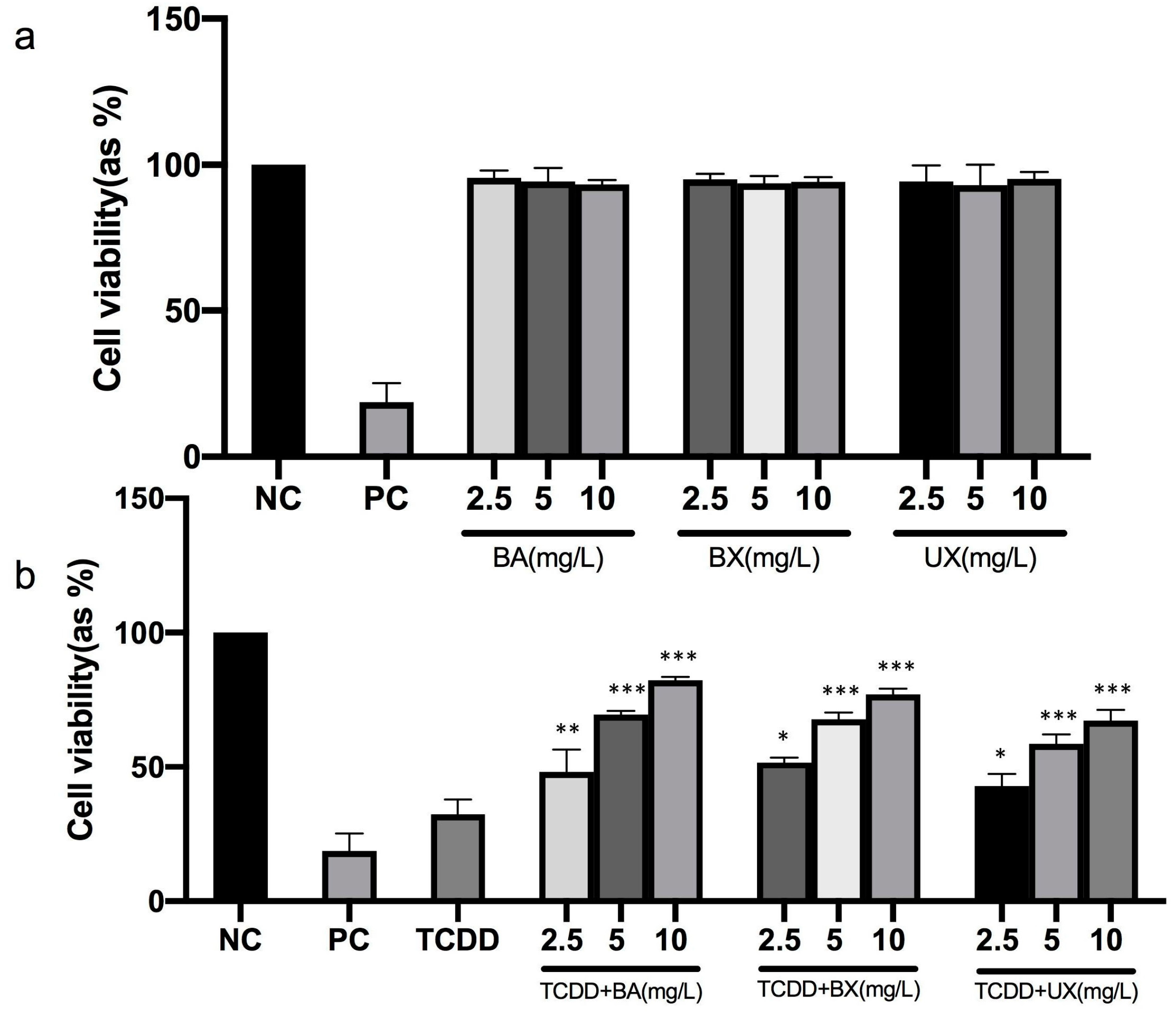
2.5. Cell Viability Assay
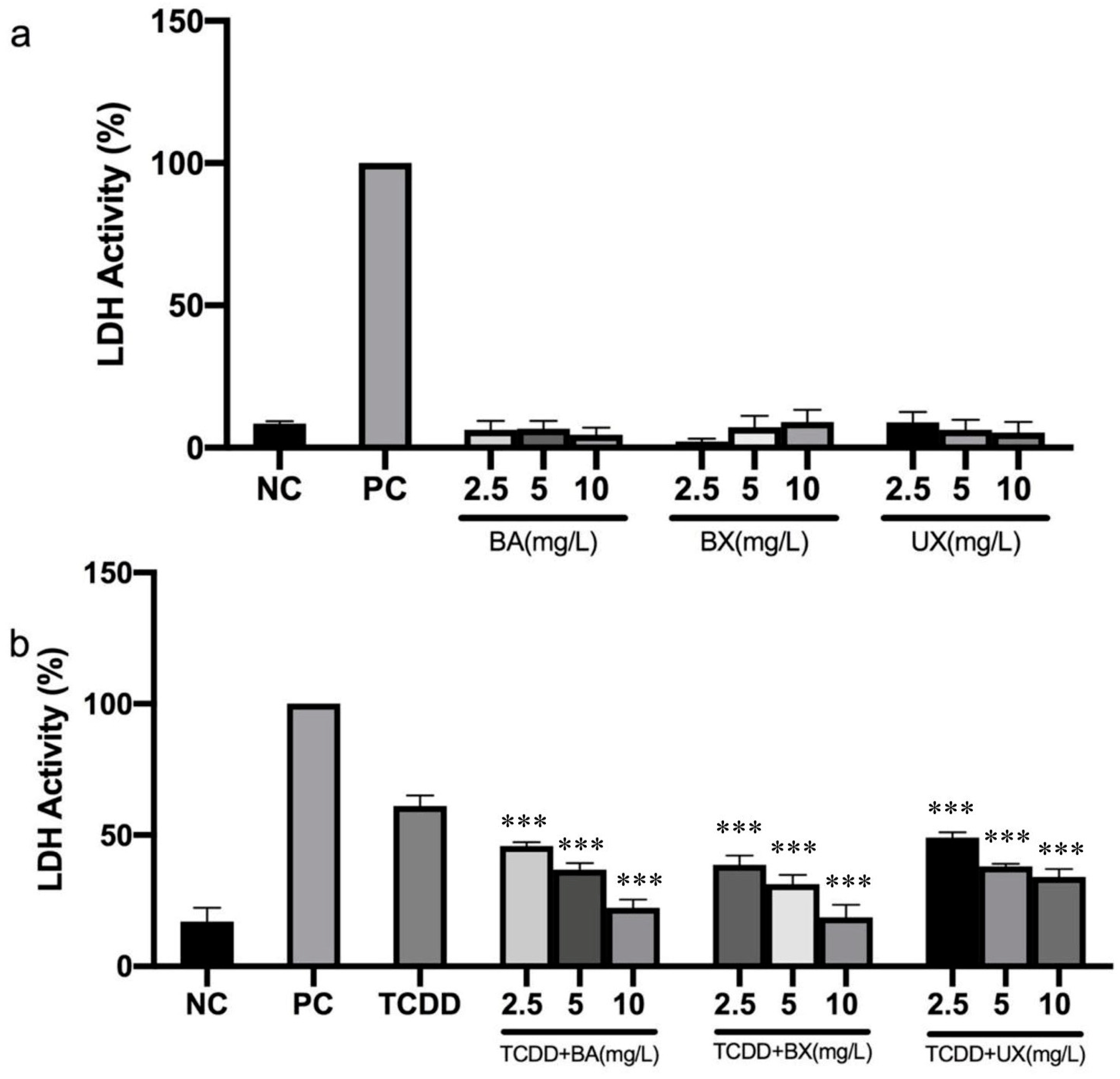
2.6. Membrane Integrity Assay
2.7. Total Antioxidant Capacity Assay
2.8. Membrane Lipid Peroxidation Assay
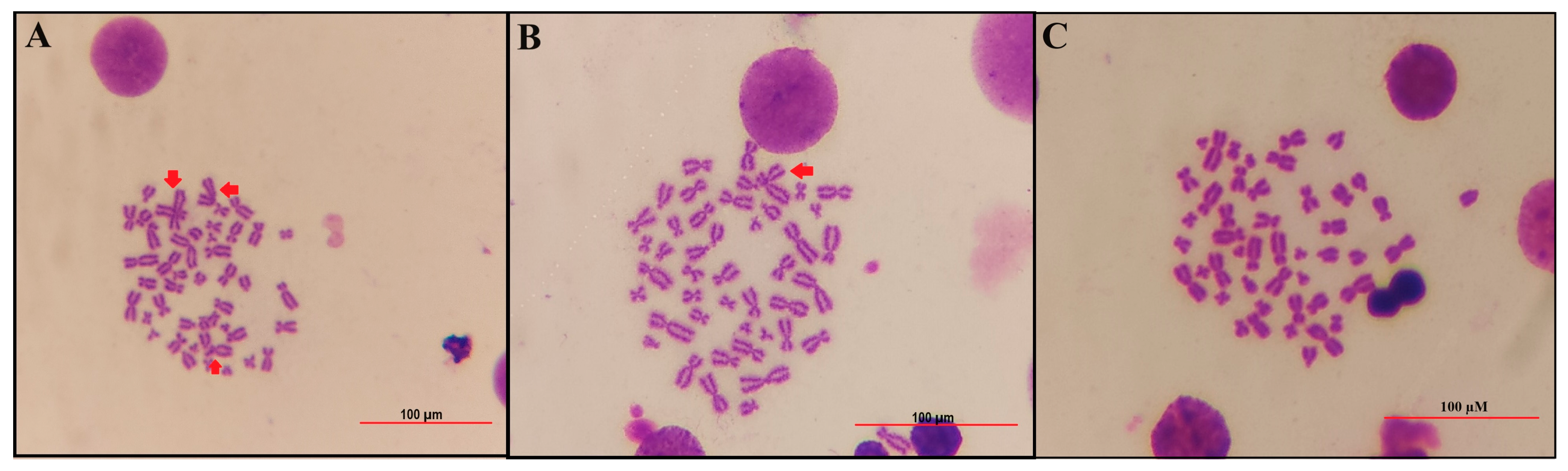
2.9. Chromosomal Aberration (CA) Assay
2.10. 8-Hydroxy-2′-deoxyguanosine Assay
2.11. Biochemical Analyses
2.12. Statistical Analysis
3. Results
3.1. Effects of B Compounds on TCDD-Mediated Cytotoxicity
3.2. Effects of B Compounds on TCDD-Mediated Oxidative Stress
3.3. Effects of B Compounds on TCDD-Mediated Genotoxicity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sha, R.; Chen, Y.; Wang, Y.; Luo, Y.; Liu, Y.; Ma, Y.; Li, Y.; Xu, L.; Xie, H.Q.; Zhao, B. Gestational and lactational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin in mice: Neurobehavioral effects on female offspring. Sci. Total Environ. 2021, 752, 141784. [Google Scholar] [CrossRef] [PubMed]
- Lang, D.S.; Becker, S.; Clark, G.C.; Devlin, R.B.; Koren, H.S. Lack of direct immunosuppressive effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) on human peripheral blood lymphocyte subsets in vitro. Arch. Toxicol. 1994, 68, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Van Gerwen, M.; Vasan, V.; Genden, E.; Saul, S.R. Human 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure and thyroid cancer risk. Toxicology 2023, 488, 153474. [Google Scholar] [CrossRef] [PubMed]
- Mohsenzadeh, M.S.; Zanjani, B.R.; Karimi, G. Mechanisms of 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced cardiovascular toxicity: An overview. Chem. Biol. Interact. 2018, 282, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Faiad, W.; Soukkarieh, C.; Hanano, A. 2,3,7,8-tetrachlorodibenzo-p-dioxin induces multigenerational testicular toxicity and biosynthetic disorder of testosterone in BALB/C mice: Transcriptional, histopathological and hormonal determinants. Ecotoxicol. Environ. Saf. 2023, 263, 115233. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.-K.; Park, J.-G.; Iwata, H.; Kim, E.-Y. 2,3,7,8-Tetrachlorodibenzo-p-dioxin prompted differentiation to CD4+CD8−CD25+ and CD4+CD8+CD25+ Tregs and altered expression of immune-related genes in the thymus of chicken embryos. Ecotoxicol. Environ. Saf. 2021, 211, 111947. [Google Scholar] [CrossRef] [PubMed]
- Novelli, M.; Beffy, P.; Masini, M.; Vantaggiato, C.; Martino, L.; Marselli, L.; Marchetti, P.; De Tata, V. Selective beta-cell toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin on isolated pancreatic islets. Chemosphere 2021, 265, 129103. [Google Scholar] [CrossRef] [PubMed]
