Application of β-Cyclodextrin Adsorbents in the Removal of Mixed Per- and Polyfluoroalkyl Substances
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
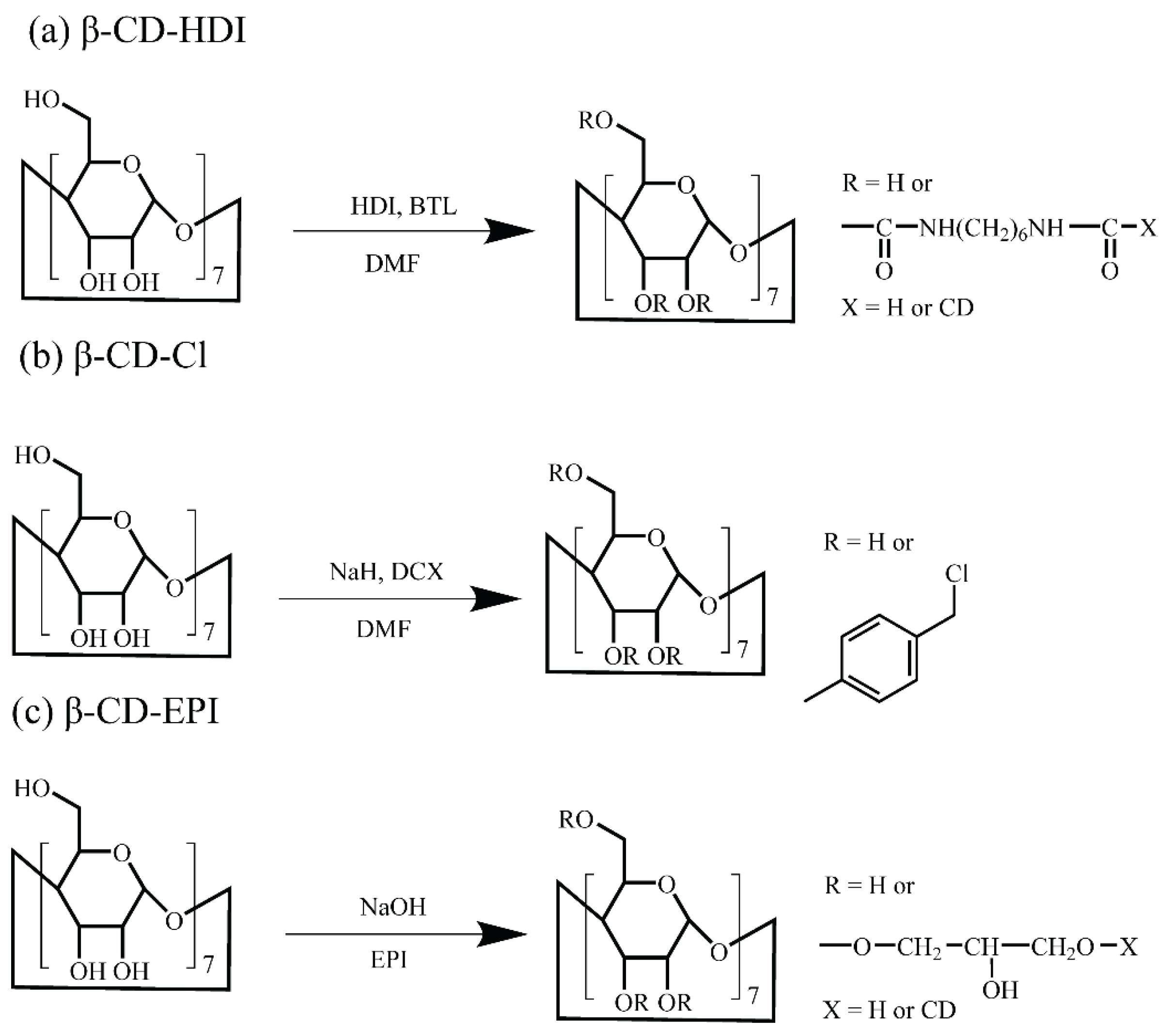
2.2. Design and Synthesis of Adsorbents
2.3. Adsorption Experiments
2.4. Sample Analysis
2.5. Quality Control
2.6. Analytical Methods
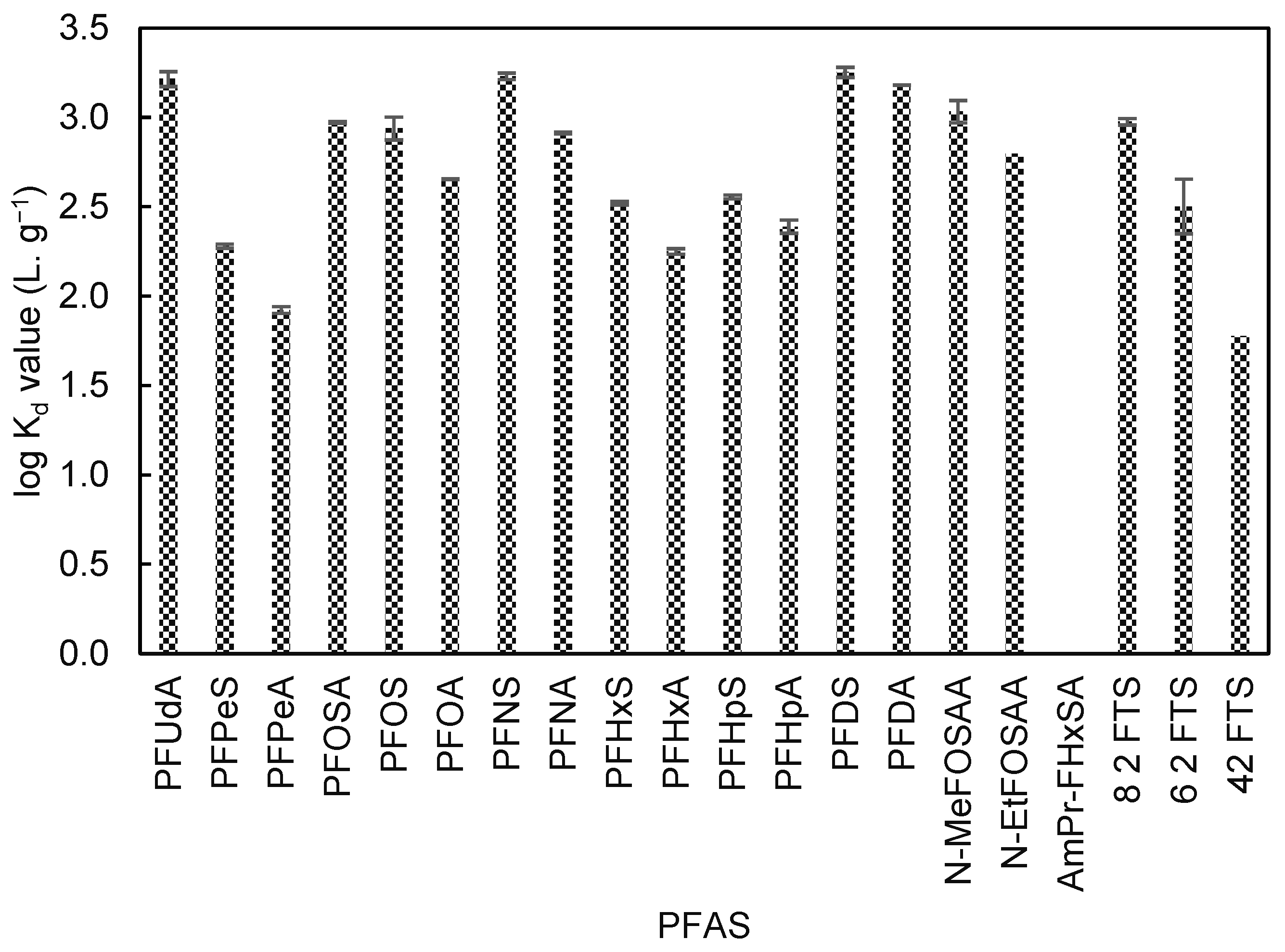
3. Results
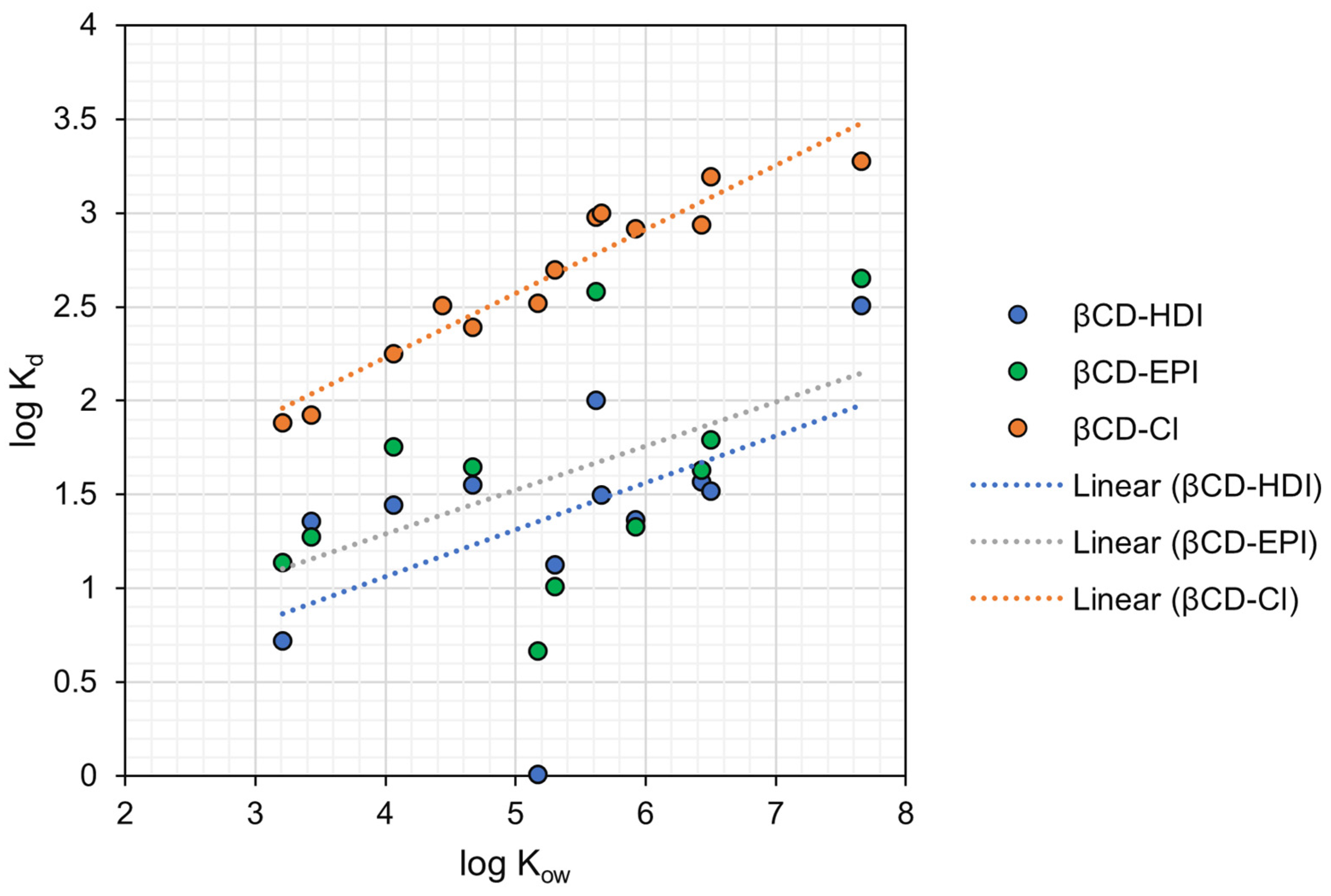
4. Discussion
4.1. Hydrophobic Interactions
4.2. Effect of Surface Charge
4.3. A Comparison between β-CD-Cl and Other Sorbents
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rahman, M.F.; Peldszus, S.; Anderson, W.B. Behaviour and fate of perfluoroalkyl and polyfluoroalkyl substances (PFASs) in drinking water treatment: A review. Water Res. 2014, 50, 318–340. [Google Scholar] [CrossRef]
- Kucharzyk, K.H.; Darlington, R.; Benotti, M.; Deeb, R.; Hawley, E. Novel treatment technologies for PFAS compounds: A critical review. J. Environ. Manag. 2017, 204, 757–764. [Google Scholar] [CrossRef]
- Ross, I.; McDonough, J.; Miles, J.; Storch, P.; Thelakkat Kochunarayanan, P.; Kalve, E.; Hurst, J.; Dasgupta, S.; Burdick, J. A review of emerging technologies for remediation of PFASs. Remediation 2018, 28, 101–126. [Google Scholar] [CrossRef]
- Barzen-Hanson, K.A.; Roberts, S.C.; Choyke, S.; Oetjen, K.; McAlees, A.; Riddell, N.; McCrindle, R.; Ferguson, P.L.; Higgins, C.P.; Field, J.A. Discovery of 40 Classes of Per- and Polyfluoroalkyl Substances in Historical Aqueous Film-Forming Foams (AFFFs) and AFFF-Impacted Groundwater. Environ. Sci. Technol. 2017, 51, 2047–2057. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Ulrich, B.A.; Chen, B.; Higgins, C.P. Sorption of Poly- and Perfluoroalkyl Substances (PFASs) Relevant to Aqueous Film-Forming Foam (AFFF)-Impacted Groundwater by Biochars and Activated Carbon. Environ. Sci. Technol. 2017, 51, 6342–6351. [Google Scholar] [CrossRef] [PubMed]
