Embryotoxicity Induced by Triclopyr in Zebrafish (Danio rerio) Early Life Stage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Test Solutions
2.2. Zebrafish Husbandry and Egg Collection
2.3. Chemical Exposure and Development Toxicity Assessment
2.4. Morphological Assessment
2.5. Locomotor Activity
2.6. Acetylcholinesterase Activity
2.7. Statistical Analysis
3. Results
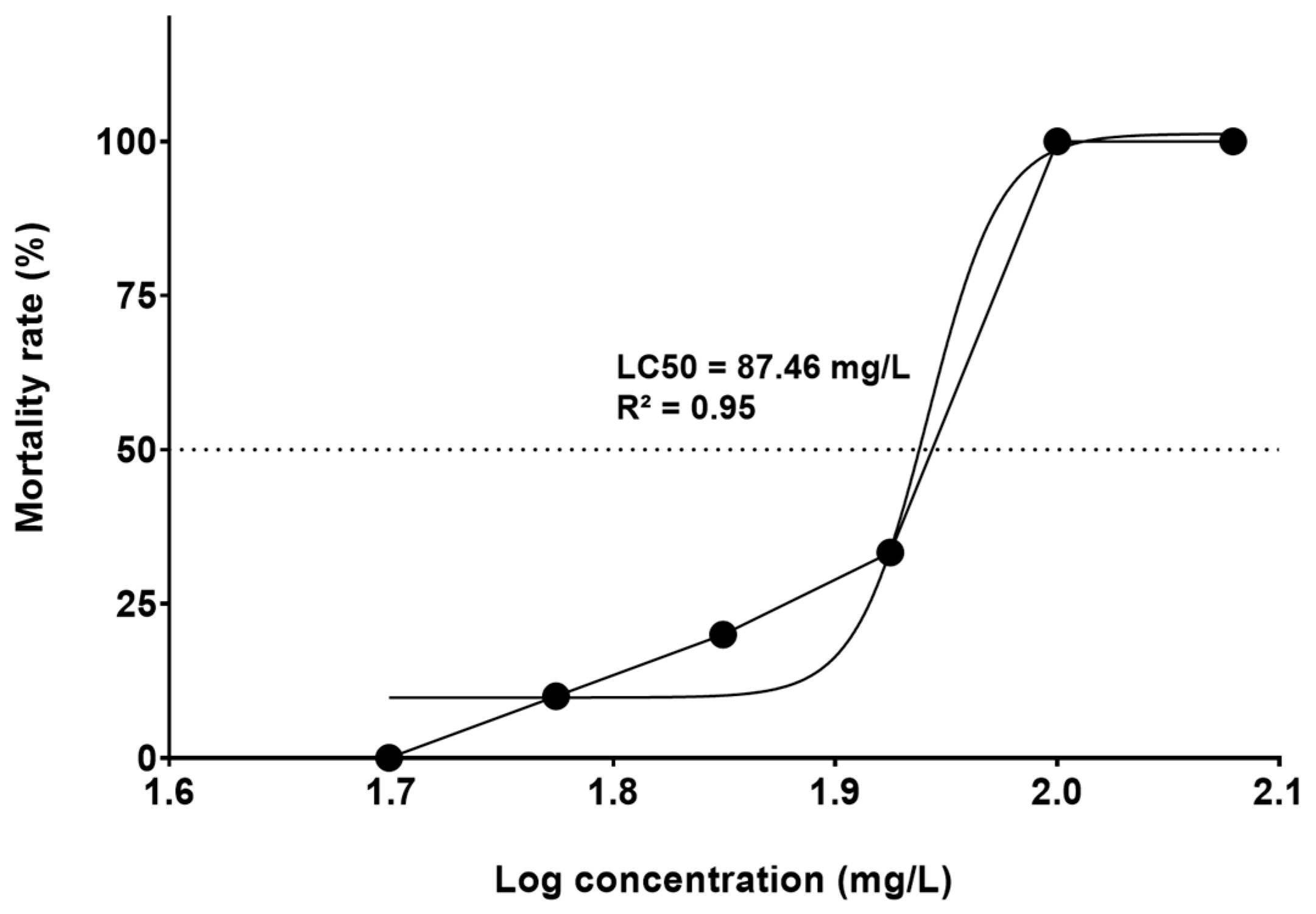
3.1. FET Test (LC50) and Development Toxicity
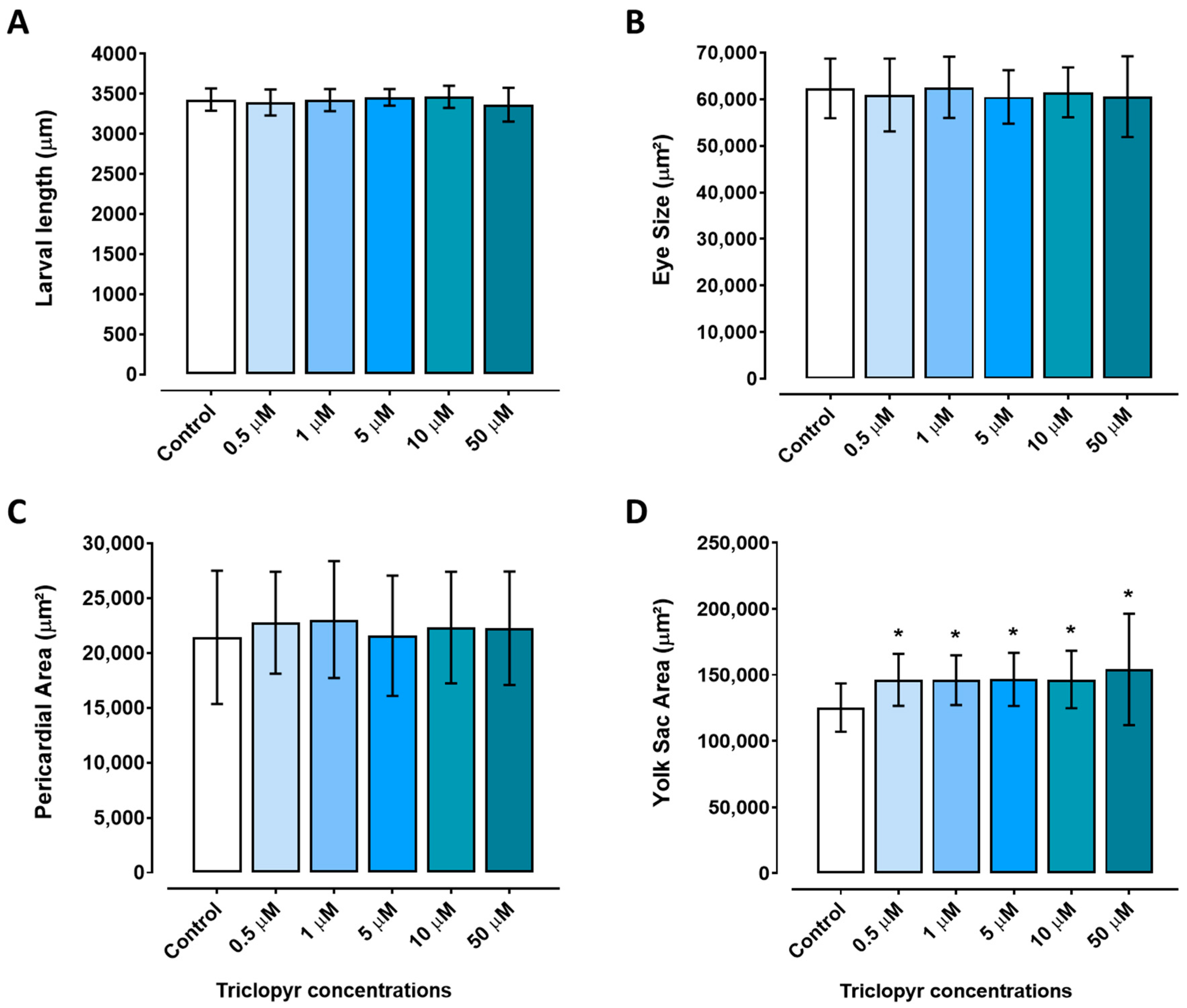
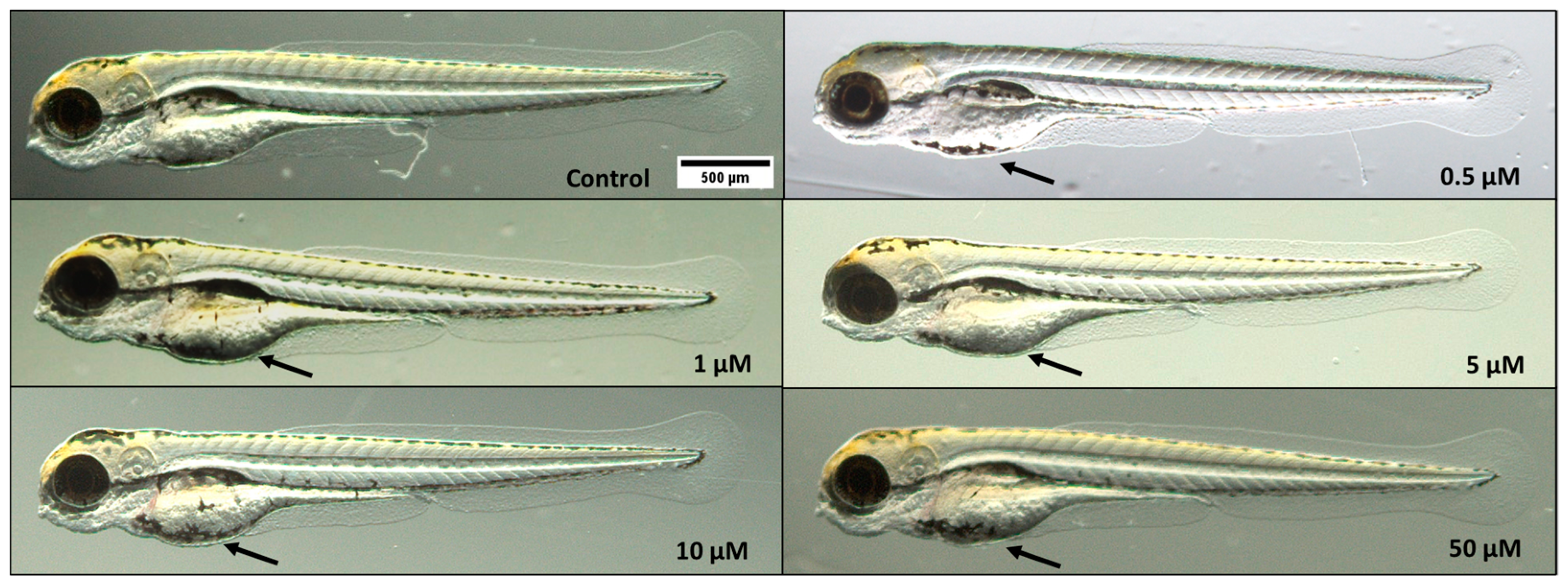
3.2. Morphological Analyses
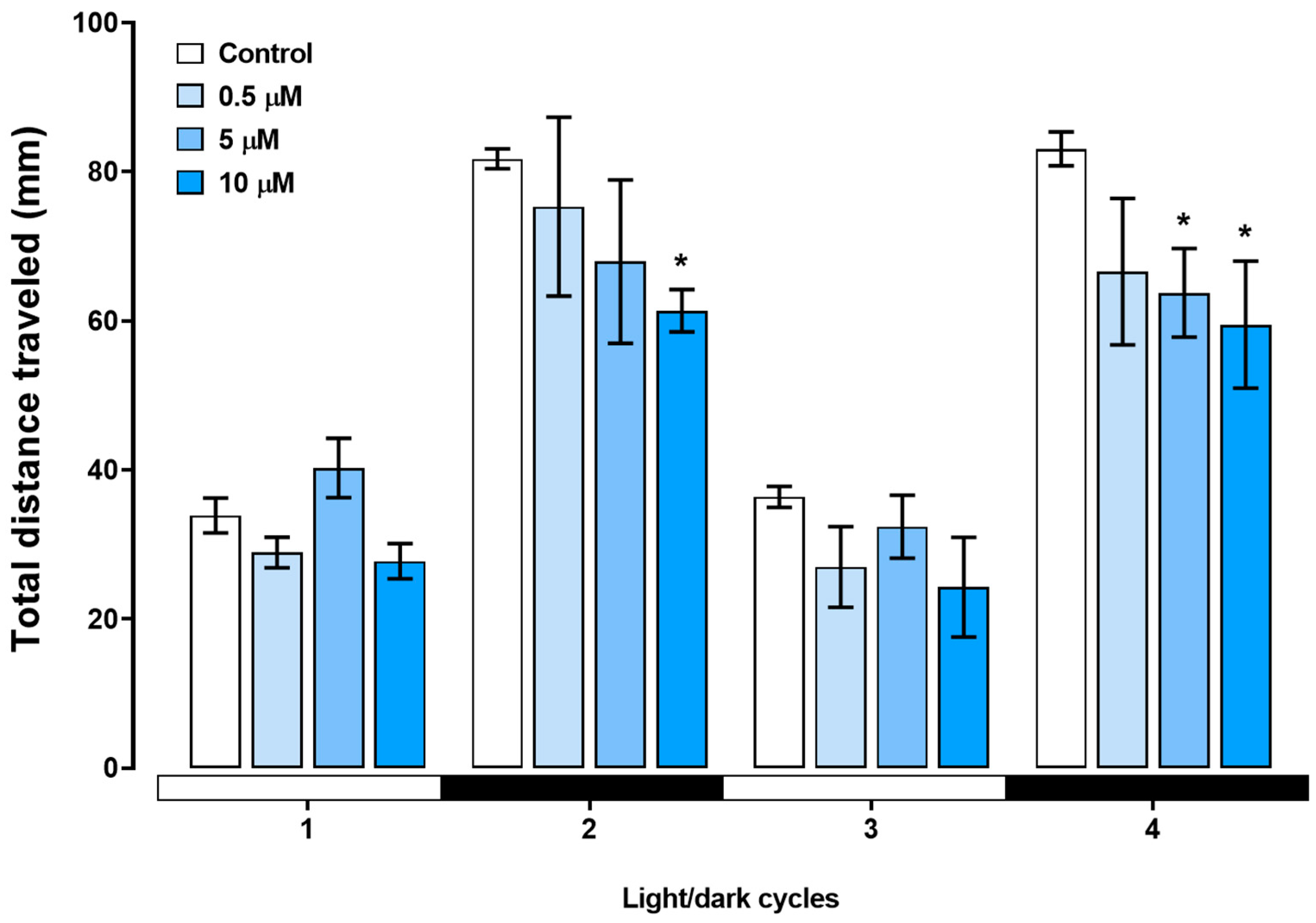
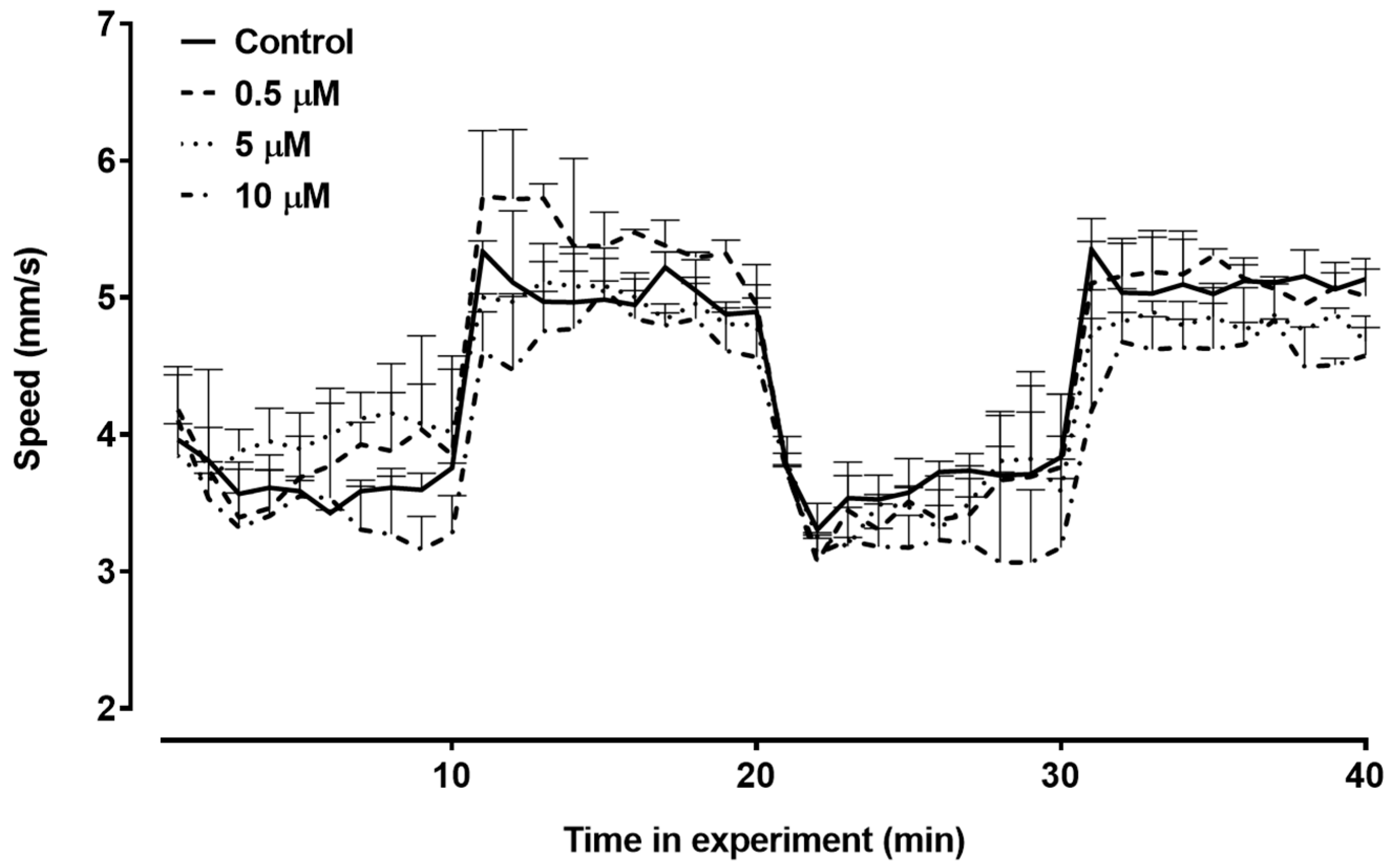
3.3. Locomotor Activity
3.4. Acetylcholinesterase Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cessna, A.J.; Grovert, R.; Waite, D.T. Environmental Fate of Triclopyr. Rev. Environ. Contam. Toxicol. 2002, 174, 19–48. [Google Scholar] [CrossRef]
- Agência Nacional de Vigilância Sanitária. Índice Monográfico: Triclopir. Available online: http://portal.anvisa.gov.br/documents/111215/117782/T28%2B%2BTriclopir.pdf/b24b997a66fb-45c0-b43a-5060db498aa4 (accessed on 22 June 2023).
- Tu, M.; Hurd, C.; Randall, J.M. Weed Control Methods Handbook. The Nature Conservancy. 2001. Available online: http://tncweeds.ucdavis.edu (accessed on 15 May 2023).
- European Food Safety Authority. Conclusion on the peer review of the pesticide risk assessment of the active substance triclopyr. EFSA J. 2006, 4, 103. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. New Pesticide Fact Sheet; PB96-181516.EPA737-F-96-005; U.S. EPA Office of Prevention, Pesticides and Toxic Substances: Washington, DC, USA, 1998; pp. 1–10, 199.
