Associations between Urinary Mercury/Cadmium Concentrations and Anthropometric Features in Korean Children
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Ethical Approval
2.3. Assessment of Anthropometry
2.4. Measurement of Urinary Mercury and Cadmium
2.5. Covariates
2.6. Adjustment of Urinary Dilution
2.7. Statistical Analysis
3. Results
3.1. General Characteristics of the Study Participants
3.2. Distribution of Urinary Mercury and Cadmium Concentrations
3.3. Association of Urinary Heavy Metal Concentrations with Height and BMI Z-Scores
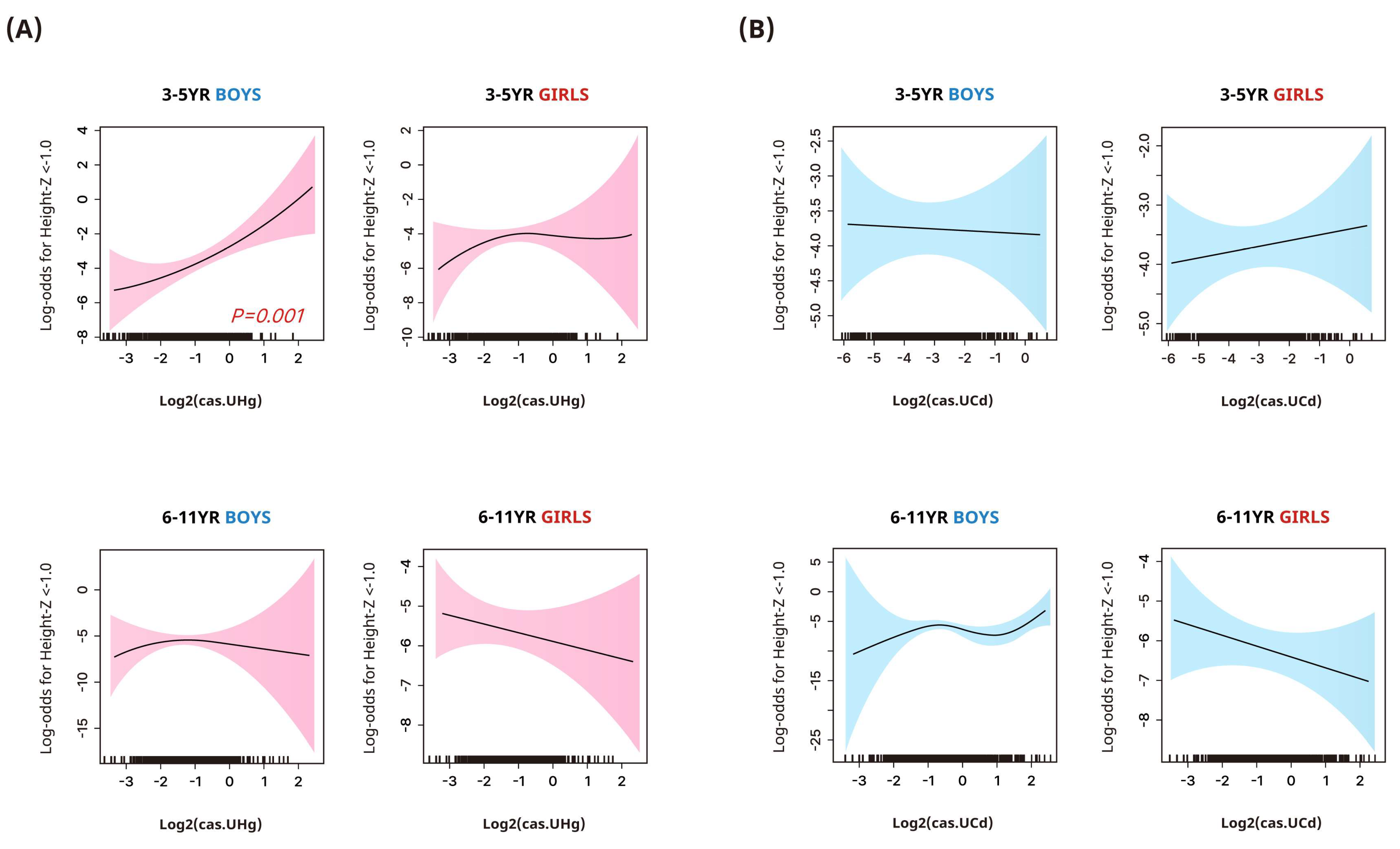
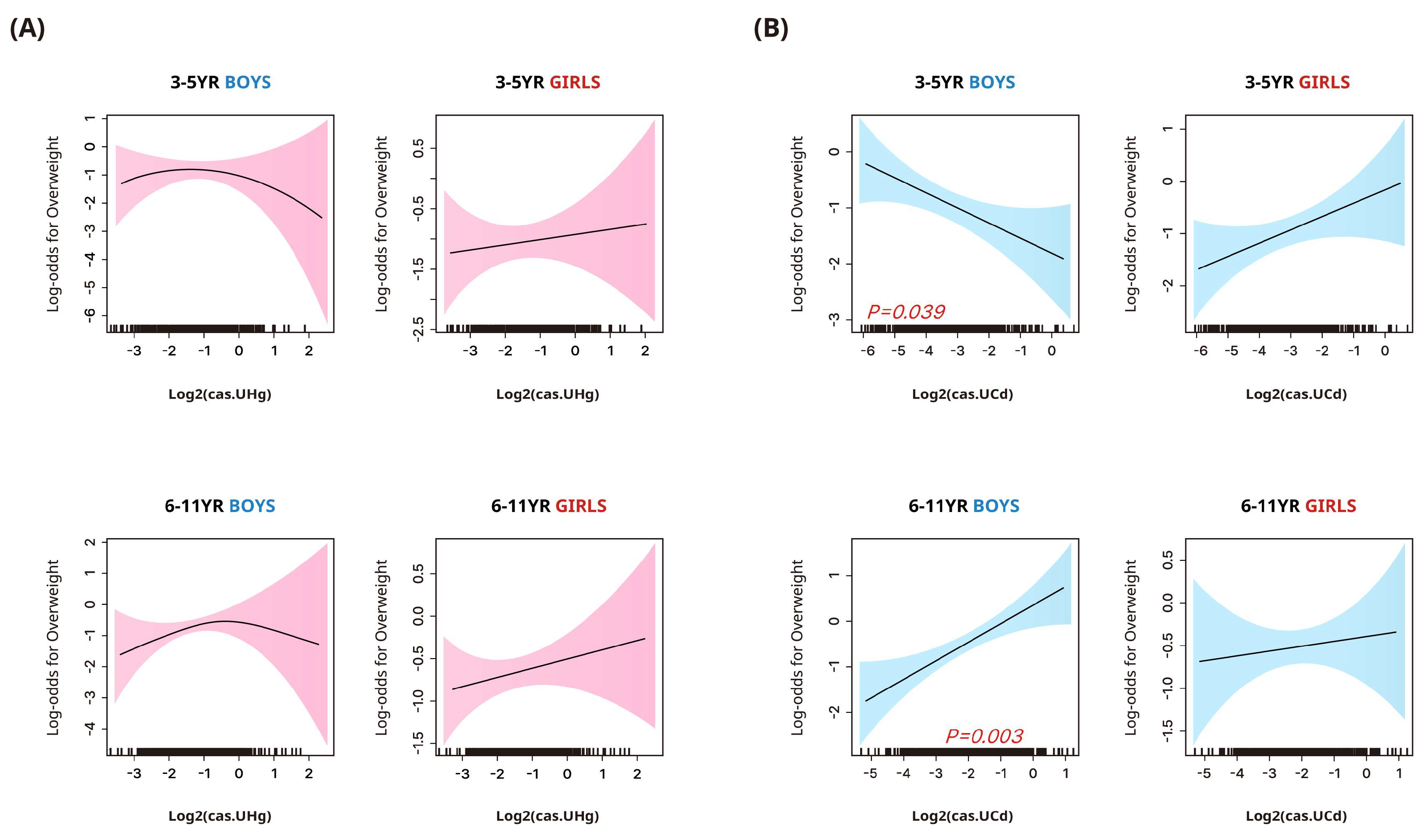
3.4. Associations between Urinary Heavy Metal Concentrations and Stunted Height or Overweight/Obesity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ali, H.; Khan, E.; Ilahi, I. Environmental chemistry and ecotoxicology of hazardous heavy metals: Environmental persistence, toxicity, and bioaccumulation. J. Chem. 2019, 2019, 6730305. [Google Scholar] [CrossRef]
- Zeng, X.; Xu, X.; Qin, Q.; Ye, K.; Wu, W.; Huo, X. Heavy metal exposure has adverse effects on the growth and development of preschool children. Environ. Geochem. Health 2019, 41, 309–321. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef] [PubMed]
- Alvarado-Cruz, I.; Alegría-Torres, J.A.; Montes-Castro, N.; Jiménez-Garza, O.; Quintanilla-Vega, B. Environmental epigenetic changes, as risk factors for the development of diseases in children: A systematic review. Ann. Glob. Health 2018, 84, 212–224. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, J.H.; Moon, H.B.; Park, J.; Choi, G.; Kim, S. Effects of mercury exposure on fetal body burden and its association with infant growth. Environ. Res. 2023, 217, 114780. [Google Scholar] [CrossRef]
