Effects of Indigo Carmine on Growth, Cell Division, and Morphology of Allium cepa L. Root Tip
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material
2.3. Experimental Design
2.4. Analysis of Cell Morphology Using Fluorescent Microscopy
2.5. Data and Statistical Analysis
3. Results
3.1. Root Growth
3.2. Mitotic Index and Mitotic Inhibition
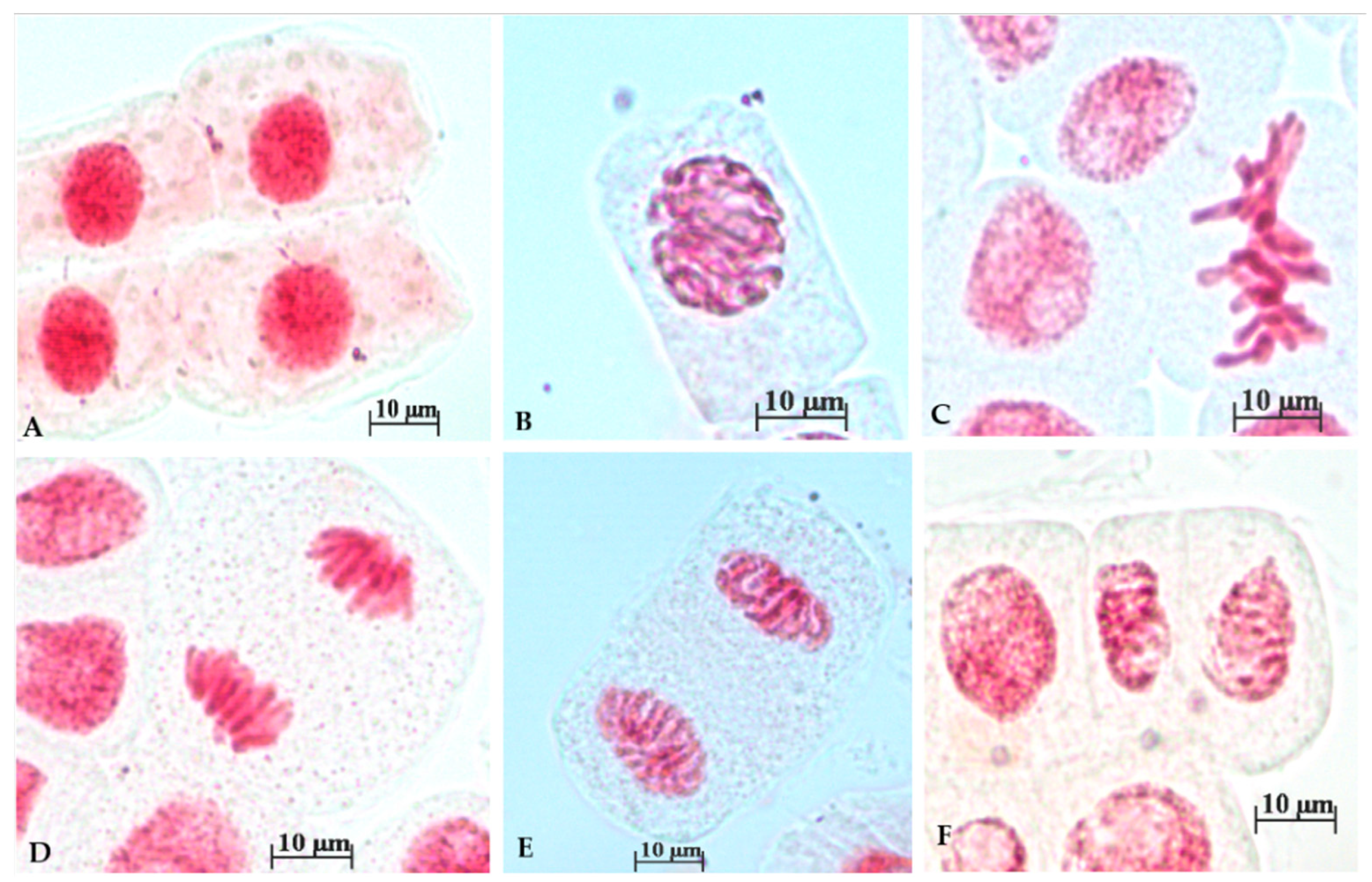
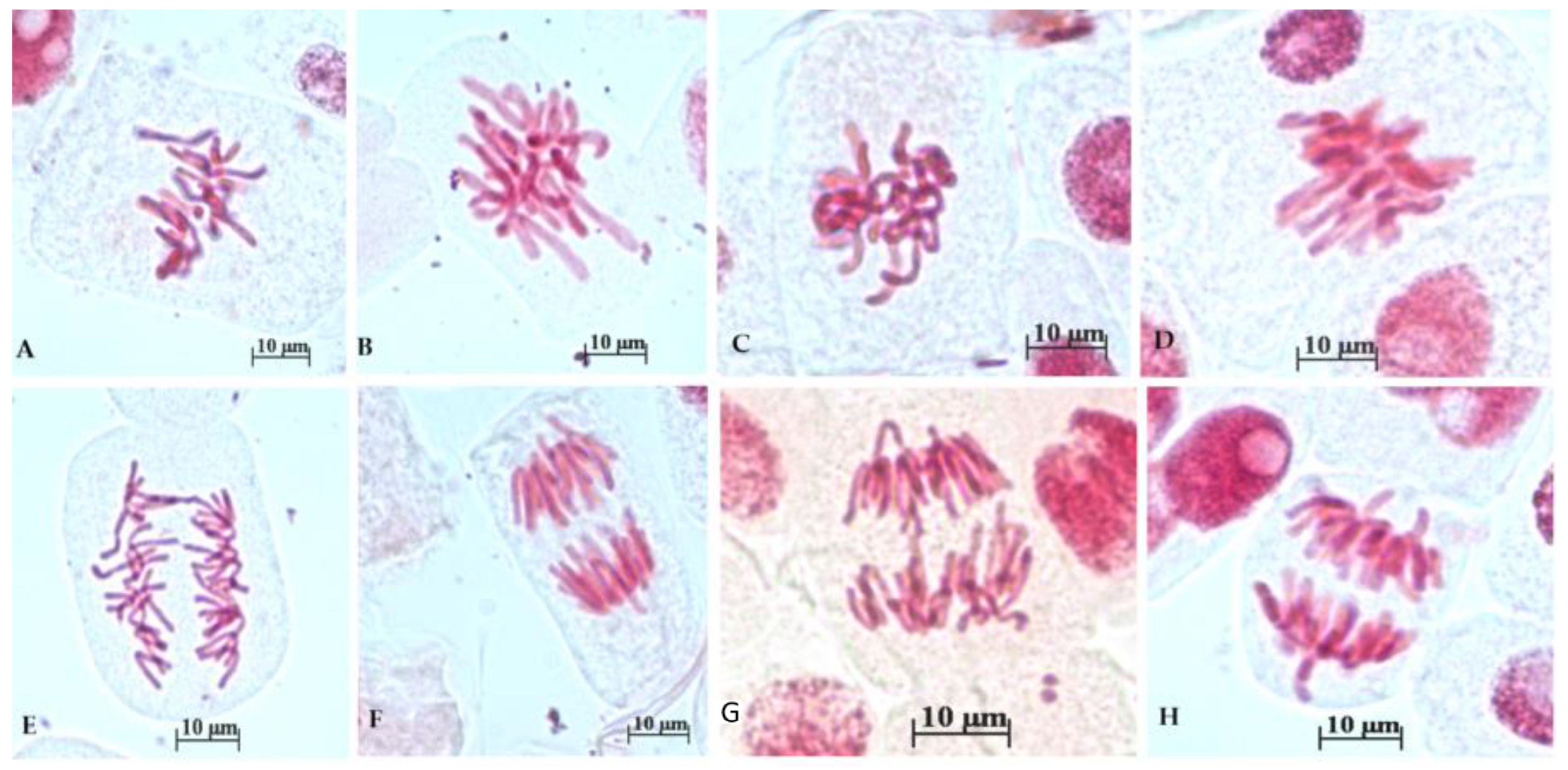
3.3. Chromosomal Anomalies
3.4. Analysis of Cell Morphology Using Fluorescent Microscopy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ristea, M.E.; Zarnescu, O. Indigo carmine: Between necessity and concern. J. Xenobiot. 2023, 13, 509–528. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.S.; Kim, K.H.; Shin, S.W.; Kim, T.K.; Kim, J.I. Indigo carmine for the selective endoscopic intervertebral nuclectomy. J. Korean Med. Sci. 2005, 20, 702–703. [Google Scholar] [CrossRef] [PubMed]
- Secula, M.S.; Cretescu, I.; Petrescu, S. An experimental study of indigo carmine removal from aqueous solution by electrocoagulation. Desalination 2011, 277, 227–235. [Google Scholar] [CrossRef]
- Kim, K.H.; Kim, Y.S.; Kuh, S.U.; Park, H.S.; Park, J.Y.; Chin, D.K.; Kim, K.S.; Cho, Y.E. Time and dose dependent cytotoxicities of ioxitalamate and indigocarmine in human nucleus pulposus cells. Spine J. 2013, 13, 564–571. [Google Scholar] [CrossRef] [PubMed]
- EFSA ANS Panel (European Food Safety Authority Panel on Food Additives and Nutrient Sources Added to Food). Scientific opinion on the re-evaluation of indigo carmine (E 132) as a food additive. EFSA J. 2014, 12, 3768. [Google Scholar] [CrossRef]