- McCleaf, P.; Englund, S.; Östlund, A.; Lindegren, K.; Wiberg, K.; Ahrens, L. Removal efficiency of multiple poly- and perfluoroalkyl substances (PFASs) in drinking water using granular activated carbon (GAC) and anion exchange (AE) column tests. Water Res. 2017, 120, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Ateia, M.; Attia, M.F.; Maroli, A.; Tharayil, N.; Alexis, F.; Whitehead, D.C.; Karanfil, T. Rapid Removal of Poly- and Perfluorinated Alkyl Substances by Poly(ethylenimine)-Functionalized Cellulose Microcrystals at Environmentally Relevant Conditions. Environ. Sci. Technol. Lett. 2018, 5, 764–769. [Google Scholar] [CrossRef]
- Murray, C.C.; Vatankhah, H.; McDonough, C.A.; Nickerson, A.; Hedtke, T.T.; Cath, T.Y.; Higgins, C.P.; Bellona, C.L. Removal of per- and polyfluoroalkyl substances using super-fine powder activated carbon and ceramic membrane filtration. J. Hazard. Mater. 2019, 366, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Rodowa, A.E.; Knappe, D.R.U.; Chiang, S.-Y.D.; Pohlmann, D.; Varley, C.; Bodour, A.; Field, J.A. Pilot scale removal of per- and polyfluoroalkyl substances and precursors from AFFF-impacted groundwater by granular activated carbon. Environ. Sci. Water Res. Technol. 2020, 6, 1083–1094. [Google Scholar] [CrossRef]
- Maimaiti, A.; Deng, S.; Meng, P.; Wang, W.; Wang, B.; Huang, J.; Wang, Y.; Yu, G. Competitive adsorption of perfluoroalkyl substances on anion exchange resins in simulated AFFF-impacted groundwater. Chem. Eng. J. 2018, 348, 494–502. [Google Scholar] [CrossRef]
- Liu, C.J.; Werner, D.; Bellona, C. Removal of per- And polyfluoroalkyl substances (PFASs) from contaminated groundwater using granular activated carbon: A pilot-scale study with breakthrough modeling. Environ. Sci. Water Res. Technol. 2019, 5, 1844–1853. [Google Scholar] [CrossRef]