- Sughis, M.; Penders, J.; Haufroid, V.; Nemery, B.; Nawrot, T.S. Bone resorption and environmental exposure to cadmium in children: A cross—Sectional study. Environ. Health 2011, 10, 104. [Google Scholar] [CrossRef]
- Nordberg, G.F.; Costa, M. Handbook on the Toxicology of Metals: Volume II; Specific Metals; Academic Press: Cambridge, MA, USA, 2021. [Google Scholar]
- American Academy of Pediatrics Council on Environmental Health. Chapter 4: Chemical and physical exposures. In Pediatric Environmental Health, 4th ed.; Etzel, R.A., Balk, S.J., Eds.; American Academy of Pediatrics: Itasca, IL, USA, 2019; pp. 585–609. [Google Scholar]
- Gardner, R.M.; Kippler, M.; Tofail, F.; Bottai, M.; Hamadani, J.; Grandér, M.; Nermell, B.; Palm, B.; Rasmussen, K.M.; Vahter, M. Environmental exposure to metals and children’s growth to age 5 years: A prospective cohort study. Am. J. Epidemiol. 2013, 177, 1356–1367. [Google Scholar] [CrossRef]
- Jedrychowski, W.A.; Perera, F.P.; Majewska, R.; Mrozek-Budzyn, D.; Mroz, E.; Roen, E.L.; Sowa, A.; Jacek, R. Depressed height gain of children associated with intrauterine exposure to polycyclic aromatic hydrocarbons (PAH) and heavy metals: The cohort prospective study. Environ. Res. 2015, 136, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Marques, R.C.; Dórea, J.G.; Leão, R.S.; Dos Santos, V.G.; Bueno, L.; Marques, R.C.; Brandão, K.G.; Palermo, E.F.; Guimarães, J.R. Role of methylmercury exposure (from fish consumption) on growth and neurodevelopment of children under 5 years of age living in a transitioning (tin-mining) area of the western Amazon, Brazil. Arch. Environ. Contam. Toxicol. 2012, 62, 341–350. [Google Scholar] [CrossRef]
- Gao, Z.Y.; Li, M.M.; Wang, J.; Yan, J.; Zhou, C.C.; Yan, C.H. Blood mercury concentration, fish consumption and anthropometry in Chinese children: A national study. Environ. Int. 2018, 110, 14–21. [Google Scholar] [CrossRef]