- Pohjanvirta, R.; Tuomisto, J. Short-term toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin in laboratory animals: Effects, mechanisms, and animal models. Pharmacol. Rev. 1994, 46, 483–549. [Google Scholar]
- Lin, P.-H.; Lin, C.-H.; Huang, C.-C.; Chuang, M.-C.; Lin, P. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) induces oxidative stress, DNA strand breaks, and poly(ADP-ribose) polymerase-1 activation in human breast carcinoma cell lines. Toxicol. Lett. 2007, 172, 146–158. [Google Scholar] [CrossRef]
- Wan, C.; Liu, J.; Nie, X.; Zhao, J.; Zhou, S.; Duan, Z.; Tang, C.; Liang, L.; Xu, G. 2,3,7,8-Tetrachlorodibenzo-P-dioxin (TCDD) induces premature senescence in human and rodent neuronal cells via ROS-dependent mechanisms. PLoS ONE 2014, 9, e89811. [Google Scholar] [CrossRef]
- Aly, H.A.A.; Domènech, Ò. Cytotoxicity and mitochondrial dysfunction of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in isolated rat hepatocytes. Toxicol. Lett. 2009, 191, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Hankinson, O. 2,3,7,8-tetrachlorodibenzo-p-dioxin suppresses the growth of human colorectal cancer cells in vitro: Implication of the aryl hydrocarbon receptor signaling. Int. J. Oncol. 2019, 54, 1422–1432. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Chun, Y.-S.; Shin, Y.J.; Ye, S.K.; Kim, M.-S.; Park, J.-W. Curcumin attenuates cytochrome P450 induction in response to 2,3,7,8-tetrachlorodibenzo-p-dioxin by ROS-dependently degrading AhR and ARNT. Cancer Sci. 2008, 99, 2518–2524. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Snyder, M.; Kenison, J.E.; Yang, K.; Lara, B.; Lydell, E.; Bennani, K.; Novikov, O.; Federico, A.; Monti, S.; et al. How the AHR Became Important in Cancer: The Role of Chronically Active AHR in Cancer Aggression. Int. J. Mol. Sci. 2020, 22, 387. [Google Scholar] [CrossRef]
- Kopf, P.G.; Walker, M.K. 2,3,7,8-tetrachlorodibenzo-p-dioxin increases reactive oxygen species production in human endothelial cells via induction of cytochrome P4501A1. Toxicol. Appl. Pharmacol. 2010, 245, 91–99. [Google Scholar] [CrossRef]
- Scorei, R. Is Boron a Prebiotic Element? A Mini-review of the Essentiality of Boron for the Appearance of Life on Earth. Orig. Life Evol. Biosph. 2012, 42, 3–17. [Google Scholar] [CrossRef]
- Murray, F.J. A comparative review of the pharmacokinetics of boric acid in rodents and humans. Biol. Trace Elem. Res. 1998, 66, 331–341. [Google Scholar] [CrossRef]
- Nielsen, F.H. Is boron nutritionally relevant? Nutr. Rev. 2008, 66, 183–191. [Google Scholar] [CrossRef]
