SiO2–TiO2 Nanoparticle Aqueous Foam for Volatile Organic Compounds’ Suppression
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples’ Preparation
2.1.1. Preparation of Bulk Foaming Solution
2.1.2. Preparation of Trimethyl Silane (TMCS)-Modified Nanosilica Particles [36]
2.1.3. Sodium Stearate (SS)-Modified Nanosilica Particle Preparation
2.1.4. Preparation of γ–Methacryloyl Oxy Propyl Trimethoxysilane (KH–570)-Modified Nanosilica Particles
2.1.5. Preparation of SiO2–TiO2 Nanoparticles [34]
2.1.6. Preparation of SiO2–TiO2 Nanoparticle Foam
2.2. Characteristics
2.2.1. Hydrophobic Performance
2.2.2. Dispersion Performance
2.2.3. Stability Performance
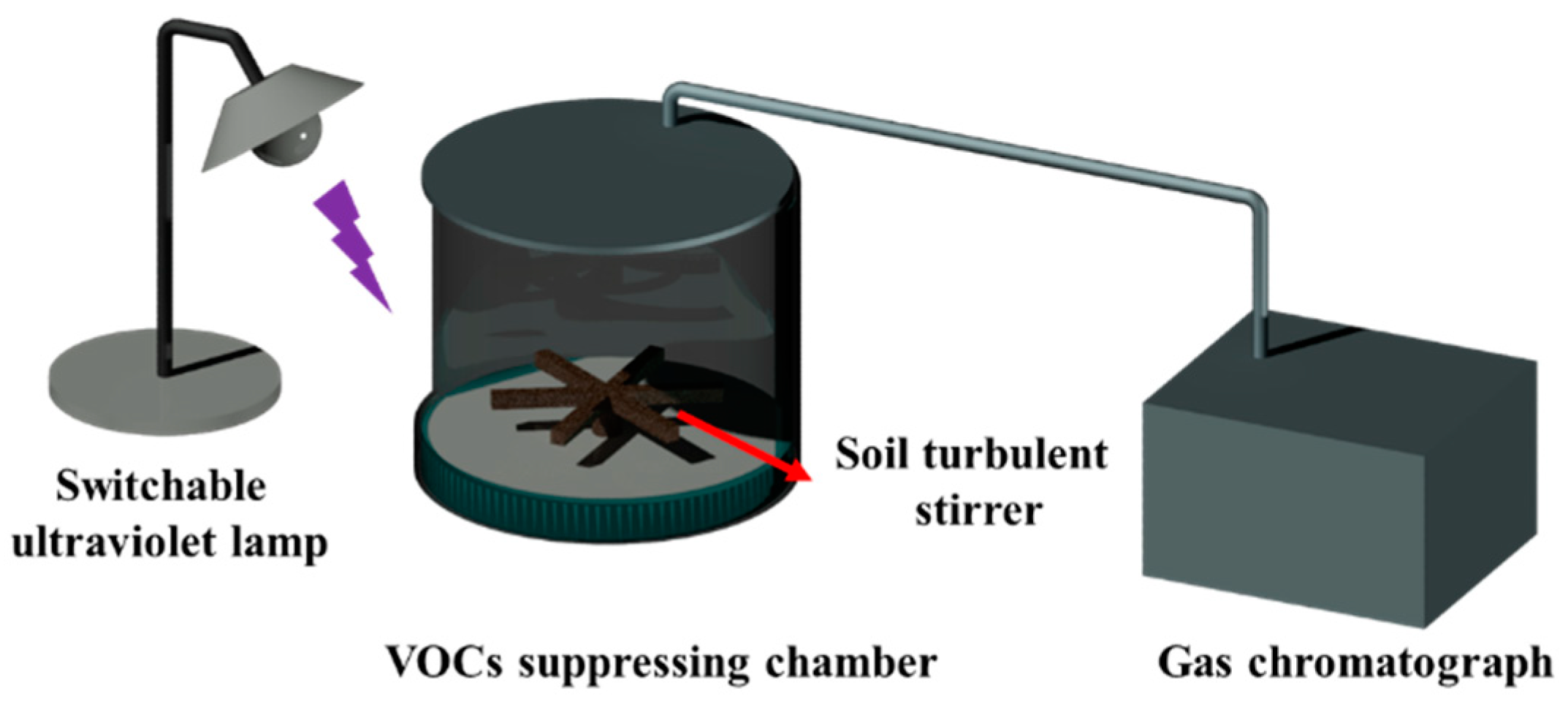
2.2.4. VOC-Suppressing Performance
2.2.5. VOCs Degradation Performance
2.2.6. Oil Resistivity of Foam
3. Results and Discussion
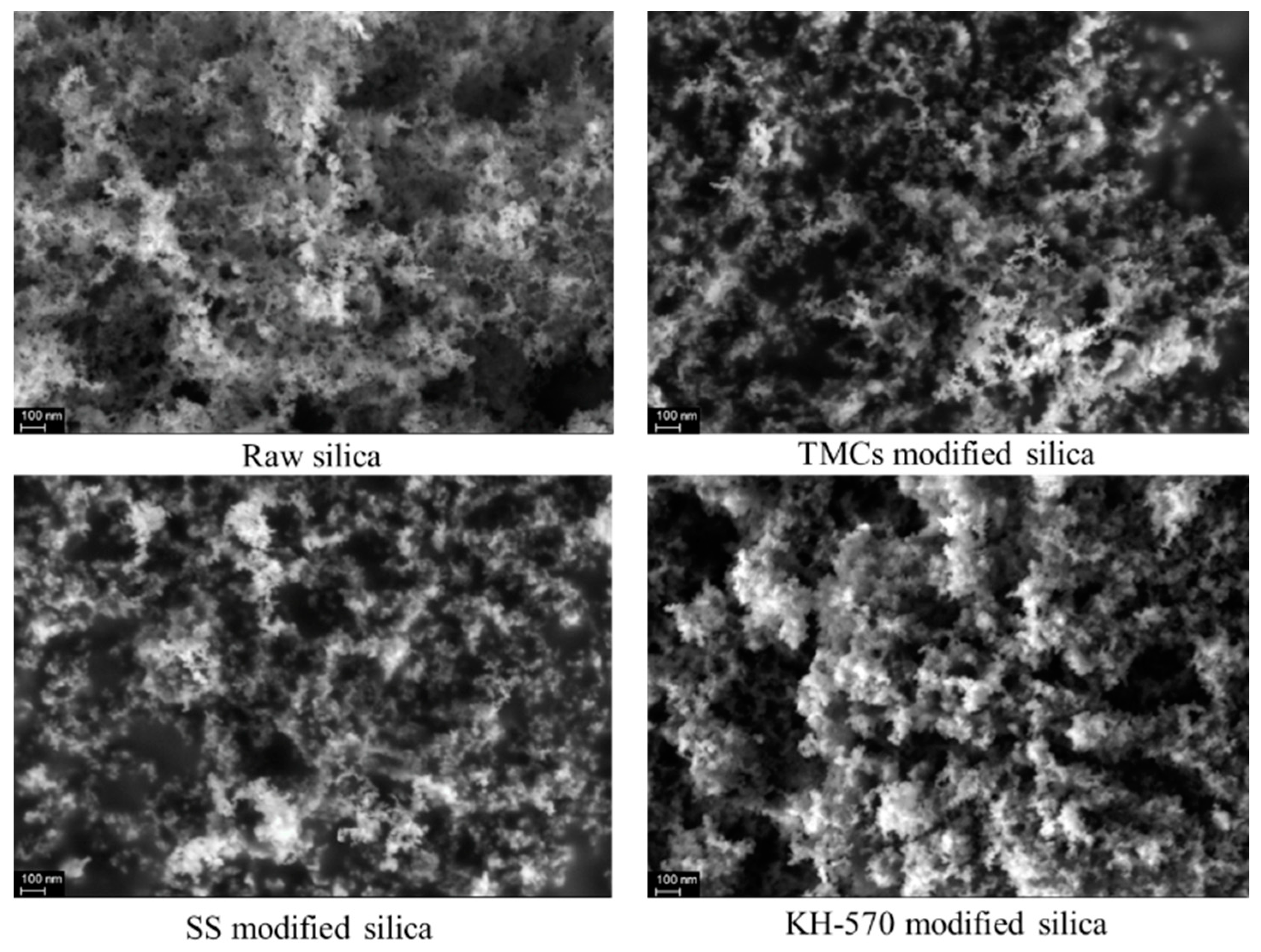
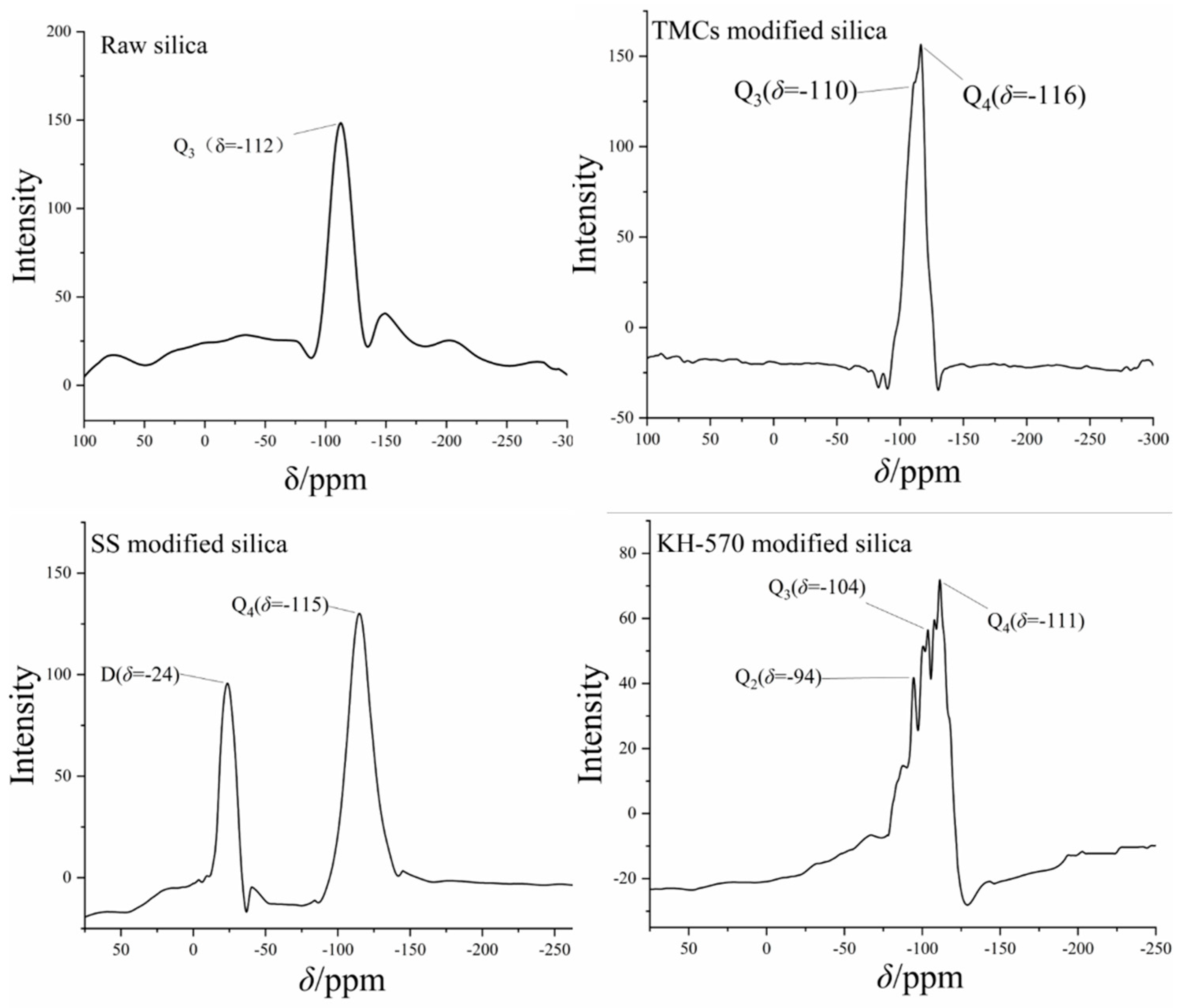
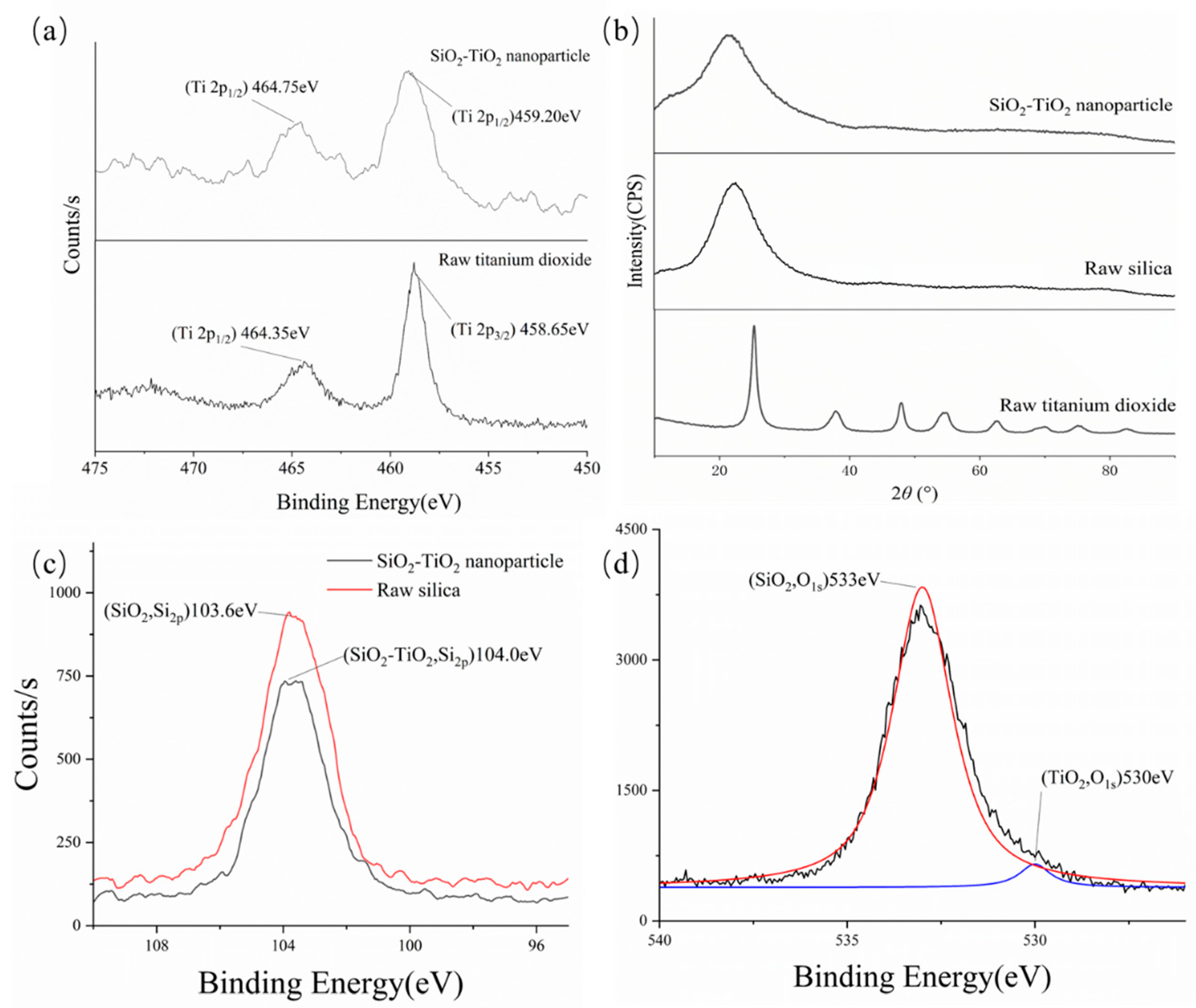
3.1. Modification of Nanosilica
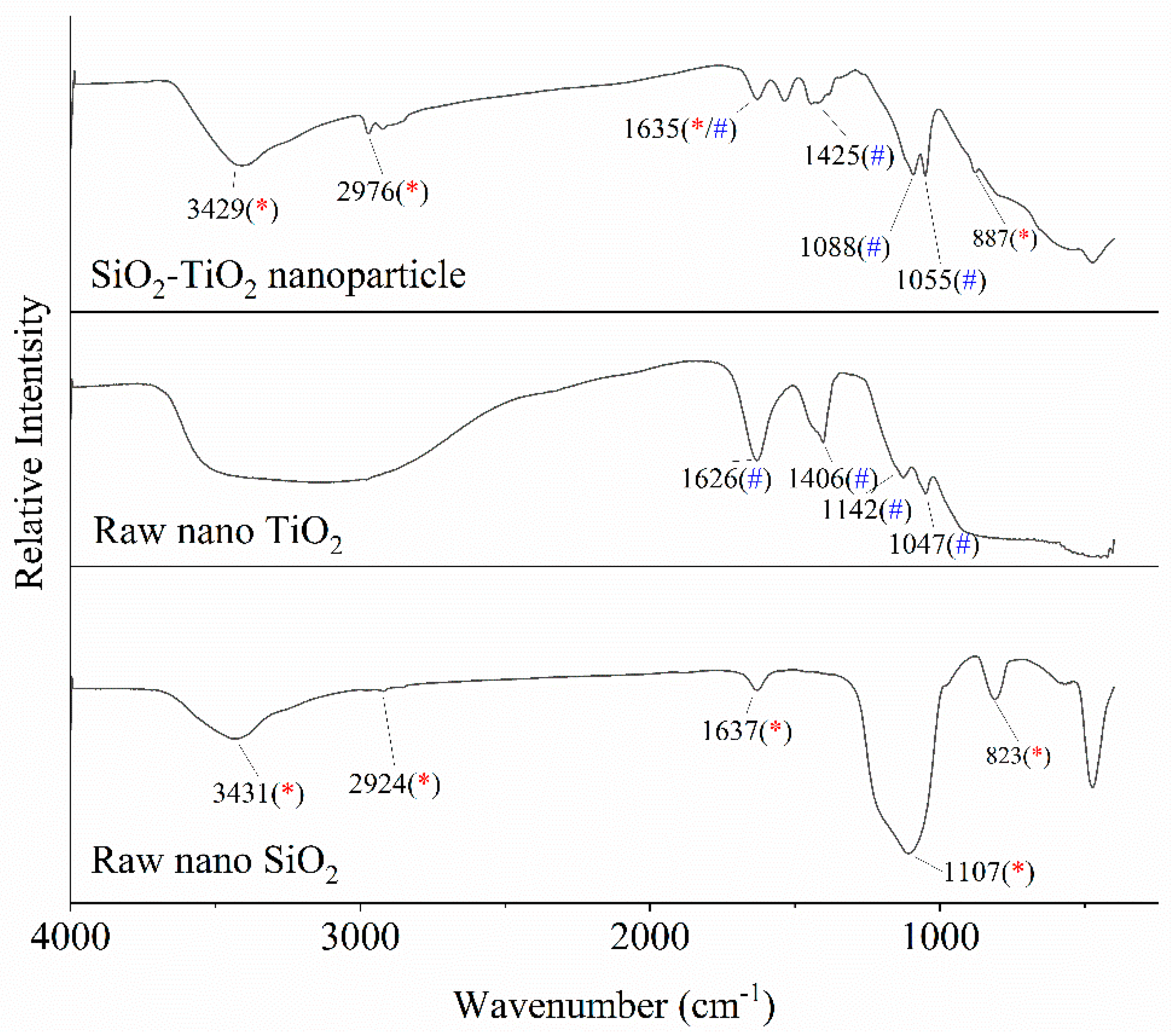
3.2. SiO2–TiO2 Nanoparticle Characterization
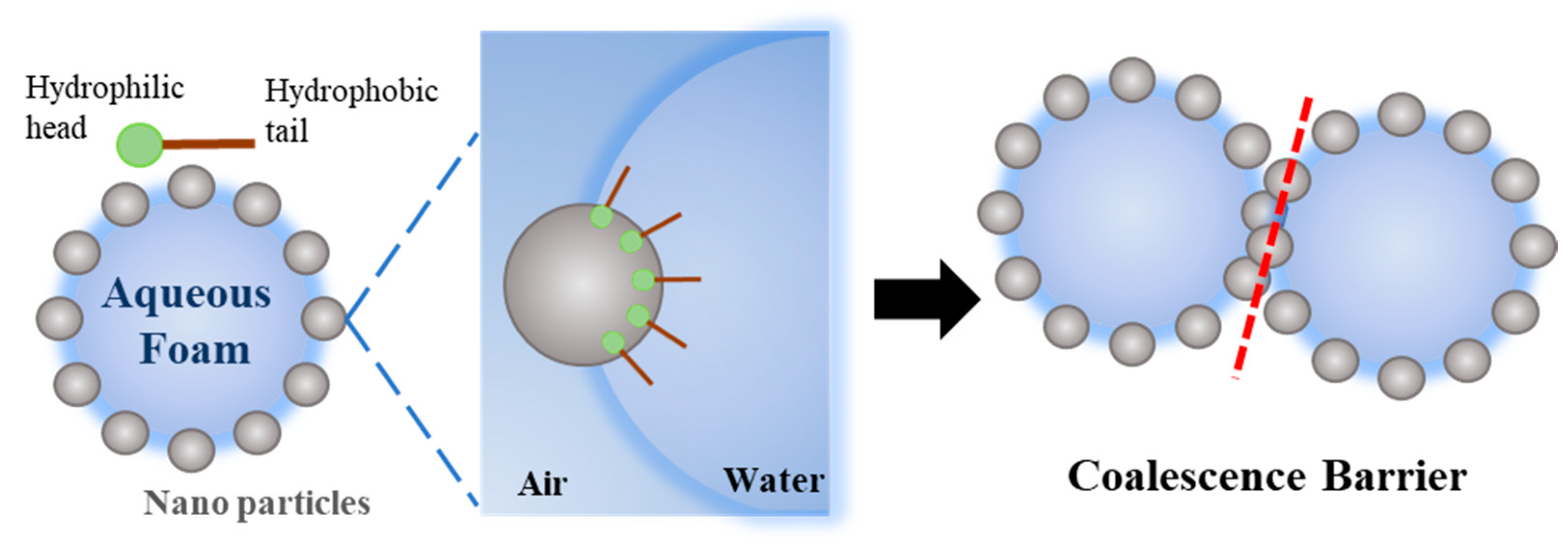