- Yang, H.; Huo, X.; Yekeen, T.A.; Zheng, Q.; Zheng, M.; Xu, X. Effects of lead and cadmium exposure from electronic waste on child physical growth. Environ. Sci. Pollut. Res. Int. 2013, 20, 4441–4447. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Yun, S.; Hwang, S.S.; Shim, J.O.; Chae, H.W.; Lee, Y.J.; Lee, J.H.; Kim, S.C.; Lim, D.; Yang, S.W.; et al. The 2017 Korean national growth charts for children and adolescents: Development, improvement, and prospects. Korean J. Pediatr. 2018, 61, 135–149. [Google Scholar] [CrossRef] [PubMed]
- NIER (National Institute of Environmental Research). Manual for Laboratory Procedures on Cycle 3 of the Korean National Environmental Health Survey (Heavy Metals); Ministry of the Environment, National Institute of Environmental Research, Environmental Health Research Division: Incheon, Republic of Korea, 2018. [Google Scholar]
- Dwyer, G.M.; Hardy, L.L.; Peat, J.K.; Baur, L.A. The validity and reliability of a home environment preschool-age physical activity questionnaire (Pre-PAQ). Int. J. Behav. Nutr. Phys. Act. 2011, 8, 86. [Google Scholar] [CrossRef] [PubMed]
- Inzaghi, E.; Pampanini, V.; Deodati, A.; Cianfarani, S. The effects of nutrition on linear growth. Nutrients 2022, 14, 1752. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, K.M.; Upson, K.; Cook, N.R.; Weinberg, C.R. Environmental chemicals in urine and blood: Improving methods for creatinine and lipid adjustment. Environ. Health Perspect. 2016, 124, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention; January 2019. Fourth National Report on Human Exposure to Environmental Chemicals. Updated Tables; 1:2019. Available online: https://stacks.cdc.gov/view/cdc/75822 (accessed on 12 January 2024).