- Caprarescu, S.; Miron, A.R.; Purcar, V.; Radu, A.L.; Sarbu, A.; Ion-Ebrasu, D.; Atanase, L.I.; Ghiurea, M. Efficient removal of indigo carmine dye by a separation process. Water Sci. Technol. 2016, 74, 2462–2473. [Google Scholar] [CrossRef]
- Pereira, P.C.G.; Reimao, R.V.; Pavesi, T.; Saggioro, E.M.; Moreira, J.C.; Correira, F.V. Lethal and sub-lethal evaluation if indigo carmine dye and byproducts after TiO2 photocatalysis in the immune system of Eisenia andrei earthworms. Ecotoxicol. Environ. Saf. 2017, 143, 275–282. [Google Scholar] [CrossRef]
- Rodriguez-Ferreras, A.; Ruiz-Salazar, J. Indigo carmine related tooth discoloration. Excipients: A pending subject. Farm. Hosp. 2019, 43, 36–38. [Google Scholar]
- Lakshmi, U.R.; Srivastava, V.C.; Mall, I.D.; Lataye, D.H. Rice husk ash as an effective adsorbent: Evaluation of adsorptive characteristics for indigo carmine dye. J. Environ. Manag. 2009, 90, 710–720. [Google Scholar] [CrossRef]
- Kekes, T.; Tzia, C. Adsorption of indigo carmine on functional chitosan and β-cyclodextrin/chitosan beads: Equilibrium, kinetics and mechanism studies. J. Environ. Manag. 2020, 262, 110372. [Google Scholar] [CrossRef]
- Edwin, D.S.S.; Manjunatha, J.G.; Raril, C.; Girish, T.; Ravishankar, D.K.; Arpitha, H.J. Electrochemical analysis of indigo carmine using polyarginine modified carbon paste electrode. J. Electrochem. Sci. Eng. 2021, 11, 87–96. [Google Scholar] [CrossRef]
