Exposure to Plasticiser DEHP Affects Eggs Spawned by Blue Mussels: A Possible Risk to Fertilisation?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Spawning Induction
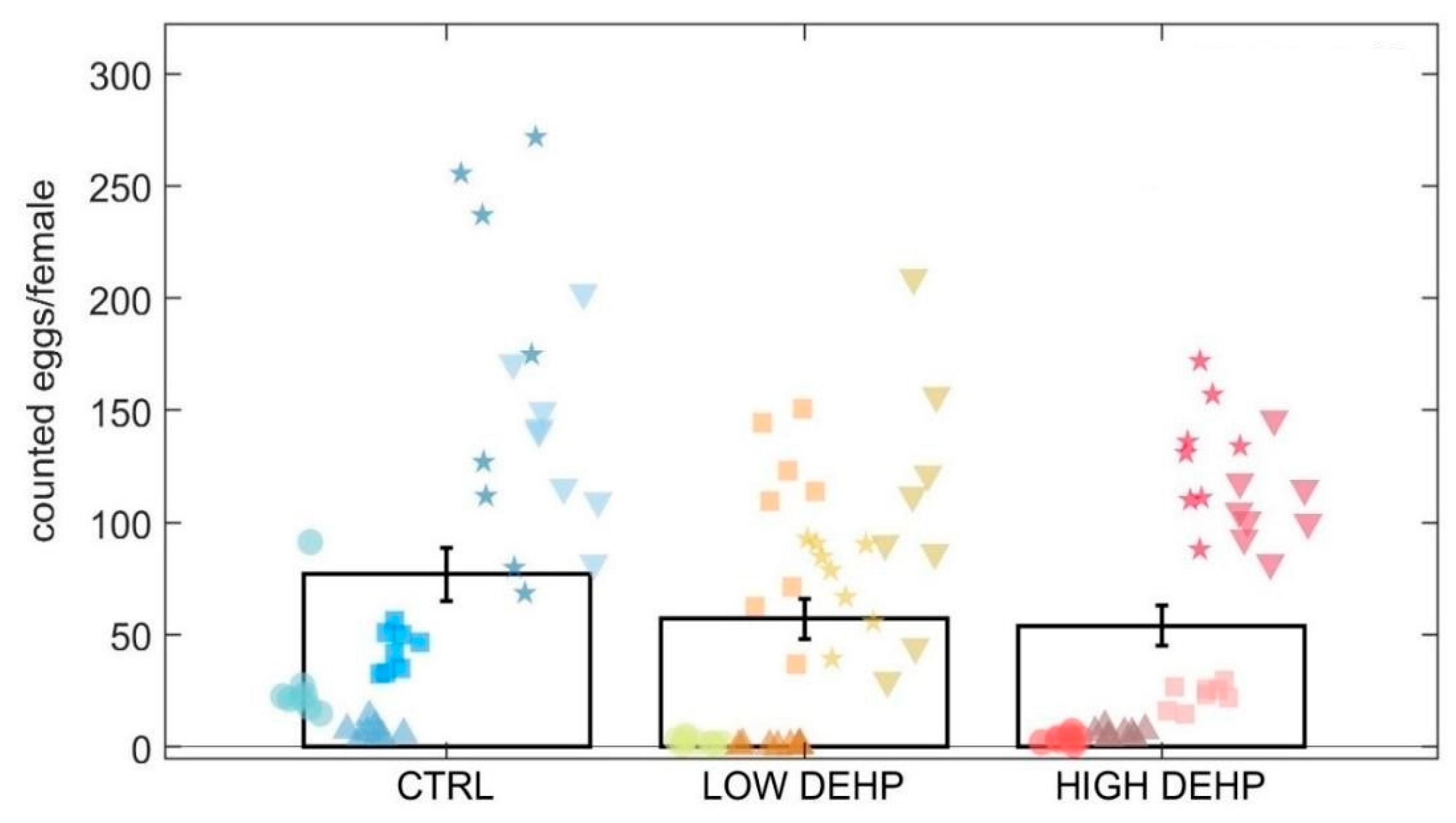
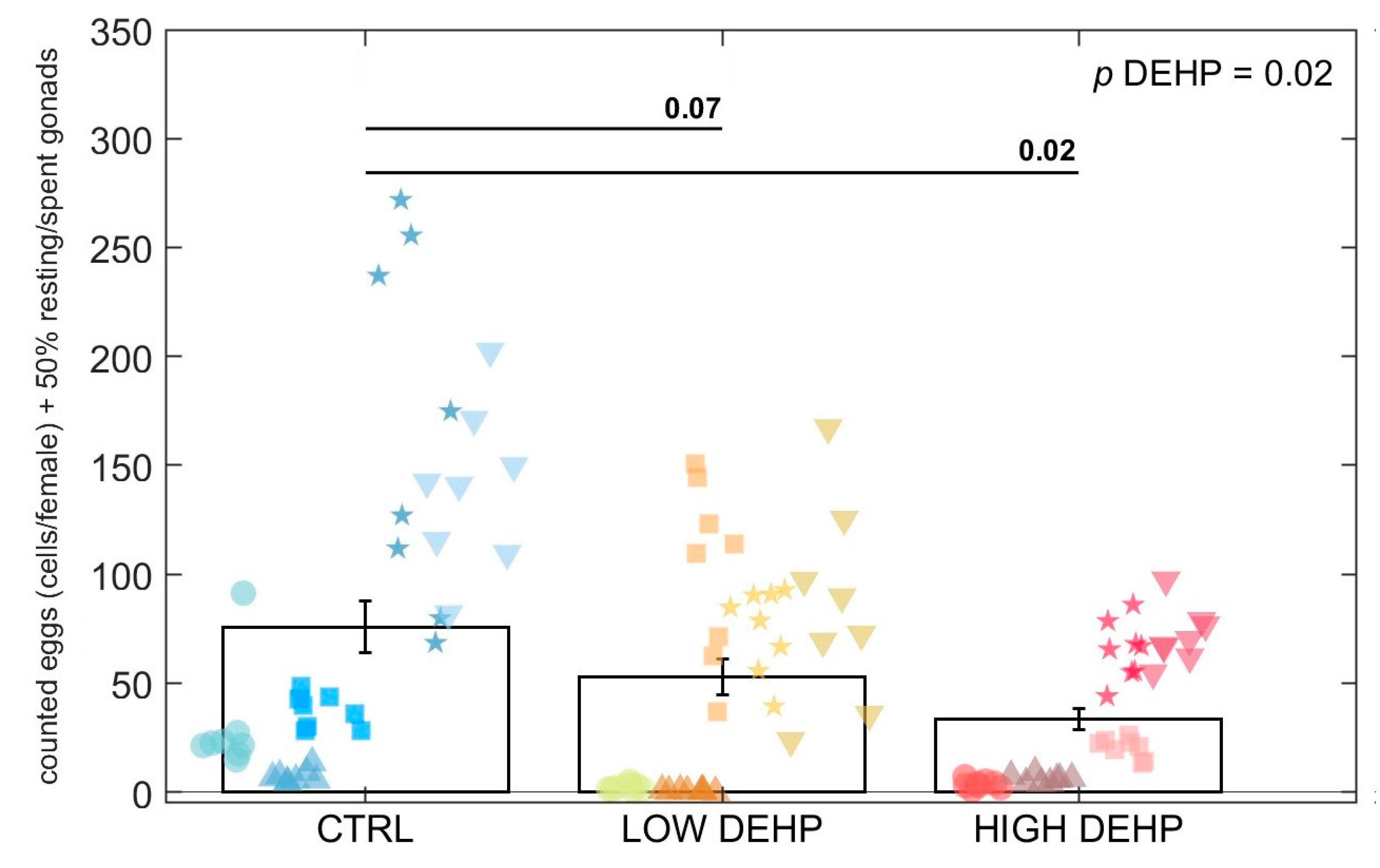
2.3. Egg Counting and Size Measurement
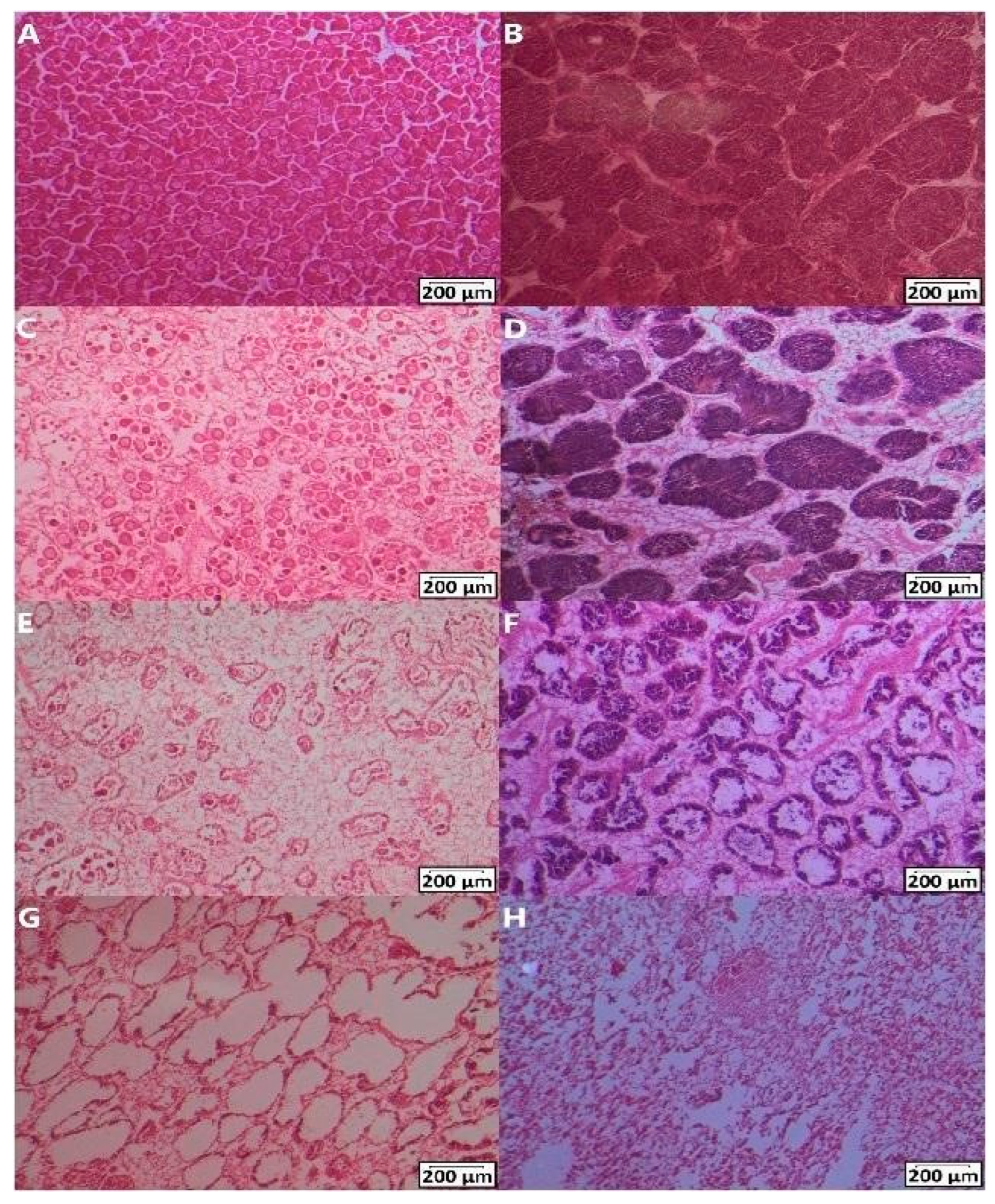
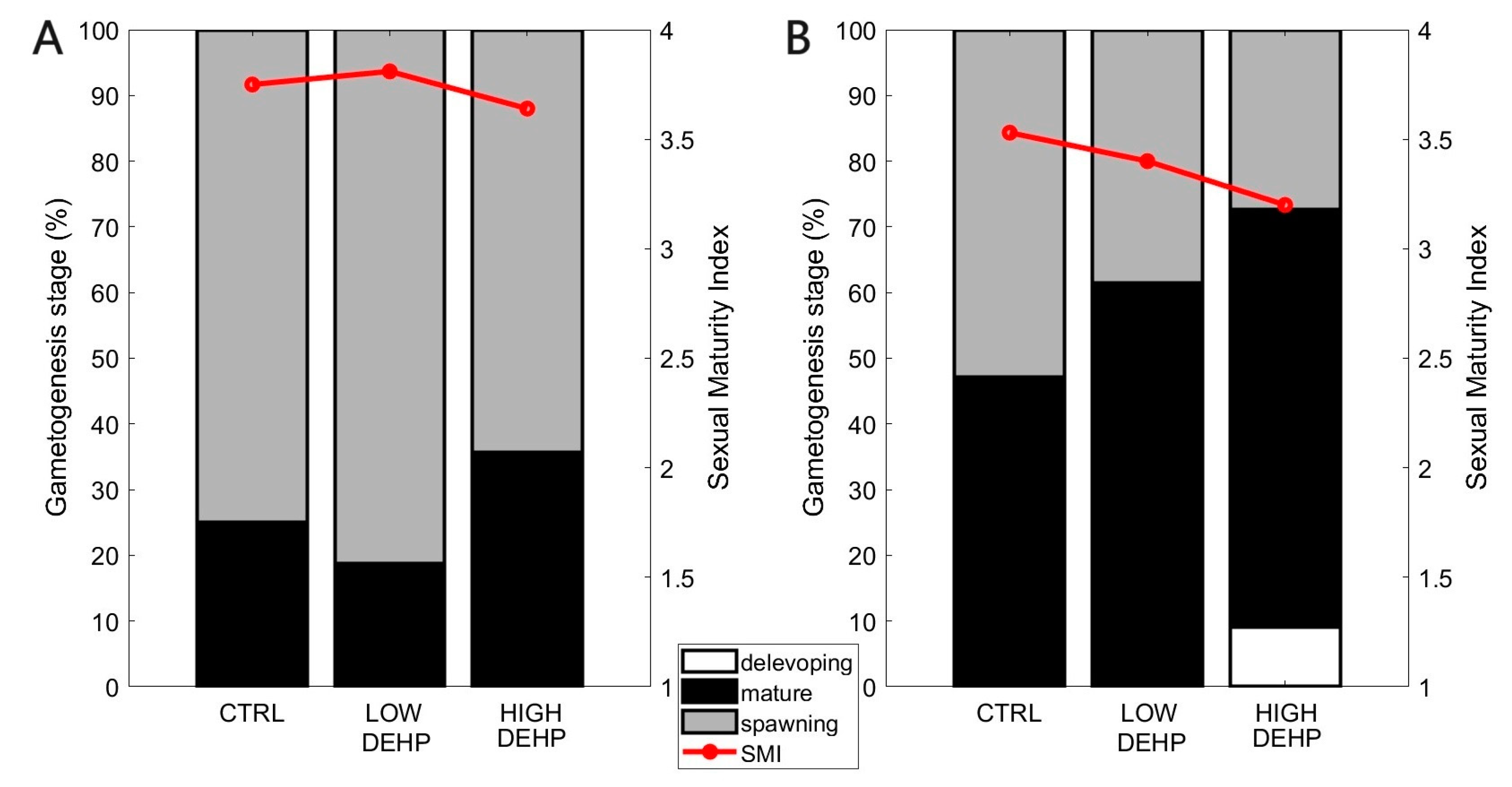
2.4. Histology Analysis to Determine Sex and Maturity Factor
2.5. Respirometer Assay and Valve Behaviours
2.6. Statistical Analysis
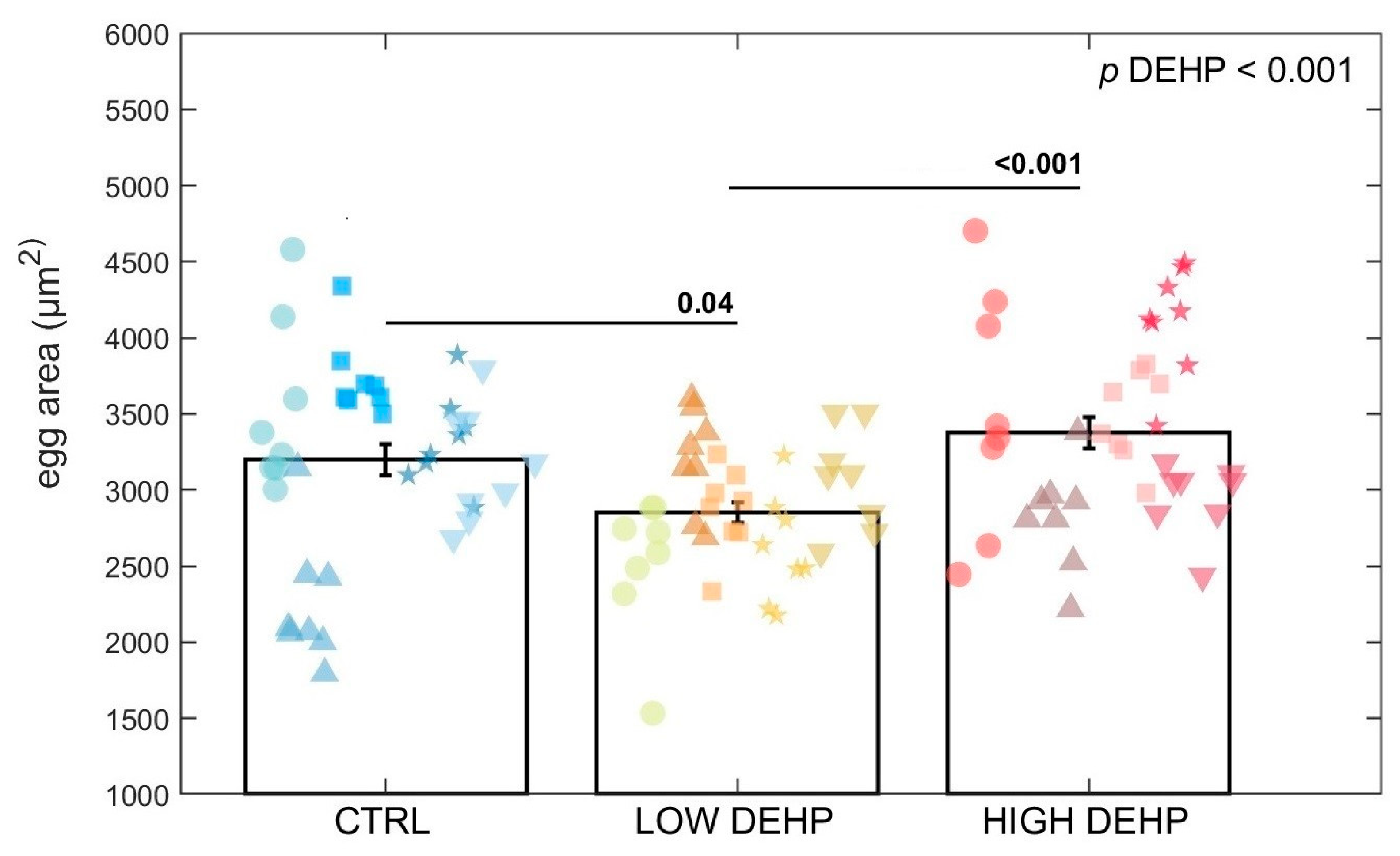
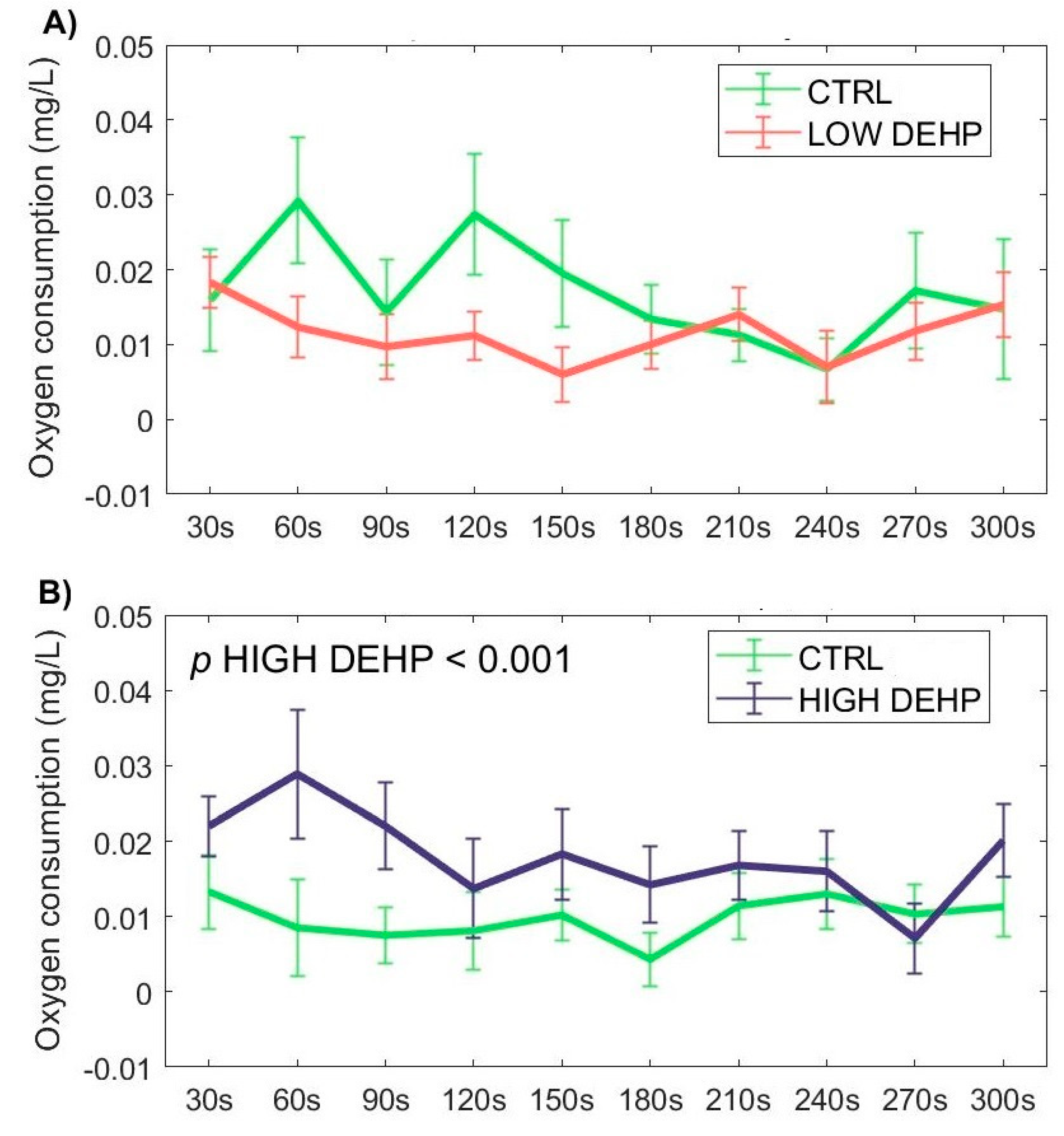
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goksøyr, A. Endocrine Disruptors in the Marine Environment: Mechanisms of Toxicity and Their Influence on Reproductive Processes in Fish. J. Toxicol. Environ. Health Part A 2006, 69, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Rotchell, J.M.; Ostrander, G.K. Molecular Markers of Endocrine Disruption in Aquatic Organisms. J. Toxicol. Environ. Health—Part B Crit. Rev. 2003, 6, 453–495. [Google Scholar] [CrossRef] [PubMed]
- Soto, A.M.; Calabro, J.M.; Prechtl, N.V.; Yau, A.Y.; Orlando, E.F.; Daxenberger, A.; Kolok, A.S.; Guillette, L.J.; le Bizec, B.; Lange, I.G.; et al. Androgenic and Estrogenic Activity in Water Bodies Receiving Cattle Feedlot Effluent in Eastern Nebraska, USA. Environ. Health Perspect. 2004, 112, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Aarab, N.; Lemaire-Gony, S.; Unruh, E.; Hansen, P.D.; Larsen, B.K.; Andersen, O.K.; Narbonne, J.F. Preliminary Study of Responses in Mussel (Mytilus edilus) Exposed to Bisphenol A, Diallyl Phthalate and Tetrabromodiphenyl Ether. Aquat. Toxicol. 2006, 78, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Bila, D.; Montalvão, A.F.; Azevedo, D.D.A.; Dezotti, M. Estrogenic Activity Removal of 17β-Estradiol by Ozonation and Identification of by-Products. Chemosphere 2007, 69, 736–746. [Google Scholar] [CrossRef] [PubMed]