- Canada, H. Fourth Report on Human Biomonitoring of Environmental Chemicals in Canada. Health Canada; 2017. Available online: https://www.canada.ca/en/health-canada/services/environmental-workplace-health/reports-publications/environmental-contaminants/fourth-report-human-biomonitoring-environmental-chemicals-canada.html (accessed on 12 January 2024).
- Vogel, N.; Murawskim, A.; Schmied-Tobies, M.I.H.; Rucic, E.; Doyle, U.; Kämpfe, A.; Höra, C.; Hildebrand, J.; Schäfer, M.; Drexler, H.; et al. Lead, cadmium, mercury, and chromium in urine and blood of children and adolescents in Germany – human biomonitoring results of the German Environmental survey 2014–2017 (GerES V). Int. J. Hyg. Environ. Health 2021, 237, 113822. [Google Scholar] [PubMed]
- Rhee, S. Control of mercury emissions: Policies, technologies, and future trends. Energy Emiss. Control Technol. 2016, 4, 1–15. [Google Scholar] [CrossRef]
- Outridge, P.M.; Mason, R.P.; Wang, F.; Guerrero, S.; Heimbürger-Boavida, L.E. Updated global and oceanic mercury budgets for the United Nations global mercury assessment 2018. Environ. Sci. Technol. 2018, 52, 11466–11477. [Google Scholar] [CrossRef]
- Han, Y.-J.; Kim, J.-E.; Kim, P.-R.; Kim, W.-J.; Yi, S.-M.; Seo, Y.-S.; Kim, S. General trends of atmospheric mercury concentrations in urban and rural areas in Korea and characteristics of high-concentration events. Atmos. Environ. 2014, 94, 754–764. [Google Scholar] [CrossRef]
- Sung, J.H.; Oh, I.; Kim, A.; Lee, J.; Sim, C.S.; Yoo, C.; Park, S.J.; Kim, G.B.; Kim, Y. Environmental and body concentrations of heavy metals at sites near and distant from industrial complexes in Ulsan, Korea. J. Korean Med. Sci. 2018, 33, e33. [Google Scholar] [CrossRef]
- Loupa, G.; Polyzou, C.; Zarogianni, A.M.; Ouzounis, K.; Rapsomanikis, S. Indoor and outdoor elemental mercury: A comparison of three different cases. Environ. Monit. Assess. 2017, 189, 72. [Google Scholar] [CrossRef] [PubMed]
- Dahmardeh Behrooz, R.; Tashakor, M.; Asvad, R.; Esmaili-Sari, A.; Kaskaoutis, D.G. Characteristics and health risk assessment of mercury exposure via indoor and outdoor household dust in three Iranian cities. Atmosphere 2022, 13, 583. [Google Scholar] [CrossRef]
- Burm, E.; Song, I.; Ha, M.; Kim, Y.M.; Lee, K.J.; Kim, H.C.; Lim, S.; Kim, S.Y.; Lee, C.G.; Kim, S.Y.; et al. Representative levels of blood lead, mercury, and urinary cadmium in youth: Korean Environmental Health Survey in Children and Adolescents (KorEHS-C), 2012–2014. Int. J. Hyg. Environ. Health 2016, 219, 412–418. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, P.; Zhao, F. Dietary cadmium exposure, risks to human health and mitigation strategies. Crit. Rev. Environ. Sci. Technol. 2023, 53, 939–963. [Google Scholar] [CrossRef]
- Cho, I.-G.; Park, M.-K.; Cho, H.-K.; Jeon, J.-W.; Lee, S.-E.; Choi, S.-D. Characteristics of metal contamination in paddy soils from three industrial cities in South Korea. Environ. Geochem. Health 2019, 41, 1895–1907. [Google Scholar] [CrossRef]
- Korea Institute of Chemical Safety. Comprehensive Chemical Information Service for Proper Management and Use of Chemicals. Available online: https://icis.me.go.kr/main.do (accessed on 11 February 2024).