- El-Kammah, M.; Elkhatib, E.; Gouveia, S.; Cameselle, C.; Aboukila, E. Enhanced removal of indigo carmine dye from textile effluent using green cost-efficient nanomaterial: Adsorption, kinetics, thermodynamics and mechanisms. Sustain. Chem. Pharm. 2022, 29, 100753. [Google Scholar] [CrossRef]
- Pasdaran, A.; Azarpira, N.; Heidari, R.; Nourinejad, S.; Zare, M.; Hamedi, A. Effects of some cosmetic dyes and pigments on the proliferation of human foreskin fibroblasts and cellular oxidative stress; potential cytotoxicity of chlorophyllin and indigo carmine on fibroblasts. J. Cosmet. Dermatol. 2022, 21, 3979–3985. [Google Scholar] [CrossRef] [PubMed]
- Tabti, S.; Benchettara, A.; Smaili, F.; Benchettara, A.; Berrabah, S.E. Electrodeposition of lead dioxide on Fe electrode application to the degradation of indigo carmine dye. J. Appl. Electrochem. 2022, 52, 1207–1217. [Google Scholar] [CrossRef]
- Okafor, S.N.; Obonga, W.; Ezeokonkwo, M.A. Assessment of the health implications of synthetic and natural food colourants—A critical review. J. Pharm. Biosci. 2016, 4, 249–254. [Google Scholar]
- Pagnacco, M.; Maksimović, J.P.; Nikolić, N.T.; Bogdanović, D.V.B.; Kragović, M.M.; Stojmenović, M.D.; Blagojević, S.N.; Senćanski, J.V. Indigo carmine in a food dye: Spectroscopic characterization and determining its micro-concentration through the clock reaction. Molecules 2022, 27, 4853. [Google Scholar] [CrossRef]
- GSFA (General Standard Food Additives). Food Additive Details: GSFA Provisions for Indigotine (Indigo Carmine). Available online: https://www.fao.org/gsfaonline/additives/details.html?id=96&d-3586470-s=2&d-3586470-o=2&print=true (accessed on 1 February 2024).