- Canesi, L.; Lorusso, L.C.; Ciacci, C.; Betti, M.; Zampini, M.; Gallo, G. Environmental Estrogens Can Affect the Function of Mussel Hemocytes through Rapid Modulation of Kinase Pathways. Gen. Comp. Endocrinol. 2004, 138, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Erythropel, H.C.; Maric, M.; Nicell, J.A.; Leask, R.L.; Yargeau, V. Leaching of the Plasticizer Di(2-Ethylhexyl)Phthalate (DEHP) from Plastic Containers and the Question of Human Exposure. Appl. Microbiol. Biotechnol. 2014, 98, 9967–9981. [Google Scholar] [CrossRef]
- Net, S.; Sempéré, R.; Delmont, A.; Paluselli, A.; Ouddane, B. Occurrence, Fate, Behavior and Ecotoxicological State of Phthalates in Different Environmental Matrices. Environ. Sci. Technol. 2015, 49, 4019–4035. [Google Scholar] [CrossRef]
- Parkerton, T.F.; Konkel, W.J. Application of Quantitative Structure I Activity Relationships for Assessing the Aquatic Toxicity of Phthalate Esters. Ecotoxicol. Environ. Saf. 2000, 45, 61–78. [Google Scholar] [CrossRef]
- ECPI. Plasticisers. 2020. Available online: https://www.plasticisers.org/factsheet/plasticisers-factsheets/ (accessed on 12 March 2021).
- Earls, A.O.; Axford, I.P.; Braybrook, J.H. Gas Chromatography-Mass Spectrometry Determination of the Migration of Phthalate Plasticisers from Polyvinyl Chloride Toys and Childcare Articles. J. Chromatogr. A 2003, 983, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Van Wezel, A.P.; Van Vlaardingen, P.; Posthumus, R.; Crommentuijn, G.H.; Sijm, D.T.H.M. Environmental Risk Limits for Two Phthalates, with Special Emphasis on Endocrine Disruptive Properties. Ecotoxicol. Environ. Saf. 2000, 46, 305–321. [Google Scholar] [CrossRef]