- Gole, V.L.; Sierra-Alvarez, R.; Peng, H.; Giesy, J.P.; Deymier, P.; Keswani, M. Sono-chemical treatment of per- and poly-fluoroalkyl compounds in aqueous film-forming foams by use of a large-scale multi-transducer dual-frequency based acoustic reactor. Ultrason. Sonochem. 2018, 45, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Freire, L.; Abad-Fernández, N.; Sierra-Alvarez, R.; Hoppe-Jones, C.; Peng, H.; Giesy, J.P.; Snyder, S.; Keswani, M. Sonochemical degradation of perfluorinated chemicals in aqueous film-forming foams. J. Hazard. Mater. 2016, 317, 275–283. [Google Scholar] [CrossRef]
- Gomez-Ruiz, B.; Gómez-Lavín, S.; Diban, N.; Boiteux, V.; Colin, A.; Dauchy, X.; Urtiaga, A. Efficient electrochemical degradation of poly- and perfluoroalkyl substances (PFASs) from the effluents of an industrial wastewater treatment plant. Chem. Eng. J. 2017, 322, 196–204. [Google Scholar] [CrossRef]
- Schmidt, B.V.K.J.; Barner-Kowollik, C. Dynamic Macromolecular Material Design—The Versatility of Cyclodextrin-Based Host–Guest Chemistry. Angew. Chem. Int. Ed. 2017, 56, 8350–8369. [Google Scholar] [CrossRef] [PubMed]
- Meegoda, J.N.; Bezerra de Souza, B.; Casarini, M.M.; Kewalramani, J.A. A Review of PFAS Destruction Technologies. Int. J. Environ. Res. Public Health 2022, 19, 16397. [Google Scholar] [CrossRef] [PubMed]
- Ching, C.; Klemes, M.J.; Trang, B.; Dichtel, W.R.; Helbling, D.E. β-Cyclodextrin Polymers with Different Cross-Linkers and Ion-Exchange Resins Exhibit Variable Adsorption of Anionic, Zwitterionic, and Nonionic PFASs. Environ. Sci. Technol. 2020, 54, 12693–12702. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Ching, C.; Ling, Y.; Nasiri, M.; Klemes, M.J.; Reineke, T.M.; Helbling, D.E.; Dichtel, W.R. Cross-linker Chemistry Determines the Uptake Potential of Perfluorinated Alkyl Substances by β-Cyclodextrin Polymers. Macromolecules 2019, 52, 3747–3752. [Google Scholar] [CrossRef]
- Li, C.; Klemes, M.J.; Dichtel, W.R.; Helbling, D.E. Tetrafluoroterephthalonitrile-crosslinked β-cyclodextrin polymers for efficient extraction and recovery of organic micropollutants from water. J. Chromatogr. A 2018, 1541, 52–56. [Google Scholar] [CrossRef]