- JECFA (Joint FAO/WHO Expert Committee on Food Additives). Safety Evaluation of Certain Food Additives: Prepared by the Eighty-Sixth Meeting of the JECFA; Joint FAO/WHO Expert Committee on Food Additives: Geneva, Switzerland, 2020. [Google Scholar]
- Park, H.J.; Lee, S.M.; Choi, J.A.; Park, N.H.; Kim, H.S.; Park, S.I. Preoperative localization of cystic lesions in the knee using ultrasound-guided injection of indigo carmine. J. Clin. Ultrasound. 2010, 38, 305–308. [Google Scholar] [CrossRef]
- Zippelius, T.; Hoburg, A.; Preininger, B.; Vörös, P.; Perka, C.; Matziolis, G.; Röhner, E. Effect of indigo carmine on human chondrocytes in vitro. Open Orthop. J. 2013, 7, 8–11. [Google Scholar] [CrossRef]
- Jeon, H.J.; Yoon, J.S.; Cho, S.S.; Kang, K.O. Indigo carmine-induced hypotension in patients undergoing general anaesthesia. Singap. Med. J. 2012, 53, 57–59. [Google Scholar]
- Naitoh, J.; Fox, B.M. Severe hypotension, bronchospasm, and urticaria from intravenous indigo carmine. Urology 1994, 44, 271–272. [Google Scholar] [CrossRef] [PubMed]
- Luketic, L.; Murji, A. Options to evaluate ureter patency at cystoscopy in a world without indigo carmine. J. Minim. Invasive Gynecol. 2016, 23, 878–885. [Google Scholar] [CrossRef]
- Higashimori, A.; Takahara, M.; Utsunomiya, M.; Fukunaga, M.; Kawasaki, D.; Mori, S.; Takimura, H.; Hirano, K.; Tsubakimoto, Y.; Nakama, T.; et al. Utility of indigo carmine angiography in patients with critical limb ischemia: Prospective multi-center intervention study (DIESEL-study). Catheter. Cardiovasc. Interv. 2019, 93, 108–112. [Google Scholar] [CrossRef]
- Fu, K.; Sano, Y.; Kato, S.; Fujii, T.; Nagashima, F.; Yoshino, T.; Okuna, T.; Yoshida, S.; Fujimori, T. Chromoendoscopy using indigo carmine dye spraying with magnifying observation is the most reliable method for differential diagnosis between non-neoplastic and neoplastic colorectal lesions: A prospective study. Endoscopy 2004, 36, 1089–1093. [Google Scholar] [CrossRef] [PubMed]
- Kiesslich, R.; Jung, M.; DiSario, J.A.; Galle, P.R.; Neurath, M.F. Perspectives of chromo and magnifying endoscopy: How, how much, when, and whom should we stain? J. Clin. Gastroenterol. 2004, 38, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Erickson, J.C.; Widmer, B.A. The vasopressor effect of indigo carmine. Anesthesiology 1968, 29, 188–189. [Google Scholar] [CrossRef]
- Kennedy, W.F.; Wirjoatmadja, K.; Akamatsu, T.J.; Bonica, J.J. Cardiovascular and respiratory effects of indigo carmine. J. Urol. 1968, 100, 775–778. [Google Scholar] [CrossRef] [PubMed]
- Craik, J.D.; Khan, D.; Afifi, R. The safety of intravenous indigo carmine to assess ureteric patency during transvaginal uterosacral suspension of the vaginal vault. J. Pelvic. Med. Surg. 2009, 15, 11–15. [Google Scholar] [CrossRef]
- Chu, J.N.; Lazar, J.; Badger, J. A postoperative blue rash: Indigo carmine dye extravasation. Int. J. Dermatol. 2015, 54, 371–372. [Google Scholar] [CrossRef]
- Rancan, E.A.; Frota, E.I.; de Freitas, T.M.N.; Jordani, M.C.; Évora, P.R.B.; Castro-E-Silva, O. Evaluation of indigo carmine onhepatic ischemia and reperfusion injury. Acta Cir. Bras. 2020, 35, 202000901. [Google Scholar] [CrossRef]
- O’Hara, J.F.; Connors, D.F.; Sprung, J.; Ballard, L.A. Upper extremity discoloration caused by subcutaneous indigo carmine injection. Anesth. Analg. 1996, 83, 1126–1128. [Google Scholar] [CrossRef]