- Lithner, D.; Larsson, A.; Dave, G. Environmental and Health Hazard Ranking and Assessment of Plastic Polymers Based on Chemical Composition. Sci. Total Environ. 2011, 409, 3309–3324. [Google Scholar] [CrossRef]
- Oehlmann, J.; Oetken, M.; Schulte-Oehlmann, U. A Critical Evaluation of the Environmental Risk Assessment for Plasticizers in the Freshwater Environment in Europe, with Special Emphasis on Bisphenol A and Endocrine Disruption. Environ. Res. 2008, 108, 140–149. [Google Scholar] [CrossRef]
- Engler, R.E. The Complex Interaction between Marine Debris and Toxic Chemicals in the Ocean. Environ. Sci. Technol. 2012, 46, 12302–12315. [Google Scholar] [CrossRef] [PubMed]
- Wittassek, M.; Koch, H.M.; Angerer, J.; Brüning, T. Assessing Exposure to Phthalates—The Human Biomonitoring Approach. Mol. Nutr. Food Res. 2011, 55, 7–31. [Google Scholar] [CrossRef]
- Zimmermann, L.; Bartosova, Z.; Braun, K.; Oehlmann, J.; Völker, C.; Wagner, M. Plastic Products Leach Chemicals That Induce In Vitro Toxicity under Realistic Use Conditions. Environ. Sci. Technol. 2021, 55, 11814–11823. [Google Scholar] [CrossRef] [PubMed]
- Schettler, T.; Skakkebæk, N.E.; De Kretser, D.; Leffers, H. Human Exposure to Phthalates via Consumer Products. Int. J. Androl. 2006, 29, 134–139. [Google Scholar] [CrossRef]
- Das, M.T.; Ghosh, P.; Thakur, I.S. Intake Estimates of Phthalate Esters for South Delhi Population Based on Exposure Media Assessment. Environ. Pollut. 2014, 189, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Wormuth, M.; Scheringer, M.; Vollenweider, M.; Hungerbühler, K. What Are the Sources of Exposure to Eight Frequently Used Phthalic Acid Esters in Europeans? Risk Anal. 2006, 26, 803–824. [Google Scholar] [CrossRef]