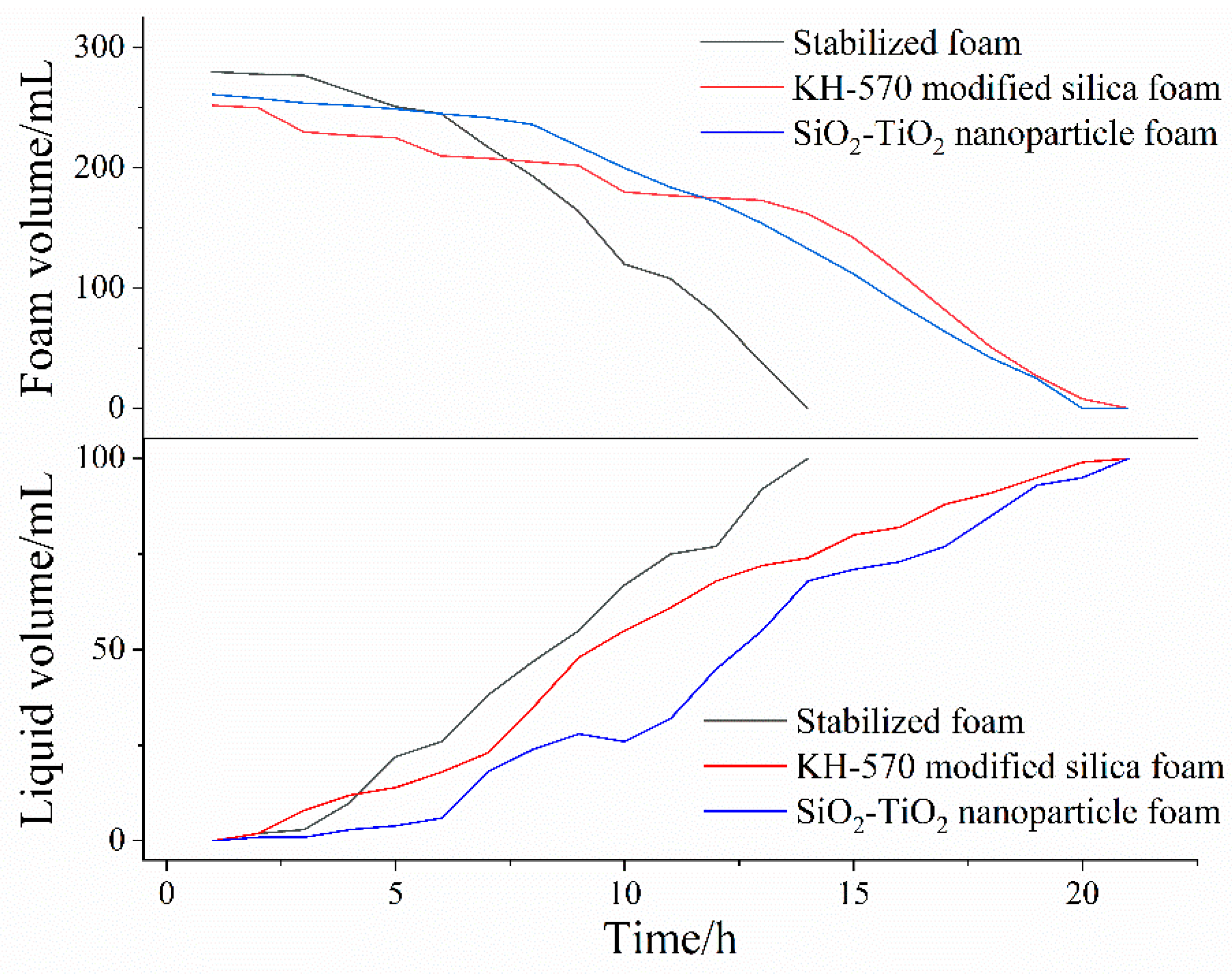
3.3. Aqueous Foam Stability
3.4. Aqueous Foam Suppressing VOCs
4. Conclusions
- (1)
- Three silicon surface modifiers: trimethylchlorosilane, sodium stearate, and KH–570 were used to carry out hydrophobic modification of nanosilica. The dispersion of nanosilica after surface hydrophobic modification was significantly improved, and KH–570-modified nanosilica nanoparticles showed superior hydrophobic and dispersion performance, with a lipophilicity degree of 13.64 and a sedimentation half-life of 13 min in ethanol solvents. From the SEM image, the distance between clusters in the nanosilica aggregate was stretched, meaning a porous structure was formed, and there was a more uniform distribution of clusters. Through a lipophilicity test, a sedimentation half-life test and surface morphology characterization, it can be found that the cross-linking between nano-silica modified by a coupling agent is significantly weakened, and the dispersion in polar solvents is stronger, making it suitable as a synthetic substrate of SiO2–TiO2 nanoparticles.