- Choi, J.W.; Lee, J.J.; Kim, G.H.; Hong, S.H. Extensive skin color change caused by extravasation of indigo carmine. Korean J. Anesthesiol. 2012, 62, 499–500. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, M.F.; Khandaker, S.; Sarker, F.; Islam, A.; Rahman, M.T.; Awual, M.R. Current treatment technologies and mechanisms for removal of indigo carmine dyes from wastewater: A review. J. Mol. Liq. 2020, 318, 114061. [Google Scholar] [CrossRef]
- Olas, B.; Bialecki, J.; Urba’nska, K.; Brys, M. The effects of natural and synthetic blue dyes on human health: A review of current knowledge and therapeutic perspectives. Adv. Nutr. 2021, 12, 2301–2311. [Google Scholar] [CrossRef]
- Kaya, M.; Cavușoğlu, K.; Yalcin, E.; Acar, A. DNA fragmentation and multifaceted toxicity induced by high-dose vanadium exposure determined by the bioindicator Allium test. Sci. Rep. 2023, 13, 8493. [Google Scholar] [CrossRef]
- Hooson, J.; Gaunt, I.F.; Kiss, I.S.; Grasso, P.; Butterworth, K.R. Long-term in toxicity carmine in mice. Food Cosmet. Toxicol. 1975, 13, 167–176. [Google Scholar] [CrossRef]
- Dixit, A.; Goyal, R.P. Evaluation of reproductive toxicity caused by indigo carmine on male swiss albino mice. Pharmacologyonline 2013, 1, 218–224. [Google Scholar]
- Gaunt, I.F.; Kiss, I.S.; Grasso, P.; Gangolli, S.D. Short-term toxicity study on indigo carmine in the pig. Food Cosmet. Toxicol. 1969, 7, 17–24. [Google Scholar] [CrossRef]
- Roychoudhury, A.; Giri, A.K. Effects of certain food dyes on chromosomes of Allium cepa. Mutat. Res. 1989, 223, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, M. Studies on mitodepressive effect of indigocarmine. Int. J. Eng. Technol. 2014, 1, 157–160. [Google Scholar]
- Bonciu, E.; Firbas, P.; Fontanetti, C.S.; Wusheng, J.; Karaismailoğlu, M.C.; Liu, D.; Menicucci, F.; Pesnya, D.S.; Popescu, A.; Romavosky, A.V.; et al. An evaluation for the standardization of the Allium cepa test as cytotoxicity and genotoxicity assay. Caryologia 2018, 71, 191–209. [Google Scholar] [CrossRef]
- Datta, S.; Singh, J.; Singh, J.; Singh, S.; Singh, S. Assessment of genotoxic effects of pesticide and vermicompost treated soil with Allium cepa test. Sustain. Environ. Res. 2018, 28, 171–178. [Google Scholar] [CrossRef]
- Pesnya, D.S.; Kurbatova, S.A.; Sharov, A.N.; Chernova, E.N.; Yershov, I.Y.; Shurganova, G.V.; Vodeneeva, E.L. Genotoxicity of natural water during the mass development of cyanobacteria evaluated by the Allium test method: A model experiment with microcosms. Toxins 2022, 14, 359. [Google Scholar] [CrossRef]
- Salazar-Mercado, S.A.; Bayona, H.A.M. Evaluation of cytotoxic potential of chlorpyrifos using Lens culinaris Med as efficient bioindicator. Ecotoxicol. Environ. Saf. 2019, 183, 109582. [Google Scholar] [CrossRef]
- Salazar-Mercado, S.A.; Caleño, J.D.Q.; Suárez, J.P.R. Cytogenotoxic effect of propanil using the Lens culinaris Med and Allium cepa L. test. Chemosphere 2020, 249, 126193. [Google Scholar] [CrossRef]
- Salazar-Mercado, S.A.; Torres-León, C.A.; Rojas-Suárez, J.P. Cytotoxic evaluation of sodium hypochlorite, using Pisum sativum L as effective bioindicator. Ecotoxicol. Environ. Saf. 2019, 30, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Tigre, R.C.; Silva, N.H.; Santos, M.G.; Honda, N.K.; Falcão, E.P.S.; Pereira, E.C. Allelopathic and bioherbicidal potential of Cladonia verticillaris on the germination and growth of Latuca sativa. Ecotoxicol. Environ. Saf. 2012, 84, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Alarcón, J.; Péréz-Sáanchez, M.; Gómez-Olivares, J.; López-Durán, R.M.; Montiel-González, R.; Valencia-Sánchez, R.; Valencia-Quitana, R. Inducción de micronúcleos en células meristemáticas de la raíz de Vicia faba tratadas con diferentes concetraciones de Marvel. Rev. Int. Contam. Ambient. 2018, 34, 95–106. [Google Scholar] [CrossRef]