- Dack, K.; Fell, M.; Taylor, C.M.; Havdahl, A.; Lewis, S.J. Mercury and prenatal growth: A systematic review. Int. J. Environ. Res. Public Health 2021, 18, 7140. [Google Scholar] [CrossRef]
- Choi, J.; Chang, J.Y.; Hong, J.; Shin, S.; Park, J.S.; Oh, S. Low-level toxic metal exposure in healthy weaning-age infants: Association with growth, dietary intake, and iron deficiency. Int. J. Environ. Res. Public Health 2017, 14, 388. [Google Scholar] [CrossRef] [PubMed]
- Flannery, B.M.; Schaefer, H.R.; Middleton, K.B. A scoping review of infant and children health effects associated with cadmium exposure. Regul. Toxicol. Pharmacol. 2022, 131, 105155. [Google Scholar] [CrossRef]
- Malin Igra, A.M.; Warnqvist, A.; Rahman, S.M.; Ekström, E.C.; Rahman, A.; Vahter, M.; Kippler, M. Environmental metal exposure and growth to 10 years of age in a longitudinal mother-child cohort in rural Bangladesh. Environ. Int. 2021, 156, 106738. [Google Scholar] [CrossRef]
- Moon, M.K.; Lee, I.; Lee, A.; Park, H.; Kim, M.J.; Kim, S.; Cho, Y.H.; Hong, S.; Yoo, J.; Cheon, G.J.; et al. Lead, mercury, and cadmium exposures are associated with obesity but not with diabetes mellitus: Korean National Environmental Health Survey (KoNEHS) 2015–2017. Environ. Res. 2022, 204, 111888. [Google Scholar] [CrossRef]
- Filippini, T.; Michalke, B.; Malagoli, C.; Grill, P.; Bottecchi, I.; Malavolti, M.; Vescovi, L.; Sieri, S.; Krogh, V.; Cherubini, A.; et al. Determinants of serum cadmium levels in a northern Italy community: A cross-sectional study. Environ. Res. 2016, 150, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Bulka, C.M.; Persky, V.W.; Daviglus, M.L.; Durazo-Arvizu, R.A.; Argos, M. Multiple metal exposures and metabolic syndrome: A cross-sectional analysis of the National Health and Nutrition Examination Survey 2011–2014. Environ. Res. 2019, 168, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.; Liu, Q.; Wu, M.; Bi, J.; Qin, X.; Fang, Q.; Wan, Z.; Lv, Y.; Wang, Y.; Song, L. Exposure to metal mixtures and overweight or obesity among Chinese adults. Biol. Trace Elem. Res. 2023, 201, 3697–3705. [Google Scholar] [CrossRef] [PubMed]
- Ba, Q.; Li, M.; Chen, P.; Huang, C.; Duan, X.; Lu, L.; Li, J.; Chu, R.; Xie, D.; Song, H.; et al. Sex-dependent effects of cadmium exposure in early life on gut microbiota and fat accumulation in mice. Environ. Health Perspect. 2017, 125, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Green, A.J.; Hoyo, C.; Mattingly, C.J.; Luo, Y.; Tzeng, J.Y.; Murphy, S.K.; Buchwalter, D.B.; Planchart, A. Cadmium exposure increases the risk of juvenile obesity: A human and zebrafish comparative study. Int. J. Obes. 2018, 42, 1285–1295. [Google Scholar] [CrossRef]