- Helvaci, C.; Alonso, R.R. Borate deposits of Turkey and Argentina; a summary and geological comparison. Turk. J. Earth Sci. 2000, 9, 1. [Google Scholar]
- Güngören, Ş. Mikrodalga Enerjisinin Kolemanit ve Üleksitin Sudaki Çözünürlüğüne Etkisinin Araştırılması. İstanbul Yerbilim. Derg. 2012, 22, 85–93. [Google Scholar]
- Alak, G.; Ucar, A.; Parlak, V.; Yeltekin, A.Ç.; Özgeriş, F.B.; Atamanalp, M.; Türkez, H. Antioxidant Potential of Ulexite in Zebrafish Brain: Assessment of Oxidative DNA Damage, Apoptosis, and Response of Antioxidant Defense System. Biol. Trace Elem. Res. 2021, 199, 1092–1099. [Google Scholar] [CrossRef] [PubMed]
- Córdova-Chávez, R.I.; Carrasco-Ruiz, M.F.; Rodríguez-Vera, D.; Pérez-Capistran, T.; Tamay-Cach, F.; Scorei, I.R.; Abad-García, A.; Soriano-Ursúa, M.A. Boron-Containing Compounds for Prevention, Diagnosis, and Treatment of Human Metabolic Disorders. Biol. Trace Elem. Res. 2023, 201, 2222–2239. [Google Scholar]
- Nielsen, F.H.; Eckhert, C.D. Boron. Adv. Nutr. 2020, 11, 461–462. [Google Scholar] [CrossRef] [PubMed]
- Biţă, A.; Scorei, I.R.; Bălşeanu, T.A.; Ciocîlteu, M.V.; Bejenaru, C.; Radu, A.; Bejenaru, L.E.; Rău, G.; Mogoşanu, G.D.; Neamţu, J.; et al. New Insights into Boron Essentiality in Humans and Animals. Int. J. Mol. Sci. 2022, 23, 9147. [Google Scholar] [CrossRef] [PubMed]
- Nandwana, V.; Nandwana, N.K.; Das, Y.; Saito, M.; Panda, T.; Das, S.; Almaguel, F.; Hosmane, N.S.; Das, B.C. The Role of Microbiome in Brain Development and Neurodegenerative Diseases. Molecules 2022, 27, 3402. [Google Scholar] [CrossRef] [PubMed]
- Hunt, C.D.; Idso, J.P. Dietary boron as a physiological regulator of the normal inflammatory response: A review and current research progress. J. Trace Elem. Exp. Med. 1999, 12, 221–233. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, M.; Mateo, P.; Bonilla, I. Boron requirement for envelope structure and function in Anabaena PCC 7119 heterocysts. J. Exp. Bot. 1991, 42, 925–929. [Google Scholar] [CrossRef]
- Türkez, H.; Geyikoğlu, F.; Tatar, A.; Keleş, S.; Ozkan, A. Effects of some boron compounds on peripheral human blood. Z. Naturforsch. C 2007, 62, 889–896. [Google Scholar] [CrossRef]
- Dai, M.; Wang, P.; Boyd, A.D.; Kostov, G.; Athey, B.; Jones, E.G.; Bunney, W.E.; Myers, R.M.; Speed, T.P.; Akil, H.; et al. Evolving gene/transcript definitions significantly alter the interpretation of GeneChip data. Nucleic Acids Res. 2005, 33, e175. [Google Scholar] [CrossRef]
- Eijssen, L.M.T.; Jaillard, M.; Adriaens, M.E.; Gaj, S.; de Groot, P.J.; Müller, M.; Evelo, C.T. User-friendly solutions for microarray quality control and pre-processing on ArrayAnalysis.org. Nucleic Acids Res. 2013, 41, W71–W76. [Google Scholar] [CrossRef]