- Fiskesjö, G. The Allium test as a standard in environmental monitoring. Hereditas 1985, 102, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Ermolaev, A.; Kudryavtseva, N.; Pivovarov, A.; Kirov, I.; Karlov, G.; Khustaleva, L. Integrating genetic and chromosome maps of Allium cepa: From markers visualization to genome assembly verification. Int. J. Mol. Sci. 2022, 23, 10486. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Tripura, K. Differential sensitivity of Allium cepa L. and Vicia faba L. to aqueous extracts of Cascabela thevetia (L.) Lippold. S. Afr. J. Bot. 2021, 139, 67–78. [Google Scholar] [CrossRef]
- Rajeshwari, A.; Suresh, S.; Chandrasekaran, N.; Mukherjee, A. Toxicity evaluation of golf nanoparticles using an Allium cepa bioassay. RSC Adv. 2016, 6, 24000–24009. [Google Scholar] [CrossRef]
- Leme, D.M.; Marin-Morales, M.A. Allium cepa test in environmental monitoring: A review on its application. Mutat. Res. 2009, 682, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.F. Chromosome aberration assays in allium: A report of the U.S. environmental protection agency gene-tox program. Mutat. Res. 1982, 99, 273–291. [Google Scholar] [CrossRef] [PubMed]
- Samarakoon, T.; Fujino, T. Modulation of phytotoxic and cytogenetic effects of cadmium by humic acid: Findings from a short-term plant-based bioassay. Water Sci. Technol. 2023, 87, 3095–3107. [Google Scholar] [CrossRef]
- Alias, C.; Feretti, D.; Viola, G.V.C.; Zerbini, I.; Bisceglie, F.; Pelosi, G.; Zani, C. Allium cepa tests: A plant-based tool for the early evaluation of toxicity and genotoxicity of newly synthetized antifungal molecules. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2023, 889, 503654. [Google Scholar] [CrossRef]
- Khan, I.S.; Ali, M.N.; Hamid, R.; Ganie, S.A. Genotoxic effect of two commonly used food dyes metanil yellow and carmoisine using Allium cepa L. as indicator. Toxicol. Rep. 2020, 7, 370–375. [Google Scholar] [CrossRef]
- Farheen, J.; Mansoor, S.; Abid, M. Geno-toxic appraisal of widely used food color additives on model plant Allium cepa root tip cells. J. Innov. Sci. 2020, 7, 174–181. [Google Scholar] [CrossRef]
- Barbério, A.; Voltolini, J.C.; Mello, M.L.S. Standardization of bulb and root samples sizes for the Allium cepa test. Ecotoxicol. 2011, 20, 927–935. [Google Scholar] [CrossRef]
- Admas, T.; Kerisew, B. Assessment of cytotoxicity and genotoxicity potential of effluents from Bahir Dar tannery using Allium cepa. Adv. Public Health 2022, 2022, 1–10. [Google Scholar] [CrossRef]
- Bellani, L.; Muccifora, S.; Barbieri, F.; Tassi, E.; Castiglione, M.R.; Giorgetti, L. Genotoxicity of the food additive E171, titanium dioxide, in the plants Lens culinaris L. and Allium cepa L. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2020, 849, 503142. [Google Scholar] [CrossRef]
- Sehgal, R.; Roy, S.; Kumar, V.L. Evaluation of cytotoxic potential of latex of Calotropis procera and podophyllotoxin in Allium cepa root model. Biocell 2006, 30, 9–13. [Google Scholar] [CrossRef]
- Alaguprathana, M.; Poonkothai, M.; Al-Ansari, M.M.; Al-Humaid, L.; Kim, W. Cytogenotoxicity assessment in Allium cepa roots exposed to methyl orange treated with Oedogonium subplagiostomum AP1. Environ. Res. 2022, 213, 113612. [Google Scholar] [CrossRef]
- Khanna, N.; Sharma, S. Allium cepa root chromosomal aberration assay: A review. IJPBR 2013, 1, 105–119. [Google Scholar] [CrossRef]