- Rawn, D.F.K.; Halldorson, T.H.J.; Woychuk, R.N.; Muir, D.C.G. Pesticides in the Red River and its tributaries in southern Manitoba: 1993–1995. Water Qual. Res. J. Can. 1999, 34, 183–219. [Google Scholar] [CrossRef]
- Getsinger, K.D.; Petty, D.G.; Madsen, J.D.; Skogerboe, J.G.; Houtman, B.A.; Haller, W.T.; Fox, A.M. Aquatic dissipation of the herbicide triclopyr in Lake Minnetonka, Minnesota. Pest. Manag. Sci. 2000, 56, 388–400. [Google Scholar] [CrossRef]
- Rodrigues, E.T.; Alpendurada, M.F.; Guimarães, A.; Avó, R.; Ferreira, B.; Pardal, M.A. The environmental condition of an estuarine ecosystem disturbed by pesticides. Environ. Sci. Pollut. Res. 2019, 26, 24075–24087. [Google Scholar] [CrossRef]
- Hope, B.K.; Pillsbury, L.; Boling, B. A state-wide survey in Oregon (USA) of trace metals and organic chemicals in municipal effluent. Sci. Total Environ. 2012, 417–418, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Cleveland, C.B.; Holbrook, D.L. A Hydrolysis Study of Tric1opyr; GH-C 2491; Dow Elanco: Indianapolis, IN, USA, 1991. [Google Scholar]
- United States Environmental Protection Agency. EPA Draft Ecological Risk Assessment for the Registration Review of Triclopyr Acid, Triclopyr Choline, Triclopyr TEA, and Triclopyr BEE; United States Environmental Protection Agency: Washington, DC, USA, 2019.
- McCall, P.J.; Gavit, P.D. Aqueous photolysis of triclopyr and its butoxyethyl ester and calculated environmental photodecomposition rates. Environ. Toxicol. Chem. 1986, 5, 879–885. [Google Scholar] [CrossRef]
- Wan, M.T.; Moul, D.J.; Watts, R.G. Acute toxicity to juvenile pacific salmonids of Garlon 3A, triclopyr, triclopyr ester and their transformation products; 3,5,6- trichloro-2-pyridinol and 2-methoxy-3,5,6-trichloropyridine. Bull. Environ. Contam. Toxicol. 1987, 39, 721–728. [Google Scholar] [CrossRef]
- Kreutzweiser, D.P.; Holmes, S.B.; Eichenberg, D.C. Influence of Exposure Duration on the Toxicity of Triclopyr Ester to Fish and Aquatic Insects. Arch. Environ. Contam. Toxicol. 1994, 26, 124–129. [Google Scholar] [CrossRef]
- Guilherme, S.; Santos, M.A.; Gaivão, I.; Pacheco, M. Genotoxicity Evaluation of the Herbicide Garlon® and Its Active Ingredient (Triclopyr) in Fish (Anguilla anguilla L.) Using the Comet Assay. Environ. Toxicol. 2015, 30, 1073–1081. [Google Scholar] [CrossRef]
- Ribeiro, O.; Pinto, M.Q.; Félix, L.; Monteiro, S.M.; Fontaínhas-Fernandes, A.; Carrola, J.S. O peixe-zebra (Danio rerio) como modelo emergente na ecotoxicologia. Rev. Ciênc. Elem. 2022, 10, 21. [Google Scholar] [CrossRef]
- Horzmann, K.A.; Freeman, J.L. Making Waves: New Developments in Toxicology with the Zebrafish. Toxicol. Sci. 2018, 163, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.J.; Teraoka, H.; Heideman, W.; Peterson, R.E. Zebrafish as a model vertebrate for investigating chemical toxicity. Toxicol. Sci. 2005, 86, 6–19. [Google Scholar] [CrossRef] [PubMed]
- Anselmo, C.S.; Sardela, V.F.; Sousa, V.P.; Pereira, H.M.G. Zebrafish (Danio rerio): A valuable tool for predicting the metabolism of xenobiotics in humans? Comp. Biochem. Physiol. Part C 2018, 212, 34–46. [Google Scholar] [CrossRef]
- Gonçalves, I.F.S.; Souza, T.M.; Vieira, L.R.; Marchi, F.C.; Nascimento, A.P.; Farias, D.F. Toxicity testing of pesticides in zebrafish—A systematic review on chemicals and associated toxicological endpoints. Environ. Sci. Pollut. Res. 2020, 27, 10185–10204. [Google Scholar] [CrossRef]
- Bambino, K.; Chu, J. Zebrafish in Toxicology and Environmental Health. In Zebrafish at the Interface of Development and Disease Research; Sadler, K.C., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 331–367. [Google Scholar]
- ISO 7346-1; Water Quality—Determination of the Acute Lethal Toxicity of Substances to a Freshwater Fish [Brachydanio rerio Hamilton-Buchanan (Teleostei, Cyprinidae)]—Part 1: Static Method. International Organization for Standardization: Geneva, Switzerland, 1996. Available online: https://www.iso.org/standard/14026.html (accessed on 14 May 2023).