- Attia, S.M.; Das, S.C.; Varadharajan, K.; Al-Naemi, H.A. White adipose tissue as a target for cadmium toxicity. Front. Pharmacol. 2022, 13, 1010817. [Google Scholar] [CrossRef]
- Lazarevic, N.; Barnett, A.G.; Sly, P.D.; Knibbs, L.D. Statistical methodology in studies of prenatal exposure to mixtures of endocrine-disrupting chemicals: A review of existing approaches and new alternatives. Environ. Health Perspect. 2019, 127, 26001. [Google Scholar] [CrossRef]
- Wells, E.M.; Herbstman, J.B.; Lin, Y.H.; Jarrett, J.; Verdon, C.P.; Ward, C.; Caldwell, K.L.; Hibbeln, J.R.; Witter, F.R.; Halden, R.U.; et al. Cord blood methylmercury and fetal growth outcomes in Baltimore newborns: Potential confounding and effect modification by omega-3 fatty acids, selenium, and sex. Environ. Health Perspect. 2016, 124, 373–379. [Google Scholar] [CrossRef]
- Ballester, F.; Iñiguez, C.; Murcia, M.; Guxens, M.; Basterretxea, M.; Rebagliato, M.; Vioque, J.; Lertxundi, A.; Fernandez-Somoano, A.; Tardon, A.; et al. Prenatal exposure to mercury and longitudinally assessed fetal growth: Relation and effect modifiers. Environ. Res. 2018, 160, 97–106. [Google Scholar] [CrossRef]
- Tan, S.W.; Meiller, J.C.; Mahaffey, K.R. The endocrine effects of mercury in humans and wildlife. Crit. Rev. Toxicol. 2009, 39, 228–269. [Google Scholar] [CrossRef]
- Romano, M.E.; Enquobahrie, D.A.; Simpson, C.; Checkoway, H.; Williams, M.A. Maternal body burden of cadmium and offspring size at birth. Environ. Res. 2016, 147, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Kippler, M.; Engström, K.; Mlakar, S.J.; Bottai, M.; Ahmed, S.; Hossain, M.B.; Raqib, R.; Vahter, M.; Broberg, K. Sex-specific effects of early life cadmium exposure on DNA methylation and implications for birth weight. Epigenetics 2013, 8, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa-Jotaki, S.; Sakurai, K.; Eguchi, A.; Tanabe, H.; Watanabe, M.; Mori, C. Association between mercury in cord serum and sex-specific DNA methylation in cord tissues. J. Dev. Orig. Health Dis. 2021, 12, 124–131. [Google Scholar] [CrossRef] [PubMed]
| Total | 3–5 Years | 6–11 Years | p-Value | |
|---|---|---|---|---|
| n | 1458 | 571 | 887 | |
| Male (%) | 51.6% | 51.4% | 51.7% | 0.839 |
| Age (year) | 6.73 ± 0.22 | 4.01 ± 0.01 | 8.49 ± 0.11 | <0.001 |
| Height z-score | 0.83 ± 0.04 | 0.74 ± 0.07 | 0.89 ± 0.05 | 0.115 |
| Height z-score < −1.0 | 5.0% | 6.9% | 3.8% | 0.020 |
| BMI (kg/m2) | 17.4 ± 0.1 | 16.1 ± 0.1 | 18.3 ± 0.1 | <0.001 |
| BMI z-score | 0.29 ± 0.04 | 0.17 ± 0.08 | 0.36 ± 0.05 | 0.073 |
| BMI status | 0.023 | |||
| Normal | 73.5% | 77.8% | 70.8% | |
| Overweight/obesity | 26.5% | 22.2% | 29.2% | |
| Family income (KRW) | 0.752 | |||
| <3,000,000 | 21.8% | 21.8% | 21.7% | |
| 3,000,000–<5,000,000 | 38.2% | 40.3% | 36.8% | |