- (2)
- After the addition of SiO2–TiO2 nanoparticles, the gas permeability between the foams was significantly reduced due to the aggregation barrier, the smaller initial size of the foam, and the delicate and uniform foam. The liquid volume half-life was increased by circa. 2 h, and the foam volume half-life was increased from circa. 9 to 14 h, which indicates greatly improved stability. The liquid volume half-life and the foam volume half-life of the SiO2–TiO2 nanoparticle foam are similar those of the KH–570-modified nanosilica, with a 12.54 h liquid volume half-life and a 14.06 h foam volume half-life.
- (3)
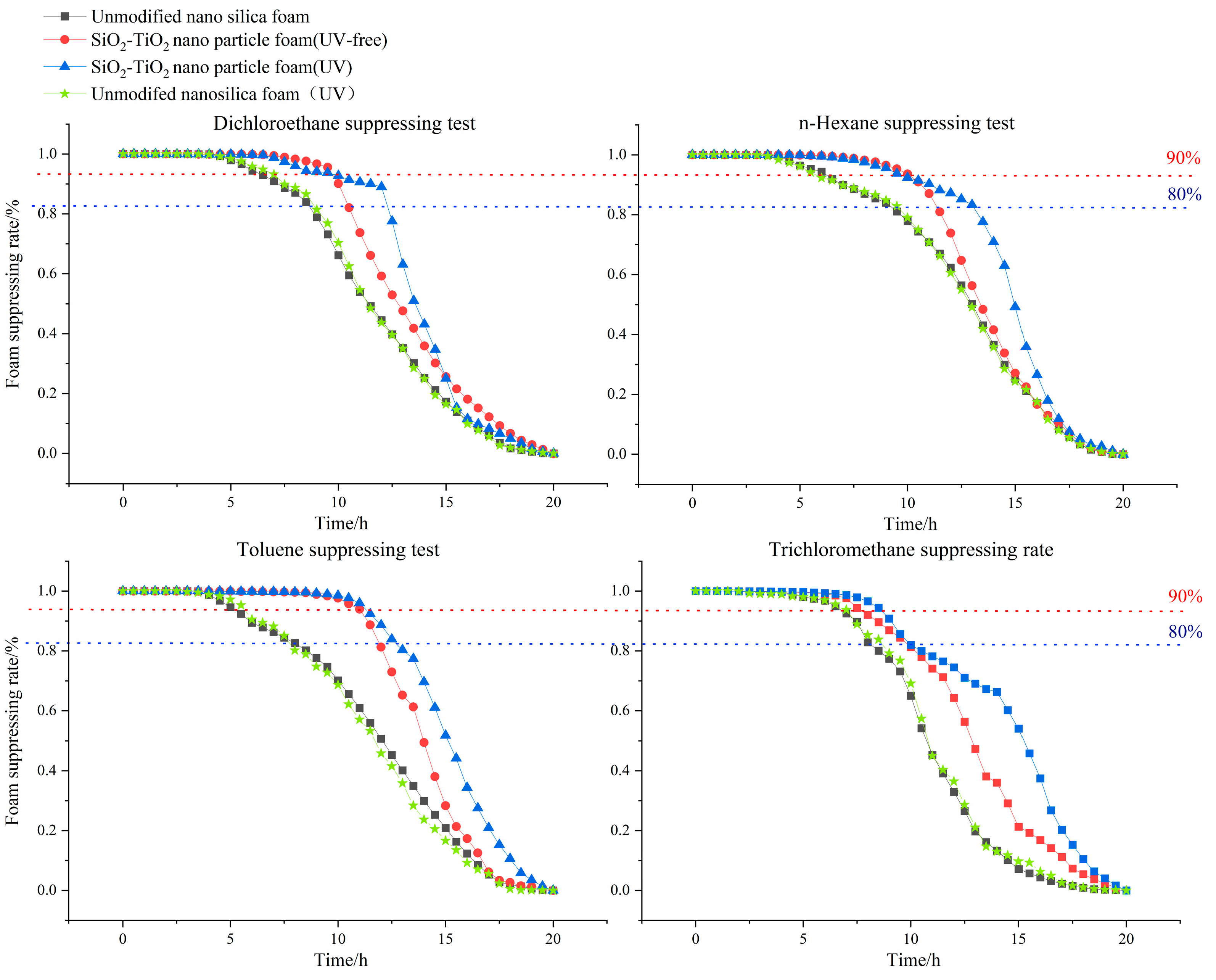
- The effective barrier time was assessed to compare the SiO2–TiO2 nanoparticle foam’s VOC suppression ability under different conditions. The VOC suppression properties of the SiO2–TiO2 nanoparticle foam (UV), SiO2–TiO2 nanoparticle foam (UV-free), original nano−SiO2 (UV-free), and original nano−SiO2 (UV) were decreased in order. Compared with unmodified nanosilica foam, the SiO2–TiO2 nanoparticle foam showed stronger suppression performances, especially under UV light irradiation, and the indices of suppression (t80, t90) of dichloroethane, n–hexane, toluene, and trichloromethane were higher than those of the former foam for 2~6 h.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Viegi, G.; Maio, S.; Pistelli, F.; Baldacci, S.; Carrozzi, L. Epidemiology of chronic obstructive pulmonary disease: Health effects of air pollution. Respirol. Off. J. Asian Pac. Soc. Respirol. 2006, 11, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Soni, V.; Singh, P.; Shree, V.; Goel, V. Effects of VOCs on Human Health. In Proceedings of the 1st International Conference on Sustainable Energy and Environmental Challenges (SEEC), Mohali, India, 26–28 February 2017; Springer: Singapore, 2017; pp. 119–142. [Google Scholar]
- Li, Y.J.; Mao, T.Y.; Tian, M.J.; Destech Publicat, I. Health Risk Assessment of VOCs Emissions from Petroleum Volatilizes. In Proceedings of the 3rd International Conference on Green Materials and Environmental Engineering (GMEE), Beijing, China, 22–23 October 2017; Destech Publications, Inc.: Beijing, China, 2017; pp. 197–201. [Google Scholar]
- Shi, J.W.; Bao, Y.Z.; Xiang, F.; Wang, Z.J.; Ren, L.; Pang, X.C.; Wang, J.; Han, X.Y.; Ning, P. Pollution Characteristics and Health Risk Assessment of VOCs in Jinghong. Atmosphere 2022, 13, 613. [Google Scholar] [CrossRef]
- Ferris Pasquini, V.; Hurtazo, H.; Quintanilla, F.; Cruz-Soto, M. 2,4-Dichlorophenol Shows Estrogenic Endocrine Disruptor Activity by Altering Male Rat Sexual Behavior. Toxics 2023, 11, 843. [Google Scholar] [CrossRef] [PubMed]
- Siegell, J.H. Exploring VOC control options. Chem. Eng. 1996, 103, 92–96. [Google Scholar]
- Zheng, G.Y.; Wei, K.X.; Kang, X.L.; Fan, W.; Ma, N.L.; Verma, M.; Ng, H.S.; Ge, S.B. A new attempt to control volatile organic compounds (VOCs) pollution—Modification technology of biomass for adsorption of VOCs gas. Environ. Pollut. 2023, 336, 18. [Google Scholar] [CrossRef]
- Li, X.; Cui, R.; Yang, B.J.; Xie, S.Y.; Zeng, G.M.; Zheng, H.W.; Zheng, H.L. Research Progress on Indoor VOC Pollution and Control. Mini-Rev. Org. Chem. 2023, 20, 124–135. [Google Scholar] [CrossRef]
- Zhang, W.P.; Li, G.Y.; Yin, H.J.; Zhao, K.; Zhao, H.J.; An, T.C. Adsorption and desorption mechanism of aromatic VOCs onto porous carbon adsorbents for emission control and resource recovery: Recent progress and challenges. Environ. Sci. Nano 2022, 9, 81–104. [Google Scholar] [CrossRef]
- Fan, N.; Liu, C.; Huang, Y.; Li, J.G. Research progress and consideration of VOC pollution control in healthy buildings in China. Chin. Sci. Bull. Chin. 2020, 65, 263–273. [Google Scholar] [CrossRef]