- Singh, N.P.; Danner, D.B.; Tice, R.R.; Brant, L.; Schneider, E.L. DNA damage and repair with age in individual human lymphocytes. Mutat. Res. 1990, 237, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Aydin, H.E.; Gunduz, M.K.; Kizmazoglu, C.; Kandemir, T.; Arslantas, A. Cytotoxic Effect of Boron Application on Glioblastoma Cells. Turk. Neurosurg. 2021, 31, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Hawthorne, M.F. The Role of Chemistry in the Development of Boron Neutron Capture Therapy of Cancer. Angew. Chem. Int. Ed. Engl. 1993, 32, 950–984. [Google Scholar] [CrossRef]
- Qian, E.A.; Wixtrom, A.I.; Axtell, J.C.; Saebi, A.; Jung, D.; Rehak, P.; Han, Y.; Moully, E.H.; Mosallaei, D.; Chow, S.; et al. Atomically precise organomimetic cluster nanomolecules assembled via perfluoroaryl-thiol SNAr chemistry. Nat. Chem. 2017, 9, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Hawthorne, M.F.; Maderna, A. Applications of Radiolabeled Boron Clusters to the Diagnosis and Treatment of Cancer. Chem. Rev. 1999, 99, 3421–3434. [Google Scholar] [CrossRef]
- Axtell, J.C.; Saleh, L.M.A.; Qian, E.A.; Wixtrom, A.I.; Spokoyny, A.M. Synthesis and Applications of Perfunctionalized Boron Clusters. Inorg. Chem. 2018, 57, 2333–2350. [Google Scholar] [CrossRef]
- Kopf, P.G.; Walker, M.K. Overview of Developmental Heart Defects by Dioxins, PCBs, and Pesticides. J. Environ. Sci. Health Part C 2009, 27, 276–285. [Google Scholar] [CrossRef]
- Latchoumycandane, C.; Chitra, K.C.; Mathur, P.P. The effect of 2,3,7,8-tetrachlorodibenzo-p-dioxin on the antioxidant system in mitochondrial and microsomal fractions of rat testis. Toxicology 2002, 171, 127–135. [Google Scholar] [CrossRef]
- Fader, K.A.; Nault, R.; Ammendolia, D.A.; Harkema, J.R.; Williams, K.J.; Crawford, R.B.; Kaminski, N.E.; Potter, D.; Sharratt, B.; Zacharewski, T.R. 2,3,7,8-Tetrachlorodibenzo- p -Dioxin Alters Lipid Metabolism and Depletes Immune Cell Populations in the Jejunum of C57BL/6 Mice. Toxicol. Sci. 2015, 148, 567–580. [Google Scholar] [CrossRef]
- Med Sci, T.J.; Çadirci, K.; Tozlu, Ö.Ö.; Türkez, H.; Mardinoğlu, A. Turkish Journal of Medical Sciences The in vitro cytotoxic, genotoxic, and oxidative damage potentials of the oral artificial sweetener aspartame on cultured human blood cells. Turk. J. Med. Sci. 2020, 50, 448–454. [Google Scholar] [CrossRef]
- Turkez, H.; Yıldırım, S.; Sahin, E.; Arslan, M.E.; Emsen, B.; Tozlu, O.O.; Alak, G.; Ucar, A.; Tatar, A.; Hacimuftuoglu, A.; et al. Boron Compounds Exhibit Protective Effects against Aluminum-Induced Neurotoxicity and Genotoxicity: In Vitro and In Vivo Study. Toxics 2022, 10, 428. [Google Scholar] [CrossRef] [PubMed]