| 5,000,000–<7,000,000 | 25.6% | 24.1% | 26.6% | |
| ≥7,000,000 | 14.4% | 13.8% | 14.9% | |
| ETS exposure at home | 6.4% | 4.9% | 7.4% | 0.082 |
| Regular physical activity | 39.0% | 33.6% | 42.4% | 0.006 |
| Grain intake per day | 0.098 | |||
| ≤2 times | 38.2% | 35.1% | 40.2% | |
| ≥3 times | 61.8% | 64.9% | 59.8% | |
| Meat consumption per week | 0.853 | |||
| ≤once | 72.5% | 71.9% | 73.0% | |
| 2–3 times | 21.9% | 22.1% | 21.7% | |
| ≥4 times | 5.6% | 6.0% | 5.3% | |
| Milk consumption per week | 0.480 | |||
| ≤3 times | 28.6% | 26.8% | 29.8% | |
| 4–6 times | 26.0% | 25.9% | 26.1% | |
| ≥7 times | 45.4% | 47.3% | 44.1% |
| Urinary Heavy Metal Concentration | |||||
|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | p-Trend | |
| Outcome: Height z-score | |||||
| Mercury | |||||
| 3–5 years | |||||
| Boys | Reference | −0.373 (−0.766, 0.021) | −0.467 (−0.854, −0.081) | −0.799 (−1.124, −0.475) | <0.001 |
| Girls | Reference | −0.051 (−0.513, 0.412) | −0.336 (−0.825, 0.154) | −0.264 (−0.775, 0.247) | 0.204 |
| 6–11 years | |||||
| Boys | Reference | −0.265 (−0.518, −0.012) | −0.198 (−0.451, 0.056) | −0.020 (−0.326, 0.286) | 0.939 |
| Girls | Reference | 0.029 (−0.309, 0.367) | 0.135 (−0.197, 0.466) | −0.229 (−0.564, 0.106) | 0.344 |
| Cadmium | |||||
| 3–5 years | |||||
| Boys | Reference | −0.409 (−0.845, 0.027) | 0.280 (−0.133, 0.693) | −0.005 (−0.366, 0.356) | 0.298 |
| Girls | Reference | 0.338 (−0.106, 0.781) | 0.670 (0.320, 1.020) | 0.433 (0.008, 0.857) | 0.015 |
| 6–11 years | |||||
| Boys | Reference | 0.199 (−0.128, 0.527) | 0.119 (−0.228, 0.466) | −0.073 (−0.405, 0.259) | 0.532 |
| Girls | Reference | 0.328 (−0.019, 0.636) | 0.11 (−0.165, 0.386) | 0.019 (−0.316, 0.353) | 0.806 |
| Outcome: BMI z-score | |||||
| Mercury | |||||
| 3–5 years | |||||
| Boys | Reference | −0.312 (−0.883, 0.259) | 0.133 (−0.497, 0.763) | −0.013 (−0.542, 0.517) | 0.652 |
| Girls | Reference | −0.378 (−0.796, 0.039) | −0.023 (−0.486, 0.44) | 0.082 (−0.421, 0.585) | 0.486 |
| 6–11 years | |||||
| Boys | Reference | 0.108 (−0.265, 0.482) | 0.418 (0.036, 0.800) | 0.413 (0.049, 0.777) | 0.012 |
| Girls | Reference | 0.141 (−0.261, 0.543) | 0.238 (−0.142, 0.618) | 0.294 (−0.102, 0.689) | 0.098 |
| Cadmium | |||||
| 3–5 years | |||||
| Boys | Reference | −0.453 (−0.962, 0.057) | −0.138 (−0.663, 0.386) | −0.676 (−1.236, −0.116) | 0.064 |
| Girls | Reference | 0.084 (−0.355, 0.523) | 0.145 (−0.337, 0.627) | 0.519 (0.103, 0.936) | 0.026 |
| 6–11 years | |||||
| Boys | Reference | −0.065 (−0.392, 0.263) | −0.032 (−0.386, 0.321) | 0.477 (0.099, 0.855) | 0.012 |
| Girls | Reference | 0.206 (−0.159, 0.571) | −0.18 (−0.527, 0.166) | 0.371 (−0.006, 0.736) | 0.266 |
| Urinary Heavy Metal Concentration | |||||