- Klemes, M.J.; Skala, L.P.; Ateia, M.; Trang, B.; Helbling, D.E.; Dichtel, W.R. Polymerized Molecular Receptors as Adsorbents to Remove Micropollutants from Water. Acc. Chem. Res. 2020, 53, 2314–2324. [Google Scholar] [CrossRef]
- Ling, Y.; Klemes, M.J.; Xiao, L.; Alsbaiee, A.; Dichtel, W.R.; Helbling, D.E. Benchmarking Micropollutant Removal by Activated Carbon and Porous β-Cyclodextrin Polymers under Environmentally Relevant Scenarios. Environ. Sci. Technol. 2017, 51, 7590–7598. [Google Scholar] [CrossRef] [PubMed]
- Klemes, M.J.; Ling, Y.; Ching, C.; Wu, C.; Xiao, L.; Helbling, D.E.; Dichtel, W.R. Reduction of a Tetrafluoroterephthalonitrile-β-Cyclodextrin Polymer to Remove Anionic Micropollutants and Perfluorinated Alkyl Substances from Water. Angew. Chem. 2019, 131, 12177–12181. [Google Scholar] [CrossRef]
- Xiao, L.; Ling, Y.; Alsbaiee, A.; Li, C.; Helbling, D.E.; Dichtel, W.R. β-Cyclodextrin Polymer Network Sequesters Perfluorooctanoic Acid at Environmentally Relevant Concentrations. J. Am. Chem. Soc. 2017, 139, 7689–7692. [Google Scholar] [CrossRef] [PubMed]
- Ching, C.; Lin, Z.-W.; Dichtel, W.R.; Helbling, D.E. Evaluating the Performance of Novel Cyclodextrin Polymer Granules to Remove Perfluoroalkyl Acids (PFAAs) from Water. ACS EST Eng. 2023, 3, 661–670. [Google Scholar] [CrossRef]
- Ateia, M.; Alsbaiee, A.; Karan, T.; Dichtel, W. Efficient PFAS Removal by Amine-Functionalized Sorbents: Critical Review of the Current Literature. Environ. Sci. Technol. Lett. 2019, 6, 688–695. [Google Scholar] [CrossRef]
- Wang, R.; Ching, C.; Dichtel, W.R.; Helbling, D.E. Evaluating the Removal of Per- and Poly fl uoroalkyl Substances from Contaminated Groundwater with Different Adsorbents Using a Suspect Screening Approach. Environ. Sci. Technol. Lett. 2020, 7, 954–960. [Google Scholar] [CrossRef]