- Turkez, H.; Arslan, M.E.; Tatar, A.; Mardinoglu, A. Promising potential of boron compounds against Glioblastoma: In Vitro antioxidant, anti-inflammatory and anticancer studies. Neurochem. Int. 2021, 149, 105137. [Google Scholar] [CrossRef] [PubMed]
- Çadırcı, K.; Özdemir Tozlu, Ö.; Türkez, H. The in vitro cytotoxicity, genotoxicity and oxidative damage potential of dapagliflozin, on cultured human blood cells. Turk. J. Biochem. 2019, 44, 692–698. [Google Scholar] [CrossRef]
- Schmedes, A.; Hølmer, G. A new thiobarbituric acid (TBA) method for determining free malondialdehyde (MDA) and hydroperoxides selectively as a measure of lipid peroxidation. J. Am. Oil Chem. Soc. 1989, 66, 813–817. [Google Scholar] [CrossRef]
- Sun, Y.; Oberley, L.W.; Li, Y. A simple method for clinical assay of superoxide dismutase. Clin. Chem. 1988, 34, 497–500. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Jacobson, G.A.; Ives, S.J.; Narkowicz, C.; Jones, G. Plasma glutathione peroxidase (GSH-Px) concentration is elevated in rheumatoid arthritis: A case–control study. Clin. Rheumatol. 2012, 31, 1543–1547. [Google Scholar] [CrossRef]
- Beutler, E.; Kelly, B.M. The effect of sodium nitrite on red cell GSH. Experientia 1963, 19, 96–97. [Google Scholar] [CrossRef]
- Atamanalp, M.; Türkez, H.; Yeltekin, A.Ç.; Özgeriş, F.B.; Ucar, A.; Çağlar, Ö.; Parlak, V.; Oner, S.; Alak, G. Borax relieved the acrylamide-induced hematotoxic, hepatotoxic, immunotoxic and genotoxic damages in rainbow trout by regulating apoptosis and Nrf2 signaling pathway. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2022, 259, 109396. [Google Scholar] [CrossRef]
- Alak, G.; Yeltekin, A.Ç.; Uçar, A.; Parlak, V.; Türkez, H.; Atamanalp, M. Borax Alleviates Copper-Induced Renal Injury via Inhibiting the DNA Damage and Apoptosis in Rainbow Trout. Biol. Trace Elem. Res. 2019, 191, 495–501. [Google Scholar] [CrossRef]
- Türkez, H.; Geyikoǧlu, F.; Çolak, S. The protective effect of boric acid on aluminum-induced hepatotoxicity and genotoxicity in rats. Turk. J. Biol. 2011, 35, 293–301. [Google Scholar] [CrossRef]
- Erdemli, M.E.; Yigitcan, B.; Erdemli, Z.; Gul, M.; Bag, H.G.; Gul, S. Thymoquinone protection against 2,3,7,8-tetrachlorodibenzo-p-dioxin induced nephrotoxicity in rats. Biotech. Histochem. 2020, 95, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Ciftci, O.; Duman, A.S.; Turkmen, N.B.; Taslıdere, A. Beta-glucan prevents toxic effects of 2,3,7,8-TCDD in terms of oxidative and histopathological damage in heart tissue of rats. Braz. J. Pharm. Sci. 2018, 54, e17674. [Google Scholar] [CrossRef]