- Völker, J.; Ashcroft, F.; Vedøy, Å.; Zimmermann, L.; Wagner, M. Adipogenic Activity of Chemicals Used in Plastic Consumer Products. Environ. Sci. Technol. 2022, 56, 2487–2496. [Google Scholar] [CrossRef]
- Tassinari, R.; Tait, S.; Busani, L.; Martinelli, A.; Narciso, L.; Valeri, M.; Gastaldelli, A.; Deodati, A.; La Rocca, C.; Maranghi, F. Metabolic, Reproductive and Thyroid Effects of Bis(2-Ethylhexyl) Phthalate (DEHP) Orally Administered to Male and Female Juvenile Rats at Dose Levels Derived from Children Biomonitoring Study. Toxicology 2021, 449, 152653. [Google Scholar] [CrossRef]
- Zhang, T.; Li, L.; Qin, X.-S.; Zhou, Y.; Zhang, X.-F.; Wang, L.-Q.; De Felici, M.; Chen, H.; Qin, G.-Q.; Shen, W. Di-(2-Ethylhexyl) Phthalate and Bisphenol A Exposure Impairs Mouse Primordial Follicle Assembly InVitro. Environ. Mol. Mutagen. 2014, 5, 343–353. [Google Scholar] [CrossRef]
- Mu, X.; Liao, X.; Chen, X.; Li, Y.; Wang, M.; Shen, C.; Zhang, X.; Wang, Y.; Liu, X.; He, J. DEHP Exposure Impairs Mouse Oocyte Cyst Breakdown and Primordial Follicle Assembly through Estrogen Receptor-Dependent and Independent Mechanisms. J. Hazard. Mater. 2015, 298, 232–240. [Google Scholar] [CrossRef] [PubMed]
- European Union Commission. Regulation 2018/2005 Amending Annex XVII to Regulation (EC) No 1907/2006 of the European Parliament and of the Council Concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) as Regards Bis(2-Ethylhexyl) Phthalate (DEHP), Dibutyl Phthalate. 2018. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32018R2005&from=E (accessed on 5 February 2022).