- Wang, R.; Lin, Z.W.; Klemes, M.J.; Ateia, M.; Trang, B.; Wang, J.; Ching, C.; Helbling, D.E.; Dichtel, W.R. A Tunable Porous β-Cyclodextrin Polymer Platform to Understand and Improve Anionic PFAS Removal. ACS Cent. Sci. 2022, 8, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.W.; Shapiro, E.F.; Barajas-Rodriguez, F.J.; Gaisin, A.; Ateia, M.; Currie, J.; Helbling, D.E.; Gwinn, R.; Packman, A.I.; Dichtel, W.R. Trace Organic Contaminant Removal from Municipal Wastewater by Styrenic β-Cyclodextrin Polymers. Environ. Sci. Technol. 2023, 57, 19624–19636. [Google Scholar] [CrossRef] [PubMed]
- Karoyo, A.H.; Wilson, L.D. Tunable macromolecular-based materials for the adsorption of perfluorooctanoic and octanoic acid anions. J. Colloid Interface Sci. 2013, 402, 196–203. [Google Scholar] [CrossRef]
- Crini, G.; Morcellet, M. Synthesis and applications of adsorbents containing cyclodextrins. J. Sep. Sci. 2002, 25, 789–813. [Google Scholar] [CrossRef]
- Wang, Y.; Darling, S.B.; Chen, J. Selectivity of Per- and Polyfluoroalkyl Substance Sensors and Sorbents in Water. ACS Appl. Mater. Interfaces 2021, 13, 60789–60814. [Google Scholar] [CrossRef] [PubMed]
- Gagliano, E.; Sgroi, M.; Falciglia, P.P.; Vagliasindi, F.G.A.; Roccaro, P. Removal of poly- and perfluoroalkyl substances (PFAS) from water by adsorption: Role of PFAS chain length, effect of organic matter and challenges in adsorbent regeneration. Water Res. 2020, 171, 115381. [Google Scholar] [CrossRef] [PubMed]
- Shoji, M.; Satoshi, I.; Yasuyuki, M.; Keiko, T.; Kenjiro, H. Adsorption and Recovery of Nonionic Surfactants by b-Cyclodextrin Polymer. J. Colloid Interface Sci. 1996, 183, 118–123. [Google Scholar]
- Yamasaki, H.; Makihata, Y.; Fukunaga, K. Efficient phenol removal of wastewater from phenolic resin plants using crosslinked cyclodextrin particles. J. Chem. Technol. Biotechnol. 2006, 81, 1271–1276. [Google Scholar] [CrossRef]
- Li, X.; Zhou, M.; Jia, J.; Jia, Q. A water-insoluble viologen-based β-cyclodextrin polymer for selective adsorption toward anionic dyes. React. Funct. Polym. 2018, 126, 20–26. [Google Scholar] [CrossRef]