- Sert, O.S.; Fato, K.O.; Gzl, N. Lightweight and highly hydrophobic silica aerogels dried in ambient pressure for an efficient oil/organic solvent adsorption. J. Hazard. Mater. 2020, 408, 124858–124886. [Google Scholar] [CrossRef]
- Sylemez, C.; Türkmen, L.; Zen, L. Achieving Dual-Functionality by Surface Coating of Zeolite with Stearic Acid: Combining Breathability and Odor Control Properties in Polyethylene/Zeolite Composite Films. Macromol. Res. 2020, 28, 1149–1159. [Google Scholar] [CrossRef]
- Gautam, P.S.; Mohanty, K.K. Novel Aqueous Foams for Suppressing VOC Emission. Environ. Sci. Technol. 2004, 38, 2721. [Google Scholar] [CrossRef] [PubMed]
- Gautam, P.S.; Mohanty, K.K. Mass transfer of volatile organic carbons through aqueous foams. J. Colloid Interface Sci. 2004, 273, 611–625. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Ma, L.J.; Zhao, T.; Pan, T.; Zhang, P.K.; Liu, B.G.; Chen, X.R. Enhancing protein-based foam stability by xanthan gum and alkyl glycosides for the reduction of odor emissions from polluted soils. J. Clean Prod. 2023, 398, 7. [Google Scholar] [CrossRef]
- Lee, S.R.; Han, J.K.; Choi, Y.J.; Nam, K. Reduction of ammonia and hydrogen sulfide emission from swine manure using aqueous foams amended with microorganisms and chemical additives. Clean-Soil Air Water 2007, 35, 230–234. [Google Scholar] [CrossRef]
- Paria, S. Surfactant-enhanced remediation of organic contaminated soil and water. Adv. Colloid Interface 2008, 138, 24–58. [Google Scholar] [CrossRef]
- Nguyen, P.; Fadaei, H.; Sinton, D. Pore-scale assessment of nanoparticle-stabilized co 2 foam for enhanced oil recovery. Energy Fuels 2014, 28, 6221–6227. [Google Scholar] [CrossRef]
- Farhadi, H.; Riahi, S.; Ayatollahi, S.; Ahmadi, H. Experimental study of nanoparticle-surfactant-stabilized CO2 foam: Stability and mobility control. Chem. Eng. Res. Des. Trans. Inst. Chem. Eng. 2016, 111, 449–460. [Google Scholar] [CrossRef]
- Narsimhan, G. Foam formation and stabilization. Curr. Opin. Colloid Interface Sci. 1996, 1, 759–763. [Google Scholar] [CrossRef]
- Pugh, R.J. Foaming, foam films, antifoaming and defoaming. Adv. Colloid Interface Sci. 1996, 64, 67–142. [Google Scholar] [CrossRef]
- Ozbayoglu, M.E.; Kuru, E.; Miska, S.; Takach, N. A Comparative Study of Hydraulic Models for Foam Drilling. J. Can. Pet. Technol. 2000, 41, PETSOC-02-06-05. [Google Scholar]
- Xu, H.; Xu, P.; Wang, D.; Yang, Y.; Wang, X.; Wang, T.; An, W.; Xu, S.; Wang, Y.Z. A dimensional stable hydrogel-born foam with enhanced mechanical and thermal insulation and fire-retarding properties via fast microwave foaming. Chem. Eng. J. 2020, 399, 125781. [Google Scholar] [CrossRef]
- Yang, L.L.; He, X.B.; Cheng, Y.X.; Jiang, G.C.; Liu, Z.Y.; Wang, S.B.; Qiu, S.X.; Wang, J.H.; Tian, W.G. Eco-friendly aqueous foam stabilized by cellulose microfibers with great salt tolerance and high temperature resistance. Pet. Sci. 2023, 20, 2499–2511. [Google Scholar] [CrossRef]
- Adamova, T.P.; Manakov, A.Y.; Elistratov, D.S.; Pil’nik, A.A.; Chernov, A.A. Experimental study of methane hydrate formation in aqueous foam stabilized by surfactants. Int. J. Heat Mass Transf. 2021, 180, 8. [Google Scholar] [CrossRef]
- Duan, F.Z.; Zhu, Y.F.; Mu, B.; Wang, A.Q. Recent progress and future prospects on aqueous foams stabilized based on clay minerals. Appl. Clay Sci. 2023, 236, 19. [Google Scholar] [CrossRef]
- Mikhienkova, E.I.; Minakov, A.V.; Lysakov, S.V.; Neverov, A.L.; Pryazhnikov, M.I. An Investigation of the Effect of the Addition of Hydrophobic Silica Nanoparticles on the Colloidal Stability of Inverse Emulsions. Tech. Phys. Lett. 2021, 47, 766–769. [Google Scholar] [CrossRef]
- Stöckelhuber, K.W.; Schulze, H.J.; Wenger, A. Metastable Water Films on Hydrophobic Silica Surfaces; Springer: Berlin/Heidelberg, Germany, 2001; pp. 11–16. [Google Scholar]