- Carnevali, O.; Tosti, L.; Speciale, C.; Peng, C.; Zhu, Y.; Maradonna, F. DEHP Impairs Zebrafish Reproduction by Affecting Critical Factors in Oogenesis. PLoS ONE 2010, 5, e10201. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Kim, J.W.; Lee, S.K. Inhibition of Oocyte Development in Japanese Medaka (Oryzias latipes) Exposed to Di-2-Ethylhexyl Phthalate. Environ. Int. 2002, 28, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhang, P.; Li, C.; Su, X.; Jin, C.; Li, Y.; Xu, Y.; Li, T. Characterisation of Immune-Related Gene Expression in Clam (Venerupis Philippinarum) under Exposure to Di(2-Ethylhexyl) Phthalate. Fish Shellfish Immunol. 2013, 34, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Zanotelli, V.R.T.; Neuhauss, S.C.F.; Ehrengrubera, M.U. Long-Term Exposure to Bis(2-Ethylhexyl) Phthalate (DEHP) Inhibits Growth of Guppy Fish (Poecilia reticulata). J. Appl. Toxicol. 2010, 30, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Kalo, D.; Hadas, R.; Furman, O.; Ben-Ari, J.; Maor, Y.; Patterson, D.G.; Tomey, C.; Roth, Z. Carryover Effects of Acute DEHP Exposure on Ovarian Function and Oocyte Developmental Competence in Lactating Cows. PLoS ONE 2015, 10, e0130896. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y. Biosynthesis of Di-(2-Ethylhexyl) Phthalate (DEHP) and Di-n-Butyl Phthalate (DBP) from Red Alga—Bangia Atropurpurea. Water Res. 2004, 38, 1014–1018. [Google Scholar] [CrossRef] [PubMed]
- Koniecki, D.; Wang, R.; Moody, R.P.; Zhu, J. Phthalates in Cosmetic and Personal Care Products: Concentrations and Possible Dermal Exposure. Environ. Res. 2011, 111, 329–336. [Google Scholar] [CrossRef]
- Park, C.J.; Barakat, R.; Ulanov, A.; Li, Z.; Lin, P.-C.; Chiu, K.; Zhou, S.; Perez, P.; Lee, J.; Flaws, J.; et al. Sanitary Pads and Diapers Contain Higher Phthalate Contents than Those in Common Commercial Plastic Products. Reprod. Toxicol. 2019, 84, 114–121. [Google Scholar] [CrossRef]
- Peterson, D.R.; Staples, C.A. Degradation of Phthalate Esters in the Environment. Handb. Environ. Chem. 2003, 3, 85–124. [Google Scholar] [CrossRef]
- Sánchez-Avila, J.; Tauler, R.; Lacorte, S. Organic Micropollutants in Coastal Waters from NW Mediterranean Sea: Sources Distribution and Potential Risk. Environ. Int. 2012, 46, 50–62. [Google Scholar] [CrossRef]
- Jebara, A.; Albergamo, A.; Rando, R.; Potortì, A.G.; Lo Turco, V.; Mansour, H.B.; Di Bella, G. Phthalates and Non-Phthalate Plasticizers in Tunisian Marine Samples: Occurrence, Spatial Distribution and Seasonal Variation. Mar. Pollut. Bull. 2021, 163, 111967. [Google Scholar] [CrossRef]
- Markert, B.A.; Breure, A.M.; Zechmeister, H.G. Bioindicators and Biomonitors; Elsevier: Amsterdam, The Netherlands, 2003. [Google Scholar]
- Burger, J. Bioindicators: A Review of Their Use in the Environmental Literature 1970–2005. Environ. Bioindic. 2006, 1, 136–144. [Google Scholar] [CrossRef]
- Chiesa, L.M.; Nobile, M.; Malandra, R.; Pessina, D.; Panseri, S.; Labella, G.F.; Arioli, F. Food Safety Traits of Mussels and Clams: Distribution of PCBs, PBDEs, OCPs, PAHs and PFASs in Sample from Different Areas Using HRMS-Orbitrap® and Modified QuEChERS Extraction Followed by GC-MS/MS. Food Addit. Contam.—Part A Chem. Anal. Control Expo. Risk Assess. 2018, 35, 959–971. [Google Scholar] [CrossRef] [PubMed]
- Van Cauwenberghe, L.; Janssen, C.R. Microplastics in Bivalves Cultured for Human Consumption. Environ. Pollut. 2014, 193, 65–70. [Google Scholar] [CrossRef]
- Wathsala, R.H.G.R.; Franzellitti, S.; Scaglione, M.; Fabbri, E. Styrene Impairs Normal Embryo Development in the Mediterranean Mussel (Mytilus galloprovincialis). Aquat. Toxicol. 2018, 201, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Chelomin, V.P.; Mazur, A.A.; Slobodskova, V.V.; Kukla, S.P.; Dovzhenko, N.V. Genotoxic Properties of Polystyrene (PS) Microspheres in the Filter-Feeder Mollusk Mytilus Trossulus (Gould, 1850). J. Mar. Sci. Eng. 2022, 10, 273. [Google Scholar] [CrossRef]
- Balbi, T.; Franzellitti, S.; Fabbri, R.; Montagna, M.; Fabbri, E.; Canesi, L. Impact of Bisphenol A (BPA) on Early Embryo Development in the Marine Mussel Mytilus Galloprovincialis: Effects on Gene Transcription. Environ. Pollut. 2016, 218, 996–1004. [Google Scholar] [CrossRef]
- Fabbri, R.; Montagna, M.; Balbi, T.; Raffo, E.; Palumbo, F.; Canesi, L. Adaptation of the Bivalve Embryotoxicity Assay for the High Throughput Screening of Emerging Contaminants in Mytilus Galloprovincialis. Mar. Environ. Res. 2014, 99, 1–8. [Google Scholar] [CrossRef]
- Andreyeva, A.Y.; Lobko, V.V.; Gostyukhina, O.L.; Tkachuk, A.A.; Murashova, A.I.; Malakhova, L.V.; Kladchenko, E.S. Accumulation, Functional and Antioxidant Responses to Acute Exposure to Di(2-Ethylhexyl)Phthalate (DEHP) in Mytilus Galloprovincialis. Mar. Pollut. Bull. 2023, 191, 114923. [Google Scholar] [CrossRef] [PubMed]
- Xiang, N.; Zhao, C.; Diao, X.; Han, Q.; Zhou, H. Dynamic Responses of Antioxidant Enzymes in Pearl Oyster Pinctada Martensii Exposed to Di(2-Ethylhexyl) Phthalate (DEHP). Environ. Toxicol. Pharmacol. 2017, 54, 184–190. [Google Scholar] [CrossRef]
- Cancio, I.; Orbea, A.; Völkl, A.; Fahimi, H.D.; Cajaraville, M.P. Induction of Peroxisomal Oxidases in Mussels: Comparison of Effects of Lubricant Oil and Benzo(a)Pyrene with Two Typical Peroxisome Proliferators on Peroxisome Structure and Function in Mytilus Galloprovincialis. Toxicol. Appl. Pharmacol. 1998, 149, 64–72. [Google Scholar] [CrossRef]
- Orbea, A.; Ortiz-Zarragoitia, M.; Cajaraville, M.P. Interactive Effects of Benzo(a)Pyrene and Cadmium and Effects of Di(2-Ethylhexyl) Phthalate on Antioxidant and Peroxisomal Enzymes and Peroxisomal Volume Density in the Digestive Gland of Mussel Mytilus Galloprovincialis Lmk. Biomarkers 2002, 7, 33–48. [Google Scholar] [CrossRef]
- Mincarelli, L.F.; Rotchell, J.M.; Chapman, E.C.; Turner, A.P.; Wollenberg Valero, K.C. Consequences of Combined Exposure to Thermal Stress and the Plasticiser DEHP in Mytilus Spp. Differ by Sex. Mar. Pollut. Bull. 2021, 170, 112624. [Google Scholar] [CrossRef]