- Wilson, L.D.; Mohamed, M.H.; Headley, J.V. Surface area and pore structure properties of urethane-based copolymers containing β-cyclodextrin. J. Colloid Interface Sci. 2011, 357, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Zhou, W.; Du, K. Rigid crosslink improves the surface area and porosity of β-cyclodextrin beads for enhanced adsorption of flavonoids. Food Chem. 2024, 439, 138081. [Google Scholar] [CrossRef] [PubMed]
- Guardian, M.G.E.; Antle, J.P.; Vexelman, P.A.; Aga, D.S.; Simpson, S.M. Resolving unknown isomers of emerging per- and polyfluoroalkyl substances (PFASs) in environmental samples using COSMO-RS-derived retention factor and mass fragmentation patterns. J. Hazard. Mater. 2021, 402, 123478. [Google Scholar] [CrossRef]
- Wang, C.; Yan, B.; Munoz, G.; Sauvé, S.; Liu, J. Modified clays reduce leaching of per- and polyfluoroalkyl substances from AFFF-contaminated soils. AWWA Water Sci. 2021, 3, e1241. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abaie, E.; Kumar, M.; Kumar, N.; Sun, Y.; Guelfo, J.; Shen, Y.; Reible, D. Application of β-Cyclodextrin Adsorbents in the Removal of Mixed Per- and Polyfluoroalkyl Substances. Toxics 2024, 12, 264. https://doi.org/10.3390/toxics12040264
Abaie E, Kumar M, Kumar N, Sun Y, Guelfo J, Shen Y, Reible D. Application of β-Cyclodextrin Adsorbents in the Removal of Mixed Per- and Polyfluoroalkyl Substances. Toxics. 2024; 12(4):264. https://doi.org/10.3390/toxics12040264
Chicago/Turabian StyleAbaie, Elham, Manish Kumar, Naveen Kumar, Yilang Sun, Jennifer Guelfo, Yuexiao Shen, and Danny Reible. 2024. "Application of β-Cyclodextrin Adsorbents in the Removal of Mixed Per- and Polyfluoroalkyl Substances" Toxics 12, no. 4: 264. https://doi.org/10.3390/toxics12040264
APA StyleAbaie, E., Kumar, M., Kumar, N., Sun, Y., Guelfo, J., Shen, Y., & Reible, D. (2024). Application of β-Cyclodextrin Adsorbents in the Removal of Mixed Per- and Polyfluoroalkyl Substances. Toxics, 12(4), 264. https://doi.org/10.3390/toxics12040264