- Hassoun, E.A.; Al-Ghafri, M.; Abushaban, A. The role of antioxidant enzymes in TCDD-induced oxidative stress in various brain regions of rats after subchronic exposure. Free Radic. Biol. Med. 2003, 35, 1028–1036. [Google Scholar] [CrossRef] [PubMed]
- Im, S.; Kang, S.; Kim, J.H.; Oh, S.J.; Pak, Y.K. Low-Dose Dioxin Reduced Glucose Uptake in C2C12 Myocytes: The Role of Mitochondrial Oxidative Stress and Insulin-Dependent Calcium Mobilization. Antioxidants 2022, 11, 2109. [Google Scholar] [CrossRef]
- Stading, R.; Chu, C.; Couroucli, X.; Lingappan, K.; Moorthy, B. Molecular role of cytochrome P4501A enzymes in oxidative stress. Curr. Opin. Toxicol. 2020, 20–21, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Xu, Z.; Gao, Q.; Xu, Y.; Wang, B.; Dai, Y. Protective role of wogonin against cadmium induced testicular toxicity: Involvement of antioxidant, anti-inflammatory and anti-apoptotic pathways. Life Sci. 2020, 258, 118192. [Google Scholar] [CrossRef]
- Cakir, S.; Eren, M.; Senturk, M.; Sarica, Z.S. The Effect of Boron on Some Biochemical Parameters in Experimental Diabetic Rats. Biol. Trace Elem. Res. 2018, 184, 165–172. [Google Scholar] [CrossRef]
- Coban, F.K.; Liman, R.; Cigerci, I.H.; Ince, S.; Hazman, O.; Bozkurt, M.F. The antioxidant effect of boron on oxidative stress and DNA damage in diabetic rats. Fresenius Environ. Bull. 2015, 24, 4059–4066. [Google Scholar]
- Khaliq, H.; Juming, Z.; Ke-Mei, P. The Physiological Role of Boron on Health. Biol. Trace Elem. Res. 2018, 186, 31–51. [Google Scholar] [CrossRef]
- Goldbach, H.E.; Wimmer, M.A. Boron in plants and animals: Is there a role beyond cell-wall structure? J. Plant Nutr. Soil Sci. 2007, 170, 39–48. [Google Scholar] [CrossRef]
- Knerr, S.; Schrenk, D. Carcinogenicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin in experimental models. Mol. Nutr. Food Res. 2006, 50, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, Y.; Nakajima, M.; Yokoi, T. Cytochrome P450-mediated metabolism of estrogens and its regulation in human. Cancer Lett. 2005, 227, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Ilavarasi, K.; Kiruthiga, P.V.; Pandian, S.K.; Devi, K.P. Hydroxytyrosol, the phenolic compound of olive oil protects human PBMC against oxidative stress and DNA damage mediated by 2,3,7,8-TCDD. Chemosphere 2011, 84, 888–893. [Google Scholar] [CrossRef] [PubMed]
- Rodu, B.; Cole, P.; Mandel, J.S. Evaluation of the National Toxicology Program Report on Carcinogens. Regul. Toxicol. Pharmacol. 2012, 64, 186–188. [Google Scholar] [CrossRef] [PubMed]