- OECD. Test No 236: Fish Embryo Acute Toxicity (FET) Test; Organisation for Economic Co-operation and Development, OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing Service: Paris, France, 2013. [Google Scholar] [CrossRef]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef]
- Abe, F.R.; Mendonça, J.N.; Moraes, L.A.B.; Oliveira, G.A.R.D.; Gravato, C.; Soares, A.M.V.M.; Oliveira, D.P. Toxicological and behavioral responses as a tool to assess the effects of natural and synthetic dyes on zebrafish early life. Chemosphere 2017, 178, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Xia, Y.; Chen, Y.; Xu, T.; Xu, L.; Guo, Z.; Xu, H.; Xie, H.Q.; Zhao, B. Acetylcholinesterase Is a Potential Biomarker for a Broad Spectrum of Organic Environmental Pollutants. Environ. Sci. Technol. 2018, 52, 8065–8074. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Junior, V.A.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Bartels, M.; Brown, C.; Chung, G.; Chan, M.; Terry, C.; Gehen, S.; Corvaro, M. Review of the pharmacokinetics and metabolism of triclopyr herbicide in mammals: Impact on safety assessments. Regul. Toxicol. Pharmacol. 2020, 116, 104714. [Google Scholar] [CrossRef]
- Stehr, C.M.; Linbo, T.L.; Baldwin, D.H.; Scholz, N.L.; Incardona, J.P. Evaluating the Effects of Forestry Herbicides on Fish Development Using Rapid Phenotypic Screens. N. Am. J. Fish. Manag. 2009, 29, 975–984. [Google Scholar] [CrossRef]
- Burggren, W.W.; Mueller, C.A. Developmental Critical Windows and Sensitive Periods as Three-Dimensional Constructs in Time and Space. Physiol. Biochem. Zool. 2015, 88, 91–102. [Google Scholar] [CrossRef]
- Belanger, S.E.; Rawlings, J.M.; Carr, G.J. Use of fish embryo toxicity tests for the prediction of acute fish toxicity to chemicals. Environ. Toxicol. Chem. 2013, 32, 1768–1783. [Google Scholar] [CrossRef] [PubMed]
- Haendel, M.A.; Tilton, F.; Bailey, G.S.; Tanguay, R.L. Developmental toxicity of the dithiocarbamate pesticide sodium metam in Zebrafish. Fundam. Appl. Toxicol. 2004, 81, 390–400. [Google Scholar] [CrossRef]
- Ahmad, F.; Ali, S.; Richardson, M.K. Effect of pesticides and metals on zebrafish embryo development and larval locomotor activity. bioRxiv 2020. [Google Scholar] [CrossRef]
- Li, K.; Wu, J.; Jiang, L.; Shen, L.; Li, J.; He, Z.; Wei, P.; Lv, Z.; He, M. Developmental toxicity of 2,4-dichlorophenoxyacetic acid in zebrafish embryos. Chemosphere 2017, 171, 40–48. [Google Scholar] [CrossRef]
- Felisbino, K.; Kirsten, N.; Milhorini, S.S.; Marçal, I.S.; Bernert, K.; Schiessl, R.; Nominato-Oliveira, L.; Guiloski, I.C. Teratogenic effects of the dicamba herbicide in Zebrafish (Danio rerio) embryos. Environ. Poll. 2023, 334, 122187. [Google Scholar] [CrossRef]
- Berrill, M.; Bertram, S.; McGillivray, L.; Kolohon, M.; Pauli, B. Effects of low concentrations of forest-use pesticides on frog embryous and tadpoles. Environ. Toxicol. Chem. 1994, 13, 657–664. [Google Scholar] [CrossRef]
- Quinlivan, V.H.; Farber, S.A. Lipid Uptake, Metabolism, and Transport in the Larval Zebrafish. Front. Endocrinol. 2017, 8, 319. [Google Scholar] [CrossRef]
- Sant, K.E.; Timme-Laragy, A.R. Zebrafish as a Model for Toxicological Perturbation of Yolk and Nutrition in the Early Embryo. Curr. Environ. Health Rep. 2018, 5, 125–133. [Google Scholar] [CrossRef]