- Xu, G.; Kong, H.; Chang, X.; Dupont, S.; Chen, H.; Deng, Y.; Hu, M.; Wang, Y. Gonadal Antioxidant Responses to Seawater Acidification and Hypoxia in the Marine Mussel Mytilus Coruscus. Environ. Sci. Pollut. Res. 2021, 28, 53847–53856. [Google Scholar] [CrossRef] [PubMed]
- Conolly, R.B.; Lutz, W.K. Nonmonotonic Dose-Response Relationships: Mechanistic Basis, Kinetic Modeling, and Implications for Risk Assessment. Toxicol. Sci. 2004, 77, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Do, R.P.; Stahlhut, R.W.; Ponzi, D.; vom Saal, F.S.; Taylor, J.A. Non-Monotonic Dose Effects of in Utero Exposure to Di(2-Ethylhexyl) Phthalate (DEHP) on Testicular and Serum Testosterone and Anogenital Distance in Male Mouse Fetuses. Reprod. Toxicol. 2012, 34, 614–621. [Google Scholar] [CrossRef]
- Li, L.; Andersen, M.E.; Heber, S.; Zhang, Q. Non-Monotonic Dose-Response Relationship in Steroid Hormone Receptor-Mediated Gene Expression. J. Mol. Endocrinol. 2007, 38, 569–585. [Google Scholar] [CrossRef]
- Welshons, W.V.; Thayer, K.A.; Judy, B.M.; Taylor, J.A.; Curran, E.M.; vom Saal, F.S. Large Effects from Small Exposures. I. Mechanisms for Endocrine-Disrupting Chemicals with Estrogenic Activity. Environ. Health Perspect. 2003, 111, 994–1006. [Google Scholar] [CrossRef]
- Mincarelli, L.F.; Chapman, E.C.; Rotchell, J.M.; Turner, A.P.; Wollenberg Valero, K.C. Sex and Gametogenesis Stage Are Strong Drivers of Gene Expression in Mytilus edul is Exposed to Environmentally Relevant Plasticiser Levels and PH 7.7. Environ. Sci. Pollut. Res. 2022, 30, 23437–23449. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Waite, J.H.; Matsuoka, M.; Odo, S.; Harayama, S. Interspecific Variations in Adhesive Protein Sequences of Mytilus edulis, M. Galloprovincialis, and M. Trossulus. Biol. Bull. 1995, 189, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Seed, R. The Ecology of Mytilus edulis L. (Lamellibranchiata) on Exposed Rocky Shores—I. Breeding and Settlement. Oecologia 1969, 3, 277–316. [Google Scholar] [CrossRef] [PubMed]
- Gosling, E. Marine Mussels: Ecology, Physiology, Genetics and Culture; John Wiley & Sons: Hoboken, NJ, USA, 2021. [Google Scholar]
- Virgin, S.D.S.; Barbeau, M.A. Gametogenesis and Spawning of the Ribbed Mussel (Geukensia demissa) near the Northern Limit of Its Range (Maritime Canada). Estuaries Coasts 2017, 40, 1131–1141. [Google Scholar] [CrossRef]
- Fearman, J.; Moltschaniwskyj, N.A. Warmer Temperatures Reduce Rates of Gametogenesis in Temperate Mussels, Mytilus Galloprovincialis. Aquaculture 2010, 305, 20–25. [Google Scholar] [CrossRef]
- Subramaniam, T.; Lee, H.J.; Jeung, H.D.; Kang, H.S.; Kim, C.W.; Kim, H.S.; Cho, Y.G.; Choi, K.S. Report on the Annual Gametogenesis and Tissue Biochemical Composition in the Gray Mussel, Crenomytilus Grayanus (Dunker 1853) in the Subtidal Rocky Bottom on the East Coast of Korea. Ocean Sci. J. 2021, 56, 424–433. [Google Scholar] [CrossRef]
- Tessmar-Raible, K.; Raible, F.; Arboleda, E. Another Place, Another Timer: Marine Species and the Rhythms of Life. BioEssays 2011, 33, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Fearman, J.-A.; Bolch, C.J.S.; Moltschaniwskyj, N.A. Energy Storage and Reproduction in Mussels, Mytilus Galloprovincialis: The Influence of Diet Quality. J. Shellfish.Res. 2009, 28, 305–312. [Google Scholar] [CrossRef]
- Hilbish, T.J.; Zimmerman, K.M. Genetic and Nutritional Control of the Gametogenic Cycle in Mytilus edulis. Mar. Biol. 1988, 228, 223–228. [Google Scholar] [CrossRef]
- Kang, C.K.; Lee, Y.W.; Eun, J.C.; Shin, J.K.; Seo, I.S.; Hong, J.S. Microphytobenthos Seasonality Determines Growth and Reproduction in Intertidal Bivalves. Mar. Ecol. Prog. Ser. 2006, 315, 113–127. [Google Scholar] [CrossRef]
- Newell, R.I.E.; Hilbish, T.J.; Koehn, R.K.; Newell, C.J. Temporal Variation in the Reproductive Cycle of Mytilus edulis L. (Bivalvia, Mytilidae) from Localities on the East Coast of the United States. Biol. Bull. 1982, 162, 299–310. [Google Scholar] [CrossRef]
- Rodhouse, P.G.; Roden, C.M.; Burnell, G.M. Food Resource, Gametogenesis And Growth Of Mytilus edulis On The Shore And In Suspended Culture: Killary Harbour, Ireland. J. Mar. Biol. Assoc. United Kingd. 1984, 64, 513–529. [Google Scholar] [CrossRef]
- Staples, C.A.; Peterson, D.R.; Parkerton, T.F.; Adams, W.J. The Environmental Fate of Phthalate Esters: A Literature Review. Chemosphere 1997, 35, 667–749. [Google Scholar] [CrossRef]
- Helm, M.M.; Bourne, N.; Lovatelli, A. Hatchery Culture of Bivalves. A Practical Manual; FAO Fisheries Technical Paper No. 471; Food and Agriculture Organization (FAO) of the United Nations: Rome, Italy, 2004. [Google Scholar]
- Resgalla, C. Spawning and Multiple End Points of the Embryo-Larval Bioassay of the Blue Mussel Mytilus Galloprovincialis (Lmk). Ecotoxicology 2016, 25, 1609–1616. [Google Scholar] [CrossRef]
- Siah, A.; Pellerin, J.; Amiard, J.; Pelletier, E.; Viglino, L. Delayed Gametogenesis and Progesterone Levels in Soft-Shell Clams (Mya Arenaria) in Relation to in Situ Contamination to Organotins and Heavy Metals in the St. Lawrence River (Canada). Comp. Biochem. Physiol. 2003, 135, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Englund, V.; Heino, M. Valve Movement of Anodonta Anatina and Unio Tumidus (Bivalvia, Unionidae) in a Eutrophic Lake. Ann. Zool. Fennici 1994, 31, 257–262. [Google Scholar]
- García-March, J.R.; Sanchís Solsona, M.Á.; García-Carrascosa, A.M. Shell Gaping Behaviour of Pinna Nobilis L., 1758: Circadian and Circalunar Rhythms Revealed by in Situ Monitoring. Mar. Biol. 2008, 153, 689–698. [Google Scholar] [CrossRef]
- Ortmann, C.; Grieshaber, M.K. Energy Metabolism and Valve Closure Behaviour in the Asian Clam Corbicula Fluminea. J. Exp. Biol. 2003, 206, 4167–4178. [Google Scholar] [CrossRef]
- Winter, J.E. The Filtration Rate of Mytilus edulis and Its Dependence on Algal Concentration, Measured by a Continuous Automatic Recording Apparatus. Mar. Biol. 1973, 22, 317–328. [Google Scholar] [CrossRef]
- Widdows, J. Physiological Responses to Pollution. Mar. Pollut. Bull. 1985, 16, 129–134. [Google Scholar] [CrossRef]
- Mazerolle, M.M.J. Package ‘AICcmodavg’; 2019. Package AICcmodavg. R Package Version 2.2–2. Available online: https://CRAN.R-project.org/package=AICcmodavg (accessed on 21 February 2024).