- Turkez, H.; Tatar, A.; Hacimuftuoglu, A.; Ozdemir, E. Boric acid as a protector against paclitaxel genotoxicity. Acta Biochim. Pol. 2010, 57, 95–97. [Google Scholar] [CrossRef] [PubMed]
- Turkez, H.; Geyikoglu, F.; Tatar, A.; Keles, M.S.; Kaplan, I. The effects of some boron compounds against heavy metal toxicity in human blood. Exp. Toxicol. Pathol. 2012, 64, 93–101. [Google Scholar] [CrossRef]
- Üstündaǧ, A.; Behm, C.; Föllmann, W.; Duydu, Y.; Degen, G.H. Protective effect of boric acid on lead- and cadmium-induced genotoxicity in V79 cells. Arch. Toxicol. 2014, 88, 1281–1289. [Google Scholar] [CrossRef]
- Liu, M.; Sun, X.; Chen, B.; Dai, R.; Xi, Z.; Xu, H. Insights into Manganese Superoxide Dismutase and Human Diseases. Int. J. Mol. Sci. 2022, 23, 15893. [Google Scholar] [CrossRef]
- Basak, T.N.; Askin, O.D.; Taslidere, A.; Dogan, F.; Ciftci, O. Beta-glucan effects on 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) toxicity in liver and brain. Biotech. Histochem. 2022, 97, 441–448. [Google Scholar] [CrossRef]
- Smirnova, E.; Moniruzzaman, M.; Chin, S.; Sureshbabu, A.; Karthikeyan, A.; Do, K.; Min, T. A Review of the Role of Curcumin in Metal Induced Toxicity. Antioxidants 2023, 12, 243. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Chai, Y.-C.; Mieyal, J.J. Glutathione and Glutaredoxin—Key Players in Cellular Redox Homeostasis and Signaling. Antioxidants 2023, 12, 1553. [Google Scholar] [CrossRef]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell. Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef]
- Beischlag, T.V.; Morales, J.L.; Hollingshead, B.D.; Perdew, G.H. The Aryl Hydrocarbon Receptor Complex and the Control of Gene Expression. Crit. Rev. Eukaryot. Gene Expr. 2008, 18, 207–250. [Google Scholar] [CrossRef]
- Shankar, P.; Villeneuve, D.L. AOP Report: Aryl Hydrocarbon Receptor Activation Leads to Early–Life Stage Mortality via Sox9 Repression-Induced Craniofacial and Cardiac Malformations. Environ. Toxicol. Chem. 2023, 42, 2063–2077. [Google Scholar] [CrossRef]
- Winans, B.; Humble, M.C.; Lawrence, B.P. Environmental toxicants and the developing immune system: A missing link in the global battle against infectious disease? Reprod. Toxicol. 2011, 31, 327–336. [Google Scholar] [CrossRef]
- Kerkvliet, N.I. AHR-mediated immunomodulation: The role of altered gene transcription. Biochem. Pharmacol. 2009, 77, 746–760. [Google Scholar] [CrossRef]



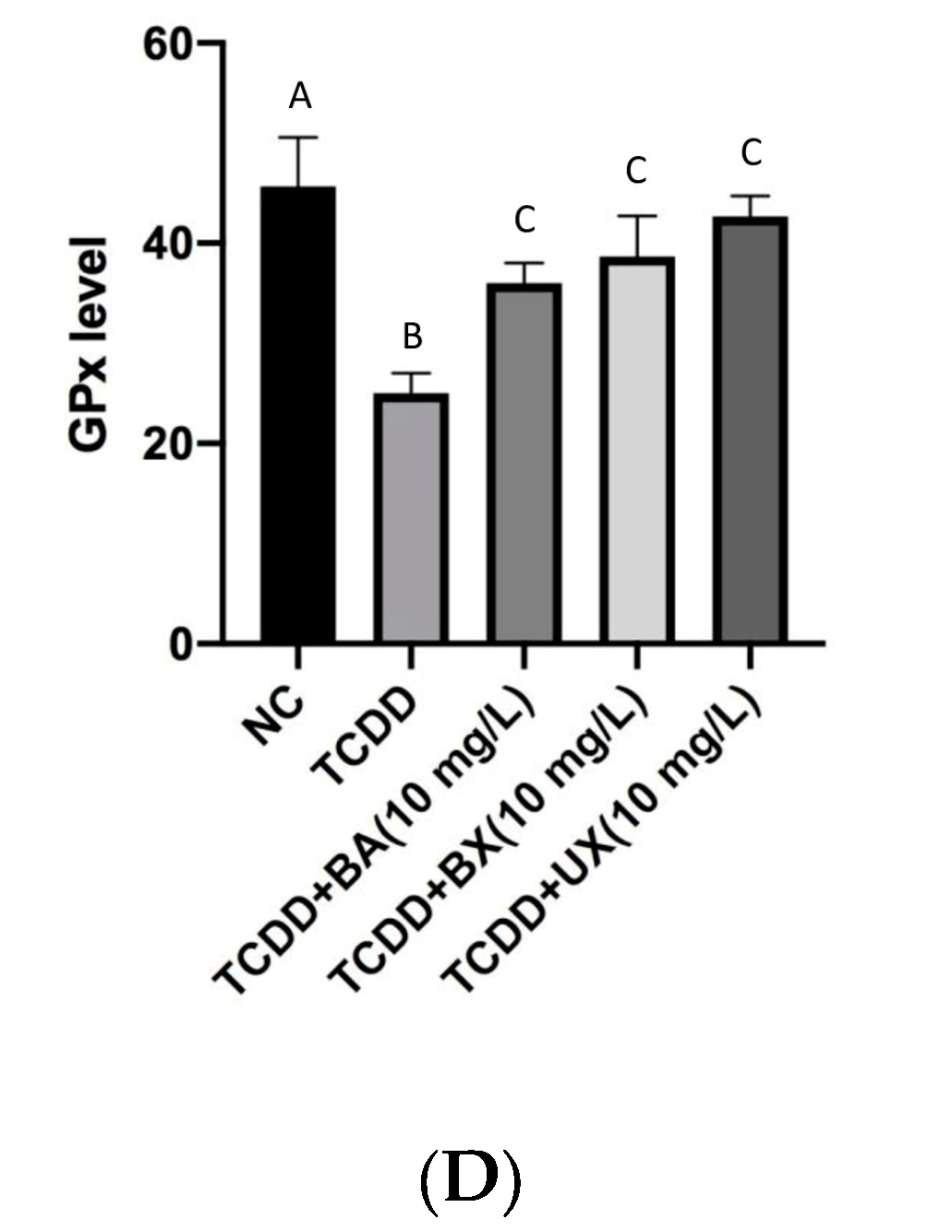
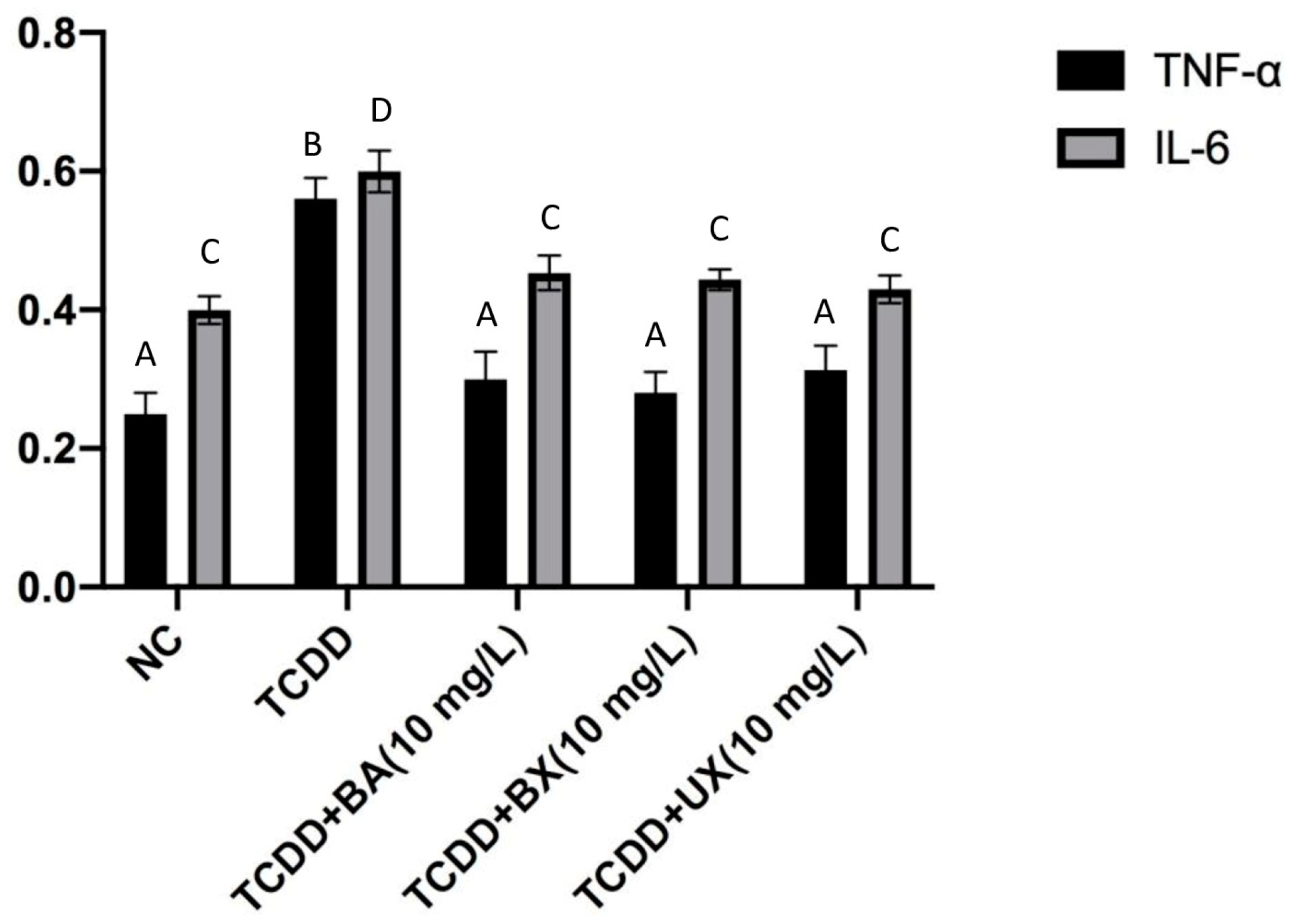
| Groups | TAC Level (mmol/L) | MDA Level (Micromol/L) |