- Caioni, G.; d’Angelo, M.; Panella, G.; Merola, C.; Cimini, A.; Amorena, M. Environmentally relevant concentrations of triclocarban affect morphological traits and melanogenesis in zebrafish larvae. Aquat. Toxicol. 2021, 236, 105842. [Google Scholar] [CrossRef]
- Nagel, R. DarT: The embryo test with the zebrafish (Danio rerio) e a general model in ecotoxicology and toxicology. Altex 2002, 19 (Suppl. S1), 38–48. [Google Scholar]
- Gee, J.H. Ecological implications of buoyancy control in fish. In Fish Biomechanics; Webb, P.W., Weihs, D., Eds.; Praeger-Scientific: New York, NY, USA, 1984; pp. 140–176. [Google Scholar]
- Tao, Y.; Li, Z.; Yang, Y.; Jiao, Y.; Qu, J.; Wang, Y.; Zhang, Y. Effects of common environmental endocrine-disrupting chemicals on zebrafish behavior. Water Res. 2022, 208, 117826. [Google Scholar] [CrossRef]
- Gaaied, S.; Oliveira, M.; Domingues, I.; Banni, M. 2,4-Dichlorophenoxyacetic acid herbicide effects on zebrafish larvae: Development, neurotransmission and behavior as sensitive endpoints. Environ. Sci. Pollut. Res. 2020, 27, 3686–3696. [Google Scholar] [CrossRef]
- Levin, E.D.; Swain, H.A.; Donerly, S.; Linney, E. Developmental chlorpyrifos effects on hatchling zebrafish swimming behavior. Neurotoxicol. Teratol. 2004, 26, 719–723. [Google Scholar] [CrossRef]
- Parker, M.O.; Brock, A.J.; Sudwarts, A.; The, M.-T.; Combe, F.J.; Brennan, C.H. Developmental role of acetylcholinesterase in impulse control in zebrafish. Front. Behav. Neurosci. 2015, 9, 271. [Google Scholar] [CrossRef]
- Jeon, H.; Park, J.; Lee, S. Developmental toxicity of chlorpyrifos-methyl and its primary metabolite, 3,5,6-trichloro-2-pyridinol to early life stages of zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 2023, 249, 114352. [Google Scholar] [CrossRef]
- Verma, R.; Choudhary, P.R.; Nirmal, N.K.; Syed, F.; Verma, R. Neurotransmitter systems in zebrafish model as a target for neurobehavioural studies. Mater. Today Proc. 2022, 69, 1565–1580. [Google Scholar] [CrossRef]
- Horzmann, K.A.; Freeman, J.L. Zebrafish Get Connected: Investigating Neurotransmission Targets and Alterations in Chemical Toxicity. Toxics 2016, 4, 19. [Google Scholar] [CrossRef]
- Padilla, S.; Glaberman, S. The zebrafish (Danio rerio) model in toxicity testing. In An Introduction to Interdisciplinary Toxicology; Pope, C.N., Liu, J., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 525–532. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertoni, Í.; Sales, B.C.P.; Viriato, C.; Peixoto, P.V.L.; Pereira, L.C. Embryotoxicity Induced by Triclopyr in Zebrafish (Danio rerio) Early Life Stage. Toxics 2024, 12, 255. https://doi.org/10.3390/toxics12040255
Bertoni Í, Sales BCP, Viriato C, Peixoto PVL, Pereira LC. Embryotoxicity Induced by Triclopyr in Zebrafish (Danio rerio) Early Life Stage. Toxics. 2024; 12(4):255. https://doi.org/10.3390/toxics12040255
Chicago/Turabian StyleBertoni, Ítalo, Bianca Camargo Penteado Sales, Cristina Viriato, Paloma Vitória Lima Peixoto, and Lílian Cristina Pereira. 2024. "Embryotoxicity Induced by Triclopyr in Zebrafish (Danio rerio) Early Life Stage" Toxics 12, no. 4: 255. https://doi.org/10.3390/toxics12040255