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, 2nd ed.; Library of Congress Cataloging-in-Publication Data; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2002; Volume 172, ISBN 978-0-387-22456-5. [Google Scholar]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Springer: New York, NY, USA, 2002; ISBN 0-387-95457-0. Available online: https://www.stats.ox.ac.uk/pub/mass4/ (accessed on 27 March 2022). [CrossRef]
- Christensen, R.H.B. Ordinal—Regression Models for Ordinal Data. R Package Version 2019.12-10. 2019. Available online: https://cran.r-project.org/package=ordinal (accessed on 27 March 2022).
- Peterson, R.A. Finding Optimal Normalizing Transformations via Best Normalize. R J. 2021, 13, 310–329. [Google Scholar] [CrossRef]
- Anderson, M.J. Permutational Multivariate Analysis of Variance (PERMANOVA). Wiley StatsRef Stat. Ref. Online 2017, 1–15. [Google Scholar] [CrossRef]
- Oksanen, J.; Legendre, P.; O’Hara, B.; Stevens, M.H.H.; Oksanen, M.J.; Suggests, M. The Vegan Package. Community Ecol. Packag. 2007, 10, 631–637. [Google Scholar]
- Honkoop, P.J.C.; Van Der Meer, J. Experimentally Induced Effects of Water Temperature and Immersion Time on Reproductive Output of Bivalves in the Wadden Sea. J. Exp. Mar. Bio. Ecol. 1998, 220, 227–246. [Google Scholar] [CrossRef]
- Sprung, M. Reproduction and Fecundity of the Mussel Mytilus edulis at Helgoland (North Sea). Helgoländer Meeresunters. 1983, 36, 243–255. [Google Scholar] [CrossRef]
- Thompson, R.J. Fecundity and Reproductive Effort in the Blue Mussel (Mytilus edulis), the Sea Urchin (Strongylocentrotus droebachiensis), and the Snow Crab (Chionoecetes opilio) from Populations in Nova Scotia and Newfoundland. J. Fish. Res. Board Canada 1979, 36, 955–964. [Google Scholar] [CrossRef]
- Dixon, D.R.; Lowe, D.M.; Miller, P.I.; Villemin, G.R.; Colaço, A.; Serrão-Santos, R.; Dixon, L.R.J. Evidence of Seasonal Reproduction in the Atlantic Vent Mussel Bathymodiolus Azoricus, and an Apparent Link with the Timing of Photosynthetic Primary Production. J. Mar. Biol. Assoc. United Kingd. 2006, 86, 1363–1371. [Google Scholar] [CrossRef]
- Kautsky, N. Quantitative Studies on Gonad Cycle, Fecundity, Reproductive Output and Recruitment in a Baltic Mytilus edulis Population. Mar. Biol. 1982, 68, 143–160. [Google Scholar] [CrossRef]
- Suárez, M.P.; Alvarez, C.; Molist, P.; Juan, F.S.A.N. Particular Aspects of Gonadal Cycle and Seasonal Distribution of Gametogenic Stages of Mytilus Galloprovincialis Cultured in the Estuary of Vigo. J. Shellfish. Res. 2005, 24, 531–540. [Google Scholar]
- Tyler, P.; Young, C.M.; Dolan, E.; Arellano, S.M.; Brooke, S.D.; Baker, M. Gametogenic Periodicity in the Chemosynthetic Cold-Seep Mussel “Bathymodiolus” Childressi. Mar. Biol. 2007, 150, 829–840. [Google Scholar] [CrossRef]
- Zardi, G.I.; McQuaid, C.D.; Nicastro, K.R. Balancing Survival and Reproduction: Seasonality of Wave Action, Attachment Strength and Reproductive Output in Indigenous Perna Perna and Invasive Mytilus Galloprovincialis Mussels. Mar. Ecol. Prog. Ser. 2007, 334, 155–163. [Google Scholar] [CrossRef]
- Ye, T.; Kang, M.; Huang, Q.; Fang, C.; Chen, Y.; Shen, H.; Dong, S. Exposure to DEHP and MEHP from Hatching to Adulthood Causes Reproductive Dysfunction and Endocrine Disruption in Marine Medaka (Oryzias melastigma). Aquat. Toxicol. 2014, 146, 115–126. [Google Scholar] [CrossRef]
- Forget-Leray, J.; Landriau, I.; Minier, C.; Leboulenger, F. Impact of Endocrine Toxicants on Survival, Development, and Reproduction of the Estuarine Copepod Eurytemora Affinis (Poppe). Ecotoxicol. Environ. Saf. 2005, 60, 288–294. [Google Scholar] [CrossRef]
- Heindler, F.M.; Alajmi, F.; Huerlimann, R.; Zeng, C.; Newman, S.J.; Vamvounis, G.; van Herwerden, L. Toxic Effects of Polyethylene Terephthalate Microparticles and Di(2-Ethylhexyl)Phthalate on the Calanoid Copepod, Parvocalanus Crassirostris. Ecotoxicol. Environ. Saf. 2017, 141, 298–305. [Google Scholar] [CrossRef]
- Matthiessen, P.; Waldock, R.; Thain, J.E.; Waite, M.E.; Scropehowe, S. Changes in Periwinkle (Littorina littorea) Populations Following the Ban on TBT-Based Antifoulings on Small Boats in the United Kingdom. Ecotoxicol. Environ. Saf. 1995, 30, 180–194. [Google Scholar] [CrossRef]
- Jensen, K.M.; Kahl, M.D.; Makynen, E.A.; Korte, J.J.; Leino, R.L.; Butterworth, B.C.; Ankley, G.T. Characterization of Responses to the Antiandrogen Flutamide in a Short-Term Reproduction Assay with the Fathead Minnow. Aquat. Toxicol. 2004, 70, 99–110. [Google Scholar] [CrossRef]
- Segner, H.; Caroll, K.; Fenske, M.; Janssen, C.R.; Maack, G.; Pascoe, D.; Schäfers, C.; Vandenbergh, G.F.; Watts, M.; Wenzel, A. Identification of Endocrine-Disrupting Effects in Aquatic Vertebrates and Invertebrates: Report from the European IDEA Project. Ecotoxicol. Environ. Saf. 2003, 54, 302–314. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.L.; Janz, M. De v Elopmental Estrogenic Exposure in Zebrafish (Danio rerio): I. Effects on Sex Ratio and Breeding Success. Aquat. Toxicol. 2003, 63, 417–429. [Google Scholar] [CrossRef]