- Amin, A.W.; Migahid, M.M. Cytogenetic effect of sea water irrigation on irradiated wheat grains. EJMHG 2000, 29, 199–213. [Google Scholar]
- Andrare-Vieira, L.F.; Palmieri, M.J.; Davide, L.C. Effects of long exposure to spent potliner on seeds, root tips, and meristematic cells of Allium cepa L. Environ. Monit. Assess. 2017, 189, 489. [Google Scholar] [CrossRef] [PubMed]
- Bhat, S.A.; Singh, J.; Vig, A.P. Vermistabilization of sugar beet (Beta vulgaris L.) waste produced from sugar factory using earthworm Eisenia fetida: Genotoxic assessment by Allium cepa test. Environ. Sci. Pollut. Res. Int. 2015, 22, 11236–11254. [Google Scholar] [CrossRef]
- Ali, M.M.; Fatima, A.; Nawaz, S.; Rehman, A.; Javed, M.; Nadeem, A. Cytotoxic and genotoxic evaluation of bisphenol S on onion root tips by Allium cepa and comet tests. Environ. Sci. Pollut. Res. Int. 2022, 29, 88803–88811. [Google Scholar] [CrossRef] [PubMed]
- Pandey, H.; Kumar, V.; Roy, B.K. Assessment of genotoxicity of some common food preservatives using Allium cepa L. as a test plant. Toxicol. Rep. 2014, 11, 300–308. [Google Scholar] [CrossRef]
- Hani, U.; Mansoor, S.; Hassan, M.; Farheen, J. Genotoxicity of heavy metals on mung bean (Vigna radiata) seedlings and its alleviation by priming with their lower concentrations. Cytologia 2020, 85, 239–244. [Google Scholar] [CrossRef]
- Schutz, D.L.; de Marco, I.G.; Teles, A.G.D.X.; Schmitz, A.P.O.; Gomes, E.M.V.; Manosso, F.C.; Tonial, I.B.; Pokrywiecki, J.C.; Lingnau, R.; Pokrywieki, T.S.; et al. Soil toxicity in a protected are in Brazil: Cytotoxic, genotoxic, and toxic effects. Sci. Total Environ. 2023, 892, 164564. [Google Scholar] [CrossRef] [PubMed]
- Rencuzogullari, E.; Kayraldiz, A.; Ila, H.B.; Cakmak, T.; Topaktaș, M. The cytogenetic effects of sodium metabisulfite, a food preservative in root tip cells of Allium cepa L. Turk. J. Biol. 2001, 25, 361–370. [Google Scholar]
- Gömürgen, A.N. Cytological effect of the potassium metabisulphite and potassium nitrate food preservative on root tips of Allium cepa L. Cytologia 2005, 70, 119–128. [Google Scholar] [CrossRef]
- Türkoğlu, Ș. Genotoxicity of five food preservatives tested on root tips of Allium cepa L. Mutat. Res. 2007, 626, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Dutta, J.; Ahmad, A.; Singh, J. Study of industrial effluents induced genotoxicity on Allium cepa L. J. Cytol. 2018, 71, 139–145. [Google Scholar] [CrossRef]
- Barman, M.; Ray, S. Cytogenotoxic effects of 3-epicaryoptin in Allium cepa L. root apical meristem cells. Protoplasma 2023, 260, 1163–1177. [Google Scholar] [CrossRef]
- Dwivedi, K.; Kumar, G. Genetic damage induced by a food coloring dye (sunset yellow) on meristematic cells of Brassica campestris L. J. Environ. Public Health 2015, 2015, 319727. [Google Scholar] [CrossRef] [PubMed]
- Haliem, A.S. Cytological effect of the herbicide sencorer on mitosis of Allium cepa. Egypt. J. Botany 1990, 33, 93–104. [Google Scholar]
- Parrotta, L.; Guerriero, G.; Sergeant, K.; Cai, G.; Hausman, J.F. Target or barrier? The cell wall of early- and later-diverging plants vs cadmium toxicity: Differences in the response mechanisms. Front. Plant Sci. 2015, 6, 133. [Google Scholar] [CrossRef]
- Jaskowiak, J.; Kwasniewska, J.; Milewska-Hendel, A.; Kurczynska, E.U.; Szurman-Zubrzycka, M.; Szarejko, I. Aluminum alters the histology and pectin cell wall composition of barley roots. Int. J. Mol. Sci. 2019, 20, 3039. [Google Scholar] [CrossRef]