|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | p-Trend | |
| Outcome: Height z-score < −1.0 | |||||
| Mercury | |||||
| 3–5 years | |||||
| Boys | 1.00 | 2.04 (0.28, 14.92) | 1.00 (0.11, 8.9) | 4.4 (0.86, 22.55) | 0.090 |
| Girls | 1.00 | 3.11 (0.70, 13.9) | 3.84 (0.7, 21.05) | 1.23 (0.18, 8.32) | 0.872 |
| 6–11 years | |||||
| Boys | 1.00 | 2.08 (0.4, 10.79) | 1.7 (0.34, 8.62) | 0.65 (0.11, 3.76) | 0.589 |
| Girls | 1.00 | 0.68 (0.17, 2.62) | 0.6 (0.16, 2.28) | 1.07 (0.34, 3.36) | 0.979 |
| Cadmium | |||||
| 3–5 years | |||||
| Boys | 1.00 | 3.47 (0.74, 16.32) | 1.31 (0.22, 7.86) | 1.68 (0.30, 9.52) | 0.946 |
| Girls | 1.00 | 0.64 (0.12, 3.42) | 0.17 (0.02, 1.28) | 1.84 (0.55, 6.13) | 0.497 |
| 6–11 years | |||||
| Boys | 1.00 | 4.19 (0.74, 23.56) | 0.99 (0.15, 6.73) | 1.11 (0.15, 8.53) | 0.346 |
| Girls | 1.00 | 0.34 (0.11, 1.08) | 0.41 (0.12, 1.48) | 0.42 (0.12, 1.44) | 0.175 |
| Outcome: Overweight/obesity | |||||
| Mercury | |||||
| 3–5 years | |||||
| Boys | 1.00 | 0.83 (0.26, 2.68) | 1.04 (0.38, 2.81) | 0.96 (0.33, 2.83) | 0.954 |
| Girls | 1.00 | 0.79 (0.26, 2.34) | 1.27 (0.45, 3.6) | 1.3 (0.39, 4.38) | 0.555 |
| 6–11 years | |||||
| Boys | 1.00 | 1.09 (0.43, 2.78) | 1.41 (0.63, 3.12) | 1.73 (0.76, 3.97) | 0.161 |
| Girls | 1.00 | 1.23 (0.51, 3) | 1.63 (0.64, 4.14) | 1.7 (0.6, 4.83) | 0.236 |
| Cadmium | |||||
| 3–5 years | |||||
| Boys | 1.00 | 0.49 (0.2, 1.18) | 0.78 (0.33, 1.85) | 0.41 (0.13, 1.28) | 0.205 |
| Girls | 1.00 | 0.86 (0.21, 3.58) | 1.42 (0.47, 4.3) | 1.85 (0.66, 5.2) | 0.200 |
| 6–11 years | |||||
| Boys | 1.00 | 0.77 (0.34, 1.76) | 1.27 (0.64, 2.51) | 2.44 (1.19, 5.00) | 0.009 |
| Girls | 1.00 | 1.20 (0.64, 2.26) | 0.86 (0.35, 2.14) | 1.13 (0.51, 2.5) | 0.985 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, M.W.; Kim, H.-B.; Kwon, A.; Park, M.J.; Kim, S.-H. Associations between Urinary Mercury/Cadmium Concentrations and Anthropometric Features in Korean Children. Toxics 2024, 12, 175. https://doi.org/10.3390/toxics12030175
Shin MW, Kim H-B, Kwon A, Park MJ, Kim S-H. Associations between Urinary Mercury/Cadmium Concentrations and Anthropometric Features in Korean Children. Toxics. 2024; 12(3):175. https://doi.org/10.3390/toxics12030175
Chicago/Turabian StyleShin, Min Won, Hyo-Bin Kim, Ahreum Kwon, Mi Jung Park, and Shin-Hye Kim. 2024. "Associations between Urinary Mercury/Cadmium Concentrations and Anthropometric Features in Korean Children" Toxics 12, no. 3: 175. https://doi.org/10.3390/toxics12030175
APA StyleShin, M. W., Kim, H.-B., Kwon, A., Park, M. J., & Kim, S.-H. (2024). Associations between Urinary Mercury/Cadmium Concentrations and Anthropometric Features in Korean Children. Toxics, 12(3), 175. https://doi.org/10.3390/toxics12030175