|---|---|---|
| Negative Control | 5.1 ± 0.91 | 342.5 ± 5.32 |
| Positive Control | 18.8 ± 1.22 | 1075.8 ± 11.23 |
| TCDD | 1.7 ± 0.08 | 892.1 ± 7.86 |
| BA 2.5 | 5.5 ± 0.54 | 325 ± 2.45 |
| BA 5 | 6.2 ± 0.45 | 329 ± 5.21 |
| BA 10 | 6.4 ± 0.68 | 290 ± 4.36 |
| BX 2.5 | 5.8 ± 0.98 | 335 ± 4.78 |
| BX 5 | 6.3 ± 0.39 | 298 ± 3.26 |
| BX 10 | 6.7 ± 1.01 | 303 ± 6.32 |
| UX 2.5 | 5.2 ± 0.43 | 331 ± 3.89 |
| UX 5 | 5.5 ± 0.56 | 325 ± 5.01 |
| UX 10 | 5.8 ± 0.32 | 319 ± 3.21 |
| TCDD + BA 2.5 | 2.4 ± 0.03 * | 741 ± 2.98 |
| TCDD + BA 5 | 2.9 ± 0.08 * | 697 ± 3.21 * |
| TCDD + BA 10 | 3.4 ± 0.27 * | 592 ± 4.69 * |
| TCDD + BX 2.5 | 2.7 ± 0.02 * | 782 ± 5.21 |
| TCDD + BX 5 | 3 ± 0.09 * | 575 ± 2.14 * |
| TCDD + BX 10 | 3. 2 ± 0.24 * | 521 ± 2.67 * |
| TCDD + UX 2.5 | 2.3 ± 0.13 * | 765 ± 4.21 |
| TCDD + UX 5 | 2.7 ± 0.19 * | 672 ± 1.98 * |
| TCDD + UX 10 | 2.9 ± 0.36 * | 603 ± 3.17 * |
| Groups | CA/Cells | 8-OH-dG Level Pmol/gDNA |
|---|---|---|
| Negative Control | 2.40 ± 0.12 | 0.87 ± 0.02 |
| Positive Controls | 8.72 ± 0.45 | 4.35 ± 0.15 |
| TCDD | 7.15 ± 0.98 | 3.92 ± 0.09 |
| BA 2.5 | 2.2 ± 0.04 | 0.78 ± 0.02 |
| BA 5 | 2.1 ± 0.08 | 0.70 ± 0.04 |
| BA 10 | 2.2 ± 0.23 | 0.83 ± 0.05 |
| BX 2.5 | 1.9 ± 0.04 | 0.80 ± 0.05 |
| BX 5 | 2.2 ± 0.67 | 0.68 ± 0.03 |
| BX 10 | 1.8 ± 0.04 | 0.74 ± 0.09 |
| UX 2.5 | 1.9 ± 0.07 | 0.72 ± 0.06 |
| UX 5 | 2 ± 0.21 | 0.77 ± 0.02 |
| UX 10 | 2.3 ± 0.34 | 0.71 ± 0.06 |
| TCDD + BA 2.5 | 6.84 ± 0.78 | 3.11 ± 0.12 * |
| TCDD + BA 5 | 5.16 ± 0.67 * | 2.62 ± 0.09 * |
| TCDD + BA 10 | 4.47 ± 0.89 * | 2.28 ± 0.45 * |
| TCDD + BX 2.5 | 6.81 ± 0.27 | 3.40 ± 0.42 * |
| TCDD + BX 5 | 6.04 ± 0.34 * | 2.52 ± 0.08 * |
| TCDD + BX 10 | 5.11 ± 0.09 * | 2.09 ± 0.54 * |
| TCDD + UX 2.5 | 6.68 ± 0.17 | 3.44 ± 0.55 * |
| TCDD + UX 5 | 6.21 ± 0.48 * | 3.08 ± 0.23 * |
| TCDD + UX 10 | 5.55 ± 0.42 * | 2.77 ± 0.22 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arslan, M.E.; Baba, C.; Tozlu, O.O. Boron Compounds Mitigate 2,3,7,8-Tetrachlorodibenzo-p-dioxin-Induced Toxicity in Human Peripheral Blood Mononuclear Cells. Toxics 2024, 12, 98. https://doi.org/10.3390/toxics12020098
Arslan ME, Baba C, Tozlu OO. Boron Compounds Mitigate 2,3,7,8-Tetrachlorodibenzo-p-dioxin-Induced Toxicity in Human Peripheral Blood Mononuclear Cells. Toxics. 2024; 12(2):98. https://doi.org/10.3390/toxics12020098
Chicago/Turabian StyleArslan, Mehmet Enes, Cem Baba, and Ozlem Ozdemir Tozlu. 2024. "Boron Compounds Mitigate 2,3,7,8-Tetrachlorodibenzo-p-dioxin-Induced Toxicity in Human Peripheral Blood Mononuclear Cells" Toxics 12, no. 2: 98. https://doi.org/10.3390/toxics12020098