- Leškovï, A.; Zvarï, K.M.; Araya, T.; Giehl, R.F.H. Nickel toxicity targets cell wall-related processes and PIN2-mediated auxin transport to inhibit root elongation and gravitropic responses in arabidopsis. Plant Cell Physiol. 2020, 61, 519–535. [Google Scholar] [CrossRef]
- Angulo-Bejarano, P.I.; Puente-Rivera, J.; Cruz-Ortega, R. Metal and metalloid toxicity in plants: An overview on molecular aspects. Plants 2021, 10, 635. [Google Scholar] [CrossRef]
- Akgündüz, M.Ç.; Çavuşoğlu, K.; Yalçın, E. The potential risk assessment of phenoxyethanol with a versatile model system. Sci. Rep. 2020, 10, 1209. [Google Scholar] [CrossRef]
- Tümer, C.; Çavuşoğlu, K.; Yalçin, E. Screening the toxicity profile and genotoxicity mechanism of excess manganese confirmed by spectral shift. Sci. Rep. 2022, 12, 20986. [Google Scholar] [CrossRef] [PubMed]
- Adamakis, I.-D.S.; Eleftheriou, E.P. Structural evidence of programmed cell death induction by tungsten in root tip cells of Pisum sativum. Plants 2019, 8, 62. [Google Scholar] [CrossRef]
- Majewska, A.; Wolska, E.; Furmanowa, M.; Urbańska, N.; Pietrosiuk, A.; Zobel, A.; Kuraś, M. Antimitotic effect, G2/M accumulation, chromosomal and ultrastructure changes in meristematic cells of Allium cepa L. root tips treated with the extract from Rhodiola rosea rhizomes. Caryologia 2003, 56, 333–347. [Google Scholar] [CrossRef]
- Ślusarczyk, J.; Wierzbicka, M.; Suchocki, P.; Kuraś, M. Ultrastructural changes in onion (Allium cepa L.) root tip meristem cells treated with Selol and sodium selenate (IV). Caryologia 2015, 68, 306–316. [Google Scholar] [CrossRef]
- Antosiewicz, D.; Wierzbicka, M. Localization of lead in Allium cepa L. cells by electron microscopy. J. Microsc. 1999, 195, 139–146. [Google Scholar] [CrossRef]


| Concentration | Root Growth (%) | Root Length (cm) |
|---|---|---|
| Control | 100% | 1.60 ± 0.51 |
| 0.0032 mg/mL | 88.75% | 1.42 ± 0.46 ** |
| 0.0064 mg/mL | 81.87% | 1.31 ± 0.31 ** |
| 0.0125 mg/mL | 74.37% | 1.19 ± 0.38 ** |
| 0.2 mg/mL | 65% | 1.04 ± 0.48 ** |
| Concentration | Mitotic Index (Mean ± Standard Deviation) | Mitotic Inhibition (%) |
|---|---|---|
| Control | 11.45 ± 6.18 | - |
| 0.0032 mg/mL | 5.5 ± 3.8 ** | 51.96% |
| 0.0064 mg/mL | 5.28 ± 2.95 ** | 53.88% |
| 0.0125 mg/mL | 6.05 ± 2.4 ** | 47.16% |
| 0.2 mg/mL | 5.30 ± 2.22 ** | 53.71% |
| Concentration | % Prophases | % Normal Metaphases | % Abnormal Metaphases | % Normal Ana/Telophases | % Abnormal Ana/Telophases |
|---|---|---|---|---|---|
| Control | 60.94 ± 14.84 | 15.15 ± 8.66 | 0.90 ± 1.85 | 21.85 ± 9.56 | 1.12 ± 2.3 |
| 0.0032 mg/mL | 79.70 ± 11.93 | 3.88 ± 7.81 | 5.59 ± 7.08 | 7.53 ± 9.29 | 2.16 ± 4.34 |
| 0.0064 mg/mL | 51.53 ± 24.58 | 3.35 ± 7.7 | 25.35 ± 15.19 | 19.74 ± 25.24 | 0 |
| 0.0125 mg/mL | 42.96 ± 14.26 | 7.96 ± 10.40 | 20.78. ± 19.45 | 19.35 ± 13.64 | 9.02 ± 4.25 |
| 0.2 mg/mL | 47.77 ± 11.31 | 7.45 ± 9.72 | 8.24 ± 8.99 | 17.31 ± 12.03 | 19.25 ± 11.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ristea, M.-E.; Zarnescu, O. Effects of Indigo Carmine on Growth, Cell Division, and Morphology of Allium cepa L. Root Tip. Toxics 2024, 12, 194. https://doi.org/10.3390/toxics12030194
Ristea M-E, Zarnescu O. Effects of Indigo Carmine on Growth, Cell Division, and Morphology of Allium cepa L. Root Tip. Toxics. 2024; 12(3):194. https://doi.org/10.3390/toxics12030194
Chicago/Turabian StyleRistea, Madalina-Elena, and Otilia Zarnescu. 2024. "Effects of Indigo Carmine on Growth, Cell Division, and Morphology of Allium cepa L. Root Tip" Toxics 12, no. 3: 194. https://doi.org/10.3390/toxics12030194