- Jung, H.-N.-R.; Lee, Y.K.; Parale, V.G.; Cho, H.H.; Mahadik, D.B.; Park, H.-H. Hydrophobic silica composite aerogels using poly(methyl methacrylate) by rapid supercritical extraction process. J. Sol-Gel Sci. Technol. 2017, 83, 692–697. [Google Scholar] [CrossRef]
- Dargahi-Zaboli, M.; Sahraei, E.; Pourabbas, B. Hydrophobic silica nanoparticle-stabilized invert emulsion as drilling fluid for deep drilling. Pet. Sci. 2017, 14, 105–115. [Google Scholar] [CrossRef]
- Sun, T.; Zhuo, Q.; Liu, X.; Sun, Z.; Wu, Z.; Fan, H. Hydrophobic silica aerogel reinforced with carbon nanotube for oils removal. J. Porous Mater. 2014, 21, 967–973. [Google Scholar] [CrossRef]
- Aguilar-Garrido, A.; Romero-Freire, A.; Paniagua-López, M.; Martínez-Garzón, F.J.; Martín-Peinado, F.J.; Sierra-Aragón, M. Technosols Derived from Mining, Urban, and Agro-Industrial Waste for the Remediation of Metal(loid)-Polluted Soils: A Microcosm Assay. Toxics 2023, 11, 854. [Google Scholar] [CrossRef]
- Ghosh, S.; Patra, R.; Majumdar, D.; Sen, K. Developing scenario of titania-based building materials for environmental remediation. Int. J. Environ. Sci. Technol. 2021, 18, 2077–2102. [Google Scholar] [CrossRef]
- Murashkevich, A.N.; Alisienok, O.A.; Zharskiy, I.M.; Novitskaya, M.S.; Maximovskikh, A.I. Titania sols as precursors in sol-gel technologies of composite materials for photocatalysis, electrorheology, sorption. J. Sol-Gel Sci. Technol. 2019, 92, 254–263. [Google Scholar] [CrossRef]
- Pittol, M.; Tomacheski, D.; Simoes, D.N.; Ribeiro, V.F.; Santana, R.M.C. Evaluation of the Toxicity of Silver/Silica and Titanium Dioxide Particles in Mammalian Cells. Braz. Arch. Biol. Technol. 2018, 61, e18160667. [Google Scholar] [CrossRef]
- Worthen, A.J.; Bagaria, H.G.; Chen, Y.; Bryant, S.L.; Huh, C.; Johnston, K.P. Nanoparticle-stabilized carbon dioxide-in-water foams with fine texture. J. Colloid Interface Sci. 2013, 391, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Il’in, A.P.; Gromov, A.A.; Tikhonov, D.V.; Yablunovskii, G.V.; Il’in, M.A. Properties of ultrafine aluminum powder stabilized by aluminum diboride. Combust. Explos. Shock Waves 2002, 38, 123–126. [Google Scholar] [CrossRef]
- Logan, B.E.; Passow, U.; Alldredge, A.L.; Grossart, H.P.; Simon, M. Rapid formation and sedimentation of large aggregates is predictable from coagulation rates (Half-Lives) of transparent exopolymer particles (TEP). Deep Sea Res. Part Ii-Top. Stud. Oceanogr. 1995, 42, 203–214. [Google Scholar] [CrossRef]
- Sheng, Y.J.; Ma, W.Z.; Yu, X.Y.; Ma, L.; Li, Y. Effect of liquid fuel on foamability and foam stability of mixtures of fluorocarbon and hydrocarbon surfactants. J. Mol. Liq. 2023, 388, 10. [Google Scholar] [CrossRef]
- Guo, J.Z.; Li, Y.; Wang, Y.X.; Lyu, Q.; Zhou, L.F. Identification of an oil-bearing layer by formation water resistivity: A case study of the Jurassic reservoir, Southwest Ordos Basin. Geophysics 2023, 88, B151–B162. [Google Scholar] [CrossRef]
- Phaodee, P.; Weston, J. Review: Implementing the hydrophilic-lipophilic deviation model when formulating detergents and other surfactant-related applications. J. Surfactants Deterg. 2023, 10, 277–286. [Google Scholar] [CrossRef]
- Zhao, F.G.; Wu, X.Q.; Ren, W.; Chen, X.F.; Shi, P.; Yao, X. Preparation and Properties of Ultra Low K Porous Silica Films Modified by Trimethylchlorosilane (TMCS). Ferroelectrics 2010, 406, 221–227. [Google Scholar]
- Teli, M.D.; Annaldewar, B.N. Superhydrophobic and ultraviolet protective nylon fabrics by modified nano silica coating. J. Text. Inst. 2017, 108, 460–466. [Google Scholar] [CrossRef]
- Egerton, T.A.; Parfitt, G.D.; Kang, Y.; Wightman, J.P. XPS analysis of uncoated and silica-coated titanium-dioxide powders. Colloids Surf. 1983, 7, 311–323. [Google Scholar] [CrossRef]