- Santos, E.M.; Paull, G.C.; Van Look, K.J.W.; Workman, V.L.; Holt, W.V.; Van Aerle, R.; Kille, P.; Tyler, C.R. Gonadal Transcriptome Responses and Physiological Consequences of Exposure to Oestrogen in Breeding Zebrafish (Danio rerio). Aquat. Toxicol. 2007, 83, 134–142. [Google Scholar] [CrossRef]
- Amaral Mendes, J.J. The Endocrine Disrupters: A Major Medical Challenge. Food Chem. Toxicol. 2002, 40, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Markey, C.M.; Rubin, B.S.; Soto, A.M.; Sonnenschein, C. Endocrine Disruptors: From Wingspread to Environmental Developmental Biology. J. Steroid Biochem. Mol. Biol. 2002, 83, 235–244. [Google Scholar] [CrossRef]
- Agathokleous, E.; Kitao, M.; Calabrese, E.J. Environmental Hormesis and Its Fundamental Biological Basis: Rewriting the History of Toxicology. Environ. Res. 2018, 165, 274–278. [Google Scholar] [CrossRef]
- Zhang, Y.; Shen, G.; Yu, Y.; Zhu, H. The Hormetic Effect of Cadmium on the Activity of Antioxidant Enzymes in the Earthworm Eisenia Fetida. Environ. Pollut. 2009, 157, 3064–3068. [Google Scholar] [CrossRef]
- Agathokleous, E. Environmental Hormesis, a Fundamental Non-Monotonic Biological Phenomenon with Implications in Ecotoxicology and Environmental Safety. Ecotoxicol. Environ. Saf. 2018, 148, 1042–1053. [Google Scholar] [CrossRef]
- Calabrese, E.J. Overcompensation Stimulation: A Mechanism for Hormetic Effects. Crit. Rev. Toxicol. 2001, 31, 425–470. [Google Scholar] [CrossRef]
- Costantini, D.; Borremans, B. The Linear No-Threshold Model Is Less Realistic than Threshold or Hormesis-Based Models: An Evolutionary Perspective. Chem. Biol. Interact. 2019, 301, 26–33. [Google Scholar] [CrossRef]
- Kim, S.A.; Lee, Y.M.; Choi, J.Y.; Jacobs, D.R.; Lee, D.H. Evolutionarily Adapted Hormesis-Inducing Stressors Can Be a Practical Solution to Mitigate Harmful Effects of Chronic Exposure to Low Dose Chemical Mixtures. Environ. Pollut. 2018, 233, 725–734. [Google Scholar] [CrossRef]
- De Nicola, E.; Meriç, S.; Gallo, M.; Iaccarino, M.; Della Rocca, C.; Lofrano, G.; Russo, T.; Pagano, G. Vegetable and Synthetic Tannins Induce Hormesis/Toxicity in Sea Urchin Early Development and in Algal Growth. Environ. Pollut. 2007, 146, 46–54. [Google Scholar] [CrossRef]
- Agathokleous, E.; Calabrese, E.J. A Global Environmental Health Perspective and Optimisation of Stress. Sci. Total Environ. 2020, 704, 135263. [Google Scholar] [CrossRef]
- Pipe, R.K. Oogenesis in the Marine Mussel Mytilus edulis: An Ultrastructural Study. Mar. Biol. 1987, 95, 405–414. [Google Scholar] [CrossRef]
- Cuenca, L.; Shin, N.; Lascarez-lagunas, L.I.; Martinez-Garcia, M.; Nadarajan, S.; Karthikraj, R.; Kannan, K. Environmentally-Relevant Exposure to Diethylhexyl Phthalate (DEHP) Alters Regulation of Double-Strand Break Formation and Crossover Designation Leading to Germline Dysfunction in Caenorhabditis Elegans. PLoS Genet. 2020, 16, e1008529. [Google Scholar] [CrossRef]
- Li, Q.; Osada, M.; Suzuki, T.; Mori, K. Changes in Vitellin during Oogenesis and Effect of Estradiol-17β on Vitellogenesis in the Pacific Oyster Crassostrea Gigas. Invertebr. Reprod. Dev. 1998, 33, 87–93. [Google Scholar] [CrossRef]
- Wang, S.; Zhong, Z.; Li, Z.; Wang, X.; Gu, H.; Huang, W.; Fang, J.K.H.; Shi, H.; Hu, M.; Wang, Y. Physiological Effects of Plastic Particles on Mussels Are Mediated by Food Presence. J. Hazard. Mater. 2021, 404, 124136. [Google Scholar] [CrossRef]


| Name of Treatment | Temperature (°C) | pH (Units) |
|---|---|---|
| CTRL (0 µg DEHP/L) | 12.8 ± 0.4 | 7.9 ± 0.1 |
| LOW DEHP (0.5 µg DEHP/L) | 12.6 ± 0.3 | 7.8 ± 0.2 |
| High DEHP (50 µg DEHP/L) | 12.7 ± 0.3 | 7.8 ± 0.1 |
| Model (K) | AICc; ΔAIC; AICcWT | Cum WT; LL |
|---|---|---|
| SEX + DEHP (4) | 118.15; 0.00; 0.47 | 0.47; −54.99 |
| SEX (3) | 118.82; 0.67; 0.67 | 0.80; −56.35 |
| SEX * DEHP (5) | 119.89; 1.74; 0.20 | 0.99; −54.82 |
| DEHP (3) | 126.92; 8.78; 0.01 | 1.0; −60.41 |
| Variable | Value; Std. Error | t-Value; p Value |
|---|---|---|
| SEX | −1.56; 0.50 | −3.14; 0.002 |
| DEHP | −0.50; 0.31 | −1.62; 0.104 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mincarelli, L.F.; Turner, A.; Anderson, G.; Wollenberg Valero, K. Exposure to Plasticiser DEHP Affects Eggs Spawned by Blue Mussels: A Possible Risk to Fertilisation? Toxics 2024, 12, 172. https://doi.org/10.3390/toxics12030172
Mincarelli LF, Turner A, Anderson G, Wollenberg Valero K. Exposure to Plasticiser DEHP Affects Eggs Spawned by Blue Mussels: A Possible Risk to Fertilisation? Toxics. 2024; 12(3):172. https://doi.org/10.3390/toxics12030172
Chicago/Turabian StyleMincarelli, Luana Fiorella, Alexander Turner, George Anderson, and Katharina Wollenberg Valero. 2024. "Exposure to Plasticiser DEHP Affects Eggs Spawned by Blue Mussels: A Possible Risk to Fertilisation?" Toxics 12, no. 3: 172. https://doi.org/10.3390/toxics12030172
APA StyleMincarelli, L. F., Turner, A., Anderson, G., & Wollenberg Valero, K. (2024). Exposure to Plasticiser DEHP Affects Eggs Spawned by Blue Mussels: A Possible Risk to Fertilisation? Toxics, 12(3), 172. https://doi.org/10.3390/toxics12030172