- Cao, T.Q.; Xia, T.Y.; Zhou, L.; Li, G.Q.; Chen, X.; Tian, H.; Zhao, J.L.; Wang, J.O.; Zhang, W.F.; Li, S.F.; et al. Distribution and concentration of surface oxygen vacancy of TiO2 and its photocatalytic activity. J. Phys. D-Appl. Phys. 2020, 53, 424001. [Google Scholar] [CrossRef]
- Li, T.T.; Tao, R.; Wang, Y.X.; Yan, T.; Fan, X.X.; Liu, K.Y. Construction of bismuth oxide iodide (BiOI)/zinc titanium oxide (Zn2TiO4) p-n heterojunction nanofibers with abundant oxygen vacancies for improving photocatalytic carbon dioxide reduction. J. Colloid Interface Sci. 2024, 655, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yu, Y.P.; Hu, H.C.; Xie, B.B.; Du, Y.; Zhu, Q.Y. Quaternized hollow TiO2-enhanced the dye adsorption capacity and photogenerated carrier separation for efficient reactive dye removal. Appl. Surf. Sci. 2024, 644, 158764. [Google Scholar] [CrossRef]
- Babyszko, A.; Wanag, A.; Kusiak-Nejman, E.; Morawski, A.W. Effect of Calcination Temperature of SiO2/TiO2 Photocatalysts on UV-VIS and VIS Removal Efficiency of Color Contaminants. Catalysts 2023, 13, 186. [Google Scholar] [CrossRef]
- Huber, S.G.; Kotte, K.; Schöler, H.F.; Williams, J. Natural Abiotic Formation of Trihalomethanes in Soil: Results from Laboratory Studies and Field Samples. Environ. Sci. Technol. 2009, 43, 4934–4939. [Google Scholar] [CrossRef]


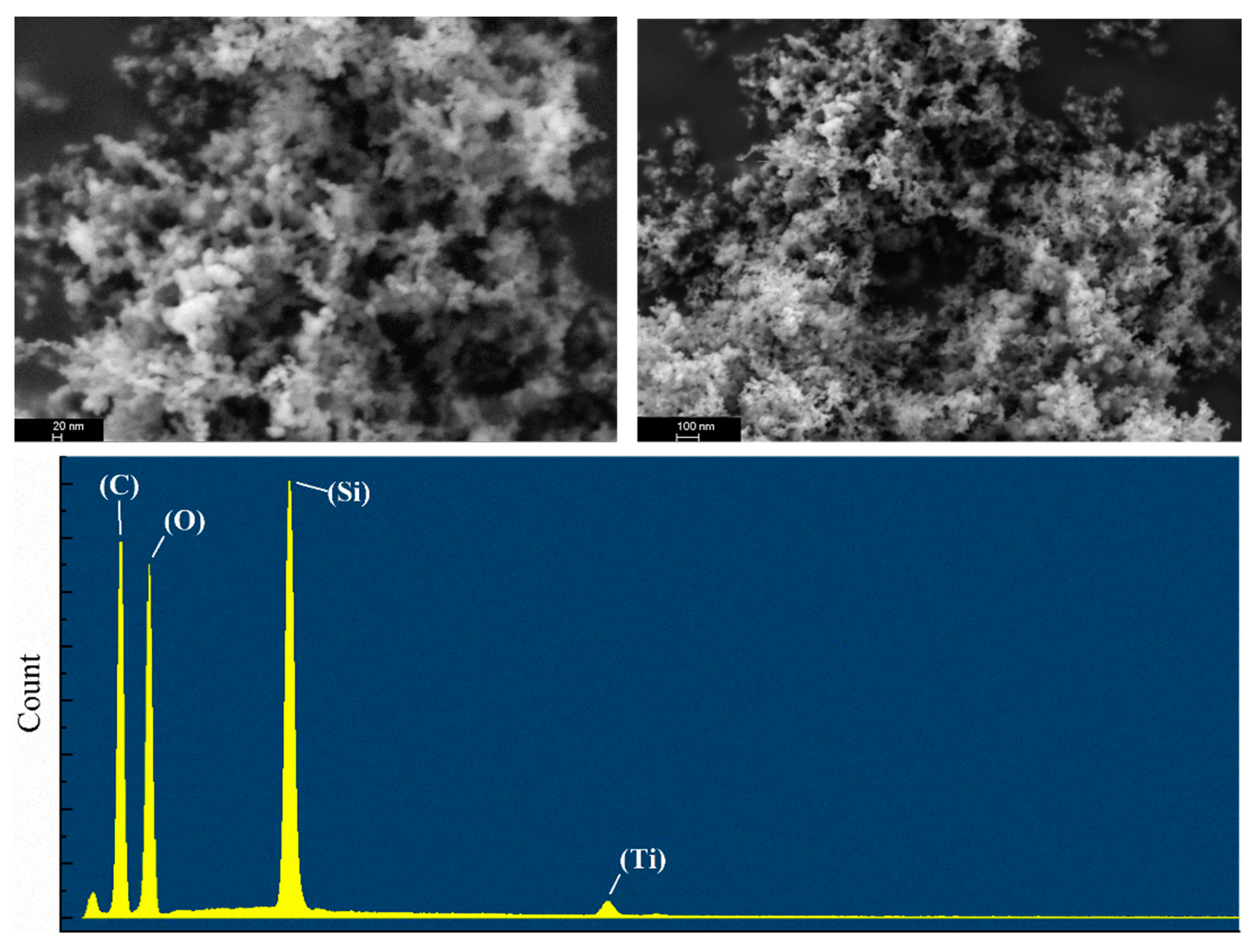



| Materials | Methanol Consumption Volume (mL) | Degree of Lipophilicity |
|---|---|---|
| Raw silica | 0.6 | 0.01 |
| TMCS-modified nanosilica | 2.6 | 4.94 |
| SS-modified nanosilica | 6.5 | 11.50 |
| KH–570-modified nanosilica | 7.9 | 13.64 |
| Materials | Sedimentation Half–Life (min) |
|---|---|
| Raw nanosilica | 2 |
| TMCS-modified nanosilica | 5 |
| SS-modified nanosilica | 10 |
| KH–570-modified nanosilica | 13 |
| Chemical Element | wt% |
|---|---|
| O | 49.19 |
| Si | 9.73 |
| Ti | 0.93 |
| Foam Stem | Liquid Volume Half-Life/h | Foam Volume Half-Life/h |
|---|---|---|
| Stabilizing foam | 8.46 | 9.62 |
| KH–570-modified silica foam | 10.34 | 14.50 |
| SiO2–TiO2 nanoparticle foam | 12.54 | 14.06 |
| Foam Type | Stem of Contaminants | |||||||
|---|---|---|---|---|---|---|---|---|
| Dichloroethane | n–Hexane | Toluene | Trichloromethane | |||||
| t80 | t90 | t80 | t90 | t80 | t90 | t80 | t90 | |
| Unmodified nanosilica foam | 8.89 | 7.24 | 9.64 | 7.08 | 8.57 | 5.90 | 8.57 | 7.52 |
| Unmodified nanosilica foam (UV) | 9.01 | 7.50 | 9.55 | 6.89 | 8.15 | 6.42 | 9.02 | 7.60 |
| SiO2–TiO2 nanoparticle foam (UV-free) | 10.65 | 10.05 | 11.59 | 10.71 | 12.11 | 11.35 | 10.28 | 8.47 |
| SiO2–TiO2 nanoparticle foam (UV) | 12.45 | 11.94 | 13.32 | 11.19 | 13.15 | 11.84 | 10.71 | 9.16 |
| Phase | Surface Tension/(mN·m−2) | Entering Index (E) | Spreading Index (S) |
|---|---|---|---|
| n–Hexane | 23.8 | 20.9 | −12.3 |
| n–Hexane/surfactant | 16.6 | ||
| Dichloroethane | 32.2 | 2.2 | −10.4 |
| Dichloroethane/surfactant | 6.3 | ||
| Toluene | 28.5 | 14.6 | −15.4 |
| Toluene/surfactant | 15.0 | ||
| Trichloromethane | 28.9 | 14.7 | −16.3 |
| Trichloromethane/surfactant | 15.5 | ||
| Surfactant solution | 28.10 | − | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Xuan, Y. SiO2–TiO2 Nanoparticle Aqueous Foam for Volatile Organic Compounds’ Suppression. Toxics 2024, 12, 99. https://doi.org/10.3390/toxics12020099
Yu J, Xuan Y. SiO2–TiO2 Nanoparticle Aqueous Foam for Volatile Organic Compounds’ Suppression. Toxics. 2024; 12(2):99. https://doi.org/10.3390/toxics12020099
Chicago/Turabian StyleYu, Jintao, and Yuning Xuan. 2024. "SiO2–TiO2 Nanoparticle Aqueous Foam for Volatile Organic Compounds’ Suppression" Toxics 12, no. 2: 99. https://doi.org/10.3390/toxics12020099
APA StyleYu, J., & Xuan, Y. (2024). SiO2–TiO2 Nanoparticle Aqueous Foam for Volatile Organic Compounds’ Suppression. Toxics, 12(2), 99. https://doi.org/10.3390/toxics12020099





